Abstract
Omega-3 fatty acids (O3FAs) are associated with lower cardiovascular disease (CVD) risk in adults. However, this association in patients with end-stage renal disease (ESRD) remains controversial prompting the need for investigation into the role of O3FAs on serum lipids and vascular inflammation markers. The present meta-analysis summarized the effects of O3FA supplementation on serum lipids and vascular inflammatory markers in patients with ESRD. PubMed, EmBase, and the Cochrane Library were searched to identify randomized controlled trials (RCTs) focused on serum lipids and vascular inflammation markers in patients with ESRD. Standard mean differences (SMD) were used to measure the effect of O3FA supplementation on serum lipids and vascular inflammatory markers. The final pooled analysis included 20 RCTs involving 1,461 patients with ESRD. The results indicated that O3FA supplementation reduced TG by 0.61, LDL by 0.35 and CRP by 0.56. However, O3FA had no significant effect on TC, HDL, albumin, hemoglobin, homocysteine, DBP, glucose, lipoprotein(a), and ferritin. O3FA supplementation is associated with lower several serum lipids and vascular inflammation markers in patients with ESRD.
Patients with end stage renal disease (ESRD) are reported to have increasing mortality attributed to cardiovascular disease (CVD). The incidence of CVD in patients undergoing dialysis is about 3 to 45 times higher than in the general population, accounting for approximately 50% of deaths1,2,3. Previous observational studies investigated the role of omega-3 fatty acid (O3FAs) in reducing the CVD risk4,5,6. Although the mechanism of action is uncertain, O3FA-derived eicosanoids may affect physiological processes including calcium transport across cell membranes, angiogenesis, apoptosis, cell proliferation, and immune cell function, all of which strongly correlate with the risk of CVD7,8,9. Observational studies often misjudge the relationships due to lack of evidence on causality. Similarly, the effects of O3FA supplementation in patients with ESRD are limited and inconclusive.
A previous meta-analysis10 of RCTs has indicated that O3FA supplementation significantly lowered the serum triglyceride (TG) levels, with no significant changes in low-density lipoprotein (LDL), total cholesterol (TC), or high-density lipoprotein (HDL) levels. Furthermore, the effects of O3FA supplementation on reducing CVD risk factors in patients with ESRD were not confirmed by any RCT. Finally, the potential role of O3FA as treatment in patients with ESRD has not been investigated in the previous meta-analysis10.
Several RCTs11,12,13,14,15 have demonstrated that supplementation of O3FA may lower CVD risk factors, whereas other RCTs have revealed inconsistent results16,17,18,19,20,21,22,23,24,25,26,27,28,29. Hence, in this meta-analysis, RCTs were used to determine the role of O3FA supplementation on associated serum lipids and vascular inflammation markers in patients with ESRD, and assess the role of O3FA supplementation in specific subpopulations.
Results
The flow chart of study selection process is outlined in Fig. 1. During the initial electronic search, 285 manuscripts were identified, of which 243 were excluded including duplicates, study with other design, and unrelated studies. The full text of 42 manuscripts was retrieved, and after detailed evaluation, 23 RCTs including 20 datasets were finally selected for meta-analysis11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33. No new eligible studies were identified in manual search. The baseline characteristics of patients (n = 1,461) with ESRD are presented in Table 1. The follow-up duration of RCTs ranged from 2 to 6 months, and the number of patients enrolled in individual RCT ranged from 15 to 206. Ten trials were conducted in Asia12,14,15,16,24,25,26,27,31,33, seven in America11,18,19,20,28,30,32, two in Europe17,23,29, and one in Africa13. The quality of RCTs was assessed using the Jadad score34 (Table 1), and the scores more than 3 were regarded as high quality trials. Two trials had a score of 517,18, six trials scored 412,16,19,31, six trials scored 311,20,23,24,26,28, four trials scored 214,15,21,25, and the remaining two trials scored 113,27.
Figure 1. Flow diagram of the literature search and trials selection process.
Table 1. Baseline characteristics of studies included in the systematic review and meta-analysis.
| Study | Country | Sample size | Mean age | Percentage male (%) | BMI (kg/m2) | Mean SBP (mm Hg) | History of CVD (%) | History of DM (%) | Disease status | Intervention | Control | Main outcomes | Duration of follow- up (months) | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Khosroshahi16 | Iran | 88 | 50.1 | 72.7 | NA | NA | NA | NA | Hemodialysis | Omega-3 (3 g/day) | Placebo | TG, TC, LDL, HDL, homocystein; hemoglobin | 2 | 4 |
| Mat Daud11 | USA | 56 | 58.5 | 50.8 | 27.7 | NA | NA | 20.0 | Hemodialysis (serum albumin ≤3.9 g/dL) | Omega-3 (2.4 g/day) | Placebo | TC, HDL, LDL, TG; albumin; hemoglobin; CRP | 6 | 3 |
| Kooshki12 | Iran | 34 | 50.0 | 61.8 | NA | NA | NA | 23.5 | Hemodialysis | Omega-3 (2.08 g/day) | Placebo | TG, TC, LDL, HDL; Lipoprotein(a); Hemoglobin; | 2.5 | 4 |
| Bowden18 | USA | 87 | 60.0 | 51.7 | NA | NA | NA | 59.8 | ESRD treated with hemodialysis | Omega-3 (1.0 g/day) | Placebo (corn oil) | HDL, LDL, TG, TC, homocysteine | 6 | 5 |
| Bouzidi13 | Algeria | 40 | 61.0 | 55.0 | 24.2 | 125 | NA | NA | Chronic renal failure and dyslipidemia | Omega-3 (2.1 g/day) | Counsel monitoring | TG, TC, HDL, LDL, Albumin | 3 | 1 |
| Lok19 | Canada | 196 | 62.9 | 50.0 | NA | NA | 33.7 | 52.6 | Stage 5 chronic kidney disease | Omega-3 (4.0 g/day) | Placebo | SBP and DBP | 6 | 4 |
| Lemos20 | Brazil | 145 | 57.0 | 58.6 | 24.6 | NA | NA | 48.3 | Hemodialysis | Omega-3 (1.0 g/day) | Placebo | TC, HDL, LDL, CRP, Hemoglobin, | 4 | 3 |
| Svensson and Rasmussen 200817,22 | Denmark | 206 | 67.0 | 64.5 | 24.4 | 151 | 100 | 23.8 | Hemodialysis and CVD | Omega-3 (1.7 g/day) | Placebo (olive oil) | TC, LDL, HDL, TG, Homocysteine | 3 | 5 |
| Svensson 200423,29 | Denmark | 58 | 59.0 | 67.2 | 28.0 | 128 | NA | NA | CRF | Omega-3 (2.4 g/day) | Placebo (olive oil) | TC, LDL, HDL, TG, DBP, SBP, Lipoprotein(a), CRP | 2 | 3 |
| Taziki24 | Iran | 33 | 53.8 | 33.3 | 24.0 | NA | NA | NA | Non-diabetic patients on hemodialysis | Omega-3 (2.0 g/day) | Control group who did not receive this drug | TG, TC, HDL, and LDL | 3 | 3 |
| Khajehdehi25 | Iran | 60 | 32.4 | 31.0 | NA | 126 | NA | NA | Hemodialysis | Omega-3 (1.5 g/day) | Placebo | TG, TC, LDL, and HDL | 2 | 2 |
| Chang26 | Korea | 50 | 64.5 | 54.0 | 21.2 | NA | 48.0 | NA | ESRD patients on hemodialysis | Omega-3 (0.6 g/day) | Placebo | TC, Albumin | 3 | 3 |
| Lee27 | Korea | 15 | 62.1 | 33.3 | NA | NA | NA | 73.3 | Hemodialysis | Omega-3 (2.4 g/day) | Placebo (olive oil) | TC, TG, HDL, LDL, glucose, Hemoglobin, Albumin, CRP, Ferritin | 3 | 1 |
| Khalatbari Soltani14 | Iran | 30 | 54.3 | 53.3 | 25.8 | NA | NA | NA | Hemodialysis and dyslipidemia | Omega-3 (13.5 g/day) | Placebo | TC, TG, LDL, HDL, CRP | 2 | 2 |
| Ando15 | Japan | 38 | 52.5 | 86.5 | 21.0 | NA | NA | 42.1 | Hemodialysis | Omega-3 (1.8 g/day) | Placebo | TC, TG, and HDL | 3 | 2 |
| Saifullah28 | USA | 23 | 57.7 | 78.3 | NA | NA | NA | 39.1 | Hemodialysis | Omega-3 (1.3 g/day) | Placebo | LDL, TC, TG, HDL, glucose, Albumin, CRP | 3 | 3 |
| Beavers21,30 | USA | 33 | 60.2 | 42.4 | NA | NA | NA | NA | ESRD | Omega-3 (1.56 g/day) | Placebo (corn-oil) | Lipoprotein(a), HDL, LDL, TG, TC, Homocysteine, CRP | 6 | 2 |
| Gharekhani31 | Iran | 45 | 56.8/57.2 | 52.0/60.0 | NA | NA | NA | 42.2 | Hemodialysis patients | Omega-3 (0.9 g/day) | Placebo | CRP; Ferritin; Albumin | 4 | 4 |
| Hung32 | USA | 34 | 50.0/53.0 | 82.0/77.0 | 30.2 | NA | NA | 3.0 | Hemodialysis patients | Omega-3 (2.9 g/day) | Placebo | CRP, Albumin | 3 | 4 |
| Naini33 | Iran | 90 | 57.7/59.4 | 53.0/60.0 | 25.3 | 144 | NA | 44.4 | Continuous ambulatory peritoneal dialysis patients | Omega-3 (3.0 g/day) | Placebo | SBP, DBP, TG, TC, LDL, HDL | 2 | 4 |
TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; NA, not applicable; ESRD, end stage renal disease; SBP, systolic blood pressure; DSP, diastolic blood pressure; CVD, cardiovascular disease; DM: diabetes mellitus; CKD, chronic kidney disease; CRF, chronic renal failure; CRP: C-Reactive Protein.
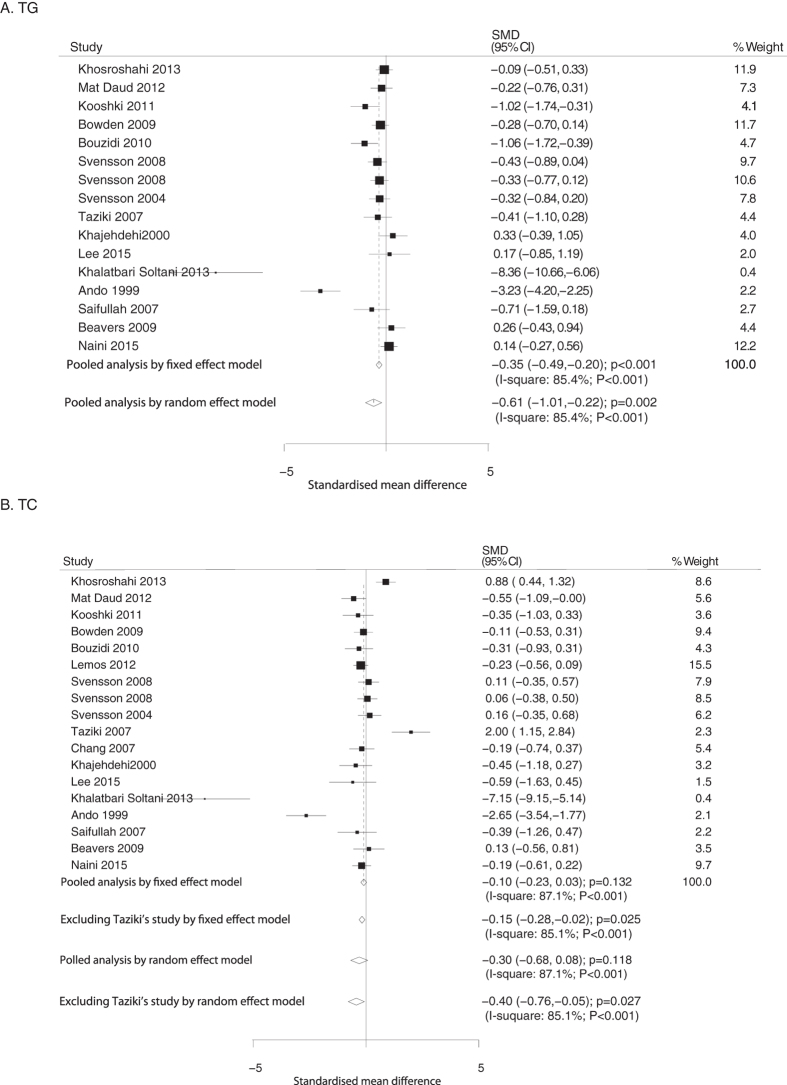
Data relating the effect of O3FA supplementation on TG were collected from 16 RCTs. Overall TG level was significantly reduced (fixed model: SMD, −0.35, 95%CI: −0.49 to −0.20, P < 0.001; random model: SMD, −0.61, 95% CI: −1.01 to −0.22, P = 0.002; Fig. 2A) in patients treated with O3FA. The extent of significant effect of O3FA was noted across all RCTs regardless of substantial heterogeneity (P < 0.001), and the conclusion was not affected after sequential exclusion of each study from pooled analyses (Table S1).
Figure 2.
Mean changes in triglyceride (A) and total cholesterol (B) based on O3FA supplementation.
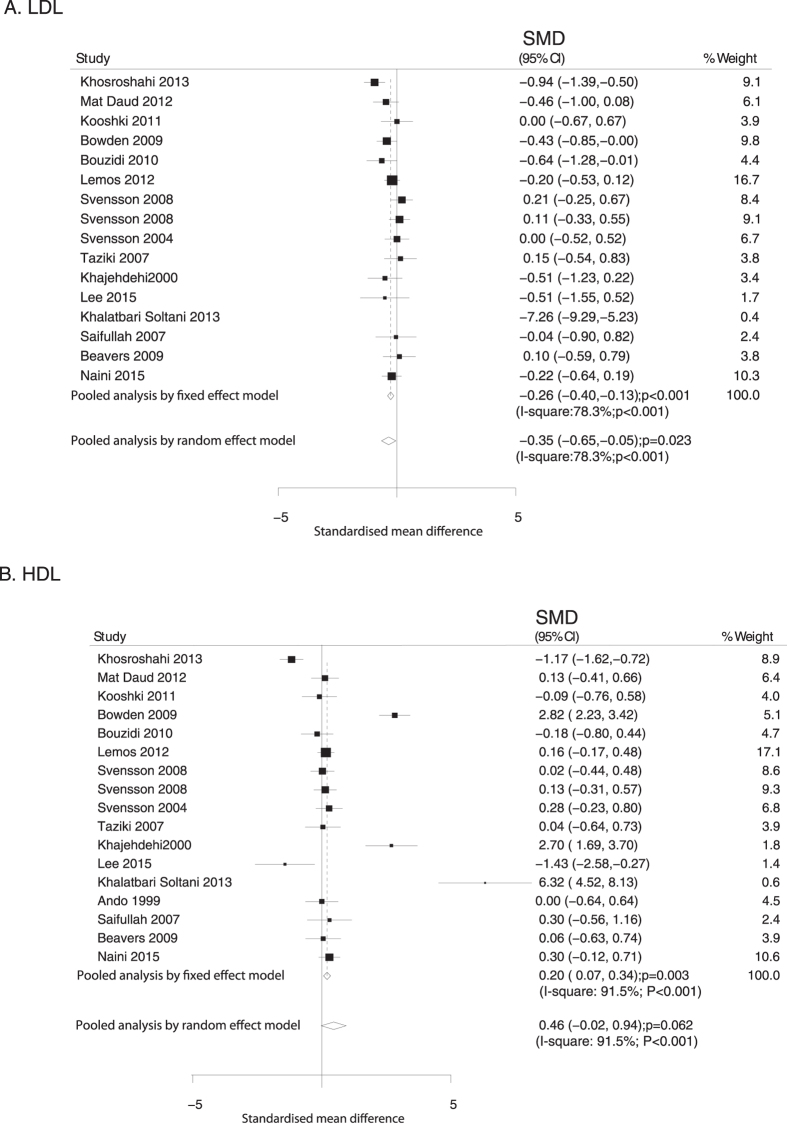
Data relating the effect of O3FA supplementation on TC were collected from 17 RCTs. No significant difference in TC level was observed in patients treated with O3FA supplementation compared to control (fixed model: SMD, −0.10, 95%CI: −0.23 to 0.03, P = 0.132; random model: SMD, −0.30, 95%CI: −0.68 to 0.08, P = 0.118; Fig. 2B). Furthermore, substantial heterogeneity was noted across the trials (P < 0.001). The Taziki’s study24 was excluded from meta-analysis as younger non-diabetic patients on maintenance hemodialysis alone were enrolled in this trial. It was found that O3FA supplementation was significantly associated with reduced TC in patients with ESRD (fixed model: SMD: −0.15, 95%CI: −0.28 to −0.02, P = 0.025; random model: SMD, −0.40, 95%CI: −0.76 to −0.05, P = 0.027; Fig. 2B). Data relating the effect of O3FA supplementation on LDL and HDL were collected from 15 and 16 trials, respectively. Overall, the O3FA supplementation was associated with lower level of LDL (fixed model: SMD, −0.26, 95%CI: −0.40 to −0.13, P < 0.001; random model: SMD, −0.35, 95%CI: −0.65 to −0.05, P = 0.023; Fig. 3A). Furthermore, the pooled analysis using the fixed model revealed that O3FA supplementation was associated with a higher level of HDL (SMD: 0.20; 95%CI: 0.07 to 0.34; P = 0.003), whereas no significant difference was observed by random model (SMD: 0.46; 95%CI: −0.02 to 0.94; P = 0.062; Fig. 3B). Substantial heterogeneity was observed in the magnitude of the effect across the studies (P < 0.001), the Khosroshahi’s study was excluded from pooled analysis and we noted O3FA supplementation has little or no significant effect on LDL; furthermore, when excluding Khosroshahi or Lee’s study, O3FA supplementation significant increased the level of HDL (Table S1).
Figure 3.
Mean changes in LDL (A) and HDL (B) based on omega-3 fatty acid supplementation.
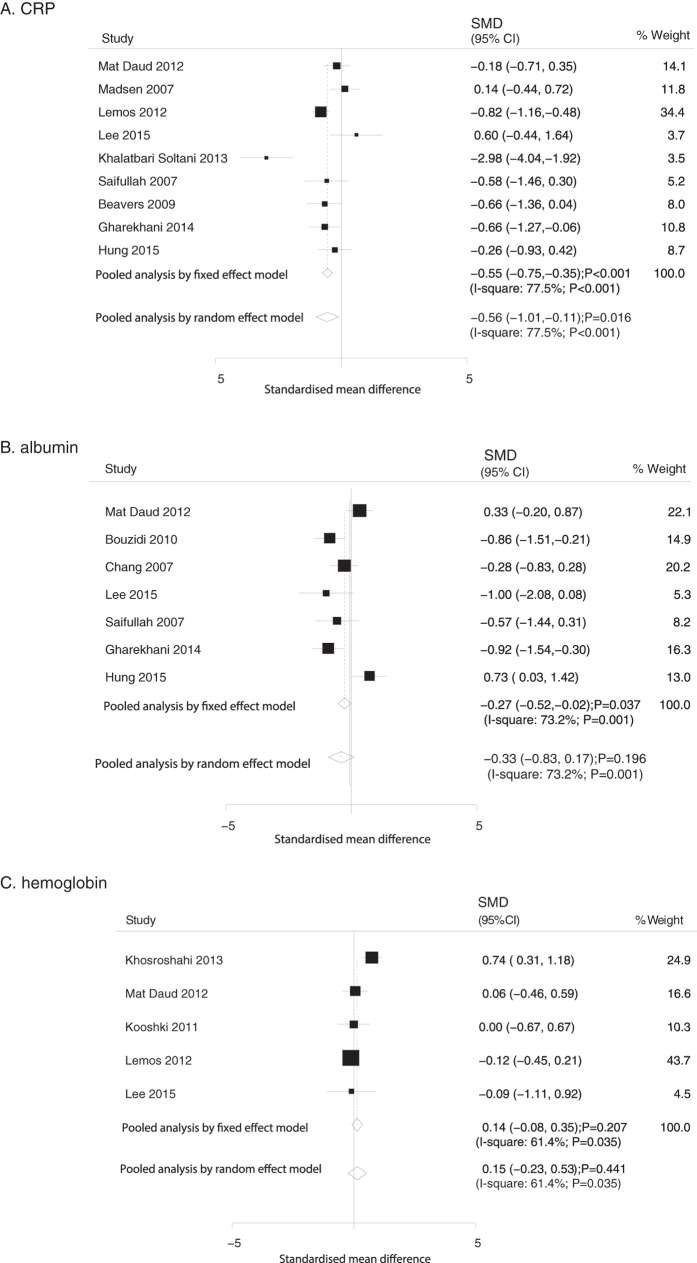
Data relating the effect of O3FA supplementation on CRP, albumin, and hemoglobin were collected from 9, 7 and 5 trials, respectively. We noted that O3FA supplementation significantly reduced the level of CRP (fixed model: SMD, −0.55, 95%CI: −0.75 to −0.35, P < 0.001; random model: SMD, −0.56, 95%CI: −1.01 to −0.11, P = 0.016; Fig. 4A). Although the summary results using fixed model indicated that O3FA supplementation was significantly associated with a reduction of albumin levels (SMD: −0.27; 95%CI: −0.52 to −0.02; P = 0.037), no significant differences were seen between O3FA and control using the random model (SMD: −0.33; 95%CI: −0.83 to 0.17; P = 0.196; Fig. 4B). In addition, no significant difference were detected in hemoglobin levels (fixed model: SMD, 0.14, 95%CI: −0.08 to 0.35, P = 0.207; random model: SMD, 0.15, 95%CI: −0.23 to 0.53, P = 0.441; Fig. 4C).
Figure 4.
Mean changes in CRP (A), albumin (B), and hemoglobin (C) based on omega-3 fatty acid supplementation.
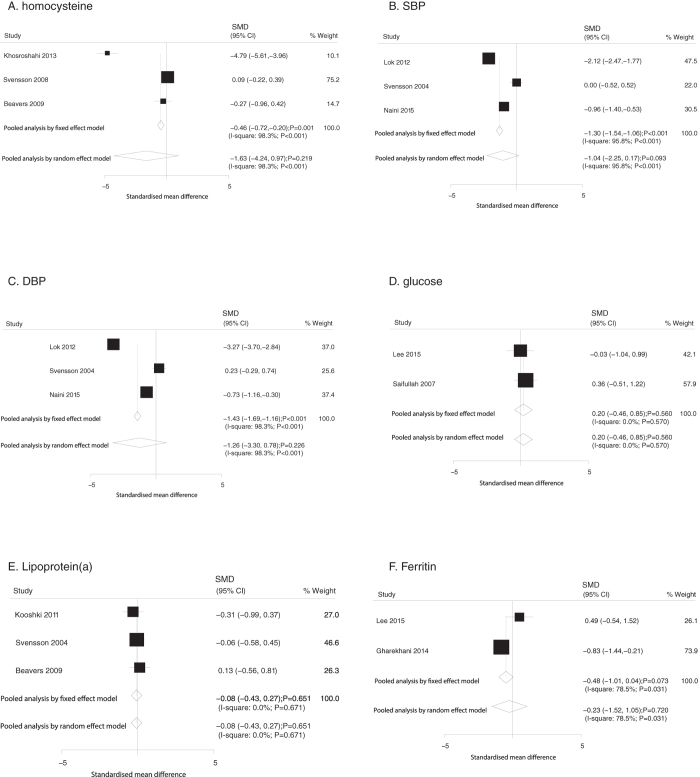
Similarly, data relating the effect of O3FA supplementation on homocysteine, SBP, DBP, glucose, lipoprotein(a), and ferritin were collected from 3, 3, 3, 2, 3, 2 trials, respectively. Overall, O3FA supplementation was not associated with altered levels of homocysteine (SMD: −1.63; 95%CI: −4.24 to 0.97; P = 0.219; Fig. 5A), SBP (SMD: −1.04; 95%CI: −2.25 to 0.17; P = 0.093; Fig. 5B), or DBP (SMD: −1.26; 95%CI: −3.30 to 0.78; P = 0.226; Fig. 5C) using the random model, whereas patients supplemented with O3FA showed significantly reduced levels of homocysteine (SMD: −0.46; 95%CI: −0.72 to −0.20; P = 0.001; Fig. 5A), SBP (SMD: −1.30; 95%CI: −1.54 to −1.06; P < 0.001; Fig. 5B), and DBP (SMD: −1.43; 95%CI: −1.69 to −1.16; P < 0.001; Fig. 5C) using the fixed model. Furthermore, O3FA supplementation had no or little effect on glucose (fixed model: SMD, 0.20; 95%CI: −0.46 to 0.85, P = 0.560; random model: SMD, 0.20; 95%CI: −0.46 to 0.85, P = 0.560; Fig. 5D), lipoprotein(a) (fixed model: SMD, −0.08, 95%CI: −0.43 to 0.27, P = 0.651; random model: SMD, −0.08, 95%CI: −0.43 to 0.27, P = 0.651; Fig. 5E), or ferritin (fixed model: SMD, −0.48, 95%CI: −1.01 to 0.04, P = 0.073; random model: SMD, −0.23, 95%CI: −1.52 to 1.05, P = 0.720; Fig. 5F) levels.
Figure 5.
Mean changes in homocysteine (A), SBP (B), DBP (C), glucose (D), lipoprotein(a) (E), and ferritin (F) based on O3FA supplementation.
In the subgroup analysis, we stratified studies into groups to evaluate the sources of heterogeneity and explore the effect of O3FA in specific subpopulations (Table 2). First, O3FA supplementation was associated with lower TG level in multiple subsets except that the duration of follow-up was greater than 3 months. Second, O3FA supplementation significantly reduced the levels of TC in patients with BMI greater than 25.0. Third, O3FA supplementation was not associated with altered levels of LDL if the study was published before 2010, and the patients were residents of other countries, with a mean age greater than 60 years, BMI less than 25.0, and the trial showed a high quality. Forth, O3FA supplementation was associated with increased level of HDL if patients’ BMI was greater than 25.0. In addition, publication year, country, and dose of O3FA contributed significant heterogeneity between subgroups to LDL and HDL; Age and follow-up duration acted as the source of heterogeneity between subgroup for the effects of HDL; Significant heterogeneity between subgroups for TG was based on BMI; Study quality produced significant heterogeneity between subgroups for TC (Table 2).
Table 2. Subgroup analysis for TG, TC, LDL, and HDL.
| Outcomes | Group | SMD and 95%CI | P value | Heterogeneity (%) | P value for heterogeneity | P value for heterogeneity between subgroups |
|---|---|---|---|---|---|---|
| TG | Publication year | |||||
| 2010 or after | −0.90 (−1.68 to −0.12) | 0.024 | 90.4 | <0.001 | 0.641 | |
| Before 2010 | −0.49 (−0.94 to −0.05) | 0.031 | 80.2 | <0.001 | ||
| Country | ||||||
| Asia | −1.14 (−2.04 to −0.24) | 0.013 | 92.6 | <0.001 | 0.921 | |
| Other | −0.36 (−0.57 to −0.15) | 0.001 | 16.8 | 0.298 | ||
| Age | ||||||
| 60 years or greater | −0.33 (−0.63 to −0.02) | 0.034 | 41.5 | 0.129 | 0.885 | |
| <60 years | −0.95 (−1.60 to −0.29) | 0.005 | 90.4 | <0.001 | ||
| BMI | ||||||
| 25 kg/m2 or greater | −1.37 (−2.65 to −0.09) | 0.035 | 94.1 | <0.001 | 0.013 | |
| <25 kg/m2 | −1.00 (−1.76 to −0.24) | 0.010 | 87.2 | <0.001 | ||
| Dose of O3FA | ||||||
| 3 g per day or greater | −1.95 (−3.72 to −0.17) | 0.031 | 96.1 | <0.001 | 0.063 | |
| <3 g per day | −0.51 (−0.86 to −0.16) | 0.004 | 75.3 | <0.001 | ||
| Follow-up duration | ||||||
| >3 months | −0.16 (−0.46 to 0.14) | 0.289 | 0.0 | 0.414 | 0.167 | |
| 3 months or less | −0.79 (−1.29 to −0.29) | 0.002 | 87.9 | <0.001 | ||
| Study quality | ||||||
| High | −0.27 (−0.52 to −0.01) | 0.041 | 45.6 | 0.102 | 0.072 | |
| Low | −1.00 (−1.75 to −0.24) | 0.010 | 90.0 | <0.001 | ||
| TC | Publication year | |||||
| 2010 or after | −0.60 (−1.24 to 0.04) | 0.067 | 90.2 | <0.001 | 0.444 | |
| Before 2010 | −0.11 (−0.59 to 0.37) | 0.644 | 84.9 | <0.001 | ||
| Country | ||||||
| Asia | −0.71 (−1.60 to 0.18) | 0.117 | 93.6 | <0.001 | 0.800 | |
| Other | −0.11 (−0.27 to 0.05) | 0.177 | 0.0 | 0.554 | ||
| Age | ||||||
| 60 years or greater | −0.06 (−0.26 to 0.14) | 0.561 | 0.0 | 0.808 | 0.625 | |
| <60 years | −0.53 (−1.16 to 0.11) | 0.106 | 92.2 | <0.001 | ||
| BMI | ||||||
| 25 kg/m2 or greater | −1.34 (−2.58 to −0.10) | 0.034 | 93.9 | <0.001 | 0.257 | |
| <25 kg/m2 | −0.16 (−0.78 to 0.47) | 0.621 | 89.7 | <0.001 | ||
| Dose of O3FA | ||||||
| 3 g per day or greater | −1.68 (−3.66 to 0.31) | 0.098 | 96.9 | <0.001 | 0.071 | |
| <3 g per day | −0.20 (−0.52 to 0.12) | 0.223 | 78.2 | <0.001 | ||
| Follow-up duration | ||||||
| >3 months | −0.21 (−0.43 to 0.01) | 0.057 | 0.0 | 0.454 | 0.209 | |
| 3 months or less | −0.39 (−0.91 to 0.13) | 0.142 | 89.8 | <0.001 | ||
| Study quality | ||||||
| High | 0.08 (−0.26 to 0.43) | 0.635 | 70.3 | 0.005 | 0.004 | |
| Low | −0.59 (−1.18 to 0.00) | 0.051 | 89.7 | <0.001 | ||
| LDL | Publication year | |||||
| 2010 or after | −0.78 (−1.33 to −0.22) | 0.006 | 86.9 | <0.001 | 0.003 | |
| Before 2010 | −0.05 (−0.24 to 0.14) | 0.612 | 0.0 | 0.453 | ||
| Country | ||||||
| Asia | −0.88 (−1.64 to −0.12) | 0.024 | 88.8 | <0.001 | 0.019 | |
| Other | −0.15 (−0.34 to 0.03) | 0.108 | 18.6 | 0.277 | ||
| Age | ||||||
| 60 years or greater | −0.15 (−0.44 to 0.15) | 0.339 | 41.2 | 0.131 | 0.130 | |
| <60 years | −0.52 (−0.98 to −0.06) | 0.028 | 84.5 | <0.001 | ||
| BMI | ||||||
| 25 kg/m2 or greater | −1.36 (−2.57 to −0.15) | 0.028 | 93.6 | <0.001 | 0.110 | |
| <25 kg/m2 | −0.06 (−0.33 to 0.20) | 0.637 | 34.8 | 0.189 | ||
| Dose of O3FA | ||||||
| 3 g per day or greater | −2.25 (−3.96 to −0.54) | 0.010 | 95.7 | <0.001 | 0.001 | |
| <3 g per day | −0.15 (−0.30 to −0.00) | 0.046 | 1.2 | 0.434 | ||
| Follow-up duration | ||||||
| >3 months | −0.28 (−0.50 to −0.05) | 0.014 | 0.0 | 0.514 | 0.885 | |
| 3 months or less | −0.44 (−0.88 to −0.01) | 0.047 | 83.5 | <0.001 | ||
| Study quality | ||||||
| High | −0.23 (−0.58 to 0.12) | 0.206 | 70.7 | 0.004 | 0.787 | |
| Low | −0.52 (−1.02 to −0.02) | 0.040 | 82.7 | <0.001 | ||
| HDL | Publication year | |||||
| 2010 or after | 0.20 (−0.49 to 0.88) | 0.575 | 91.4 | <0.001 | <0.001 | |
| Before 2010 | 0.68 (−0.00 to 1.36) | 0.051 | 91.1 | <0.001 | ||
| Country | ||||||
| Asia | 0.63 (−0.33 to 1.58) | 0.197 | 93.5 | <0.001 | 0.012 | |
| Other | 0.41 (−0.12 to 0.94) | 0.131 | 89.2 | <0.001 | ||
| Age | ||||||
| 60 years or greater | 0.28 (−0.70 to 1.26) | 0.578 | 93.8 | <0.001 | 0.044 | |
| <60 years | 0.53 (−0.03 to 1.09) | 0.066 | 90.4 | <0.001 | ||
| BMI | ||||||
| 25 kg/m2 or greater | 1.27 (0.12 to 2.41) | 0.030 | 93.0 | <0.001 | 0.067 | |
| <25 kg/m2 | 0.07 (−0.12 to 0.27) | 0.471 | 0.0 | 0.957 | ||
| Dose of O3FA | ||||||
| 3 g per day or greater | 1.50 (−0.62 to 3.62) | 0.165 | 97.3 | <0.001 | 0.005 | |
| <3 g per day | 0.35 (−0.10 to 0.80) | 0.124 | 87.9 | <0.001 | ||
| Follow-up duration | ||||||
| >3 months | 0.78 (−0.41 to 1.98) | 0.200 | 95.4 | <0.001 | <0.001 | |
| 3 months or less | 0.34 (−0.19 to 0.86) | 0.206 | 89.1 | <0.001 | ||
| Study quality | ||||||
| High | 0.33 (−0.62 to 1.27) | 0.497 | 95.5 | <0.001 | 0.714 | |
| Low | 0.52 (−0.04 to 1.07) | 0.069 | 87.2 | <0.001 | ||
TG, triglyceride; TC, total cholesterol; LDL, low-density lipoprotein; HDL, high-density lipoprotein; CI, confidence interval; SMD, standard mean difference; BMI, body mass index; O3FA, omega-3 fatty acid.
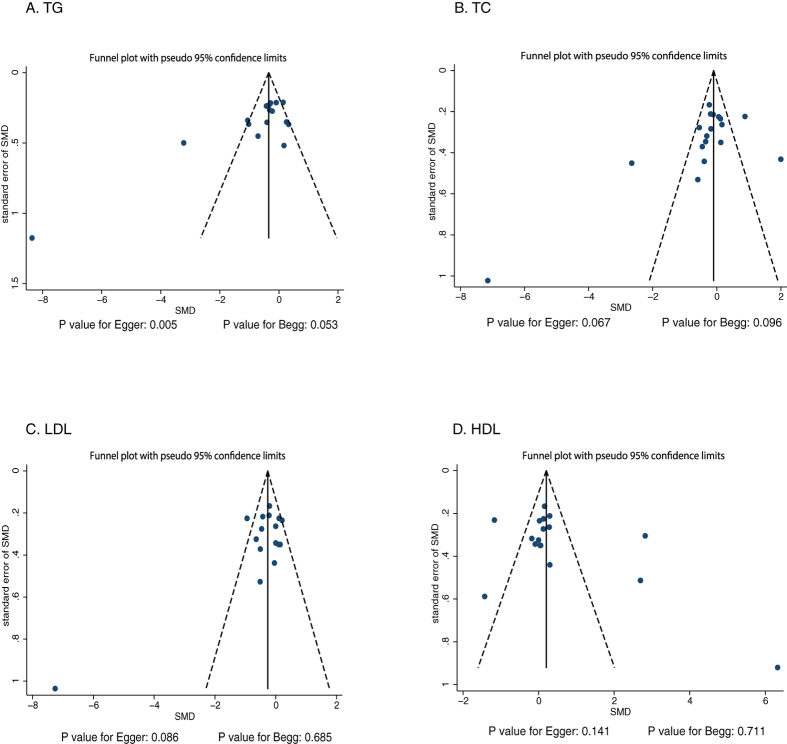
Review of the funnel plots could not rule out the potential for publication bias for TG, TC, LDL, and HDL (Fig. 6). The Egger and Begg tests revealed no publication bias for TC (P values: 0.067 and 0.096, respectively), LDL (P values: 0.086 and 0.685, respectively), and HDL (P values: 0.141 and 0.711, respectively). Although the Begg test showed no evidence of publication bias for TG (P value: 0.053), the Egger test showed potential evidence of publication bias for TG (P value: 0.005). The conclusions were not changed after adjustment for publication bias by using the trim and fill method.
Figure 6. Funnel plot for TG, TC, LDL, and HDL.
Discussion
In the present meta-analysis, the effects of O3FA supplementation on serum lipids and vascular inflammation markers such as TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin in patients with ESRD were investigated. In this comprehensive systemic review, 1,461 patients with ESRD in 20 RCTs were included. In the absence of statistical heterogeneity (I2 < 50%), the fixed-effect model was used, otherwise the random effects model was applied. Therefore, the meta-analysis indicated that O3FA supplementation significantly reduced TG, LDL and CRP levels, whereas no significant effect was found in TC, HDL, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a), and ferritin levels using random models. According to sensitivity analysis, O3FA supplementation was beneficial in regulating TC. Subgroup analyses revealed that O3FA supplementation significantly reduced the TG and LDL levels in several subpopulations. Furthermore, O3FA supplementation was associated with a reduced TC, LDL and increased HDL levels in patients with BMI greater than 25.0.
The methodological evaluation of each included study was limited by randomization, blinding, allocation concealment, withdrawals and dropouts, and use of intention-to-treat analysis. Our meta-analysis of RCTs provides clear randomization, blinding, and allocation concealment. Although most trials reported withdrawals and dropouts, and use of intention-to-treat analysis, the other forms of bias contributed to heterogeneity in every study. Ultimately, considering the unsatisfactory quality of included studies, we critically analyzed our recommendations for the treatment of patients with ESRD.
A previous meta-analysis10 suggested that O3FA significantly lowered the serum TG levels. However, there was no significant effect on LDL, TC, and HDL. The inherent limitations of the previous review were as follows several important factors such as CRP, homocysteine, SBP, DBP, glucose, albumin, hemoglobin, lipoprotein(a), and ferritin were not summarized, and the study failed to reveal the effect of O3FA supplementation on serum lipids and vascular inflammation markers in several specific subpopulations. Furthermore, a previous meta-analysis35,36 evaluated the effect of O3FA supplementation on the risk of major cardiovascular events. The effects of treatment on serum lipids and vascular inflammation markers remain unclear. Hence, a comprehensive systematic review and meta-analysis was undertaken to assess the effect of O3FA supplementation on serum lipids and vascular inflammation markers in patients with ESRD. Further subgroup analyses were performed to evaluate the effect of O3FA in subpopulations. In the present meta-analysis, the pooled SMD was <0 for TG, TC, LDL, CRP, albumin, homocysteine, SBP, DBP, lipoprotein(a) and ferritin whereas it was >0 for HDL hemoglobin, and glucose, which reflected a potential protective effect of O3FA. However, these trends were not obvious and require further validation.
Several RCTs included in this systemic review have reported inconsistent results. Many RCTs have reported that there is no significant difference between O3FAs and control on major CVD risk factors. Kooshki et al.12 conducted a RCT including 34 hemodialysis patients and found that marine O3FA supplementation could reduce serum TG, whereas it could not affect other serum lipids, lipoprotein, and hematologic factors among hemodialysis patients. Furthermore, Bouzidi et al.13 study indicated that O3FA supplementation could improve hypertriglyceridemia and oxidative stress in patients with chronic renal failure, which might lead to a decreased rate of cardiovascular complications. Khalatbari Soltani et al.14 study indicated that flax seed consumption could improve lipid abnormalities and reduce systemic inflammation in hemodialysis patients. Ando et al.15 concluded that eicosapentanoic acid administration was an effective and safe treatment to decrease plasma remnant lipoproteins. Khosroshahi et al.16 study indicated that O3FA supplementation could significantly reduce the serum homocysteine level; however, in contrast, high serum TC levels also reported. Similarly, Taziki et al.24 pointed out that O3FA was associated with higher TC levels. Khosroshahi et al.16 and Bowden et al.18 suggested that O3FA significantly reduced the LDL, whereas other trials reported no significant difference13,14. The reason could be that the sample population size was smaller than expected, and these trials were designed to evaluate other lipid factors as primary end point. Hence, clinically significant differences in LDL were not found. Similarly, Khajehdehi et al.25 and Lee et al.27 reported inconsistent results relevant to HDL when compared to other studies14,16,18. Some RCTs suggested that O3FA supplementation was associated with elevated HDL level14,18,25. On the contrary, other two trials reported that the O3FA supplementation was related with reduced HDL level16,27. The reason might be that the enrolled patients had significant heterogeneity in disease status. Moreover, two trials reported that O3FA supplementation resulted in significant reduction of homocysteine levels16,21. Lok et al.19 study suggested that O3FA was correlated with lower DBP and SBP. It was also found that O3FA supplementation could reduce the risk of intravascular clots, indicating its potential on reducing the risk of CVD.
The subgroup analysis indicated that O3FA supplementation had a significant relationship in reducing TG among multiple subsets. Notably, O3FA had no significant effect on LDL if the study published before 2010, the patients were residents of other countries, with a mean age greater than 60 years, BMI less than 25.0, and the study was a high-quality trial probably because of fewer number of trials included in these subsets. In addition, it was found that O3FA played a different role on serum lipids in different follow-up subsets. The possible reason could be that patients had different platelet count, alkaline phosphatase, serum sodium and potassium, and total iron binding capacity affected the treatment effects37. Another important reason could be that long-term O3FA supplementation might result in reduction of platelet activity and elimination of free radicals38. Finally, O3FA was associated with lower levels of TC and LDL, whereas elevated HDL level in patients with BMI was greater than 25.0. The reason for these could be that overweight participants had significant higher levels of TC and LDL than normal weight individuals, and O3FA supplementation showed reductions in BMI due to 03FA could promote fat oxidation and impair adipogenesis39.
A few advantages of the present meta-analysis were as follows: only RCTs were included for evaluation; the effect of O3FA supplementation in patients with ESRD was quantitatively determined using large pooled sample size; and the study provided evidence supporting the effects of O3FA supplementation on serum lipids and vascular inflammation markers such as TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin.
The present meta-analysis has certain limitations. First, a plenty of substantial heterogeneity among the included trials was identified in view of patients with different baseline characteristics. Second, publication bias could not be avoided when meta-analyzing published studies. Finally, more detailed relevant analysis and more comprehensive results could be restricted by conducting analysis using pooled data instead of individual data.
In summary, the findings of this meta-analysis suggested that O3FA supplementation was associated with lower serum TG, LDL, and CRP levels. Furthermore, sensitivity or subgroup analysis suggested that O3FA might play an important role on regulating TC levels. However, there was no significant difference between the effects of O3FA and control on HDL, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin levels. Future trials should focus on specific disease status and benefits of O3FA treatment for patients with ESRD.
Methods
Data Sources, Search Strategy, and Selection Criteria
The present meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis protocol, 200940. The RCTs designed to evaluate the influence of omega-3 supplementation on serum lipids and vascular inflammation markers were included in our study, regardless of language and publication status. Meanwhile, the impact of omega-3 supplementation on TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, systolic blood pressure (SBP), diastolic blood pressure (DBP), glucose, lipoprotein(a) and ferritin were examined. The relevant RCTs to be included in this meta-analysis were identified as follows: (1) Screening of electronic databases: PubMed, Embase, and the Cochrane Central Register of Controlled Trials were searched for studies from their inception until April 2016, using (“linolenic acid” OR “timnodonic acid” OR “ALA” OR “δ-amino linolenic acid” OR “EPA” OR “eicosapentaenoic Acid” OR “docosahexaenoic acid” OR “DHA” OR “docosahexaenoic acid” OR “omega-3 fatty acid” OR “fish oil” OR “n-3 fatty acids” OR “fatty acid” OR “omega-3” OR “α-linolenic acid” OR “eicosapentanoic acid”) AND (“kidney failure” OR “chronic renal failure” OR “dialysis” OR “hemodialysis” OR “peritoneal dialysis”) as the search terms.
(2)Other sources: ongoing (completed but not published) RCTs were identified from the metaRegister of Controlled Trials. Data pertaining to registered RCTs was obtained from the website http://clinicaltrials.gov/ (US NIH). Besides, manual searches were carried out from the reference lists within the entire relevant original and review articles in order to identify the additional eligible trials.
Two authors followed a standardized approach for conducting literature research. In case of disagreements between the two authors, mutual consensus was arrived after discussion. Since observational studies were susceptible to confounding variables and bias, the present systemic review was limited to RCTs. Subsequently, eligible studies were identified based on the following criteria: (1) patients with ESRD; (2) RCTs; (3) omega-3 fatty acid supplementation; and (4) at least one of the following variables: TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin. Studies were excluded if: (1) patients were diagnosed with other diseases; (2) the study was an observational study; (3) the study with inappropriate control; and (4) the mean difference was not obtained or calculated.
Data Collection and Quality Assessment
A standardized protocol was adopted by two authors to extract all the data from included trials. The data including first author, publication year, country, sample size, mean patient’s age, sex ratio of participants, disease condition, intervention, control, outcomes, and follow-up duration were collected. In addition, data of serum lipids and vascular inflammation markers including TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin were collected. Simultaneously, the quality of included RCTs were assessed using Jadad score34, ranging from 0 to 5, on the basis of parameters including randomization, blinding, allocation concealment, withdrawals and dropouts, and use of intention-to-treat analysis.
Statistical Analysis
The results of each RCT was considered as continuous data, and standard mean difference (SMD) and 95% confidence intervals (CIs) of each individual study were calculated from mean, standard deviation, and sample size in each group in individual RCT. Furthermore, SMD with 95%CIs were calculated for serum lipids and vascular inflammation markers, including TG, TC, LDL, HDL, CRP, albumin, hemoglobin, homocysteine, SBP, DBP, glucose, lipoprotein(a) and ferritin in patients with ESRD receiving omega-3 supplementation. The pooled SMDs of O3FA supplementation and control were compared using the fixed-effect (Mantel-Haenszel method) and random-effect models (DerSimonian-Laird method)41,42. In addition, to investigate the potential heterogeneity exist between RCTs, a subgroup analysis was performed based on the country, control, follow-up duration, and study quality. Besides, each individual trial was removed for carrying out a sensitivity analysis in the meta-analysis43. The heterogeneity of the treatment effects among RCTs was assessed using Cochrane Q-test; meanwhile, a P value of less than 0.10 was considered statistically significant44,45. P value for heterogeneity between subgroups were calculated by using Chi-square test46. Visual inspections of funnel plots for TG, TC, LDL, and HDL were conducted. The publication bias for TG, TC, LDL, and HDL parameters was also statistically assessed using Egger47 and Begg48 tests, and P values less than 0.05 was considered to have a significant publication bias. If significant publication bias was detected, trim and fill method were used to adjustment for publication bias49. STATA software (Version 10.0; StataCorp, Texas, United States of America) was used to perform the statistical analyses.
Additional Information
How to cite this article: Xu, T. et al. Effect of omega-3 fatty acid supplementation on serum lipids and vascular inflammation in patients with end-stage renal disease: a meta-analysis. Sci. Rep. 6, 39346; doi: 10.1038/srep39346 (2016).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This work was supported by grants from the following sources: National Science & Technology Support Program during the 12th Five-year Plan Period (Grant No. 2011BAI10B02), the scientific research project for the Universities of Educational Commission of Liaoning Province of China (Grant No. L2011134), Key Social Development Program of Science and Technology Commission of Liaoning Province of China (Grant No. 2013225303), Key Social Development Program of Science and Technology Commission of Shenyang of China (Grant No. F16-206-9-04), and Key Social Development Program of Science and Technology Commission, Liaoning Province of China (Grant No. 201404046).
Footnotes
Author Contributions Tianhua Xu, Yiting Sun and Li Yao contributed to conception and design; Tianhua Xu, Yiting Sun, Wei Sun, Li Yao, Li Sun, Linlin Liu, Jianfei Ma and Lining Wang contributed to acquisition, analysis and interpretation of data; Tianhua Xu, Yiting Sun, Wei Sun, Li Yao, Li Sun, Linlin Liu, Jianfei Ma and Lining Wang were involved in drafting or critical revision of the manuscript. All the authors approved the final version.
References
- Giray B., Kan E., Bali M., Hincal F. & Basaran N. The effect of vitamin E supplementation on antioxidant enzyme activities and lipid peroxidation levels in hemodialysis patients. Clin Chim Acta 338, 91–98 (2003). [DOI] [PubMed] [Google Scholar]
- Himmelfarb J. et al. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J Ren Nutr 17, 296–304 (2007). [DOI] [PubMed] [Google Scholar]
- Vernaglione L., Cristofano C. & Chimienti S. Omega-3 polyunsaturated fatty acids and proxies of cardiovascular disease in hemodialysis: a prospective cohort study. Journal of nephrology 21, 99 (2008). [PubMed] [Google Scholar]
- Hu F. B. et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 287, 1815–1821 (2002). [DOI] [PubMed] [Google Scholar]
- Benito P. et al. Effects of milk enriched with ω-3 fatty acid, oleic acid and folic acid in patients with metabolic syndrome. Clin Nutr 25, 581–587 (2006). [DOI] [PubMed] [Google Scholar]
- Nóbrega O. T. et al. Usual dietary intake and cardiovascular risk factors in older Brazilian women. Aging Clin Exp Res 24, 669–674 (2012). [DOI] [PubMed] [Google Scholar]
- Baronzio G. et al. Omega-3 fatty acids can improve radioresponse modifying tumor interstitial pressure, blood rheology and membrane peroxidability. Anticancer Res 14, 1145–1154 (1993). [PubMed] [Google Scholar]
- Avula C. R., Lawrence R. A., Jolly C. A. & Fernandes G. Role of n-3 polyunsaturated fatty acids (PUFA) in autoimmunity, inflammation, carcinogenesis, and apoptosis. Recent research developments in lipids 4, 303–319 (2000). [Google Scholar]
- Troyer D. & Fernandes G. Nutrition and apoptosis. Nutrition Research 16, 1959–1987 (1996). [Google Scholar]
- Chi H. et al. Omega-3 Fatty Acid Supplementation on Lipid Profiles in Dialysis Patients: Meta-analysis. Arch Med Res 45, 469–477 (2014). [DOI] [PubMed] [Google Scholar]
- Daud Z. A. M. et al. Effects of protein and omega-3 supplementation, provided during regular dialysis sessions, on nutritional and inflammatory indices in hemodialysis patients. Vascular health and risk management 8, 187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooshki A., Taleban F. A., Tabibi H. & Hedayati M. Effects of omega-3 fatty acids on serum lipids, lipoprotein (a), and hematologic factors in hemodialysis patients. Ren Fail 33, 892–898 (2011). [DOI] [PubMed] [Google Scholar]
- Bouzidi N. et al. Effects of omega-3 polyunsaturated fatty-acid supplementation on redox status in chronic renal failure patients with dyslipidemia. J Ren Nutr 20, 321–328 (2010). [DOI] [PubMed] [Google Scholar]
- Khalatbari Soltani S. et al. Effects of flaxseed consumption on systemic inflammation and serum lipid profile in hemodialysis patients with lipid abnormalities. Hemodialysis International 17, 275–281 (2013). [DOI] [PubMed] [Google Scholar]
- Ando M., Sanaka T. & Nihei H. Eicosapentanoic acid reduces plasma levels of remnant lipoproteins and prevents in vivo peroxidation of LDL in dialysis patients. J Am Soc Nephrol 10, 2177–2184 (1999). [DOI] [PubMed] [Google Scholar]
- Khosroshahi H. T. et al. Effect of omega-3 supplementation on serum level of homocysteine in hemodialysis patients. Iran J Kidney Dis 7, 479 (2013). [PubMed] [Google Scholar]
- Rasmussen L. E., Jørgensen K. A., Schmidt E. B. & Christensen J. H. The content of docosahexaenoic acid in serum phospholipid is inversely correlated with plasma homocysteine levels in patients with end-stage renal disease. Nutrition research 30, 535–540 (2010). [DOI] [PubMed] [Google Scholar]
- Bowden R. G., Jitomir J., Wilson R. L. & Gentile M. Effects of omega-3 fatty acid supplementation on lipid levels in endstage renal disease patients. J Ren Nutr 19, 259–266 (2009). [DOI] [PubMed] [Google Scholar]
- Lok C. E. et al. Effect of fish oil supplementation on graft patency and cardiovascular events among patients with new synthetic arteriovenous hemodialysis grafts: a randomized controlled trial. JAMA 307, 1809–1816 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos J. R., de Alencastro M. G., Konrath A. V., Cargnin M. & Manfro R. C. Flaxseed oil supplementation decreases C-reactive protein levels in chronic hemodialysis patients. Nutrition Research 32, 921–927 (2012). [DOI] [PubMed] [Google Scholar]
- Beavers K. M., Beavers D. P., Bowden R. G., Wilson R. L. & Gentile M. Omega‐3 fatty acid supplementation and total homocysteine levels in end‐stage renal disease patients. Nephrology 13, 284–288 (2008). [DOI] [PubMed] [Google Scholar]
- Schmidt E. B., Jørgensen K. A. & Christensen J. H. The effect of n-3 fatty acids on lipids and lipoproteins in patients treated with chronic haemodialysis: a randomized placebo-controlled intervention study. Nephrology Dialysis Transplantation 23, 2918–2924 (2008). [DOI] [PubMed] [Google Scholar]
- Christensen J. H., Sølling J. & Schmidt E. B. The effect of n-3 fatty acids on plasma lipids and lipoproteins and blood pressure in patients with CRF. Am J Kidney Dis 44, 77–83 (2004). [DOI] [PubMed] [Google Scholar]
- Taziki O., Lessan-Pezeshki M., Akha O. & Vasheghani F. The effect of low dose omega-3 on plasma lipids in hemodialysis patients. Saudi J Kidney Dis Transpl 18, 571 (2007). [PubMed] [Google Scholar]
- Khajehdehi P. Lipid-lowering effect of polyunsaturated fatty acids in hemodialysis patients. J Ren Nutr 10, 191–195 (2000). [DOI] [PubMed] [Google Scholar]
- Chang J. W. et al. Effects of α-lipoic acid on the plasma levels of asymmetric dimethylarginine in diabetic end-stage renal disease patients on hemodialysis: a pilot study. Am J Nephrol 27, 70–74 (2007). [DOI] [PubMed] [Google Scholar]
- Lee S. M., Son Y. K., Kim S. E. & An W. S. The effects of omega-3 Fatty Acid on vitamin d activation in hemodialysis patients: a pilot study. Mar Drugs 13, 741–755 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saifullah A. et al. Oral fish oil supplementation raises blood omega-3 levels and lowers C-reactive protein in haemodialysis patients—a pilot study. Nephrology Dialysis Transplantation 22, 3561–3567 (2007). [DOI] [PubMed] [Google Scholar]
- Madsen T., Schmidt E. B. & Christensen J. H. The effect of n-3 fatty acids on C-reactive protein levels in patients with chronic renal failure. J Ren Nutr 17, 258–263 (2007). [DOI] [PubMed] [Google Scholar]
- Beavers K. M., Beavers D. P., Bowden R. G., Wilson R. L. & Gentile M. Effect of over-the-counter fish-oil administration on plasma Lp(a) levels in an end-stage renal disease population. J Ren Nutr 19, 443–449 (2009). [DOI] [PubMed] [Google Scholar]
- Gharekhani A. et al. The effect of omega-3 fatty acids on depressive symptoms and inflammatory markers in maintenance hemodialysis patients: a randomized, placebo-controlled clinical trial. Eur J Clin Pharmacol 70, 655–665 (2014). [DOI] [PubMed] [Google Scholar]
- Hung A. M. et al. Omega-3 fatty acids inhibit the up-regulation of endothelial chemokines in maintenance hemodialysis patients. Nephrol Dial Transplant 30, 266–274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naini A. E., Keyvandarian N., Mortazavi M., Taheri S. & Hosseini S. M. Effect of Omega-3 fatty acids on blood pressure and serum lipids in continuous ambulatory peritoneal dialysis patients. J Res Pharm Pract 4, 135–41 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadad A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17, 1–12 (1996). [DOI] [PubMed] [Google Scholar]
- Rizos E. C., Ntzani E. E., Bika E., Kostapanos M. S. & Elisaf M. S. Association between omega-3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta-analysis. JAMA 308, 1024–33 (2012). [DOI] [PubMed] [Google Scholar]
- Casula M., Soranna D., Catapano A. L. & Corrao G. Long-term effect of high dose omega-3 fatty acid supplementation for secondary prevention of cardiovascular outcomes: A meta-analysis of randomized, placebo controlled trials [corrected]. Atheroscler Suppl 14, 243–51 (2013). [DOI] [PubMed] [Google Scholar]
- Fiedler R., Mall M., Wand C. & Osten B. Short-term administration of omega-3 fatty acids in hemodialysis patients with balanced lipid metabolism. J Ren Nutr 15, 253–256 (2005). [DOI] [PubMed] [Google Scholar]
- Bowden R. G., Wilson R. L., Deike E. & Gentile M. Fish oil supplementation lowers C-reactive protein levels independent of triglyceride reduction in patients with end-stage renal disease. Nutr Clin Pract 24, 508–512 (2009). [DOI] [PubMed] [Google Scholar]
- Abeywardena M. Y. & Belobrajdic D. P. Long-Chain Omega-3 Polyunsaturated Fatty Acids and Obesity. Obesity. Springer International Publishing pp 29–44 (2016). [Google Scholar]
- Moher D., Liberati A., Tetzlaff J. & Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 151, 264–269 (2009). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Ades A., Lu G. & Higgins J. The interpretation of random-effects meta-analysis in decision models. Med Decis Making 25, 646–654 (2005). [DOI] [PubMed] [Google Scholar]
- Tobias A. Assessing the influence of a single study in meta-analysis. Stata Tech Bull 47, 15–17 (1999). [Google Scholar]
- Higgins J. P., Thompson S. G., Deeks J. J. & Altman D. G. Measuring inconsistency in meta-analyses. BMJ: British Medical Journal 327, 557 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J. J., Higgins J. & Altman D. G. Analysing Data and Undertaking Meta‐Analyses. Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series 243–296 (2008). [Google Scholar]
- Deeks J. J., Altman D. G. & Bradburn M. J. Statistical methods for examining heterogeneity and combining results from several studies in meta-analysis. In Egger M., Davey Smith G., Altman D. G. eds Systematic Reviews in Health Care: Metaanalysis in Context. 2nd ed. London: BMJ Books, 285–312 (2001). [Google Scholar]
- Egger M., Smith G. D., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1088–1101 (1994). [PubMed] [Google Scholar]
- Duvall S. & Tweedie R. A nonparametric “trim and fill” method for assessing publication bias in meta-analysis. J Am Stat Assoc 95, 89–98 (2000). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.