Abstract
Background/objectives
Given the potent role of sex hormones on brain chemistry and function, we investigated the association of reproductive history indicators of hormonal exposures, including reproductive period, pregnancy, and use of hormonal contraceptives, on mid- and late-life cognition in postmenopausal women.
Design
Analysis of baseline data from two randomized clinical trials, the Women’s Isoflavone Soy Health (WISH) and the Early vs Late Intervention Trial of Estradiol (ELITE).
Setting
University academic research center
Participants
830 naturally menopausal women
Measurements
Participants were uniformly evaluated with a cognitive battery and a structured reproductive history. Outcomes were composite scores for verbal episodic memory, executive functions, and global cognition. Reproductive variables included ages at pregnancies, menarche, and menopause, reproductive period, number of pregnancies, and use of hormones for contraception and menopausal symptoms. Multivariable linear regression evaluated associations between cognitive scores (dependent variable) and reproductive factors (independent variables), adjusting for age, race/ethnicity, income and education.
Results
On multivariable modeling, age at menarche ≥ 13 years of age was inversely associated with global cognition (p= 0.05). Last pregnancy after age 35 was positively associated with verbal memory (p=0.03). Use of hormonal contraceptives was positively associated with global cognition (p trend=0.04), and verbal memory (p trend=0.007). The association between hormonal contraceptive use and verbal memory and executive functions was strongest for more than 10 years of use. Reproductive period was positively associated with global cognition (p=0.04) and executive functions (p=0.04).
Conclusion
In this sample of healthy postmenopausal women, reproductive life events related to sex hormones, including earlier age at menarche, later age at last pregnancy, length of reproductive period, and use of oral contraceptives are positively related to aspects of cognition in later life.
Keywords: reproductive history, cognition, postmenopausal women
INTRODUCTION
Estrogens play a key role in regulating neuronal biochemistry1–3 and brain function related to cognitive processes 2–6. Given the potent role of sex hormones on brain chemistry and function, the impact of the sex hormone milieu during reproductive life on mid- and late-life cognition in women has been a topic of interest.
Reproductive history is an important aspect of reproductive hormonal dynamics that women experience between menarche and menopause 7,8. Proxies for lifelong cumulative exposure to estrogen, including length of the reproductive period (from menarche to menopause), number of pregnancies, age at first pregnancy, and duration of breast feeding, have been evaluated in relation to late-life cognition 8–12. However, age at last pregnancy, which can be a marker of later surge of pregnancy-related hormones, has not been investigated in relation to cognitive functions. Older age at last pregnancy has been linked with elevated breast and reduced endometrial cancer risks 13–16. In addition to factors associated with endogenous sex hormones, exogenous hormones, particularly menopausal hormone therapy (HT), have been investigated in relation to cognitive function 11,17. However, the impact of hormonal contraceptive use on later-life cognition is not conclusive 9,12,18,19.
Studies evaluating the impact of reproductive history on later-life cognition have primarily focused on older postmenopausal women over the age of 60 years. Given recent evidence suggesting a cognitive decline during the menopausal transition 20, such associations should also be evaluated among younger postmenopausal women. While many studies have reported associations of reproductive period with cognitive outcomes 9–12, fewer have reported cognitive associations with other reproductive factors that are markers of hormonal exposures, including pregnancy history and use of hormonal contraceptives; even fewer studies have evaluated the joint effects of these variables in multivariable models 9,11,12. Furthermore, most such studies have used general screening tests for cognitive dysfunction, or a limited number of cognitive tests, as measures of cognitive function. 10,11 With these limitations in mind, we investigated the association of reproductive history including reproductive years, a detailed pregnancy history, hormonal contraceptive use, and use of menopausal hormone therapy with mid- and late-life cognition using a comprehensive cognitive battery in postmenopausal women, with an average age of 60 years, ranging from 41–92 years of age.
METHODS
Design and Participants
We used baseline data from 830 naturally menopausal women who were participants in two randomized, double-blind, placebo-controlled clinical trials, the Women’s Isoflavone Soy Health trial (WISH, conducted from April 2004 through March 2009) and the Early vs Late Intervention Trial of Estradiol (ELITE, conducted from June 2005 through February 2013). Details of the WISH and ELITE trial designs have been described 21,22. Postmenopausal women in the WISH trial were randomly assigned to daily 25 g soy protein or daily total milk protein matched placebo, while those in the ELITE study were randomly assigned to oral 17β-estradiol (1 mg daily) or matched placebo. Study participants in both trials were healthy postmenopausal women, currently non-smoking and HT non-users, free of cardiovascular disease or any other chronic disease conditions. Both WISH and ELITE trials were conducted at the Atherosclerosis Research Unit in the University at Southern California, Los Angeles, CA, applying almost identical study design, patient characteristics (demographic and clinical), and data collection methods. Hence we compiled the baseline data from both trial participants for this cross-sectional study.
Reproductive History
A detailed history on reproductive factors was collected from participant self-reports at baseline, using the same structured questionnaire that included uniformly worded questions to elicit age at menarche, date of and age at last menstrual period, total number of pregnancies (including miscarriages and abortions), age at first and last pregnancy, history and dates of hysterectomy and oophorectomy, and history of postmenopausal and contraceptive hormone therapy (ever use, duration of use, age of first and last use). Length of reproductive period was calculated as the years between ages at menarche and menopause. For trial eligibility, postmenopausal status was defined as a serum level of total estradiol (E2) <25 pg/ml and an absence of vaginal bleeding for at least six months (natural menopause) or bilateral oophorectomy (surgical menopause). Surgically menopausal women were excluded from the current study. While the women were not currently using any menopausal hormone therapy (HT) by trial eligibility, history of past HT use (ending at least 1 month prior to trial randomization) was recorded at baseline.
Cognitive Assessment
Cognitive skills assessed at baseline were used in the current study as the outcomes of interest. A comprehensive neuropsychological battery emphasizing standardized tests sensitive to age-associated change in middle-age and older adults were used for cognitive assessment 21,22. Neuropsychological tests and corresponding cognitive skills included Symbol Digit Modalities Test, complex scanning and visual tracking, attention, and psychomotor speed; Trail Making Test, Part B, visuomotor tracking, planning, cognitive flexibility, and psychomotor speed; Shipley Institute of Living Scale, Abstraction scale, concept formation; Letter-Number Sequencing, working memory, attention, and concentration; Block Design, visuospatial perception, nonverbal concept formation, planning, and visuoconstructive ability; Judgment of Line Orientation, visuospatial perception; animal naming, verbal fluency and semantic memory; Boston Naming Test, naming and semantic memory; California Verbal Learning Test, verbal episodic memory, word list learning and concept formation; East Boston Memory Test, verbal episodic memory and logical memory; and Faces I and II, visual episodic memory, memory for faces, and visuoperceptual processing. The verbal intelligence quotient was estimated with the Wechsler Test of Adult Reading.
In the current study, composite scores for verbal episodic memory, executive functions, and overall cognitive performance (global cognition) were the outcomes of interest. Details on the methods for obtaining the composite scores have been described 21. Briefly, each composite score was calculated as the average of component standardized scores weighted by the inverse inter-test correlation matrix. The verbal memory composite was defined a priori by California Verbal Learning Test and East Boston Memory Test immediate and delayed recall scores, and the global composite by scores from all neuropsychological measures. Tests used for the executive functions composite were determined by a principal components analysis of baseline scores; this composite used scores from the Symbol Digit Modalities Test, the Trail Making Test, the Shipley Abstraction scale, Letter-Number Sequencing, and category fluency.
Statistical Analysis
For the current analysis, dependent variables were the global composite cognitive score, and the verbal memory and executive functions composite scores. Reproductive history variables included age at first and last pregnancy, age at menarche, age at menopause, duration of reproductive period (age at menopause minus age at menarche), duration of use of hormonal contraception, ever pregnant, number of full-term and non full-term pregnancies, and past use of menopausal hormone therapy. Correlations between the reproductive variables were evaluated using Spearman correlation coefficients.
All cognitive outcome variables followed a normal distribution. Multivariable linear regression was used to evaluate the associations between cognitive scores (dependent variable) and reproductive factors (independent variables). Women with complete data contributed to the multivariable analyses. The categorization for the age at last pregnancy (≤ 35 vs. > 35 years) was based on literature reporting lower risk of moderate or severe hot flashes among women with older age at last pregnancy compared to women who were 35 years or less at their last pregnancy 23. Age at last pregnancy of 35 years also represented the 75th percentile for the distribution of age at last pregnancy in our study participants. In a sensitivity analysis, age at last pregnancy was also analyzed as a continuous variable. Reproductive variables with p<0.15 on univariable models were selected as candidates for multivariable modeling. Age at cognitive testing, race or ethnicity, income and education were included in all multivariable models, as they were confounders of associations between the reproductive variables of interest and cognitive dependent variables. Years since menopause was not included in the multivariable models because of its strong correlation with age. The multivariable analysis was further stratified by age at cognitive assessment categorized as midlife (<60 years) vs later life (≥60 years) postmenopausal women. Formal tests of interaction were performed to evaluate the statistical significance of any interaction between the reproductive factors and age on cognitive outcomes. All statistical analyses used SAS 9.4 statistical software (SAS Institute Inc., Cary, NC).
RESULTS
A total of 830 (324 WISH and 506 ELITE) postmenopausal women contributed to this analysis. The majority of the women were non-Hispanic white (67%), 40% were 10 years or more past menopause, with an average (SD) age at cognitive testing of 60 (6.9) years ranging from 41 to 92 years, with 16 (2.2) years of education (Table 1). Annual income was <$50,000 for 28%, $50,000 – $89,990 for 27%, and ≥$90,000 for 37% of the participants. The average ages at menarche and menopause were 13 (1.5) and 50 (4.6) years, respectively, with an average reproductive period of 37 (4.8) years. Sixteen percent of the women had never been pregnant, 47% had their first pregnancy before 24 years of age, and 21% had their last pregnancy after the age of 35 years. Seventy-nine percent of the women used hormonal contraceptives sometime during their reproductive lives, and 68% used HT. All cognitive composite outcomes were lower in women ≥ 60 years compared to women <60 years old.
Table 1.
Characteristics of Study Participants (n=830)
| Variablesa | Total Sample (n=830) | Age<60 (n=426) | Age ≥ 60 (n=404) |
|---|---|---|---|
| Age | 60.0 (6.9) | 54.6 (3.2) | 65.6 (4.9) |
| Race or Ethnicity | |||
| White non-Hispanic | 558 (67%) | 265 (62%) | 293 (73%) |
| Black non-Hispanic | 71 (9%) | 35 (8%) | 36 (9%) |
| Hispanic | 115 (14%) | 71 (17%) | 44 (11%) |
| Asian or Pacific Islander | 72 (9%) | 48 (11%) | 24 (6%) |
| Other | 14 (2%) | 7 (2%) | 7 (2%) |
| Education (years) | 16 (2.2) | 16 (2.2) | 16 (2.2) |
| Annual Income (thousands) | |||
| <$50K | 236 (28%) | 93 (22%) | 143 (35%) |
| $50K–$89.99K | 224 (27%) | 121 (28%) | 103 (25%) |
| ≥$90K | 310 (37%) | 195 (46%) | 115 (28%) |
| Not Reported | 60 (7%) | 17 (4%) | 43 (11%) |
| Age at Menarche | 13 (1.5) | 13 (1.4) | 13 (1.5) |
| ≥13 years | 455 (55%) | 234 (55%) | 221 (55%) |
| < 13 years | 375 (45%) | 192 (45%) | 183 (45%) |
| Age at Menopause | 50 (4.6) | 49 (4.6) | 51 (4.6) |
| >52 years | 250 (30%) | 121 (28%) | 129 (32%) |
| ≤52 years | 520 (63%) | 291 (68%) | 229 (57%) |
| Undetermined | 60 (7%) | 14 (3%) | 46 (11%) |
| Reproductive Period (years)b | 37 (4.8) | 37 (4.7) | 38 (4.9) |
| Number of Full-Term Pregnancies | 3. (1.2) | 3 (1.3) | 3 (1.1) |
| Never Pregnant | 130 (16%) | 81 (19%) | 49 (12%) |
| No full-term pregnancies | 90 (11%) | 59 (14%) | 31 (8%) |
| 1 full-term | 147 (18%) | 77 (18%) | 70 (17%) |
| 2 full-term | 241 (29%) | 122 (29%) | 119 (29%) |
| > 2 full-term | 222 (27%) | 87 (20%) | 135 (33%) |
| Age at First Pregnancy | 25 (5.7) | 25 (5.9) | 24 (5.4) |
| Never Pregnant | 130 (16%) | 81 (19%) | 49 (12%) |
| ≤24 years | 390 (47%) | 167 (39%) | 223 (55%) |
| >24 years | 307 (37%) | 176 (41%) | 131 (32%) |
| Missing | 3 (<1%) | 2 (<1%) | 1 (<1%) |
| Age at Last Pregnancy | 31 (7.3) | 31 (7.6) | 30 (7.1) |
| Never Pregnant | 130 (16%) | 81 (19%0 | 49 (12%) |
| ≤ 35 years | 518 (62%) | 243 (57%) | 275 (68%) |
| > 35 years | 171 (21%) | 96 (23%) | 75 (19%) |
| Missing | 11 (1%) | 6 (1%) | 5 (1%) |
| Duration of Hormonal Contraception (years) | 8 (6.6) | 8 (6.7) | 8 (6.6) |
| None | 175 (21%) | 66 (15%) | 109 (27%) |
| 1 – 4 years | 244 (29%) | 137 (32%) | 107 (26%) |
| 5 – 10 years | 228 (27%) | 118 (28%) | 110 (27%) |
| >10 years | 179 (22%) | 102 (24%) | 77 (19%) |
| Missing | 4 (<1%) | 3 (<1%) | 1 (<1%) |
| Menopausal Hormone Therapy | |||
| No | 263 (32%) | 185 (43%) | 78 (19%) |
| Yes | 567 (68%) | 241 (57%) | 326 (81%) |
| Years Since Menopausec | 10 (7.5) | 5 (4.7) | 15 (6.9) |
| Cognitive Scores | |||
| Global Composite | −0.02 (1.72) | 0.17 (1.77) | −0.23 (1.65) |
| Verbal Memory | 0.03 (2.93) | 0.39 (2.98) | −0.36 (2.83) |
| Executive Functions | 0.11 (3.63) | 0.77 (3.68) | −0.58 (3.44) |
Numbers in table are mean (SD) or n (%)
Reproductive period determined in 771 women
Years since menopause determined in 764 women
In univariate analysis, older age at menarche (≥13 years) was weakly associated with lower global cognition compared to women having menarche at <13 years of age (p = 0.08; Table 2). Although age at menopause was not significantly associated with any of the cognitive outcomes, a longer reproductive period was associated with higher global cognition (p = 0.019). Cognitive scores did not significantly differ by pregnancy status (ever vs. never pregnant). Relative to women who had 1 full-term pregnancy, women who had been pregnant with no full-term pregnancy or had 2 full-term pregnancies had higher global cognitive, verbal memory, and executive functions scores. Women reporting their first pregnancy at or after the age of 24 years had significantly better executive functions compared to women who had their first pregnancy before 24 years of age (p = 0.02). Women having their last pregnancy after the age of 35 years had significantly better global cognition (p = 0.02) and verbal memory (p = 0.008) scores compared to women with last pregnancy at or before 35 years of age. Compared to non-users, women using hormonal contraceptives for any length of time during their reproductive period had significantly better performance on all three cognitive measures (all p<0.001). Women with more than 10 years of hormonal contraceptive use benefited most cognitively compared to women with shorter duration (all p<0.001).
Table 2.
Univariate Associations of Reproductive Factors with Cognitive Composite Scores
| Variables | Global Composite | Verbal Memory | Executive Functions | |||
|---|---|---|---|---|---|---|
| Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | |
| Age (years) | −0.04 (0.01) | <0.001 | −0.07 (0.01) | <0.001 | −0.13 (0.02) | <0.001 |
| Race or Ethnicity | ||||||
| White non-Hispanic | Ref | <0.001a | Ref | <0.001a | Ref | <0.001a |
| Black non-Hispanic | −0.89 (0.21) | −0.82 (0.36) | −2.01 (0.44) | |||
| Hispanic | −1.20 (0.17) | −1.54 (0.30) | −3.35 (0.36) | |||
| Asian or Pacific Islander | −0.93 (0.21) | −1.46 (0.36) | −1.62 (0.43) | |||
| Other | −1.09 (0.45) | −2.04 (0.77) | −1.84 (0.92) | |||
| Education (years) | 0.25 (0.03) | <0.001 | 0.32 (0.04) | <0.001 | 0.61 (0.05) | <0.001 |
| Annual Income (thousands) | ||||||
| <$50K | Ref | <0.001a | Ref | <0.001a | Ref | <0.001a |
| $50K–$89.99K | 0.64 (0.15) | 1.03 (0.27) | 1.49 (0.32) | |||
| ≥$90K | 1.17 (0.14) | 1.79 (0.25) | 2.80 (0.30) | |||
| Not Reported | 0.03 (0.24) | 0.01 (0.41) | 0.55 (0.51) | |||
| Age at Menarche | ||||||
| ≥13 years | −0.21 (0.12) | 0.08 | −0.23 (0.21) | 0.27 | −0.18 (0.26) | 0.48 |
| Age at Menopause | ||||||
| >52 years | 0.15 (0.13) | 0.26 | 0.02 (0.23) | 0.92 | 0.22 (0.26) | 0.43 |
| Reproductive Period (years) | 0.03 (0.01) | 0.019 | 0.03 (0.02) | 0.16 | 0.04 (0.03) | 0.11 |
| Number of Full-Term Pregnancies | ||||||
| 1 full-term | Ref | 0.003a | Ref | 0.004a | Ref | 0.003a |
| 2 full-term | 0.37 (0.19) | 0.04 | 0.60 (0.31) | 0.05 | 0.76 (0.38) | 0.05 |
| > 2 full-term | 0.10 (0.18) | 0.59 | 0.21 (0.31) | 0.51 | −0.19 (0.39) | 0.63 |
| Never Pregnant | 0.21 (0.21 ) | 0.32 | 0.38 (0.39) | 0.28 | 0.49 (0.44) | 0.27 |
| No full-term pregnancies | 0.95 (0.23) | <0.001 | 1.41 (0.39) | <0.001 | 1.37 (0.49) | 0.005 |
| Never Pregnant | 0.08 (0.16) | 0.66 | 0.08 (0.28) | 0.78 | −0.11 (0.35) | 0.75 |
| Age at First Pregnancy | ||||||
| ≤24 years | Ref | 0.31a | Ref | 0.43a | Ref | 0.02a |
| >24 years | 0.19 (0.13) | 0.14 | 0.29 (0.22) | 0.20 | 0.80 (0.28) | 0.004 |
| Never Pregnant | 0.01 (0.17) | 0.94 | 0.05 (0.30) | 0.87 | 0.46 (0.37) | 0.21 |
| Age at Last Pregnancy | ||||||
| ≤35 years | Ref | 0.055a | Ref | 0.03a | Ref | 0.69a |
| >35 years | 0.36 (0.15) | 0.02 | 0.69 (0.26) | 0.008 | 0.26 (0.32) | 0.42 |
| Never Pregnant | 0.009 (0.17) | 0.96 | 0.09 (0.29) | 0.74 | 0.17 (0.36) | 0.63 |
| Duration of Hormonal Contraceptive Use | ||||||
| None | Ref | <0.001a | Ref | <0.001a | Ref | <0.001a |
| 1 – 4 years | 0.92 (0.17) | <0.001 | 1.45 (0.28) | <0.001 | 1.72 (0.36) | <0.001 |
| 5 – 10 years | 0.87 (0.17) | <0.001 | 1.29 (0.28) | <0.001 | 1.71 (0.36) | <0.001 |
| >10 years | 1.09 (0.19) | <0.001 | 1.92 (0.31) | <0.001 | 2.31 (0.38) | <0.001 |
| Any Hormonal Contraception | 0.95 (0.14) | <0.001 | 1.52 (0.25) | <0.001 | 1.89 (0.31) | <0.001 |
| Any Menopausal Hormone Therapy | −0.11 (0.13) | 0.40 | −0.18 (0.22) | 0.42 | −0.60 (0.27) | 0.03 |
| Years Since Menopause | −0.04 (0.01) | <0.001 | −0.06 (0.01) | <0.001 | −0.12 (0.02) | <0.001 |
Global p-value (race/ethnicity, income, number of full-term pregnancies, age at first and last pregnancy) or p-value for trend (duration of hormonal contraceptive use)
We examined the correlation among the reproductive factors in order to avoid inclusion of variables with high collinearity in multivariable models. Chronological age was highly positively correlated with years since menopause (r = 0.79, p <0.001, Table 3); we therefore did not include years since menopause in multivariable models including chronological age. Length of reproductive period was strongly positively correlated with age at menopause (r = 0.93, p <0.001), but was only moderately inversely correlated with age at menarche (r = −0.32, p <0.001). To evaluate the extent of overlap, the three cognitive outcome measures were correlated using Pearson’s correlation. Global cognition score was significantly positively associated with verbal memory and executive function scores (r = 0.74, 0.73, respectively; p-value for both <.0001). Verbal memory was significantly associated with executive function (r = 0.51, p-value <.0001).
Table 3.
Correlation between Reproductive Factors, Age at Testing, and Years since Menopausea
| Age at menarche |
Age at menopause |
Reproductive period |
Years since menopause |
Number of pregnancies |
Age at first pregnancy |
Age at last pregnancy |
Hormonal contraceptive use |
|
|---|---|---|---|---|---|---|---|---|
| Age at testing | 0.01 (0.73) | 0.24 (<0.001) | 0.23 (<0.001) | 0.79 (<0.001) | 0.12 (<0.001) | −0.08 (0.03) | −0.07 (0.09) | 0.05 (0.22) |
| Age at menarche | 0.005 (0.91) | −0.32 (<0.001) | −0.02 (0.67) | 0.03 (0.43) | 0.03 (0.39) | 0.04 (0.35) | 0.01 (0.85) | |
| Age at menopause | 0.93 (<0.001) | −0.31 (<0.001) | 0.12 (0.006) | −0.03 (0.48) | 0.01 (0.83) | 0.06 (0.15) | ||
| Reproductive period | −0.29 (<0.001) | 0.11 (0.003) | −0.03 (0.42) | 0.01 (0.81) | 0.04 (0.33) | |||
| Years since menopause | 0.11 (0.003) | −.03 (0.41) | 0.01 (0.81) | 0.04 (0.33) | ||||
| Number of pregnancies | −0.33 (<0.001) | 0.34 (<0.001) | −0.11 (0.007) | |||||
| Age at first pregnancy | 0.45 (<0.001) | −0.33 (<0.001) | ||||||
| Age at last pregnancy | −0.03 (0.50) |
Spearman’s correlation coefficient (p-value)
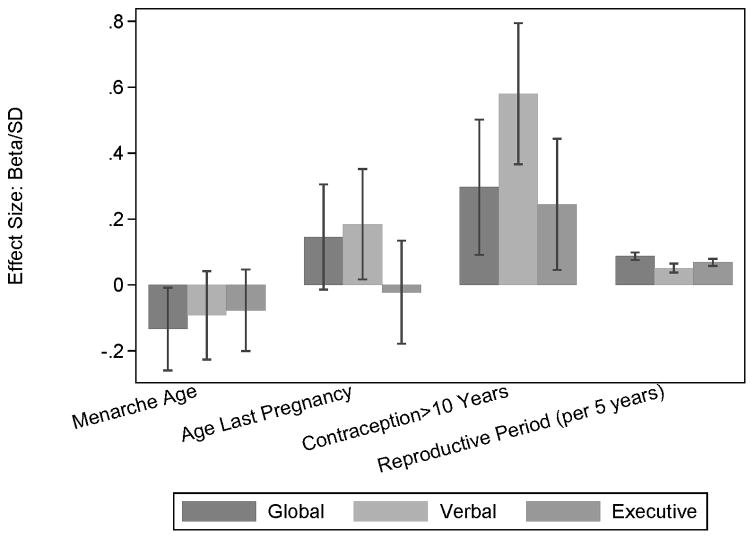
Multivariable analysis included reproductive factors that were univariately associated with any of the cognitive outcomes at a significance level of ≤ 0.15; age, race or ethnicity, income and education were included as model covariates (Table 4). Age at menarche, age at last pregnancy, full term pregnancy status, reproductive period, and duration of hormonal contraception were significant independent correlates of one or more cognitive outcomes. Onset of menarche at or above 13 years of age was inversely associated with global weighted cognition (p = 0.05), but not with verbal memory or executive functions. Last pregnancy after the age of 35 years was significantly positively associated with verbal memory scores (p = 0.03), and with global cognition at borderline significance (p = 0.07), but not with executive functions. Age at last pregnancy was not associated with global (p = 0.35) or verbal memory (p = 0.39) scores when analyzed as a continuous variable. Duration of hormonal contraceptive use was significantly positively associated with global cognitive score (p trend = 0.04), verbal memory (p trend = 0.007) and at borderline significance with executive functions (p trend = 0.06). The association between hormonal contraceptive use and verbal memory and executive functions was strongest for more than 10 years of use. The length of the reproductive period was significantly positively associated with global cognition (p = 0.04) and executive functions (p = 0.04). Multivariable models replacing age with years since menopause showed similar results (data not shown). Figure 1 displays the effect sizes for reproductive variables (age at menarche, age at last pregnancy, hormonal contraception use > 10 years, and length of reproductive period); the effect size was calculated as the beta estimate (Table 4) divided by the standard deviation (SD) of the corresponding cognitive outcome (Table 1). The effect sizes allow interpretation of the beta estimates on an SD metric (proportion of SD). For each cognitive composite score, multivariable adjusted means by reproductive factor groupings, along with the overall sample ranges and 5th, 95th percentiles, are provided in Supplementary Table S1.
Table 4.
Multivariable Associations of Reproductive Factors with Cognitive Composite Scoresa
| Global Composite (n=755) | Verbal Memory (n=748) | Executive Functions (n=739) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate (SE) | p-value | Estimate (SE) | p-value | Estimate (SE) | p-value | |
| Age (years) | −0.03 (0.01) | 0.006 | −0.05 (0.02) | <0.001 | −0.12 (0.02) | <0.001 |
| Race or Ethnicity | ||||||
| White non-Hispanic | Ref | <0.001b | Ref | <0.001b | Ref | <0.001b |
| Black non-Hispanic | −0.83 (0.21) | −0.74 (0.36) | −1.91 (0.42) | |||
| Hispanic | −1.00 (0.18) | −1.33 (0.31) | −2.99 (0.36) | |||
| Asian or Pacific Islander | −0.85 (0.20) | −1.40 (0.36) | −1.69 (0.41) | |||
| Other | −1.02 (0.45) | −1.76 (0.79) | −1.42 (0.90) | |||
| Education (years) | 0.15 (0.03) | <0.001 | 0.15 (0.05) | 0.003 | 0.40 (0.06) | <0.001 |
| Income (annual) | ||||||
| Less than 50,000 | Ref | Ref | ||||
| 50,000 – 89.9000 | 0.41 (0.15) | 0.008 | 0.76 (0.27) | 0.006 | 0.67 (0.31) | 0.03 |
| More then 90,0000 | 0.71 (0.15) | <0.001 | 1.26 (0.27) | <0.001 | 1.25 (0.31) | <0.001 |
| Not reported | −0.04 (0.24) | 0.88 | 0.11 (0.42) | 0.79 | 0.05 (0.48) | 0.92 |
| Age at Menarche | ||||||
| <13 years | Ref | Ref | Ref | |||
| ≥13 years | −0.23 (0.11) | 0.05 | −0.27 (0.20) | 0.19 | −0.28 (0.23) | 0.23 |
| Age at Last Pregnancy | ||||||
| ≤35 years | Ref | Ref | Ref | |||
| >35 years | 0.25 (0.14) | 0.07 | 0.54 (0.25) | 0.03 | −0.08 (0.28) | 0.77 |
| Never Pregnant | 0.21 (0.20) | 0.29 | 0.53 (0.35) | 0.13 | 0.26 (0.41) | 0.52 |
| Duration of Hormonal Contraception | ||||||
| None | Ref | 0.04b | Ref | 0.007b | Ref | 0.06b |
| <5 years | 0.59 (0.17) | <0.001 | 0.93 (0.30) | 0.002 | 0.65 (0.34) | 0.06 |
| 5–10 years | 0.44 (0.17) | 0.01 | 0.73 (0.30) | 0.02 | 0.41 (0.35) | 0.24 |
| >10 years | 0.51 (0.18) | 0.005 | 1.07 (0.32) | <0.001 | 0.89 (0.37) | 0.02 |
| Reproductive Period (years) | 0.03 (0.01) | 0.04 | 0.03 (0.02) | 0.11 | 0.05 (0.02) | 0.04 |
| Number of Full-Term Pregnancies | ||||||
| 1 full-term | Ref | Ref | Ref | |||
| 2 full-term | 0.17 (0.17) | 0.33 | 0.42 (0.30) | 0.17 | 0.45 (0.35) | 0.19 |
| >2 full-term | 0.24 (0.17) | 0.17 | 0.45 (0.31) | 0.15 | 0.42 (0.36) | 0.23 |
| No full-term | 0.61 (0.22) | 0.005 | 1.00 (0.39) | 0.01 | 0.50 (0.45) | 0.26 |
Adjusted for age at cognitive testing, education, and race/ethnicity
Global p-value (race/ethnicity) or p-value for trend (duration of hormonal contraceptive use)
Figure 1.
Associations of reproductive factors with cognitive outcomes. Estimates of association are presented as effect sizes and 95% confidence intervals (beta estimates from Table 4, per standard deviation of cognitive outcome).
Among women younger than 60 years on stratified analysis, age at menarche ≥ 13 years was inversely associated with global cognition at borderline significance (p=0.06), longer reproductive period was significantly positively associated with executive functions (p=0.02), and having no full term pregnancies was significantly positively associated with global cognition and verbal memory (Supplementary Table S2a). Among women 60 years or older, age at last pregnancy ≥ 35 years was positively associated with verbal memory, and hormonal contraception use was associated with verbal memory (p=0.002) (Supplementary Table S2b). In general, statistical tests for interaction indicated that the associations of the included reproductive factors with cognition did not differ in midlife and later life women (p for interaction > 0.05). Two exceptions to this generalization were: (1) the association of a non-full term pregnancy with verbal memory differed between the two age groups (p for interaction=0.01), with a positive association evident in midlife, but not in later life; (2) the positive association of reproductive period and executive functions differed between the two age groups with borderline significance (p for interaction=0.065), with a positive association evident in midlife but not in later life women.
DISCUSSION
Our data show significant associations of reproductive factors with midlife to late-life cognition among a reasonably large sample of healthy naturally postmenopausal women. In particular, later age at last pregnancy had a beneficial association with verbal and global cognitive performance in later life. We also documented a beneficial association of hormonal contraceptives with verbal and global cognition, as well as a beneficial association on executive function with more than 10 years of hormonal contraceptive use. Longer reproductive life was associated with better cognitive performance. All reproductive findings were mutually adjusted and controlled for several non-reproductive variables related to cognition.
Reproductive factors in women are considered a reflection of cumulative exposures to endogenous and exogenous sex steroid hormones. A considerable body of research indicates a significant role of sex steroid hormones, particularly estrogen, on cognition 2,3,17,22,24 and progesterone on neurogenesis 25. Accumulating evidence from neuroscience 2–6 and animal behavioral research provide a compelling rationale for the hypothesis that reproductive events have a long-term impact on cognition 26.
Years of reproductive capacity reflects duration of exposure to premenopausal levels of endogenous sex steroid hormones. Our results related to age at menarche and reproductive period are consistent with previous studies 8–12,27. The inverse association between later age of menarche and global cognition and positive association of reproductive period with global cognition and executive functions were evident with adjustment for reproductive events that alter hormonal levels. Although the reproductive period is a function of age at menarche, the associations of these factors with cognition were independent of each other. In a population-based cohort of French women aged 65 and older, later menarche was inversely associated with visual memory and psychomotor speed, whereas longer reproductive period was positively associated with verbal fluency 12. The risk of cognitive impairment was increased with later menarche and younger menopause (but not with reproductive period) in a case-control study among Swedish twin pairs aged 65–84 years 11. Our results indicate that earlier gonadal hormone exposures associated with earlier age at menarche may contribute to cognitive function later in life independently of its contribution to a longer reproductive period. In our sample, scores on the Wechsler Adult Reading Test were not correlated with age at menarche (r=−0.05, p=0.14), suggesting that this inverse association of age at menarche with cognition in later life does not merely reflect an association with genetic factors and early life exposures that influence cognitive abilities in adult life. Other studies also reported a positive association between longer reproductive period and cognition 9,10. Later menarche was also linked with increased risk of Alzheimer disease 28.
A last pregnancy after age 35 was positively associated with verbal memory and to some extent global cognition. This association was not evident when age at last pregnancy was modeled as a continuous variable, suggesting an age-specificity of this association. Although age at last pregnancy has been investigated in relation to other endogenous hormone-related conditions13–16, our findings in relation to cognitive function are novel. Both pregnancy and post-partum contribute robust changes in the sex steroid hormonal milieu. Pregnancy induces a tremendous surge in estradiol and progesterone levels 29 . Animal studies show improvement in learning and memory during pregnancy, post-partum, and even later 30–32. Human studies have failed to confirm these animal studies 33,34 and have even suggested impaired verbal memory 35, word fluency and word list learning 35 in pregnant compared to non-pregnant women. Few studies have evaluated long-term changes in cognitive function in relation to pregnancy. Animal studies show that transient hormonal during pregnancy induce neurogenesis in brain regions involved with cognition 36–38. Functional brain changes induced by reproductive experiences have been suggested to have lifelong effects 39, particularly in terms of improvement in memory and learning. Therefore, it is biologically plausible that a late pregnancy might offer protection from cognitive decline in later life. Alternatively, late pregnancy may reflect socioeconomic and lifestyle factors associated with better cognitive function. Our results were adjusted for race, education, and income, important components of socioeconomic status that are associated with both hormonal contraceptive use and cognitive performance.
Hormonal contraception, predominantly with oral contraceptives, was beneficially associated with global cognition, verbal memory, and executive functions. Only a handful of studies have evaluated oral contraceptive use in relation to late life cognition, with all reporting null associations 9–12,19. Prior studies were characterized by relatively small sample size9,19, limited neurocognitive assessment 10,11, low prevalence of hormonal contraception use 10–12, and lack of data on duration of use 9–12,19. Only one study among 261 healthy middle-aged women (both pre- and postmenopausal) reported significant beneficial associations between longer duration of hormonal contraceptive use and cognition in the visuospatial domain and speed and flexibility 18. While it is possible that postmenopausal women who used hormonal contraception are different from non-users for reasons beyond the factors we adjusted for in our study (age, race, education and income), it is biologically plausible that hormonal contraception may beneficially impact cognition later in life. Hormonal contraception maintains a regular menstrual cycle, primarily through cyclical low levels of estrogen and progesterone, which in turn regulate other sex steroid hormone concentrations through the hypothalamo-pituitary-gonadal axis feedback mechanism and help to maintain a higher level of estrogen than normal state 40. Although the positive association between hormonal contraception and executive functions was nominally significant only among long-term users (>10 years), somewhat smaller positive associations were observed for shorter-term use. The trend test (for duration of use) was significant for verbal memory and near-significant (p=0.06) for executive functions. These findings may simply reflect a dose effect in both domains: larger cognitive effects with longer exposures. If so, we would then predict significant findings for executive functions for the shorter exposures, if our sample size were larger. It is difficult to compare the sensitivity, specificity, and precision of test instruments used to measure memory versus executive functions, and our data do not allow us to disentangle domain effects from measurement issues.
Our study adds significantly to the existing knowledge relating reproductive factors and mid- to late-life cognition. Our primary contribution lies in the multivariable modeling of several key reproductive factors in relation to different aspects of cognitive function obtained from an extensive battery of cognitive tests. In addition to domain-specific cognitive measures, we used a global composite measure to provide a robust indicator of the net cognitive effect, which in most instances would be most important clinically. Although composites of verbal episodic memory and executive functions were correlated with global cognition and with each other, these provide robust markers of domain-specific effects that are most often hypothesized to benefit from estrogen exposures, most often impacted during normal aging, and most often impaired early in the course of Alzheimer disease. Our finding of a significant beneficial association of later age at last pregnancy with verbal memory in later life is novel. Age at last pregnancy was not associated with executive function, which further justifies the use of a global composite score as it is possible that an exposure might enhance one aspect of cognition but have a neutral or deleterious effect on another. We also documented a beneficial duration-dependent association of hormonal contraceptive use on cognition, adding to the limited knowledge in this area. While almost all existing studies on this topic have been limited to women over the age of 60, our study population is unique in that almost half of the women were less than age 60.
Major strengths of our study include the large ethnically diverse sample of mid- to later life cognitively healthy postmenopausal women. All participants were evaluated with an extensive cognitive battery, with standardized collection of reproductive history through a structured questionnaire, allowing analysis of an array of reproductive factors in multivariable models. Collection of a broad array of other demographic and lifestyle variables allowed for control of many possible confounders.
Study limitations included reliance on self-reported recall rather than real-time assessment or other documentation of reproductive events. In addition, the study participants were volunteers in randomized clinical trials, and cannot be considered representative of the general population. Women in this study were well educated with relatively high income levels, and were sufficiently healthy to participate in a clinical trial. It is also possible that some degree of healthy volunteer bias contributed to study findings even after controlling for demographic factors. Results should therefore be interpreted with these cautions in mind.
In conclusion, in this sample of healthy postmenopausal women, reproductive life events related to sex hormones, including earlier age at menarche, later age at last pregnancy, length of reproductive period, and use of oral contraceptives were positively associated with multiple aspects of cognitive function in later life.
Supplementary Material
Supplementary Table S1: Adjusted Mean Cognitive Composite Scores by Reproductive Factors
Supplementary Table S2a: Multivariable Associations of Reproductive Factors with Cognitive Composite Scores: Women Below Age 60 at Baseline
Supplementary Table S2b: Multivariable Associations of Reproductive Factors with Cognitive Composite Scores: Women Age 60 and Older at Baseline
Acknowledgments
Grants/funds: Work was supported by NIH grants U01AT001653, R01AG024154, P01AG026572
Conflict of Interest Disclosures
| Elements of Financial/Personal Conflicts | Karim R | Dang H | Henderson VW | Hodis HN | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | x | ||||
| Grants/Funds | x | x | x | x | ||||
| Honoraria | x | x | x | x | ||||
| Speaker Forum | x | x | x | x | ||||
| Consultant | x | x | x | x | ||||
| Stocks | x | x | x | x | ||||
| Royalties | x | x | x | x | ||||
| Expert Testimony | x | x | x | x | ||||
| Board Member | x | x | x | X | ||||
| Patents | x | x | x | x | ||||
| Personal Relationship | x | x | x | x | ||||
| Elements of Financial/Personal Conflicts | St John J | Brinton R | Mack WJ | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | x | x | x | |||||
| Grants/Funds | x | x | x | |||||
| Honoraria | x | x | x | |||||
| Speaker Forum | x | x | x | |||||
| Consultant | x | x | x | |||||
| Stocks | x | x | x | |||||
| Royalties | x | x | x | |||||
| Expert Testimony | x | x | x | |||||
| Board Member | x | x | x | |||||
| Patents | x | x | x | |||||
| Personal Relationship | x | x | x | |||||
For “yes” x mark(s): give brief explanation below:
Footnotes
Author contributions. Karim: study design, statistical analysis, data interpretation, manuscript preparation; Dang: statistical analysis; Henderson: study design, study conduct, data collection, data interpretation; manuscript preparation; Hodis: study design, study conduct, data collection, data interpretation; manuscript preparation; St. John: data collection, manuscript preparation; Brinton: data interpretation, manuscript preparation; Mack: study design, study conduct, data collection; statistical analysis, data interpretation, manuscript preparation. All authors critically reviewed and approved the final version of the manuscript.
Sponsor’s role. Sponsors had no role in study design or conduct, statistical analysis or preparation of this manuscript.
Employment or affiliation: All authors completed work as part of employment at University of Southern California or Stanford University.
Board Member: Drs. Hodis and Henderson serve/served as board members of North American Menopause Society
References
- 1.Brann DW, Dhandapani K, Wakade C, Mahesh VB, Khan MM. Neurotrophic and neuroprotective actions of estrogen: basic mechanisms and clinical implications. Steroids. 2007;72:381–405. doi: 10.1016/j.steroids.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31:529–37. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brinton RD. Estrogen-induced plasticity from cells to circuits: predictions for cognitive function. Trends Pharmacol Sci. 2009;30:212–22. doi: 10.1016/j.tips.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 5.Amin Z, Epperson CN, Constable RT, Canli T. Effects of estrogen variation on neural correlates of emotional response inhibition. Neuroimage. 2006;32:457–64. doi: 10.1016/j.neuroimage.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 6.Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Hum Brain Mapp. 2014;35:847–65. doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clavel-Chapelon F, Gerber M. Reproductive factors and breast cancer risk. Do they differ according to age at diagnosis? Breast Cancer Res Treat. 2002;72:107–15. doi: 10.1023/a:1014891216621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith CA, McCleary CA, Murdock GA, et al. Lifelong estrogen exposure and cognitive performance in elderly women. Brain Cogn. 1999;39:203–18. doi: 10.1006/brcg.1999.1078. [DOI] [PubMed] [Google Scholar]
- 9.Tierney MC, Ryan J, Ancelin ML, et al. Lifelong estrogen exposure and memory in older postmenopausal women. J Alzheimers Dis. 2013;34:601–8. doi: 10.3233/JAD-122062. [DOI] [PubMed] [Google Scholar]
- 10.Heys M, Jiang C, Cheng KK, et al. Life long endogenous estrogen exposure and later adulthood cognitive function in a population of naturally postmenopausal women from Southern China: the Guangzhou Biobank Cohort Study. Psychoneuroendocrinology. 2011;36:864–73. doi: 10.1016/j.psyneuen.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 11.Rasgon NL, Magnusson C, Johansson AL, Pedersen NL, Elman S, Gatz M. Endogenous and exogenous hormone exposure and risk of cognitive impairment in Swedish twins: a preliminary study. Psychoneuroendocrinology. 2005;30:558–67. doi: 10.1016/j.psyneuen.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Ryan J, Carriere I, Scali J, Ritchie K, Ancelin ML. Life-time estrogen exposure and cognitive functioning in later life. Psychoneuroendocrinology. 2009;34:287–98. doi: 10.1016/j.psyneuen.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Setiawan VW, Pike MC, Karageorgi S, et al. Age at last birth in relation to risk of endometrial cancer: pooled analysis in the epidemiology of endometrial cancer consortium. Am J Epidemiol. 2012;176:269–78. doi: 10.1093/aje/kws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albrektsen G, Heuch I, Tretli S, Kvale G. Breast cancer incidence before age 55 in relation to parity and age at first and last births: a prospective study of one million Norwegian women. Epidemiology. 1994;5:604–11. doi: 10.1097/00001648-199411000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh CC, Chan HW, Lambe M, Ekbom A, Adami HO, Trichopoulos D. Does age at the last birth affect breast cancer risk? Eur J Cancer. 1996;32A:118–21. doi: 10.1016/0959-8049(95)00528-5. [DOI] [PubMed] [Google Scholar]
- 16.Lambe M, Hsieh CC, Chan HW, Ekbom A, Trichopoulos D, Adami HO. Parity, age at first and last birth, and risk of breast cancer: a population-based study in Sweden. Breast Cancer Res Treat. 1996;38:305–11. doi: 10.1007/BF01806150. [DOI] [PubMed] [Google Scholar]
- 17.Henderson VW, Popat RA. Effects of endogenous and exogenous estrogen exposures in midlife and late-life women on episodic memory and executive functions. Neuroscience. 2011;191:129–38. doi: 10.1016/j.neuroscience.2011.05.059. [DOI] [PubMed] [Google Scholar]
- 18.Egan KR, Gleason CE. Longer duration of hormonal contraceptive use predicts better cognitive outcomes later in life. J Womens Health (Larchmt) 2012;21:1259–66. doi: 10.1089/jwh.2012.3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLay RN, Maki PM, Lyketsos CG. Nulliparity and late menopause are associated with decreased cognitive decline. J Neuropsychiatry Clin Neurosci. 2003;15:161–7. doi: 10.1176/jnp.15.2.161. [DOI] [PubMed] [Google Scholar]
- 20.Epperson CN, Sammel MD, Freeman EW. Menopause effects on verbal memory: findings from a longitudinal community cohort. J Clin Endocrinol Metab. 2013;98:3829–38. doi: 10.1210/jc.2013-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henderson VW, St John JA, Hodis HN, et al. Long-term soy isoflavone supplementation and cognition in women: a randomized, controlled trial. Neurology. 2012;78:1841–8. doi: 10.1212/WNL.0b013e318258f822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson VW, St John JA, Hodis HN, et al. Cognition, mood, and physiological concentrations of sex hormones in the early and late postmenopause. Proc Natl Acad Sci U S A. 2013;110:20290–5. doi: 10.1073/pnas.1312353110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakano K, Pinnow E, Flaws JA, Sorkin JD, Gallicchio L. Reproductive history and hot flashes in perimenopausal women. J Womens Health (Larchmt) 2012;21:433–9. doi: 10.1089/jwh.2011.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan J, Stanczyk FZ, Dennerstein L, et al. Hormone levels and cognitive function in postmenopausal midlife women. Neurobiol Aging. 2012;33:617, e11–22. doi: 10.1016/j.neurobiolaging.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Wang J, Zhao L, et al. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150:3186–96. doi: 10.1210/en.2008-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinton RD, Yao J, Yin F, Mack WJ, Cadenas E. Perimenopause as a neurological transition state. Nat Rev Endocrinol. 2015;11:393–405. doi: 10.1038/nrendo.2015.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hesson J. Cumulative estrogen exposure and prospective memory in older women. Brain Cogn. 2012;80:89–95. doi: 10.1016/j.bandc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Paganini-Hill A, Henderson VW. Estrogen deficiency and risk of Alzheimer’s disease in women. Am J Epidemiol. 1994;140:256–61. doi: 10.1093/oxfordjournals.aje.a117244. [DOI] [PubMed] [Google Scholar]
- 29.Berg FD, Kuss E. Serum concentration and urinary excretion of “classical” estrogens, catecholestrogens and 2-methoxyestrogens in normal human pregnancy. Arch Gynecol Obstet. 1992;251:17–27. doi: 10.1007/BF02718274. [DOI] [PubMed] [Google Scholar]
- 30.Kinsley CH, Madonia L, Gifford GW, et al. Motherhood improves learning and memory. Nature. 1999;402:137–8. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 31.Lemaire V, Billard JM, Dutar P, et al. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci. 2006;23:3368–74. doi: 10.1111/j.1460-9568.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- 32.Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–15. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Christensen H, Leach LS, Mackinnon A. Cognition in pregnancy and motherhood: prospective cohort study. Br J Psychiatry. 2010;196:126–32. doi: 10.1192/bjp.bp.109.068635. [DOI] [PubMed] [Google Scholar]
- 34.Glynn LM. Giving birth to a new brain: hormone exposures of pregnancy influence human memory. Psychoneuroendocrinology. 2010;35:1148–55. doi: 10.1016/j.psyneuen.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29:793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 36.Kinsley CH, Trainer R, Stafisso-Sandoz G, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–42. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Levy F, Gheusi G, Keller M. Plasticity of the parental brain: a case for neurogenesis. J Neuroendocrinol. 2011;23:984–93. doi: 10.1111/j.1365-2826.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- 38.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 39.Kinsley CH, Bardi M, Karelina K, et al. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Arch Sex Behav. 2008;37:43–56. doi: 10.1007/s10508-007-9277-x. [DOI] [PubMed] [Google Scholar]
- 40.Atwood CS, Meethal SV, Liu T, et al. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Adjusted Mean Cognitive Composite Scores by Reproductive Factors
Supplementary Table S2a: Multivariable Associations of Reproductive Factors with Cognitive Composite Scores: Women Below Age 60 at Baseline
Supplementary Table S2b: Multivariable Associations of Reproductive Factors with Cognitive Composite Scores: Women Age 60 and Older at Baseline