Abstract
Bordetella parapertussis causes the prolonged coughing illness known as pertussis or whooping cough, persisting for weeks within the respiratory tracts of infected hosts but inducing a very poor T cell response relative to that induced by Bordetella pertussis, the more common cause of pertussis. In this study, we examine the contributions of cytokines involved in the clearance of B. parapertussis and immunomodulation that delays effective clearance. The slow elimination of this pathogen from the respiratory tracts of mice coincides with the gradual accumulation of CD4+ T cells in the lungs and B. parapertussis-responsive IFN-γ–producing cells in the spleen. IFN-γ–deficient mice were defective in the accumulation of leukocytes in lungs and in clearance of B. parapertussis from the lungs. In vitro B. parapertussis-stimulated macrophages produced IL-10, which inhibited the generation of the IFN-γ response that is required for protection in vivo. As compared with wild-type mice, IL-10–deficient mice produced significantly higher levels of IFN-γ, had higher numbers of leukocytes accumulated in the lungs, and cleared B. parapertussis more rapidly. Together, these data indicate that B. parapertussis induces the production of IL-10, which facilitates its persistence within infected hosts by limiting a protective IFN-γ response.
Despite the intricate defense mechanisms used by the mammalian immune system, the threat of disease is ongoing and acts as a stark reminder of the fact that successful microbial pathogens appear to have developed ways of manipulating and evading them. For example, several pathogens are known to promote the production of factors such as IL-10, an anti-inflammatory cytokine and an important regulator of the host immune response, to avoid induction of the host inflammatory response (reviewed in (1, 2)). This cytokine is produced by APCs, such as macrophages and dendritic cells, and results in a T cell response skewed toward a Th2 or Treg phenotype (3). IL-10 is also produced in large quantities by Treg cells and serves as a negative regulator of both Th1 and Th2 responses (4). Importantly, IL-10 inhibits the production of proinflammatory cytokines such as IFN-γ (5, 6). This inhibitory effect is mediated by the suppressive effects of IL-10 on APCs as well as by the upregulation of SOCS1, the gene responsible for the majority of the physiological inhibition of IFN-γ–induced signaling (7, 8).
IFN-γ, which is largely produced by NK cells and activated T cells, contributes to protective immunity against microbial pathogens in part by enhancing both the recruitment of leukocytes to the site of infection and the activation of these cells (9–11). IFN-γ also upregulates the expression of MHC molecules, in addition to other costimulatory molecules, on APCs (12, 13) and drives the differentiation of naive T cells to Th1 cells (14). Although this cytokine is protective against a wide range of pathogens (15, 16), excessive production can lead to severe, uncontrolled inflammation and subsequent damage to host tissues (17, 18). IL-10–mediated inhibition of IFN-γ is crucial for protection against immune-mediated inflammatory disorders such as colitis and experimental autoimmune encephalitis (19–21). Notably, several bacterial pathogens dampen the IFN-γ response, often limiting the protection conferred by this proinflammatory cytokine (22–26). For instance, the inhibition of IFN-γ production by low calcium response toxin enhances the lethality of Yersinia pestis infection (27). Furthermore, some pathogens can modulate specific aspects of IFN-γ–induced signaling pathways to optimize their interactions with the host. For example, Chlamydia species inhibit IFN-γ–mediated MHC class II upregulation, limiting the capacity of CD4+ T cells to respond to the infection (28).
Bordetella pertussis and Bordetella parapertussis are the etiologic agents of whooping cough in humans, which is a severe coughing illness that is re-emerging in even highly vaccinated populations (29–33). These bacteria spread between hosts via cough-produced aerosolized droplets, and the ability to persist within the host respiratory tract increases the amount of time during which the bacteria are shed, thereby enhancing the opportunity for transmission. Previous studies have addressed how B. pertussis persists within its host; pertussis toxin (PTX) prevents the migration of neutrophils to the lungs, allowing B. pertussis to grow to higher numbers in both naive and immune hosts (34, 35). Although not as well studied as B. pertussis, B. parapertussis behaves similarly in a mouse model of infection despite notable differences from B. pertussis, such as the absence of PTX. Inefficient stimulation of proinflammatory TLR4 responses appear to allow it to grow rapidly over the first few days, after which point bacterial numbers slowly decline over a month or more (36). This persistence led us to investigate how B. parapertussis modulates immune responses to extend its survival within infected hosts.
Both B. pertussis and B. bronchiseptica induce IL-10 as a means of limiting the host immune response to allow for persistence within the host, although these bacteria use different subsets of virulence factors not expressed by B. parapertussis or by one another, indicating that the mechanisms by which these pathogens modulate host immunity may differ between species. B. bronchiseptica induces IL-10 via the type III secretion system (25), whereas B. pertussis stimulates the production of this cytokine through the synergistic action of adenylate cyclase toxin and LPS (37). B. parapertussis, although closely related to these pathogens, differs greatly in its expression of virulence factors. For instance, the LPS of B. parapertussis is significantly less stimulatory of TLR4 than that of either B. pertussis or B. bronchiseptica, and human-adapted B. parapertussis strains do not express a type III secretion system (25, 38, 39). In our studies, we evaluate whether the induction of IL-10 contributes to the persistence of B. parapertussis in the host respiratory tract.
We have previously documented that CD4+ T cells are important for the elimination of B. parapertussis from the lower respiratory tract (40). In this study, we show that the reduction of bacterial numbers corresponds to an accumulation of CD4+ T cells in the lungs, as well as to the production of IFN-γ by T cells. Furthermore, we show that this proinflammatory cytokine is required for both the efficient recruitment of leukocytes and the clearance of B. parapertussis from the lungs. However, the IFN-γ response generated against B. parapertussis is delayed by the production of IL-10, which decreases leukocyte accumulation and delays the clearance of B. parapertussis from the lungs. Together, these data suggest that the induction of an IL-10 response, which subsequently limits protective IFN-γ production, allows B. parapertussis to persist within the host respiratory tract.
Materials and Methods
Bacterial strains and growth
B. parapertussis strain 12822 was isolated from German clinical trials and has previously been described (41). B. parapertussis strain 12822G is an isogenic gentamicin-resistant derivative of 12822 (40). Bacteria were maintained on Bordet‑Gengou agar (Difco, Albany, NY) containing 10% defibrinated sheep blood (Hema Resources, Aurora, OR) and appropriate antibiotics. Liquid cultures of bacteria were grown at 37°C overnight on a roller drum to midlog phase (an OD of ∼0.3) in Stainer-Scholte broth. Liquid cultures were incubated at 70°C for 30 min to generate preparations of heat-killed bacteria. These cultures were plated before and after incubation to determine the number of viable bacteria present.
Inoculation of mice
C57BL/6, TCRβδ−/− (B6.129P2-Tcrbtm1MomTcrdtm1Mom/J), IFN-γ−/− (B6.129S7-Ifngtm1Ts/J), and IL-10−/− (B6.129P2-Il10tm1Cgn/J) mice were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred in our Bordetella-free, specific pathogen-free facilities at The Pennsylvania State University. Bacteria grown overnight in liquid culture were diluted in PBS to ∼1 × 107 CFU/ml. Groups of 4- to 6-wk-old mice were lightly sedated with 5% isoflurane in oxygen and inoculated by pipetting 50 μl of the inoculum (5 × 105 CFU) onto the external nares. Heat-killed bacteria were diluted to 109 CFU/ml and delivered by pipetting 50 μl (5 × 107 CFU) onto the external nares. All of the protocols were reviewed by the university’s Institutional Animal Care and Use Committee, and all of the animals were handled in accordance with institutional guidelines.
Quantification of bacteria and leukocytes in the lungs
To quantify bacteria, lungs were excised on day 0, 3, 7, 14, or 28 post-inoculation and homogenized in 1 ml of PBS, and the homogenate was serially diluted and plated onto Bordet‑Gengou agar plates. CFUs were counted after incubating for 3 d at 37°C. To quantify leukocytes, bronchoalveolar lavage fluid (BALF) was collected from B. parapertussis-infected mice on day 0 (uninfected mice), 3, 7, 14, or 28 postinoculation. RBCs were lysed by treatment with 0.83% ammonium chloride as previously described (18). Leukocytes were counted on a hemocytometer to quantify total numbers in the BALF. Aliquots of cells were then stained with FITC-labeled anti–Ly-6G (eBioscience, San Diego, CA) for neutrophils, FITC-labeled anti-F4/80 (eBioscience) for macrophages, or FITC-labeled anti-CD4 (eBioscience) for CD4+ T cells, and the percentage of FITC-positive cells was multiplied by the total number of leukocytes to calculate the numbers of neutrophils, macrophages, and CD4+ T cells, respectively.
Splenocyte restimulations
Restimulation of splenocytes was performed as previously described (18). Briefly, splenocytes were isolated by homogenizing spleens through a wire sieve, spinning at 400 × g for 5 min at 4°C, lysing the RBCs with 0.83% ammonium chloride, and then washing the cells with DMEM (HyClone, Logan, UT). Cells were resuspended in DMEM supplemented with 10% FCS (HyClone), 1 mM sodium pyruvate (HyClone), and 100 μg/ml penicillin and streptomycin (HyClone) at a concentration of 1 × 107cells/ml. Two hundred microliters of this suspension (2 × 106 cells) were placed into each well of a 96-well plate. Splenocytes were stimulated with medium alone or 1 × 107 CFU of heat-killed (HK) B. parapertussis (multiplicity of infection (MOI) of 5). After 3 d, the supernatant was collected and analyzed for IFN-γ and IL-10 production as specified by the manufacturers of ELISA kits (R&D Systems, Minneapolis, MN).
Stimulation of macrophage cell lines
The murine macrophage cell line MH-S was obtained from American Type Culture Collection (Manassas, VA) and cultured in RPMI 1640 supplemented with 10% FBS, penicillin, and streptomycin. Cells were placed in 96-well plates with ∼105 cells/well and stimulated with either live B. parapertussis or HK B. parapertussis at MOIs of 1, 10, or 100 or 1 or 100 ng/well of purified B. parapertussis LPS to equal a total volume of 100 μl/well (19). HK B. parapertussis was made by heating cultures at 65°C for 30 min. Proteinase K was added to cultures at a concentration of 50 μg/ml for 30 min at 37°C. Both trypsin and RNase were added at a concentration of 200 μg/ml at 37°C for 10 and 30 min, respectively. Cell culture supernatants were collected after 24 h, and the concentration of IL-10 was measured via ELISA. These results were also reproduced using J774 cells, a murine peritoneal macrophage cell line.
Intracellular cytokine staining
Peritoneal exudate cells were analyzed by flow cytometry for intracellular IL-10. Briefly, cells were collected, RBCs were lysed with 0.83% ammonium chloride solution, and leukocytes were resuspended in DMEM before being counted on a hemocytometer. Approximately 2 × 106 cells were incubated at 37°C in 5% CO2 with 1 × 107 CFU of HK B. parapertussis and brefeldin A for 12 h. Cells were stained with a FITC-labeled surface marker for macrophages (F4/80), fixed, permeabilized, and stained with PE-labeled IL-10 Abs according to the manufacturer’s protocol (eBioscience). Cells were analyzed by flow cytometry for the quantification of double-positive cells.
Histological examination
Lungs were removed and inflated with 10% neutral buffered formalin, embedded in paraffin, and sectioned. Slides were prepared and stained with H&E, and an assessment of microscopic lesions was made by one of the authors (M.J.K.), who is experienced in rodent pathology and was blinded to the experimental treatments. Descriptive evaluations of the lesions were recorded, and lung lesions were graded by using a scale of 0–4. Sections with no lesions and no inflammation were given a score of 0, a score of 1 indicated slight inflammation with few or scattered lesions and <10% of lung fields affected, a score of 2 indicated mild lesions with 10–20% of lung fields affected, a score of 3 indicated moderate lesions with 20–30% of the lung fields affected, and those given a score of 4 were characterized by extensive lesions, marked inflammation, and 31–50% of the lung affected. Plus marks indicated scores with a severity that fell between categories. Photographs were taken at ×100 magnification with an Olympus (Center Valley, PA) BH-2 light microscope fitted with a Nikon (Melville, NY) digital camera (DXM1200F).
Statistical analysis
Three to four mice were used per group per individual time point in each experiment. The mean for each group of mice ± SD (error bars) was determined for CFU, leukocytes, and cytokines. Two-tailed, unpaired Student t tests were used to determine statistical significance between groups. All of the experiments were performed at least twice with similar results, and figures shown are representative of one experiment. p < 0.05 were taken to be statistically significant.
Results
T cells required for the clearance of B. parapertussis are slowly recruited to the lungs
B. parapertussis persists in the respiratory tract of its host for a month or more but is unlikely to do so by the same mechanism as B. pertussis because it lacks PTX. To study the mechanism by which B. parapertussis may manipulate host immune responses, we first examined the kinetics of the generation of responses that are involved in protection. C57BL/6 mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 postinoculation to quantify bacterial numbers in the lungs and CD4+ T cell numbers in the BALF, because these cells are essential to the clearance of B. parapertussis (40). B. parapertussis numbers peaked at ∼4 × 106 CFU on day 3 postinoculation and slowly declined thereafter until bacteria were nearly undetectable by day 28 postinoculation (Fig. 1). CD4+ T cells were almost undetectable on days 0 and 3 postinoculation but had increased to ∼3 × 103 cells/ml of BALF by days 7 and 14 postinoculation and to 2 × 104 cells/ml of B. parapertussis correlates with the slow accumulation of CD4+ T cells in the BALF, although they do not define the numbers of T cells in tissues or nodes or the function of those cells.
FIGURE 1.
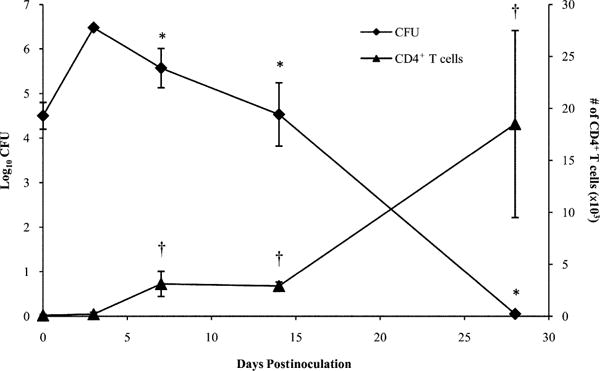
B. parapertussis and CD4+ T cell numbers in murine lungs over time. C57BL/6 mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 postinoculation to quantify bacterial numbers in the lungs or CD4+ T cells in the BALF. Bacterial numbers are expressed as the log10 mean CFU of each group of four mice ± SD. CD4+ T cell numbers are expressed as the mean number of cells per lung ± SD. *p < 0.05 when comparing bacterial numbers to the peak observed on day 3 postinoculation; †p < 0.05 when comparing CD4+ T cell numbers to those of naive mice.
T cells produce IFN-γ, which facilitates the clearance of B. parapertussis
IFN-γ is a proinflammatory cytokine required for protection against the closely related pathogens B. pertussis (42, 43) and B. bronchiseptica (25). Because activated T cells are a major source of IFN-γ, we examined the IFN-γ response against B. parapertussis in wild-type and T cell-deficient mice. First, we investigated the presence of IFN-γ in the lung homogenates during infection and found the levels to be very low. Therefore, we examined the systemic cytokine response generated against B. parapertussis through restimulation of splenocytes over the course of infection, which provided a more robust representation of the Ag-specific cytokine response. C57BL/6 and TCRβδ−/− mice were inoculated with B. parapertussis, and their spleens excised for ex vivo assessment of IFN-γ production on days 0, 7, 14, and 28 postinoculation. IFN-γ was produced at low levels (<50 pg/ml) by media-stimulated splenocytes, which serves as the unstimulated control to determine background cytokine levels, from either mouse strain (Fig. 2). Upon stimulation with HK B. parapertussis, splenocytes from wild-type mice produced IFN-γ, which peaked on days 7 and 28 postinoculation at concentrations of 1000 and 200 pg/ml, respectively, with low levels observed between these time points (Fig. 2). Similar results were observed upon examination of lung homogenate cytokines in C57BL/6 mice during infection (data not shown). HK B. parapertussis stimulated splenocytes from T cell-deficient mice produced low levels of IFN-γ (<50 pg/ml) at all time points (Fig. 2), indicating that T cells are required for the efficient production of this cytokine in response to B. parapertussis.
FIGURE 2.
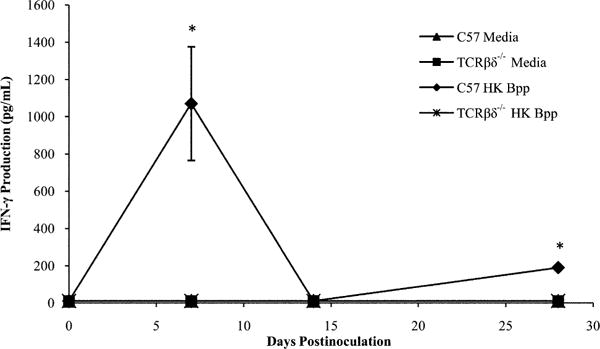
Production of IFN-γ in response to B. parapertussis. C57BL/6 and TCRβδ−/− mice were inoculated with B. parapertussis and sacrificed 0, 7, 14, or 28 d later. Splenocytes were exposed to media or HK B. parapertussis and monitored for the production of IFN-γ. IFN-γ levels are expressed as the mean pictograms per milliliter produced by each group of mice ± SD. *p < 0.05 when compared with media-stimulated controls.
We hypothesized that IFN-γ contributes to the clearance of B. parapertussis from the lungs. To test this, C57BL/6 and IFN-γ −/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 postinoculation for the enumeration of bacterial numbers in the lungs. In wild-type mice, B. parapertussis numbers peaked at ∼5 × 106 CFU on day 3 postinoculation and declined thereafter until nearly undetectable by day 28 postinoculation (Fig. 3). Bacterial numbers in the lungs of IFN-γ −/− mice were similar to those of wild-type mice on days 3 and 7 postinoculation. However, the lungs of IFN-γ−/− mice harbored ∼100-fold more bacteria as compared with those of wild-type mice on days 14 and 28 postinoculation (Fig. 3). Overall, these data indicate that IFN-γ is required for the efficient elimination of B. parapertussis from the lungs of mice.
FIGURE 3.
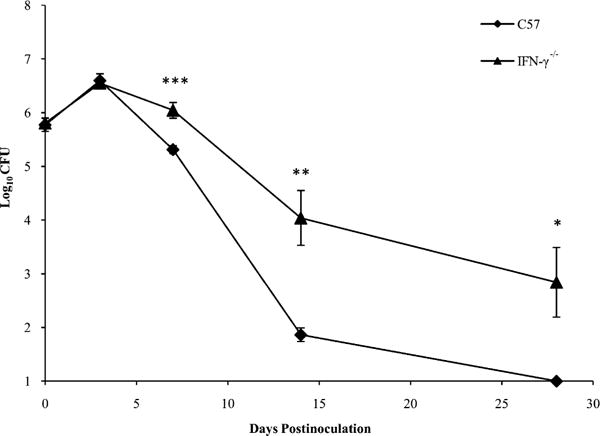
Effect of IFN-γ on clearance of B. parapertussis. C57BL/6 and IFN-γ−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 for the quantification of bacterial numbers in the lungs. CFU are expressed as the log10 mean of each group of mice ± SD. *p < 0.05; **p < 0.01; ***p < 0.001.
IFN-γ is required for the efficient recruitment of leukocytes to the lungs upon B. parapertussis infection
IFN-γ can mediate the recruitment of leukocytes to the site of infection by inducing the production of chemokines by immune cells (44, 45). To assess the role of IFN-γ in the recruitment of leukocytes during B. parapertussis infection, C57BL/6 and IFN-γ−/− mice were inoculated with B. parapertussis and sacrificed on day 3, 7, 14, or 28 for BALF leukocyte enumeration. In wild-type mice, few leukocytes were recovered from the BALF by day 3 postinoculation (∼5 × 105). Leukocyte numbers rose to peak levels on day 7 postinoculation (∼2 × 106) and declined thereafter (Fig. 4A). Leukocyte numbers in the BALF of IFN-γ−/− mice were also low on day 3 postinoculation (∼5 × 105) but remained low even on day 7 postinoculation (∼8 × 105) (Fig. 4A). Similarly, more neutrophils and macrophages were recovered from the lungs of wild-type mice as compared with those of IFN-γ−/− mice (Fig. 4B, 4C), although IFN-γ did not appear to affect the accumulation of T cells (data not shown). Thus, IFN-γ contributes to the efficient recruitment of leukocytes to the lungs in response to B. parapertussis infection.
FIGURE 4.
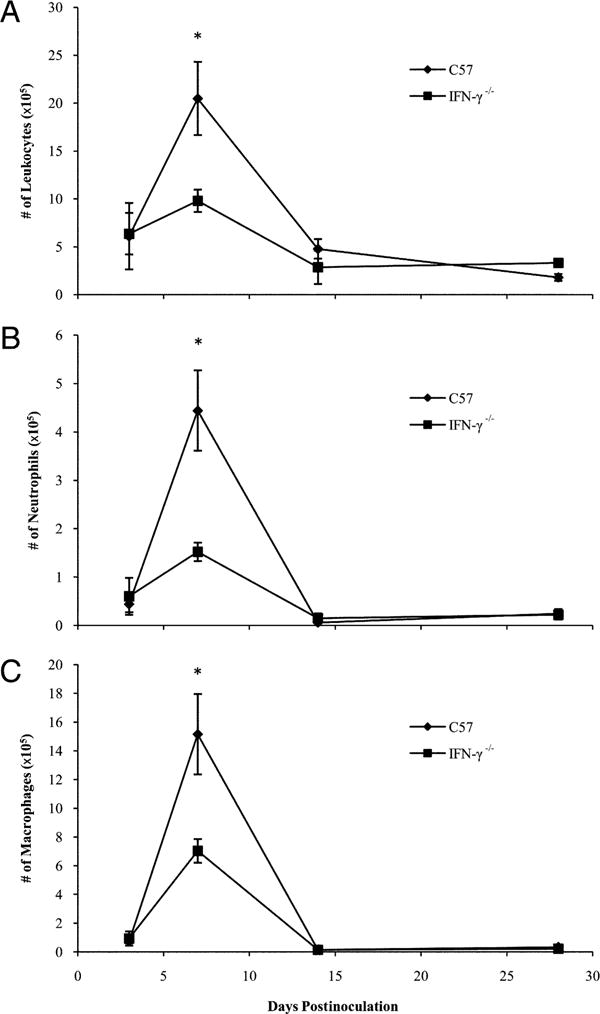
Leukocyte accumulation in response to B. parapertussis infection of wild-type and IFN-γ–deficient mice. C57BL/6 and IFN-γ−/− mice were inoculated with B. parapertussis and sacrificed on day 3, 7, 14, or 28 to quantify (A) total leukocytes, (B) neutrophils, or (C) macrophages in the BALF. WBC numbers are expressed as the mean number of cells per lung ± SD. *p < 0.05 when comparing wild-type versus IFN-γ−/− mice.
IL-10 is produced by macrophages in response to B. parapertussis
IFN-γ is important for the efficient clearance of B. parapertussis from the lungs, but its production can be modulated by IL-10 (46), an anti-inflammatory cytokine known to be induced by other Bordetella species (25, 37). Therefore, we measured IL-10 production in response to B. parapertussis infection and assessed the cell type responsible for the production of this cytokine. C57BL/6 and TCRβδ−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 postinoculation. Splenocytes were isolated and stimulated with HK B. parapertussis to assess IL-10 production by these cells. IL-10 was nearly undetectable in the supernatants of unstimulated splenocytes at any time point. In contrast, IL-10 was produced by HK B. parapertussis stimulated splenocytes from wild-type and T cell-deficient mice (200 and 350 pg/ml, respectively) on day 3 postinoculation, whereas lower levels (<50 pg/ml) were observed in the supernatants of stimulated splenocytes from mice that had been infected for 7 d (Fig. 5A). After day 7 postinoculation, IL-10 was produced in large amounts, but wild-type splenocytes produced significantly more than T cell-deficient splenocytes (day 14, 700 and 400 pg/ml; day 28, 4000 and 600 pg/ml, respectively). Together, these data indicate that some non-T cell produces IL-10 early in response to B. parapertussis, whereas T cells produce larger amounts of this cytokine after the first week of infection.
FIGURE 5.
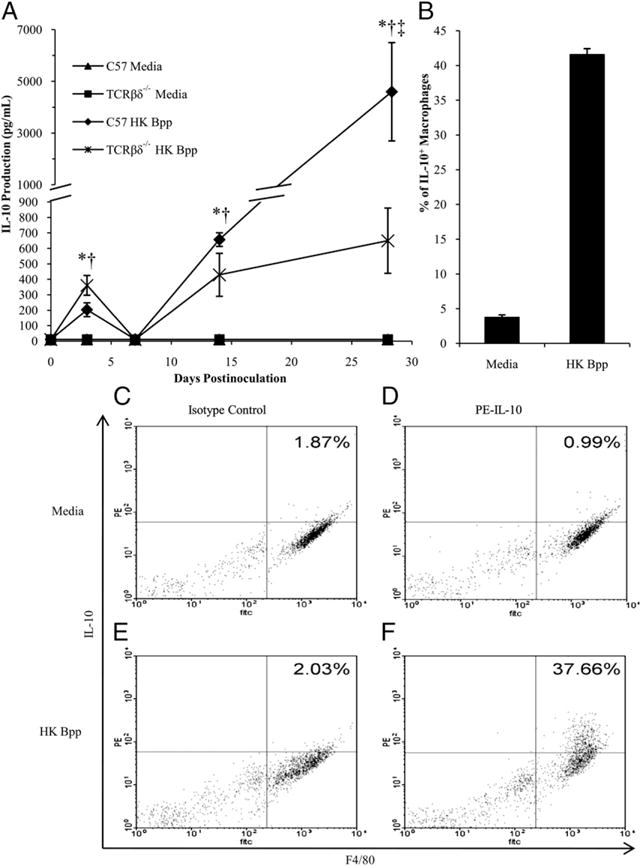
IL-10 production in response to B. parapertussis. A, C57BL/6 and TCRβδ−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 postinoculation for the quantification of IL-10 production by splenocytes. IL-10 levels are expressed as the mean picograms per milliliter of each group of mice ± SD. p < 0.05 when comparing media control to HK B. parapertussis stimulated are denoted by asterisks for C57BL/6 or dagger for TCRβδ−/−, and double dagger represents p < 0.05 when comparing HK B. parapertussis stimulated C57BL/6 and TCRβδ−/− mice. The lower limit of detection was 50 pg. B–F, Peritoneal macrophages from C57BL/6 mice were stimulated with media (C, D) or HK B. parapertussis (E, F) at an MOI of 5, and the percentages of IL-10-positive macrophages were quantified (B). Peritoneal exudate cells were stained with a PE-labeled isotype control Ab (C, E) or PE-labeled anti–IL-10 (D, F).
Because macrophages provide a first line of defense against infection and can produce IL-10, we examined whether B. parapertussis induces the production of IL-10 by these cells. Peritoneal exudate cells isolated from wild-type mice were incubated with HK B. parapertussis and intracellularly stained to assess IL-10 production induced upon primary Ag exposure. Although only 3% of macrophages stimulated with the media control were positive for intracellular IL-10, ∼40% of macrophages stimulated with B. parapertussis were positive for this cytokine after 12 h (Fig. 5B–F), indicating that macrophages produce IL-10 in response to B. parapertussis.
MH-S cells, a murine alveolar macrophage cell line, were then stimulated with live B. parapertussis, HK B. parapertussis, or purified B. parapertussis LPS. Live bacteria induced dose-dependent production of IL-10, with an MOI of 100 eliciting ∼50 pg/ml of IL-10 (Fig. 6A). In contrast, an equivalent number of heat-killed bacteria induced much lower levels of IL-10 at a concentration of only 20 pg/ml (Fig. 6A). Therefore, live B. parapertussis and to a lesser extent HK B. parapertussis induce the production of IL-10 by macrophages. B. parapertussis LPS (1 and 100 ng/well) did not induce levels of IL-10 that were measurably different from those induced by PBS alone (data not shown). In addition, when live B. parapertussis was treated with formalin, trypsin, or proteinase K prior to stimulation of the MH-S cells, IL-10 production was markedly decreased to only 13 pg/ml or less at an MOI of 100 (Fig. 6A). To further understand the mechanism by which B. parapertussis induces IL-10 production in macrophage cells, MH-S cells were also stimulated with culture supernatants. Macrophage cells treated with B. parapertussis culture supernatants produced IL-10 at levels comparable to those of live B. parapertussis-stimulated cells, although this effect was abrogated when supernatants were treated with formalin, trypsin, or proteinase K (Fig. 6B). Strikingly, when B. parapertussis culture supernatants were treated with RNase, IL-10 production was only slightly decreased as compared with live B. parapertussis or B. parapertussis culture supernatants alone (Fig. 6B). On the basis of these data, B. parapertussis appears to secrete a heat-labile protein factor that induces the production of IL-10 by macrophages.
FIGURE 6.
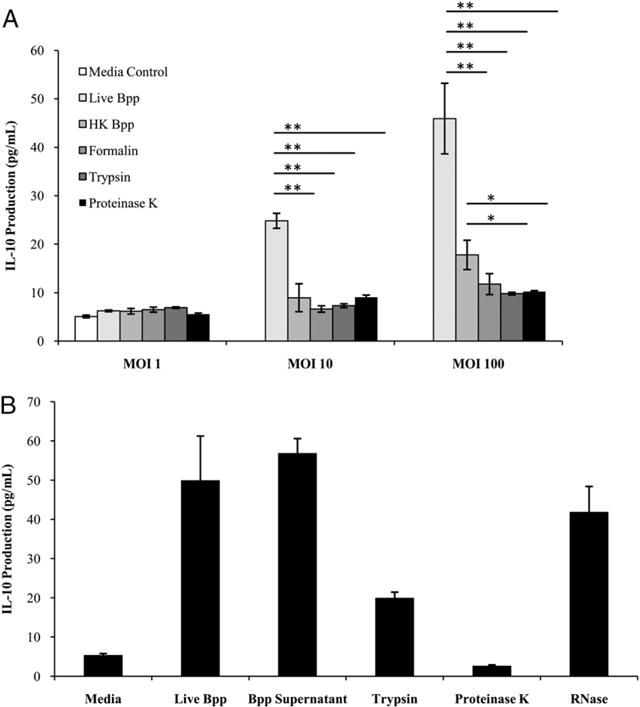
IL-10 production by macrophages in response to live versus HK B. parapertussis. A, MH-S cells were stimulated with live B. parapertussis, HK B. parapertussis, or live B. parapertussis treated with heat, formalin, trypsin, or proteinase K at varying MOIs. IL-10 levels are expressed as the mean picograms per milliliter ± SD. *p < 0.05; **p < 0.01. B, MH-S cells were stimulated with live B. parapertussis, bacterial culture supernatants, and supernatants that were treated with proteinase K, trypsin, or RNase. Cells were stimulated with these groups for 24 h before supernatants were collected and analyzed for IL-10 quantification via ELISA. IL-10 levels are expressed as the mean picograms per milliliter ± SD.
IL-10 inhibits IFN-γ production and the resulting inflammatory response to B. parapertussis
To investigate the possibility that IL-10 limits the IFN-γ–mediated leukocyte recruitment important for the clearance of B. parapertussis infection, C57BL/6 and IL-10−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, or 14 postinoculation for the quantification of leukocytes in the BALF. Again, the peak of leukocyte accumulation in the lungs occurred around day 7 postinoculation in wild-type mice, although this peak was significantly higher in the lungs of IL-10−/− mice (Fig. 7A). Similarly, more neutrophils were recovered from the lungs of IL-10−/− mice (6 × 105) compared with those of wild-type mice (1.5 × 105) on day 7 postinoculation (Fig. 7B), indicating that IL-10 does limit the accumulation of neutrophils in the lungs during B. parapertussis infection. In addition, histological examination of wild-type versus IL-10–deficient lungs was performed to further assess the inflammatory infiltrate induced by B. parapertussis infection. Little inflammation was observed in the lungs of naive wild-type (Fig. 7C) or IL-10–deficient mice (Fig. 7D). By day 3 postinoculation, there were few signs of inflammation in the lungs of wild-type mice (Fig. 7E), but foci of inflammation, largely composed of neutrophils, were observed in the lungs of IL-10−/− mice (Fig. 7F). The lungs of wild-type mice contained numerous mild lesions by day 7 postinoculation (Fig. 7G), whereas those of IL-10−/− mice presented with lesions in addition to regions of pneumonia and leukocyte infiltration (Fig. 7H). Together, these data indicate that IL-10 inhibits the inflammatory response against B. parapertussis infection.
FIGURE 7.
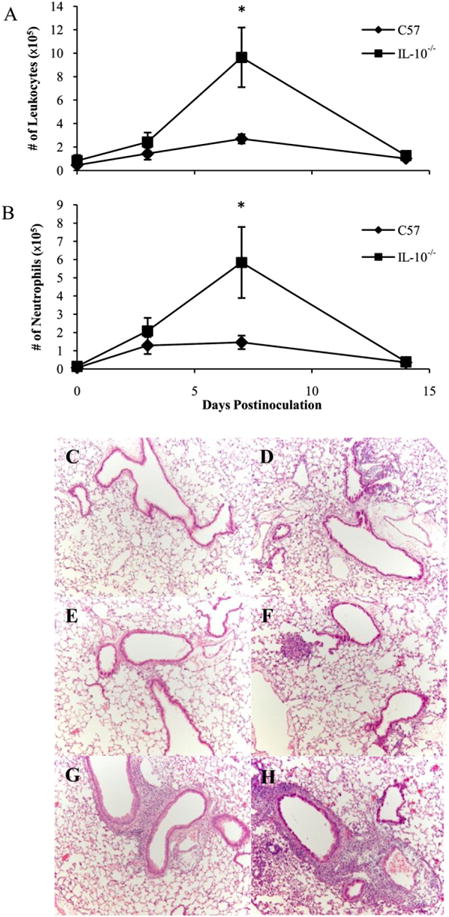
Effect of IL-10 on the inflammatory response to B. parapertussis infection. C57BL/6 and IL-10−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, or 14 to quantify leukocytes (A) and neutrophils (B) in the BALF. Cell numbers are expressed as the mean number of cells per lung ± SD; *p < 0.05. C–H, Mice were also sacrificed on day 0 (C, D), 3 (E, F), or 7 (G, H) to evaluate pathology of the lungs of C57BL/6 (C, E, G) and IL-10−/− (D, F, H) mice. C–H, Sections stained with H&E are shown at ×100 magnification.
IL-10 inhibits the clearance of B. parapertussis from the respiratory tract
If inhibiting protective leukocyte influx via IL-10 induction is a strategy used by B. parapertussis to persist in its host, then we would expect that this pathogen would be more rapidly eliminated from IL-10-deficient hosts. To test this, C57BL/6 and IL-10−/− mice were inoculated with B. parapertussis and sacrificed on days 0, 3, 7, 14, or 28 postinoculation for the quantification of bacterial numbers in the lungs. B. parapertussis numbers peaked on day 3 (4 × 106 CFU) in the lungs of wild-type mice and were reduced to 3 × 104 and <100 CFU by days 14 and 28, respectively (Fig. 8). In the lungs of IL-10−/− mice, bacterial numbers also peaked at 4 × 106 CFU on day 3 postinoculation but were reduced to <100 CFU by day 14 and were undetectable by day 28 postinoculation (Fig. 8). Therefore, the 100-fold higher CFU in C57BL/6 versus IL-10−/− mice indicates that IL-10 delays the elimination of B. parapertussis from murine lungs.
FIGURE 8.
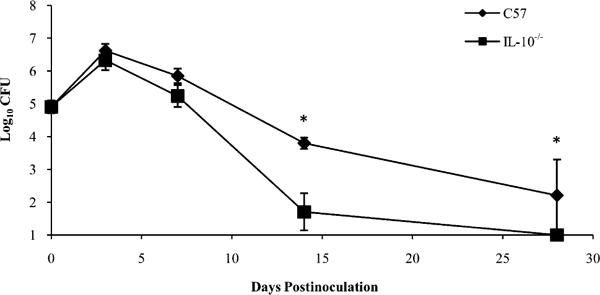
Effect of IL-10 on the clearance of B. parapertussis from the lungs. C57BL/6 and IL-10−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 3, 7, 14, or 28 to quantify bacterial numbers in the lungs. CFU are expressed as the log10 mean of each group of mice ± SD; *p < 0.05.
Because an innate IL-10 response could skew the T cell response toward a Th2 or Treg phenotype, we assessed the effect of IL-10 on the IFN-γ response generated against B. parapertussis (47). The amounts of IFN-γ produced by splenocytes from C57BL/6 versus IL-10−/− mice in response to B. parapertussis after 0, 7, and 28 d of infection were compared. Unstimulated splenocytes produced <50 pg/ml of IFN-γ. Similarly, B. parapertussis-stimulated splenocytes from naive mice produced low levels of this cytokine. B. parapertussis-stimulated splenocytes from C57BL/6 mice infected with B. parapertussis for 7 or 28 d produced ∼1750 and 250 pg of IFN-γ, respectively (Fig. 9), whereas those from IL-10−/− mice produced ∼12,500 and 6000 pg of IFN-γ, respectively, at these times (Fig. 9). Similar results were also observed upon examination of lung homogenate cytokines (data not shown). Overall, these data indicate that B. parapertussis induces much higher IFN-γ production in the absence of IL-10.
FIGURE 9.
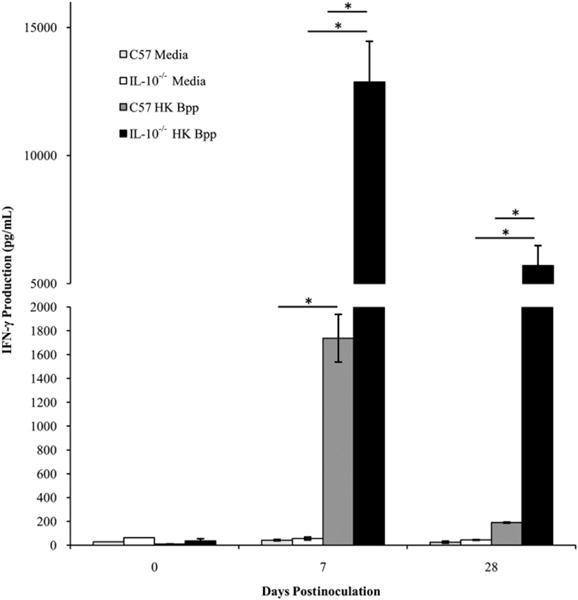
Effect of IL-10 on the IFN-γ response to B. parapertussis. C57BL/6 and IL-10−/− mice were inoculated with B. parapertussis and sacrificed on day 0, 7, or 28. Splenocytes were exposed to media or HK B. parapertussis and monitored for the production of IFN-γ. IFN-γ production was quantified and expressed as the mean picograms per milliliter of each group of mice ± SD. The lower limit of detection was 50 pg; *p < 0.05.
Discussion
Acute pathogens have a limited opportunity to transmit to new hosts during short infectious periods, providing a strong selective advantage to those that can develop mechanisms to prolong infection. Furthermore, many pathogens, including the bordetellae, are capable of modulating aspects of the immune system to promote their persistence within hosts. Because IL-10 is an anti-inflammatory cytokine that significantly dampens critical immune effectors known to be involved in bacterial clearance, there should be strong selective advantage to pathogens that have evolved mechanisms by which they can influence the production of this cytokine to avoid immune-mediated clearance. There are examples of both microbial pathogens and commensals that induce IL-10 production by tissue resident macrophages and dendritic cells as a means of dampening host immune responses (48).
Interestingly, IL-10–deficient mice clear both B. bronchiseptica and B. parapertussis from their respiratory tracts more rapidly than wild-type mice (25; Fig. 8), although the absence of IL-10 does not appear to affect the course of B. pertussis infection (data not shown), an observation likely due to the expression of PTX by this species. During B. bronchiseptica or B. parapertussis infection, IL-10 limits the IFN-γ–mediated recruitment of leukocytes to the lungs. Consequently, in the absence of IL-10, a stronger inflammatory response may result in more efficient clearance of these pathogens. Although more IFN-γ could also be produced during B. pertussis infection in IL-10–deficient hosts, the expression of PTX inhibits the recruitment of phagocytic cells (35, 49, 50) and may, therefore, overcome the inflammatory effects of increased IFN-γ on bacterial clearance.
The protective immune response against infection by B. parapertussis should be considered when creating the next generation of whooping cough vaccines. Although the incidence of whooping cough dropped upon the introduction of whole-cell whooping cough vaccines in the 1940s and 1950s (51, 52), the switch from whole-cell to acellular vaccines has roughly correlated with the recent resurgence of whooping cough. Importantly, neither vaccine is very effective against B. parapertussis, with the acellular vaccine being even less effective than whole-cell vaccines (53, 54). Whole-cell vaccines stimulate a Th1-skewed response characterized by large amounts of IFN-γ, but current acellular vaccines induce a Th2-skewed T cell response with little Bordetella-specific IFN-γ (53–55). Because this cytokine is now known to be protective against both causative agents of whooping cough, B. pertussis (43) and B. parapertussis (Fig. 2), it seems likely that modifying vaccines to induce more of a Th1 response could help to combat the resurgence of this disease.
Although B. parapertussis is able to cause the same respiratory illness as B. pertussis despite the absence of PTX, little is known regarding the virulence factors that are important to its pathogenesis. Recent work has illuminated the importance of O Ag, which is not expressed by B. pertussis, in B. parapertussis pathogenesis. We have previously reported that O Ag enables B. parapertussis to avoid B. pertussis-induced Ab responses (56), potentially providing an explanation as to how B. parapertussis causes disease in B. pertussis-immunized individuals (57). Also, O Ag enhances the evasion of complement-mediated control by this pathogen (58). Despite its protective role, the fact that O Ag is a component of LPS makes it an unlikely candidate to be included in future vaccines. Although it is unclear which B. parapertussis protein toxins and/or adhesins could be effectively used in immunizations, our observation that IL-10 induction facilitates its persistence by modulating IFN-γ provides important insight into the pathogenesis of B. parapertussis disease and the type of immune response required for efficient protection against this respiratory pathogen.
Acknowledgments
We thank Dr. Anne Buboltz and Dr. Elizabeth Goebel for a critical reading of the manuscript, Dr. Avery August and Dr. Sandeep Prabhu for the use of reagents and equipment, and the staff of The Pennsylvania State University flow cytometry facility for technical assistance.
This work was funded by National Institutes of Health Grant AI 053075 (to E.T.H.).
Abbreviations used
- BALF
bronchoalveolar lavage fluid
- HK
heat-killed
- MOI
multiplicity of infection
- PTX
pertussis toxin
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-β: the role of T regulatory cells. Immunology. 2006;117:433–442. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Garra A, Vieira PL, Vieira P, Goldfeld AE. IL-10‑producing and naturally occurring CD4+ Tregs: limiting collateral damage. J Clin Invest. 2004;114:1372–1378. doi: 10.1172/JCI23215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- 4.Groux H, O’Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997;389:737–742. doi: 10.1038/39614. [DOI] [PubMed] [Google Scholar]
- 5.Fiorentino DF, Zlotnik A, Vieira P, Mosmann TR, Howard M, Moore KW, O’Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- 6.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174:1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander WS, Starr R, Fenner JE, Scott CL, Handman E, Sprigg NS, Corbin JE, Cornish AL, Darwiche R, Owczarek CM, et al. SOCS1 is a critical inhibitor of interferon γ signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 8.Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- 9.Ellis TN, Beaman BL. Interferon-γ activation of polymorphonuclear neutrophil function. Immunology. 2004;112:2–12. doi: 10.1111/j.1365-2567.2004.01849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun K, Salmon SL, Lotz SA, Metzger DW. Interleukin-12 promotes gamma interferon-dependent neutrophil recruitment in the lung and improves protection against respiratory Streptococcus pneumoniae infection. Infect Immun. 2007;75:1196–1202. doi: 10.1128/IAI.01403-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper AM, Adams LB, Dalton DK, Appelberg R, Ehlers S. IFN-γ and NO in mycobacterial disease: new jobs for old hands. Trends Microbiol. 2002;10:221–226. doi: 10.1016/s0966-842x(02)02344-2. [DOI] [PubMed] [Google Scholar]
- 12.Boss JM. Regulation of transcription of MHC class II genes. Curr Opin Immunol. 1997;9:107–113. doi: 10.1016/s0952-7915(97)80166-5. [DOI] [PubMed] [Google Scholar]
- 13.Thomas HE, Parker JL, Schreiber RD, Kay TWH. IFN-γ action on pancreatic beta cells causes class I MHC upregulation but not diabetes. J Clin Invest. 1998;102:1249–1257. doi: 10.1172/JCI2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magram J, Connaughton SE, Warrier RR, Carvajal DM, Wu CY, Ferrante J, Stewart C, Sarmiento U, Faherty DA, Gately MK. IL-12-deficient mice are defective in IFN γ production and type 1 cytokine responses. Immunity. 1996;4:471–481. doi: 10.1016/s1074-7613(00)80413-6. [DOI] [PubMed] [Google Scholar]
- 15.Jouanguy E, Döffinger R, Dupuis S, Pallier A, Altare F, Casanova JL. IL-12 and IFN-γ in host defense against mycobacteria and salmonella in mice and men. Curr Opin Immunol. 1999;11:346–351. doi: 10.1016/s0952-7915(99)80055-7. [DOI] [PubMed] [Google Scholar]
- 16.Novelli F, Casanova JL. The role of IL-12, IL-23 and IFN-γ in immunity to viruses. Cytokine Growth Factor Rev. 2004;15:367–377. doi: 10.1016/j.cytogfr.2004.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hazlett LD. Pathogenic mechanisms of P. aeruginosa keratitis: a review of the role of T cells, Langerhans cells, PMN, and cytokines. DNA Cell Biol. 2002;21:383–390. doi: 10.1089/10445490260099665. [DOI] [PubMed] [Google Scholar]
- 18.Breuer K, Wittmann M, Kempe K, Kapp A, Mai U, Dittrich-Breiholz O, Kracht M, Mrabet-Dahbi S, Werfel T. Alpha-toxin is produced by skin colonizing Staphylococcus aureus and induces a T helper type 1 response in atopic dermatitis. Clin Exp Allergy. 2005;35:1088–1095. doi: 10.1111/j.1365-2222.2005.02295.x. [DOI] [PubMed] [Google Scholar]
- 19.Berg DJ, Davidson N, Kühn R, Müller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4+ TH1-like responses. J Clin Invest. 1996;98:1010–1020. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh UP, Singh S, Taub DD, Lillard JW., Jr Inhibition of IFN-γ-inducible protein-10 abrogates colitis in IL-10−/− mice. J Immunol. 2003;171:1401–1406. doi: 10.4049/jimmunol.171.3.1401. [DOI] [PubMed] [Google Scholar]
- 21.Yang J, Lindsberg PJ, Hukkanen V, Seljelid R, Gahmberg CG, Meri S. Differential expression of cytokines (IL-2, IFN-γ, IL-10) and adhesion molecules (VCAM-1, LFA-1, CD44) between spleen and lymph nodes associates with remission in chronic relapsing experimental autoimmune encephalomyelitis. Scand J Immunol. 2002;56:286–293. doi: 10.1046/j.1365-3083.2002.01132.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoshizawa S, Tateda K, Matsumoto T, Gondaira F, Miyazaki S, Standiford TJ, Yamaguchi K. Legionella pneumophila evades gamma interferon-mediated growth suppression through interleukin-10 induction in bone marrow-derived macrophages. Infect Immun. 2005;73:2709–2717. doi: 10.1128/IAI.73.5.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuirk P, McCann C, Mills KH. Pathogen-specific T regulatory 1 cells induced in the respiratory tract by a bacterial molecule that stimulates interleukin 10 production by dendritic cells: a novel strategy for evasion of protective T helper type 1 responses by Bordetella pertussis. J Exp Med. 2002;195:221–231. doi: 10.1084/jem.20011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Yamaguchi K. Induction of interleukin-10 and down-regulation of cytokine production by Klebsiella pneumoniae capsule in mice with pulmonary infection. J Med Microbiol. 2001;50:456–461. doi: 10.1099/0022-1317-50-5-456. [DOI] [PubMed] [Google Scholar]
- 25.Pilione MR, Harvill ET. The Bordetella bronchiseptica type III secretion system inhibits gamma interferon production that is required for efficient antibody-mediated bacterial clearance. Infect Immun. 2006;74:1043–1049. doi: 10.1128/IAI.74.2.1043-1049.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skinner JA, Pilione MR, Shen H, Harvill ET, Yuk MH. Bordetella type III secretion modulates dendritic cell migration resulting in immunosuppression and bacterial persistence. J Immunol. 2005;175:4647–4652. doi: 10.4049/jimmunol.175.7.4647. [DOI] [PubMed] [Google Scholar]
- 27.Brubaker RR. Interleukin-10 and inhibition of innate immunity to Yersiniae: roles of Yops and LcrV (V antigen) Infect Immun. 2003;71:3673–3681. doi: 10.1128/IAI.71.7.3673-3681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong G, Fan T, Liu L. Chlamydia inhibits interferon γ-inducible major histocompatibility complex class II expression by degradation of upstream stimulatory factor 1. J Exp Med. 1999;189:1931–1938. doi: 10.1084/jem.189.12.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Melker HE, Schellekens JF, Neppelenbroek SE, Mooi FR, Rümke HC, Conyn-van Spaendonck MA. Reemergence of pertussis in the highly vaccinated population of the Netherlands: observations on surveillance data. Emerg Infect Dis. 2000;6:348–357. doi: 10.3201/eid0604.000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skowronski DM, De Serres G, MacDonald D, Wu W, Shaw C, Macnabb J, Champagne S, Patrick DM, Halperin SA. The changing age and seasonal profile of pertussis in Canada. J Infect Dis. 2002;185:1448–1453. doi: 10.1086/340280. [DOI] [PubMed] [Google Scholar]
- 31.von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–750. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 32.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE, EUVAC-NET Group Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24:761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Pertussis—United States, 1997–2000. JAMA. 2002;287:977–979. [PubMed] [Google Scholar]
- 34.Carbonetti NH, Artamonova GV, Andreasen C, Bushar N. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect Immun. 2005;73:2698–2703. doi: 10.1128/IAI.73.5.2698-2703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirimanjeswara GS, Agosto LM, Kennett MJ, Bjornstad ON, Harvill ET. Pertussis toxin inhibits neutrophil recruitment to delay antibody-mediated clearance of Bordetella pertussis. J Clin Invest. 2005;115:3594–3601. doi: 10.1172/JCI24609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolfe DN, Buboltz AM, Harvill ET. Inefficient Toll-like receptor-4 stimulation enables Bordetella parapertussis to avoid host immunity. PLoS One. 2009;4:e4280. doi: 10.1371/journal.pone.0004280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross PJ, Lavelle EC, Mills KH, Boyd AP. Adenylate cyclase toxin from Bordetella pertussis synergizes with lipopolysaccharide to promote innate interleukin-10 production and enhances the induction of Th2 and regulatory T cells. Infect Immun. 2004;72:1568–1579. doi: 10.1128/IAI.72.3.1568-1579.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mann PB, Wolfe D, Latz E, Golenbock D, Preston A, Harvill ET. Comparative Toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect Immun. 2005;73:8144–8152. doi: 10.1128/IAI.73.12.8144-8152.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parkhill J, Sebaihia M, Preston A, Murphy LD, Thomson N, Harris DE, Holden MTG, Churcher CM, Bentley SD, Mungall KL, et al. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat Genet. 2003;35:32–40. doi: 10.1038/ng1227. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe DN, Kirimanjeswara GS, Harvill ET. Clearance of Bordetella parapertussis from the lower respiratory tract requires humoral and cellular immunity. Infect Immun. 2005;73:6508–6513. doi: 10.1128/IAI.73.10.6508-6513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heininger U, Cotter PA, Fescemyer HW, Martinez de Tejada G, Yuk MH, Miller JF, Harvill ET. Comparative phenotypic analysis of the Bordetella parapertussis isolate chosen for genomic sequencing. Infect Immun. 2002;70:3777–3784. doi: 10.1128/IAI.70.7.3777-3784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahon BP, Sheahan BJ, Griffin F, Murphy G, Mills KH. Atypical disease after Bordetella pertussis respiratory infection of mice with targeted disruptions of interferon-γ receptor or immunoglobulin μ chain genes. J Exp Med. 1997;186:1843–1851. doi: 10.1084/jem.186.11.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahon BP, Mills KH. Interferon-γ mediated immune effector mechanisms against Bordetella pertussis. Immunol Lett. 1999;68:213–217. doi: 10.1016/s0165-2478(99)00070-x. [DOI] [PubMed] [Google Scholar]
- 44.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-γ. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 45.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 46.Conti P, Kempuraj D, Kandere K, Di Gioacchino M, Barbacane RC, Castellani ML, Felaco M, Boucher W, Letourneau R, Theoharides TC. IL-10, an inflammatory/inhibitory cytokine, but not always. Immunol Lett. 2003;86:123–129. doi: 10.1016/s0165-2478(03)00002-6. [DOI] [PubMed] [Google Scholar]
- 47.Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy—review of a new approach. Pharmacol Rev. 2003;55:241–269. doi: 10.1124/pr.55.2.4. [DOI] [PubMed] [Google Scholar]
- 48.Rigby RJ, Knight SC, Kamm MA, Stagg AJ. Production of interleukin (IL)-10 and IL-12 by murine colonic dendritic cells in response to microbial stimuli. Clin Exp Immunol. 2005;139:245–256. doi: 10.1111/j.1365-2249.2004.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molski TF, Naccache PH, Marsh ML, Kermode J, Becker EL, Sha’afi RI. Pertussis toxin inhibits the rise in the intracellular concentration of free calcium that is induced by chemotactic factors in rabbit neutrophils: possible role of the “G proteins” in calcium mobilization. Biochem Biophys Res Commun. 1984;124:644–650. doi: 10.1016/0006-291x(84)91603-6. [DOI] [PubMed] [Google Scholar]
- 50.Bradford PG, Rubin RP. Pertussis toxin inhibits chemotactic factor-induced phospholipase C stimulation and lysosomal enzyme secretion in rabbit neutrophils. FEBS Lett. 1985;183:317–320. doi: 10.1016/0014-5793(85)80801-2. [DOI] [PubMed] [Google Scholar]
- 51.Lautrop H. Epidemics of parapertussis 20 years’ observations in Denmark. Lancet. 1971;297:1195–1198. doi: 10.1016/s0140-6736(71)91713-2. [DOI] [PubMed] [Google Scholar]
- 52.Cherry JD. The epidemiology of pertussis and pertussis immunization in the United Kingdom and the United States: a comparative study. Curr Probl Pediatr. 1984;14:7–77. doi: 10.1016/0045-9380(84)90016-1. [DOI] [PubMed] [Google Scholar]
- 53.Mahon BP, Brady MT, Mills KH. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J Infect Dis. 2000;181:2087–2091. doi: 10.1086/315527. [DOI] [PubMed] [Google Scholar]
- 54.Barnard A, Mahon BP, Watkins J, Redhead K, Mills KH. Th1/Th2 cell dichotomy in acquired immunity to Bordetella pertussis: variables in the in vivo priming and in vitro cytokine detection techniques affect the classification of T-cell subsets as Th1, Th2 or Th0. Immunology. 1996;87:372–380. doi: 10.1046/j.1365-2567.1996.497560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mahon BP, Ryan MS, Griffin F, Mills KH. Interleukin-12 is produced by macrophages in response to live or killed Bordetella pertussis and enhances the efficacy of an acellular pertussis vaccine by promoting induction of Th1 cells. Infect Immun. 1996;64:5295–5301. doi: 10.1128/iai.64.12.5295-5301.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wolfe DN, Goebel EM, Bjornstad ON, Restif O, Harvill ET. The O antigen enables Bordetella parapertussis to avoid Bordetella pertussis-induced immunity. Infect Immun. 2007;75:4972–4979. doi: 10.1128/IAI.00763-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Restif O, Wolfe DN, Goebel EM, Bjornstad ON, Harvill ET. Of mice and men: asymmetric interactions between Bordetella pathogen species. Parasitology. 2008;135:1517–1529. doi: 10.1017/S0031182008000279. [DOI] [PubMed] [Google Scholar]
- 58.Goebel EM, Wolfe DN, Elder K, Stibitz S, Harvill ET. O antigen protects Bordetella parapertussis from complement. Infect Immun. 2008;76:1774–1780. doi: 10.1128/IAI.01629-07. [DOI] [PMC free article] [PubMed] [Google Scholar]


