Abstract
Background
Self-management interventions are widely implemented in care for patients with heart failure (HF). Trials however show inconsistent results and whether specific patient groups respond differently is unknown. This individual patient data meta-analysis assessed the effectiveness of self-management interventions in HF patients and whether subgroups of patients respond differently.
Methods and Results
Systematic literature search identified randomized trials of self-management interventions. Data of twenty studies, representing 5624 patients, were included and analyzed using mixed effects models and Cox proportional-hazard models including interaction terms. Self-management interventions reduced risk of time to the combined endpoint HF-related hospitalization or all-cause death (hazard ratio [HR], 0.80; 95% confidence interval [CI], 0.71–0.89), time to HF-related hospitalization (HR, 0.80; 95%CI, 0.69–0.92), and improved 12-month HF-related quality of life (standardized mean difference 0.15; 95%CI, 0.00–0.30). Subgroup analysis revealed a protective effect of self-management on number of HF-related hospital days in patients <65 years (mean number of days 0.70 days vs. 5.35 days; interaction p=0.03). Patients without depression did not show an effect of self-management on survival (HR for all-cause mortality, 0.86; 95%CI, 0.69–1.06), while in patients with moderate/severe depression self-management reduced survival (HR, 1.39; 95%CI, 1.06–1.83, interaction p=0.01).
Conclusions
This study shows that self-management interventions had a beneficial effect on time to HF-related hospitalization or all-cause death, HF-related hospitalization alone, and elicited a small increase in HF-related quality of life. The findings do not endorse limiting self-management interventions to subgroups of HF patients, but increased mortality in depressed patients warrants caution in applying self-management strategies in these patients.
Keywords: heart failure, individual patient data meta-analysis, self-management, subgroup analysis
Heart failure (HF) is one of the most prevalent chronic conditions1 and despite advances in medical treatment, patients diagnosed with HF face an increased risk of hospitalization and mortality.2 The impact of HF on patients’ lives is substantial, as they are expected to adhere daily to drug treatment, lifestyle changes and monitoring of signs and symptoms to prevent decompensation.3 Self-management interventions, which aim at improving patients’ knowledge and skills to perform those behaviors and manage their condition, have received increasing attention in care for patients with HF.
A meta-analysis on the effects of self-management interventions in patients with HF showed significant reductions of all-cause and HF-related hospitalization in patients receiving the self-management intervention, although there were no effects on mortality and quality of life (QoL).4 A more recent systematic review, however, emphasized the heterogeneous findings across studies.5 Several recently conducted large randomized controlled trials (RCTs) were unable to show beneficial effects of self-management interventions on mortality or hospitalization rates,6–9 further illustrating heterogeneity in observed effects.
Part of this heterogeneity may be attributable to varying trial designs, intervention components, follow-up periods, or outcome assessments. Since individual RCTs included different groups of patients, variations in patient characteristics are another likely source of heterogeneity. Specific subgroups of patients might benefit more, or even might not benefit, from self-management interventions. Such knowledge will contribute to targeting self-management interventions to those groups anticipated to benefit most, which may become indispensable in times of decreasing resources.
Sample sizes in individual trials are generally too small to identify factors modifying the success of self-management interventions. By combining data from multiple trials, individual patient data (IPD) meta-analysis allows a reliable identification of patient subgroups with a differential treatment response. Furthermore, IPD meta-analysis enables a uniform definition of subgroups across studies, uniform imputation of missing data and statistical analysis, and analysis of unreported endpoints.10 Additionally, the main effects of included self-management interventions can be pooled and analyzed in a uniform manner.
This IPD meta-analysis aimed to evaluate effectiveness of self-management interventions regarding HF-related or generic quality of life, HF-related or all-cause hospitalization, and all-cause mortality and to identify subgroups of patients with HF that respond differently to such interventions.
Methods
Data Sources and Study Selection
The electronic databases of PubMed, EMBASE, CENTRAL, PsycINFO and CINAHL were searched from January 1985 through June 2013, as well as reference lists of systematic reviews.
Studies were included if they (1) met the definition of self-management intervention, (2) had a RCT design, (3) included patients with an established diagnosis of HF, (4) compared the self-management intervention to usual care or another self-management intervention, (5) reported data on one or more of the selected outcomes, (6) followed patients for at least six months, and (7) were reported in English, Dutch, French, German, Italian, Portuguese, or Spanish. Self-management interventions were defined as interventions providing information to patients and minimally two of the following components: (1) stimulation of sign/symptom monitoring, (2) education in problem solving skills, and enhancement of (3) medical treatment adherence, (4) physical activity, (5) dietary intake, or (6) smoking cessation. Studies were independently assessed by two researchers (NHJ and HW) on risk of bias (low/unclear/high) using three criteria based on the ‘Risk of bias’ tool from the Cochrane Collaboration11: (1) random concealed allocation to treatment, (2) intention-to-treat analysis, and (3) other deviances (e.g., high drop-out, imbalances between groups). Any discrepancies were solved through consensus with a third researcher (JCAT). Studies scoring a high risk of bias on one or more criteria used from the ‘Risk of bias’ tool11 were defined as ‘high risk of bias’. Those studies were included in the analysis, but the impact of studies of lower methodological quality was assessed in a sensitivity analysis by excluding these studies.
Data collection
The principal investigators of selected studies were invited to participate in this IPD meta-analysis and share their de-individualized raw trial data. For details on the search syntax, collaboration with principal investigators, and a list of all requested variables, we refer to the study protocol.12 Data from each trial were checked on range, extreme values, internal consistency, missing values, and consistency with published reports. When recoding of categorical variables was needed to create uniform categories, principal investigators were consulted to ensure correct interpretation of variables. This IPD meta-analysis is exempt from formal approval by the Medical Research Ethics Committee of University Medical Center Utrecht, since it re-analyzes de-identified data from trials in which informed consent has been obtained by principal investigators.
Outcomes
This study focused in the analysis on 8 main outcomes, divided into HF-related outcomes and general outcomes. HF-related outcomes were time to the combined endpoint of HF-related hospitalization or all-cause death, time to first HF-related hospitalization, total days of HF-related hospital stay at 12 months, and HF-related quality of life (HF-QoL) at 12 months (measured with Heart Failure Symptom Scale,13 Kansas City Cardiomyopathy Questionnaire,14 MacNew Heart Disease Health-related Quality of Life Instrument,15 or Minnesota Living With Heart Failure Questionnaire16). General outcomes were generic QoL at 12 months (measured with Short Form Health Survey 1217 or 3618), time to all-cause death, time to first all-cause hospitalization, and total days of all-cause hospital stay at 12 months. In addition, outcomes at 6 months and binary outcomes for mortality and hospitalization at 6 and 12 months were collected and analyzed, but are presented in Supplemental Tables 1 and 2 as subordinate outcomes.
Patient-specific effect modifiers
Clinically relevant potential effect modifiers (i.e., variables, such as sex or age, that modify the effect of self-management interventions) were selected based on the self-management literature in HF patients19 and availability of comparable data across trials. The selected patient characteristics are presented along with the baseline data in Table 1. We assumed that these characteristics could modify the effect of interventions; e.g., self-management interventions might be more effective in patients with only primary education compared to higher educated patients.
Table 1.
Baseline characteristics of heart failure patients included in individual patient data meta-analysis.
| Control | Intervention | Total | |
|---|---|---|---|
| Sample size, n | 2674 | 2950 | 5624 |
| Sex | |||
| Male | 1505 (56.2) | 1711 (58.0) | 3126 (57.2) |
| Female | 1169 (43.7) | 1239 (42.0) | 2408 (42.8) |
| Age, y | 69.9 ± 12.3 | 69.6 ± 12.4 | 69.7 ± 12.4 |
| <65 years | 796 (29.8) | 917 (31.1) | 1713 (30.5) |
| 65–80 years | 1358 (50.8) | 1491 (50.5) | 2849 (50.7) |
| >80 years | 520 (19.4) | 542 (18.4) | 1062 (18.9) |
| Systolic dysfunction: LVEF | 39.7 ± 18.4 | 38.7 ± 18.1 | 39.2 ± 18.2 |
| >35% LVEF | 805 (48.8) | 903 (47.3) | 1708 (48.0) |
| ≤35% LVEF | 846 (51.2) | 1008 (52.7) | 1854 (52.0) |
| NYHA class | |||
| NYHA I & II | 1141 (45.2) | 1317 (47.0) | 2458 (46.1) |
| NYHA III | 899 (35.6) | 1065 (38.0) | 1964 (36.9) |
| NYHA IV | 484 (19.2) | 422 (15.0) | 906 (17.0) |
| Comorbidity index* | |||
| No comorbid conditions | 401 (16.7) | 556 (20.7) | 957 (18.8) |
| Comorbid conditions in 1 cluster | 925 (38.6) | 991 (36.9) | 1916 (37.7) |
| Comorbid conditions in >1 cluster | 1070 (44.7) | 1136 (42.3) | 2206 (43.4) |
| Depression† | |||
| No/mild depression | 959 (73.9) | 1169 (68.8) | 2128 (71.0) |
| Moderate/severe depression | 339 (26.1) | 531 (31.2) | 870 (29.0) |
| Level of education | |||
| Primary education or below | 807 (42.3) | 910 (39.4) | 1717 (40.7) |
| Secondary education | 711 (37.3) | 939 (40.6) | 1650 (39.1) |
| Higher education | 388 (20.4) | 461 (20.0) | 849 (20.1) |
| Years since diagnosis (median and interquartile range) | 2.0 (0.1–6.0) | 1.3 (0.1–5.2) | 1.6 (0.1–5.4) |
| <1 year diagnosed | 400 (41.3) | 619 (46.2) | 1019 (44.1) |
| 1–2 years diagnosed | 118 (12.2) | 171 (12.8) | 289 (12.5) |
| >2 years diagnosed | 451 (46.5) | 551 (41.1) | 1002 (43.4) |
| Living status | |||
| Living with others | 1064 (75.2) | 1076 (73.2) | 2140 (74.2) |
| Living alone | 350 (24.8) | 393 (26.8) | 743 (25.8) |
| Body mass index | 28.2 ± 6.9 | 27.9 ± 6.4 | 28.0 ± 6.6 |
| <25 | 483 (34.2) | 647 (36.1) | 1130 (35.3) |
| 25–29.99 | 508 (36.0) | 611 (34.1) | 1119 (35.0) |
| ≥30 | 420 (29.8) | 532 (29.7) | 952 (29.7) |
| Smoking status | |||
| Current non-smoker | 933 (79.9) | 993 (82.1) | 1926 (81.1) |
| Current smoker | 234 (20.1) | 216 (17.9) | 450 (18.9) |
LVEF indicates left ventricular ejection fraction; and NYHA, New York Heart Association.
Values are n(%), mean±SD or median(interquartile range).
Categories in the present IPD meta-analysis are based on clusters of the Cumulative Illness Rating Scale.20
Based on validated cut-off scores of instrument used in each specific study.
Statistical analyses
Principal investigators were involved in designing a detailed plan for the statistical analysis and agreed upon this prior to data analysis (see Supplemental Methods for detailed statistical plan). Data from individual studies were merged to create one database. Using multiple imputation by chained equations (25 imputations),21 missing values for baseline variables and outcomes were imputed within studies. The imputed datasets were analyzed using a one-stage approach (i.e., simultaneously analyzing all observations while accounting for clustering of observations within studies).22 Results of imputed datasets were pooled using Rubin’s rules and presented as the primary results.23
All analyses were performed according to the intention-to-treat principle. For time-to-event endpoints, effects of self-management were quantified by estimating hazard ratios (HR) using Cox proportional-hazard models, including a frailty term to account for clustering within studies. The continuous outcomes (HF-QoL and generic QoL) were quantified by standardized mean differences (SMD) between intervention arms and analyzed using linear mixed effects models. To correctly model the presence of overdispersion in count data of total days of hospital stay, negative binomial mixed effects models were used to estimate relative length of stay. Binary outcome data (all-cause mortality, all-cause, and HF-related hospitalization) were analyzed with log-binomial mixed effects models, which estimated risk ratios (RR). In case of non-convergence of a model, odds ratios (OR) were estimated using a logistic mixed effects model, which is an addition to the published protocol.12 All mixed effects models included a random intercept and random slope for the treatment effect to take clustering within studies into account.
To assess whether the effect of self-management was modified by patient characteristics, the aforementioned models were extended with interaction terms for categorical patient characteristics included in Table 1. This was performed for each characteristic separately. If there were two or more effect modifiers with p<0.10 for the interaction (likelihood ratio test), the interaction terms were included in a multivariable model to estimate the effect of self-management within subgroups independent of other relevant effect modifiers. Effect modification was considered significant if the interaction term showed p<0.05 in the final model.
As a sensitivity analysis, we investigated potential retrieval bias (i.e., selective inclusion of studies in the IPD meta-analysis). Published main effects of studies for which we could not obtain the original data (and thus were not included in the IPD meta-analysis) were pooled in a random effects meta-analysis, together with the main effects of included studies. We repeated the main effects analysis by excluding the studies with enhanced usual care. To assess the impact of studies of lower methodological quality, a sensitivity analysis was performed excluding studies with a high risk of bias. Three additional sensitivity analyses assessed the robustness of the effect modifier analysis: (1) complete-case analysis to assess the effect of imputing data, (2) analyses restricted to newer studies (recruitment since 2000), and (3) excluding studies one-by-one to assess if the observed subgroup effects are attributable to a specific study. All analyses were done in R for Windows version 3.1.1 (R Development Core Team, Vienna).
Results
Thirty-two studies (n=8737) met the inclusion criteria and principal investigators were approached to participate in this IPD meta-analysis. The investigators of five studies could not be contacted, IPD of three studies were no longer available, and investigators of four studies were not willing to participate. This resulted in inclusion of data of 20 RCTs, representing 5624 patients in total.
Patient characteristics for which baseline data were available are presented in Table 1. A majority of patients was male (57.2%) and mean age was 69.7 years (SD 12.4). Mean left-ventricular ejection fraction (LVEF) was 39.2% (SD 18.2) and 26.0% of patients had a preserved ejection fraction (≥50%). Median time since diagnosis of HF was 1.6 years (IQR 0.1–5.4). Baseline characteristics of patients included in this IPD meta-analysis were similar to those of patients in eligible studies that did not provide original data, except for the percentage males and current smokers (resp. 63.8% and 11.2% in non-participating studies).
Characteristics of included studies are presented in Table 2. Sample size ranged from 4231 to 1023 patients.7 The majority of interventions were delivered by a specialized nurse, two interventions used a group approach,29,39 and two interventions consisted of telephonic case management.36,37 One trial included two intervention arms.7 Duration of the interventions ranged from 0.525,30 to 187 months. Two studies provided “enhanced care” to the control patients,6,29 consisting of some educational components. These components were judged marginal and in line with the education delivered to HF patients in usual care. Consequently, these two studies were included in the analysis.
Table 2.
Description of trials on self-management in heart failure patients included in individual patient data meta-analysis (N=20).
| Study | Country | Sample size |
Setting | Intervention group | Control group | Duration (months)* |
|---|---|---|---|---|---|---|
| Agren, 201224 | Sweden | 155 | Clinic/hospital or home | 3 individual sessions for patient and partner by nurse | Usual care | 3 |
| Aldamiz, 200725 |
Spain | 279 | Clinic/hospital and home | 4 home visits by nurse/physician | Usual care | 0.5 |
| Atienza, 200426 |
Spain | 338 | Clinic/hospital | 1 individual session before discharge by nurse, 1 visit to physician, 3-monthly follow-up visits, and tele-monitoring |
Usual care | 12 |
| Blue, 200127 | United Kingdom |
165 | Clinic/hospital and home | Home visits by nurse, follow-up telephone calls with intensity based on patient's needs |
Usual care | 12 |
| Bruggink, 200728 |
Netherlands | 240 | Clinic/hospital | 2 individual sessions by nurse/physician, 1 telephone call, follow-up 6 visits |
Usual care | 12 |
| DeWalt, 20126 | United States |
605 | Clinic/hospital | 1 individual session by health educator, follow-up multiple telephone calls |
Usual care + 1 session on self-management and educational manual |
12 |
| Heisler, 201329 | United States |
266 | Clinic/hospital and home | 1 group session by lay peer tutor, weekly telephone contact with matched peer, follow-up 3 optional group sessions |
Usual care + 1 group session on self-management |
6 |
| Jaarsma, 199930 | Netherlands | 179 | Clinic/hospital and home | 1 home visit and 1 telephone call after discharge by nurse | Usual care | 0.5 |
| Jaarsma, 20087 | Netherlands | 1023 | Clinic/hospital |
1: 2 individual sessions by cardiologist, 9 visits to nurse, possibility to contact nurse 2: 2 individual sessions by cardiologist, 18 visits to nurse, 2 home visits, 2 multidisciplinary sessions, follow-up regular telephone contact by nurse |
Usual care | 18 |
| Leventhal, 201131 |
Switzerland | 42 | Clinic/hospital and home | 1 home visit by nurse, educational booklet, follow-up 17 telephone calls |
Usual care + booklet | 12 |
| Martensson, 200532 |
Sweden | 153 | Home (recruitment general practice) |
1 individual session by nurse, follow-up educational CD- ROM and telephone contact |
Usual Care | 12 |
| Otsu, 201133 | Japan | 102 | Clinic/hospital | 6 individual sessions by nurse | Usual care | 6 |
| Peters-Klimm, 201034 |
Germany | 197 | Home (recruitment general practice) |
1 individual session by nurse/physician, follow-up 3 home visits and telephone calls |
Usual care | 12 |
| Rich, 199535 | United States |
282 | Clinic/hospital and home | Daily visits by multidisciplinary professionals during hospitalization, follow-up home visits and telephone calls by nurse at decreasing intensity |
Usual care | 3 |
| Riegel, 200236 | United States |
358 | Telephonic case- management |
Telephone calls by nurse at decreasing intensity | Usual care | 6 |
| Riegel, 200637 | United States |
135 | Telephonic case- management |
Telephone calls by nurse at decreasing intensity | Usual care | 6 |
| Sisk, 200638 | United States |
406 | Clinic/hospital | 1 individual session by nurse, follow-up telephone calls | Usual care | 12 |
| Smeulders, 200939 |
Netherlands | 317 | Clinic/hospital | 6 group sessions by lay peer tutor and nurse, handbook, follow-up telephone contact with co-participants |
Usual care | 1.5 |
| Stromberg. 200340 |
Sweden | 106 | Clinic/hospital and home | 1 visit after discharge to nurse, follow-up based on patient’s status and needs (face-to-face and/or telephone) |
Usual care | 12 |
| Tsuyuki. 200441 |
Canada | 276 | Clinic/hospital | 1 individual session by pharmacist, follow-up 7 telephone calls by nurse |
Usual care + general heart failure brochure |
6 |
Duration of the self-management intervention evaluated.
Main effects of self-management interventions
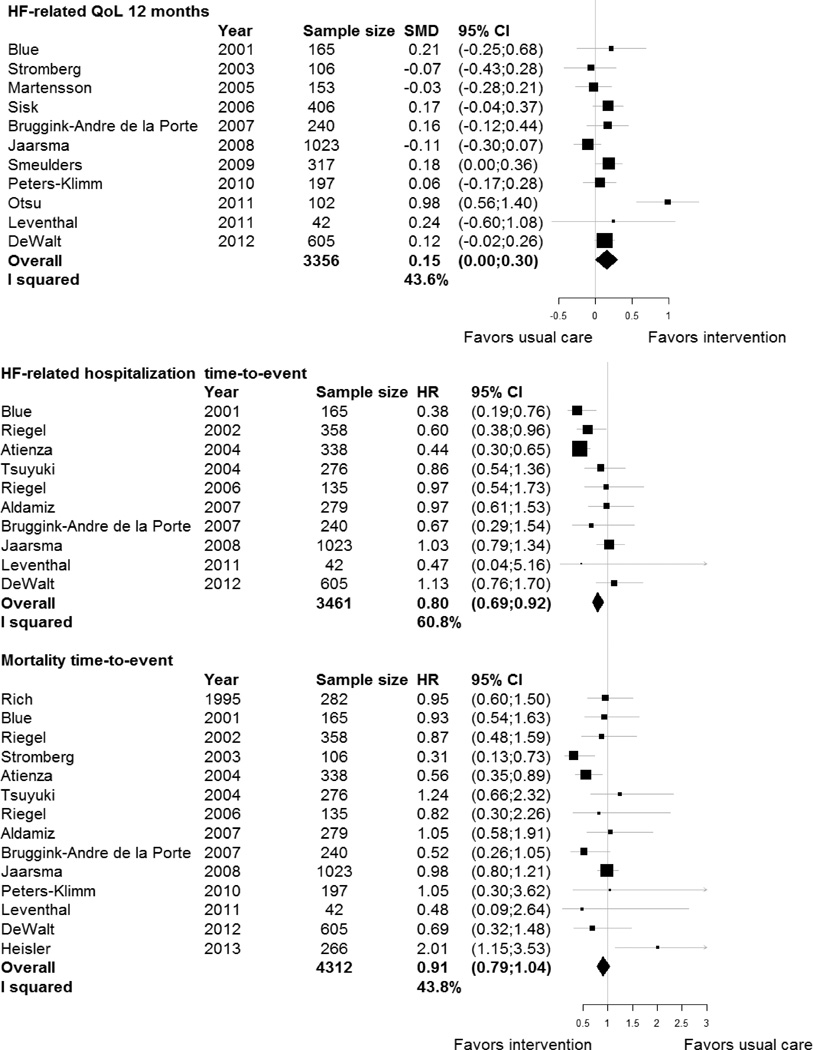
Self-management interventions showed significant effects on several HF-related outcomes (Table 3). Interventions reduced risk of time to the combined endpoint of HF-related hospitalization or all-cause death (HR, 0.80; 95% confidence interval [CI], 0.71–0.89) and time to HF-related hospitalization alone (HR, 0.80; 95% CI, 0.69–0.92). There was a small improvement in HF-QoL at 12 months in patients receiving the intervention (SMD, 0.15; 95% CI, 0.00–0.30). No effects were found for total days in hospital due to HF readmissions or any of the general outcomes. Figure 1 shows the effects across studies for HF-QoL, HF-related hospitalization, and all-cause mortality.
Table 3.
Effects of self-management interventions in patients with heart failure included in individual patient data meta-analysis.
| Outcome | Effect size |
N studies |
n patients |
Treatment effect (95% CI) |
Subgroups Age |
n patients |
Treatment effect (95% CI) |
p-value for interaction |
Subgroups Depression |
n patients |
Treatment effect (95% CI) |
p-value for interaction |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Heart failure-related outcomes | ||||||||||||
| HF-related hospitalization/ mortality time-to-event |
HR | 10 | 3461 | 0.80 (0.71–0.89) | <65 years | 1086 | 0.84 (0.66–1.07) | 0.77 | No/mild | 1274 | 0.81 (0.66–0.99) | 0.12 |
| 65–80 years | 1739 | 0.81 (0.69–0.95) | Moderate/severe | 696 | 1.05 (0.81–1.36) | |||||||
| >80 years | 636 | 0.74 (0.58–0.95) | ||||||||||
| HF-related QoL 12 months |
SMD | 11 | 3356 | 0.15 (0.00–0.30) | <65 years | 1208 | 0.20 (0.02–0.38) | 0.65 | No/mild | 1832 | 0.16 (0.14–0.19) | 0.41 |
| 65–80 years | 1607 | 0.12 (−0.04–0.29) | Moderate/severe | 772 | 0.25 (−0.01–0.50) | |||||||
| >80 years | 541 | 0.09 (−0.12–0.30) | ||||||||||
| HF-related hospitalization time-to-event |
HR | 10 | 3461 | 0.80 (0.69–0.92) | <65 years | 1086 | 0.81 (0.62–1.07) | 0.88 | No/mild | 1274 | 0.92 (0.71–1.18) | 0.64 |
| 65–80 years | 1739 | 0.78 (0.64–0.94) | Moderate/severe | 696 | 1.00 (0.74–1.35) | |||||||
| >80 years | 636 | 0.85 (0.63–1.15) | ||||||||||
| Total days HF-related hospital stay 12 months |
RLOS | 5 | 892 | 0.86 (0.44–1.67) | <65 years | 139 | 0.09 (0.02–0.38) | 0.03 | No/mild | 228 | 0.49 (0.13–1.84) | 0.94 |
| 65–80 years | 521 | 0.95 (0.46–1.94) | Moderate/severe | 39 | 0.37 (0.01–9.70) | |||||||
| >80 years | 232 | 0.96 (0.31–2.97) | ||||||||||
| General outcomes | ||||||||||||
|
Generic QoL-PCS 12 months |
MD | 8 | 1739 | 0.95 (−1.15–3.05) | <65 years | 561 | 1.84 (−0.74–4.42) | 0.63 | No/mild | 796 | 0.41 (0.09–0.73) | 0.45 |
| 65–80 years | 882 | 0.41 (−1.80–2.61) | Moderate/severe | 191 | −1.29 (−5.67–3.09) | |||||||
| >80 years | 296 | 1.13 (−2.01–4.26) | ||||||||||
| Generic QoL-MCS 12 months |
MD | 8 | 1739 | 0.27 (−2.53–3.08) | <65 years | 561 | 2.07 (−1.54–5.68) | 0.37 | No/mild | 796 | −0.88(−1.36–−0.39) | 0.52 |
| 65–80 years | 882 | −0.26 (−3.49–2.97) | Moderate/severe | 191 | −2.91 (−9.36–3.54) | |||||||
| >80 years | 296 | −1.19 (−5.62–3.24) | ||||||||||
| Mortality time-to-event |
HR | 14 | 4312 | 0.91 (0.79–1.04) | <65 years | 1232 | 1.12 (0.80–1.56) | 0.25 | No/mild | 1619 | 0.86 (0.69–1.06) | 0.01 |
| 65–80 years | 2224 | 0.93 (0.78–1.11) | Moderate/severe | 814 | 1.39 (1.04–1.87) | |||||||
| >80 years | 856 | 0.79 (0.62–1.00) | ||||||||||
| All-cause hospitalization time-to-event |
HR | 12 | 3833 | 0.93 (0.85–1.03) | <65 years | 1188 | 1.09 (0.91–1.31) | 0.07 | No/mild | 1469 | 0.99 (0.84–1.15) | 0.10 |
| 65–80 years | 1928 | 0.92 (0.81–1.05) | Moderate/severe | 767 | 1.22 (1.00–1.49) | |||||||
| >80 years | 717 | 0.79 (0.64–0.97) | ||||||||||
| Total days all-cause hospital stay 12 months |
RLOS | 9 | 2304 | 0.97 (0.77–1.23) | <65 years | 741 | 1.14 (0.80–1.63) | 0.39 | No/mild | 1036 | 1.06 (0.72–1.56) | 0.45 |
| 65–80 years | 1110 | 0.98 (0.74–1.31) | Moderate/severe | 359 | 0.90 (0.49–1.64) | |||||||
| >80 years | 453 | 0.77 (0.49–1.20) | ||||||||||
CI indicates confidence interval; HF, heart failure; HR, hazard ratio; MCS, mental component scale; MD, mean difference; PCS, physical component scale; QoL, quality of life; RLOS, relative length of stay; and SMD, standardized mean difference.
Figure 1.
Forest plot of effects of self-management interventions on heart failure-related quality of life, heart failure-related hospitalization, and all-cause mortality.
CI indicates confidence interval; HR, hazard ratio; and SMD, standardized mean difference.
Effects in patient subgroups
In the HF-related outcomes, subgroup analysis revealed significant effect modification by age on days in hospital due to HF (Table 3). For younger patients (<65 years), mean number of days in hospital due to HF in the intervention group was 0.70 days, while this was 5.35days in the control group (relative length of stay, 0.09; 95% CI, 0.02–0.38). This difference was not found in patients aged 65–80 years (3.30 days in intervention group vs. 3.84 days in control group, interaction p=0.03). For general outcomes (Table 3), there was significant effect modification by comorbid depression on time to all-cause death. While no significant effect of self-management was found in patients with no/mild depression on all-cause death (HR, 0.86; 95% CI, 0.69–1.06), there was a negative effect in patients with moderate/severe depression on all-cause death (HR, 1.39; 95% CI, 1.06–1.83, interaction p=0.01). In univariable analysis, level of education showed significant effect modification on time to first all-cause hospitalization with lower educated patients showing a positive effect of the self-management intervention (HR, 0.82; 95% CI, 0.71–0.96, Supplemental Table 3), while there was no effect in patients who had completed secondary education (HR, 0.98; 95% CI, 0.82–1.17), or higher education (HR, 1.26; 95% CI, 0.99–1.60; interaction p=0.02). After adjustment for potential effect modification by age, effect modification by level of education was no longer significant (interaction p=0.07). Additional analyses of outcomes measured at 6 months did not yield different insights (Supplemental Tables 1 and 2).
Sensitivity analyses
Including published effects of eligible studies for which original data could be obtained, did not change the primary findings (Supplemental Table 4), neither did the sensitivity analysis of excluding studies with enhanced usual care (Supplemental Table 5). The other sensitivity analyses also yielded similar effects. Only when subgroup analysis was repeated without the trial by Jaarsma and colleagues,7 effect modification by depression on time to all-cause death was no longer statistically significant (interaction p=0.22) and the negative effect for patients with moderate/severe depression on all-cause death was no longer present (HR, 0.63, 95% CI, 0.29–1.34).
Discussion
To our knowledge, this study is the first IPD meta-analysis including sufficiently large numbers of HF patients to be able to identify subgroups of patients that respond differently to self-management interventions. We observed protective effects of self-management interventions on time to the combined endpoint of HF-related hospitalization or all-cause death, HF-related hospitalization alone and HF-QoL. Subgroup analyses showed that younger patients responded better to self-management in terms of reduced total days of HF-related hospitalization, and that HF patients with depression showed a reduced survival following the self-management intervention.
The beneficial effects found on time to the combined endpoint of HF-related hospitalization or all-cause death and on HF-related hospitalization alone have also been reported by previous (aggregate data) meta-analyses on similar interventions.4,42 Earlier systematic reviews consistently stressed the large heterogeneity across studies regarding effects of self-management on health-related QoL.5 Our study included several recent large neutral trials6,7 and was the first to pool the results for HF-QoL and compute an overall effect. Although 95% confidence intervals were rather wide, we observed a small positive effect for HF-QoL at 12 months. In contrast to HF-related outcomes, we found no effects of self-management interventions on general outcomes (i.e., generic QoL, all-cause mortality, all-cause hospitalization). This is in line with previous meta-analyses.4,42 Thus, it seems that self-management interventions are particularly effective in HF patients for improving outcomes directly related to their disease.
The subgroup analysis showed that younger patients (<65 years) benefited more from self-management interventions than older patients. Younger patients in intervention groups were discharged sooner from hospitalization for HF during follow-up than their counterparts in control groups. There was no intervention effect in older patients. Older hospitalized patients have an increased risk of functional decline, cognitive dysfunction and generally suffer from more comorbid conditions, complicating their overall functioning and recovery time once hospitalized.43 Especially older persons are at high risk in the period after hospitalization due to deprived sleep, poor nutrition, stress, symptoms, new treatments, and inactivity. Equipping patients with self-management skills might not be sufficient in such complex situations. Post-discharge instability may need new approaches not only targeting HF itself for a safer transition from hospital to home.44 Still, the effect modification by age was not consistent across other health outcomes studied and the number of patients aged <65 included in the analysis was relatively small (n=139). The findings should therefore be considered hypothesis-generating.
Self-management interventions increased the risk of all-cause mortality in patients with moderate/severe depression. Sensitivity analyses indicated that this effect was driven by the largest study included in this IPD meta-analysis.7 The authors of that study reported a similar trend of their intervention for patients with depressive symptoms in their subgroup analysis.45 These findings question the suitability of generic self-management interventions in HF patients with depressive symptoms. Depression is often associated with reduced motivation, which might compromise adherence to medication regimen and lifestyle changes,46 particularly if multiple comorbid conditions (and treatment) need to be self-managed. These patients may be burdened with self-managing their HF. Increased mortality following self-management interventions might therefore be caused by suboptimal (self-)management of their illnesses, including HF. Interestingly, the negative effect was limited to all-cause mortality. In the five studies that measured depression, self-management interventions showed an overall HR of 0.95 on time to HF-related hospitalization (95% CI, 0.94–0.97) and subgroup analysis did not reveal a differential treatment effect between patients with and without depression (HR depression, 1.00; 95% CI, 0.74–1.35; HR without depression, 0.92; 95% CI, 0.71–1.18; interaction p=0.64). With no clear explanation for reduced survival in HF patients with depression, caution is warranted before applying self-management strategies in care for those patients. Patients with depressive symptoms might need additional psychological interventions or medication before initiating self-management interventions.47 Screening HF patients on symptoms of depression might help to determine to what extent attention should be paid to self-management skills or additional psychological interventions in the treatment plan.
Previous subgroup analyses in three large RCTs have shown that self-management interventions might be more effective for patients with low socio-economic status. DeWalt and colleagues found that only patients with low literacy showed a positive effect on HF-related hospitalizations after self-management support.6 A Dutch self-management trial found greatest improvements in health-related QoL in patients with lower education.48 The third trial showed that patients with reduced income benefitted most from self-management.8 The pattern across studies generates the hypothesis that patients with a lower socio-economic status may benefit most from self-management interventions. Similarly, our analyses indicate a protective effect of self-management on time to first all-cause hospitalization in patients with lower education. However, after adjusting for other potential effect modifiers, this effect did not reach statistical significance.
This IPD meta-analysis was one of the first attempts to pool individual patient data on self-management interventions for patients with HF. The study included sufficient patients (n=5624) to analyze treatment effects in patient subgroups and applied robust statistical modelling according to a pre-specified plan. Reported effects were found across cultures and healthcare settings. Nevertheless, this study has several limitations that deserve further discussion. First, despite numerous efforts to reach all principal investigators, we were unable to include all 32 eligible trials. Inclusion of 62.5% (20/32) of eligible trials is relatively high compared to IPD meta-analyses on similar interventions.49 Including published results of trials for which no IPD were available did not change main effects, but this could not be checked for the subgroup analysis due to limited published subgroup data. Second, included self-management interventions differed in terms of intensity, duration, mode, and content. Although reported effects were found for self-management interventions in any setting, specific types of interventions might work better for specific subgroups of patients. Addressing the question “what works for whom?” deserves attention in subsequent research. Third, this IPD meta-analysis was highly dependent on data previously collected in individual studies which limited choice of potential effect modifiers to be studied. Individual trials indicated that self-management interventions might be more effective in non-adherers to regimens25 or in patients with better cognitive status.48 We could not analyze those potential effect modifiers, since variables were not collected in all studies. If uniform standards for baseline variables were established, a meaningful comparison of patient subgroups across studies may provide further insight into patient characteristics modifying treatment effects. Finally, although all (subgroup) analyses were pre-planned and documented in our protocol,12 their large number increases the risk of false-positive findings. Our subgroup analysis was exploratory in nature and not intended to demonstrate causal mechanisms. Causal mechanisms of subgroup effects need to be completely understood before any final conclusions can be drawn. Validation of our findings in large trial databases may confirm our subgroup findings.
Conclusion
We found that despite diversity in intensity, content, and personnel delivering the intervention, self-management interventions in patients with HF improve outcomes directly related to their disease. Although self-management interventions might be more effective in younger patients in reducing length of hospital stay, we did not observe consistent subgroup effects across different health outcomes. This study does not endorse limiting self-management interventions to specific subgroups of HF patients, but increased mortality in depressed patients warrants caution in applying self-management strategies in these patients.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by The Netherlands Organisation for Health Research and Development, ZonMw (grant number 520001002).
FA is in the advisory board of Medtronic, Sorin. DAD reports grants from NIH during the conduct of the study, outside the submitted work. MH reports grants from MDRTC during the conduct of the study, outside the submitted work. RTT reports grants from Merck Canada Inc., AstraZeneca Canada, and personal fees from Merck Canada Inc., outside the submitted work.
Footnotes
Conflict of Interest Disclosures: The other authors have no conflict of interest to declare.
References
- 1.Ambrosy AP, Fonarow GC, Butler J, Chionel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–1133. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 2.Curtis LH, Greiner MA, Hammill BG, Kramer JM, Whellan DJ, Schulman KA, Hernandez AF. Early and long-term outcomes of heart failure in elderly persons, 2001–2005. Arch Intern Med. 2008;168:2481–2488. doi: 10.1001/archinte.168.22.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 4.Jovicic A, Holroyd-Leduc JM, Straus SE. Effects of self-management intervention on health outcomes of patients with heart failure: a systematic review of randomized controlled trials. BMC Cardiovasc Disord. 2006;6:43. doi: 10.1186/1471-2261-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ditewig JB, Blok H, Havers J, van Veenendaal H. Effectiveness of self-management interventions on mortality, hospital readmissions, chronic heart failure hospitalization rate and quality of life in patients with chronic heart failure: a systematic review. Patient Educ Couns. 2010;78:297–315. doi: 10.1016/j.pec.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 6.DeWalt DA, Schillinger D, Ruo B, Bibbins-Domingo K, Baker DW, Holmes GM, Weinberger M, abasco-O'Connell A, Broucksou K, Hawk V, Grady KL, Erman B, Sueta CA, Chang PP, Cene CW, Wu JR, Jones CD, Pignone M. Multisite randomized trial of a single-session versus multisession literacy-sensitive self-care intervention for patients with heart failure. Circulation. 2012;125:2854–2862. doi: 10.1161/CIRCULATIONAHA.111.081745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jaarsma T, van der Wal MH, Lesman-Leegte I, Luttik ML, Hogenhuis J, Veeger NJ, Sanderman R, Hoes AW, van Gilst WH, Lok DJ, Dunselman PH, Tijssen JG, Hillege HL, van Veldhuisen DJ. Effect of moderate or intensive disease management program on outcome in patients with heart failure: Coordinating Study Evaluating Outcomes of Advising and Counseling in Heart Failure(COACH) Arch Intern Med. 2008;168:316–324. doi: 10.1001/archinternmed.2007.83. [DOI] [PubMed] [Google Scholar]
- 8.Powell LH, Calvin JE, Jr, Richardson D, Janssen I, Mendes de Leon CF, Flynn KJ, Grady KL, Rucker-Whitaker CS, Eaton C, Avery E. Self-management counseling in patients with heart failure: the heart failure adherence and retention randomized behavioral trial. JAMA. 2010;304:1331–1338. doi: 10.1001/jama.2010.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dracup K, Moser DK, Pelter MM, Nesbitt TS, Southard J, Paul SM, Robinson S, Cooper LS. Randomized, controlled trial to improve self-care in patients with heart failure living in rural areas. Circulation. 2014;130:256–264. doi: 10.1161/CIRCULATIONAHA.113.003542. [DOI] [PubMed] [Google Scholar]
- 10.Riley RD, Lambert PC, Abo-Zaid G. Meta-analysis of individual participant data: rationale, conduct, and reporting. BMJ. 2010;340:c221. doi: 10.1136/bmj.c221. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0. The Cochrane Collaboration; 2011. [Google Scholar]
- 12.Jonkman NH, Westland H, Trappenburg JC, Groenwold RHH, Effing-Tijdhof TW, Troosters T, van der Palen J, Bourbeau J, Jaarsma T, Hoes AW, Schuurmans MJ. Towards tailoring of self-management for patients with chronic heart failure or chronic obstructive pulmonary disease: a protocol for an individual patient data meta-analysis. BMJ Open. 2014;4:e005220. doi: 10.1136/bmjopen-2014-005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker DW, Brown J, Chan KS, Dracup KA, Keeler EB. A telephone survey to measure communication, education, self-management, and health status for patients with heart failure: the Improving Chronic Illness Care Evaluation(ICICE) J Card Fail. 2005;11:36–42. doi: 10.1016/j.cardfail.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35:1245–1255. doi: 10.1016/s0735-1097(00)00531-3. [DOI] [PubMed] [Google Scholar]
- 15.Hofer S, Lim L, Guyatt G, Oldridge N. The MacNew Heart Disease health-related quality of life instrument: a summary. Health Qual Life Outcomes. 2004;2:3. doi: 10.1186/1477-7525-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rector TS, Kubo SH, Cohn JN. Patients' self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure Questionnaire. Heart Failure. 1987;3:198–209. [Google Scholar]
- 17.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey(SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 19.Bos-Touwen I, Jonkman N, Westland H, Schuurmans M, Rutten F, de Wit N, Trappenburg J. Tailoring of self-management interventions in patients with heart failure. Curr Heart Fail Rep. 2015;12:223–235. doi: 10.1007/s11897-015-0259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 21.Groothuis-Oudshoorn K, van Buuren S. MICE: Multivariate Imputation by Chained Equations in R. Journal of Statistical Software. 2011;45 [Google Scholar]
- 22.Simmonds MC, Higgins JP, Stewart LA, Tierney JF, Clarke MJ, Thompson SG. Meta-analysis of individual patient data from randomized trials: a review of methods used in practice. Clin Trials. 2005;2:209–217. doi: 10.1191/1740774505cn087oa. [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Multiple imputation for non-response in surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 24.Agren S, Evangelista LS, Hjelm C, Stromberg A. Dyads affected by chronic heart failure: a randomized study evaluating effects of education and psychosocial support to patients with heart failure and their partners. J Card Fail. 2012;18:359–366. doi: 10.1016/j.cardfail.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aldamiz-Echevarría I, Muñiz J, Rodríguez-Fernández JA, Vidán-Martínez L, Silva-César M, Lamelo-Alfonsín F, Díaz-Díaz JL, Ramos-Polledo V, Castro-Beiras A. Randomized controlled clinical trial of a home care unit intervention to reduce readmission and death rates in patients discharged from hospital following admission for heart failure. Revista española de cardiología. 2007;60:914–922. doi: 10.1157/13109644. [DOI] [PubMed] [Google Scholar]
- 26.Atienza F, Anguita M, Martinez-Alzamora N, Osca J, Ojeda S, Almenar L, Ridocci F, Valles F, de Velasco JA. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. Eur J Heart Fail. 2004;6:643–652. doi: 10.1016/j.ejheart.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 27.Blue L, Lang E, McMurray JJ, Davie AP, McDonagh TA, Murdoch DR, Petrie MC, Connolly E, Norrie J, Round CE, Ford I, Morrison CE. Randomised controlled trial of specialist nurse intervention in heart failure. BMJ. 2001;323:715–718. doi: 10.1136/bmj.323.7315.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruggink-Andre de la Porte PW, Lok DJ, van Veldhuisen DJ, van Wijngaarden J, Cornel JH, Zuithoff NPA, Badings E, Hoes AW. Added value of a physician-and-nurse-directed heart failure clinic: results from the Deventer–Alkmaar heart failure study. Heart. 2007;93:819–825. doi: 10.1136/hrt.2006.095810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heisler M, Halasyamani L, Cowen ME, Davis MD, Resnicow K, Strawderman RL, Choi H, Mase R, Piette JD. A Randomized Controlled Effectiveness Trial of Reciprocal Peer Support in Heart Failure. Circ Heart Fail. 2013;6:246–253. doi: 10.1161/CIRCHEARTFAILURE.112.000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaarsma T, Halfens R, Huijer Abu-Saad H, Dracup K, Gorgels T, van Ree J, Stappers J. Effects of education and support on self-care and resource utilization in patients with heart failure. Eur Heart J. 1999;20:673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 31.Leventhal ME, Denhaerynck K, Brunner-La Rocca HP, Burnand B, Conca-Zeller A, Bernasconi AT, Mahrer-Imhof R, Froelicher ES, De Geest S. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF): a randomised controlled trial study of an outpatient inter-professional management programme for heart failure patients in Switzerland. Swiss Med Wkly. 2011;141:w13171. doi: 10.4414/smw.2011.13171. [DOI] [PubMed] [Google Scholar]
- 32.Martensson J, Stromberg A, Dahlstrom U, Karlsson JE, Fridlund B. Patients with heart failure in primary health care: effects of a nurse-led intervention on health-related quality of life and depression. Eur J Heart Fail. 2005;7:393–403. doi: 10.1016/j.ejheart.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 33.Otsu H, Moriyama M. Effectiveness of an educational self-management program for outpatients with chronic heart failure. Jpn J Nurs Sci. 2011;8:140–152. doi: 10.1111/j.1742-7924.2010.00166.x. [DOI] [PubMed] [Google Scholar]
- 34.Peters-Klimm F, Campbell S, Hermann K, Kunz CU, Muller-Tasch T, Szecsenyi J. Case management for patients with chronic systolic heart failure in primary care: the HICMan exploratory randomised controlled trial. Trials. 2010;11:56. doi: 10.1186/1745-6215-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 36.Riegel B, Carlson B, Kopp Z, LePetri B, Glaser D, Unger A. Effect of a standardized nurse case-management telephone intervention on resource use in patients with chronic heart failure. Arch Intern Med. 2002;162:705–712. doi: 10.1001/archinte.162.6.705. [DOI] [PubMed] [Google Scholar]
- 37.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006;12:211–219. doi: 10.1016/j.cardfail.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Sisk JE, Hebert PL, Horowitz CR, McLaughlin MA, Wang JJ, Chassin MR. Effects of nurse management on the quality of heart failure care in minority communities: a randomized trial. Ann Intern Med. 2006;145:273–283. doi: 10.7326/0003-4819-145-4-200608150-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smeulders ES, van Haastregt JC, Ambergen T, Janssen-Boyne JJ, van Eijk JT, Kempen GI. The impact of a self-management group programme on health behaviour and healthcare utilization among congestive heart failure patients. Eur J Heart Fail. 2009;11:609–616. doi: 10.1093/eurjhf/hfp047. [DOI] [PubMed] [Google Scholar]
- 40.Stromberg A, Martensson J, Fridlund B, Levin LA, Karlsson JE, Dahlstrom U. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure: results from a prospective, randomised trial. Eur Heart J. 2003;24:1014–1023. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 41.Tsuyuki RT, Fradette M, Johnson JA, Bungard TJ, Eurich DT, Ashton T, Gordon W, Ikuta R, Kornder J, Mackay E, Manyari D, O'Reilly K, Semchuk W. A multicenter disease management program for hospitalized patients with heart failure. J Card Fail. 2004;10:473–480. doi: 10.1016/j.cardfail.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Gonseth J, Guallar-Castillon P, Banegas JR, Rodriguez-Artalejo F. The effectiveness of disease management programmes in reducing hospital re-admission in older patients with heart failure: a systematic review and meta-analysis of published reports. Eur Heart J. 2004;25 doi: 10.1016/j.ehj.2004.04.022. 1570-159. [DOI] [PubMed] [Google Scholar]
- 43.Bagshaw SM, Stelfox HT, McDermid RC, Rolfson DB, Tsuyuki RT, Baig N, Artiuch B, Ibrahim Q, Stollery DE, Rokosh E, Majumdar SM. Association between frailty and short- and long-term outcomes among critically ill patients: a multicentre prospective cohort study. CMAJ. 2014;186:E96–E102. doi: 10.1503/cmaj.130639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dharmarajan K, Masoudi FA, Spertus JA, Li SX, Krumholz HM. Contraindicated initiation of beta-blocker therapy in patients hospitalized for heart failure. JAMA Intern Med. 2013;173:1547–1549. doi: 10.1001/jamainternmed.2013.7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaarsma T, Lesman-Leegte I, Hillege HL, Veeger NJ, Sanderman R, van Veldhuisen DJ. Depression and the usefulness of a disease management program in heart failure: insights from the COACH (Coordinating study evaluating Outcomes of Advising and Counseling in Heart failure) study. J Am Coll Cardiol. 2010;55:1837–1843. doi: 10.1016/j.jacc.2009.11.082. [DOI] [PubMed] [Google Scholar]
- 46.De Jong MJ, Chung ML, Wu JR, Riegel B, Rayens MK, Moser DK. Linkages between anxiety and outcomes in heart failure. Heart Lung. 2011;40:393–404. doi: 10.1016/j.hrtlng.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whalley B, Rees K, Davies P, Bennett P, Ebrahim S, Liu Z, West R, Moxham T, Thompson DR, Taylor RS. Psychological interventions for coronary heart disease. Cochrane Database Syst Rev. 2011;8:CD002902. doi: 10.1002/14651858.CD002902.pub3. [DOI] [PubMed] [Google Scholar]
- 48.Smeulders ES, van Haastregt JC, Ambergen T, Stoffers HE, Janssen-Boyne JJ, Uszko-Lencer NH, Gorgels AP, Lodewijks-van der Bolt CL, van Eijk JT, Kempen GI. Heart failure patients with a lower educational level and better cognitive status benefit most from a self-management group programme. Patient Educ Couns. 2010;81:214–221. doi: 10.1016/j.pec.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 49.Heneghan C, Ward A, Perera R Self-Monitoring Trialist Collaboration. Self-monitoring of oral anticoagulation: systematic review and meta-analysis of individual patient data. Lancet. 2012;379:322–334. doi: 10.1016/S0140-6736(11)61294-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.