Abstract
Background:
In order to better define the pathogenic role of cerebrospinal fluid (CSF) drainage catheters in postoperative patients, we comparatively analyze the clinical course of device and non-device-related meningitis.
Methods:
This is an observational, partially prospective, study on consecutive adult patients who developed meningitis after undergoing neurosurgical procedures at the Neurosurgery and Neurointensive care Departments, Spedali Civili, Brescia, Italy, between January 1999 and August 2007.
Results:
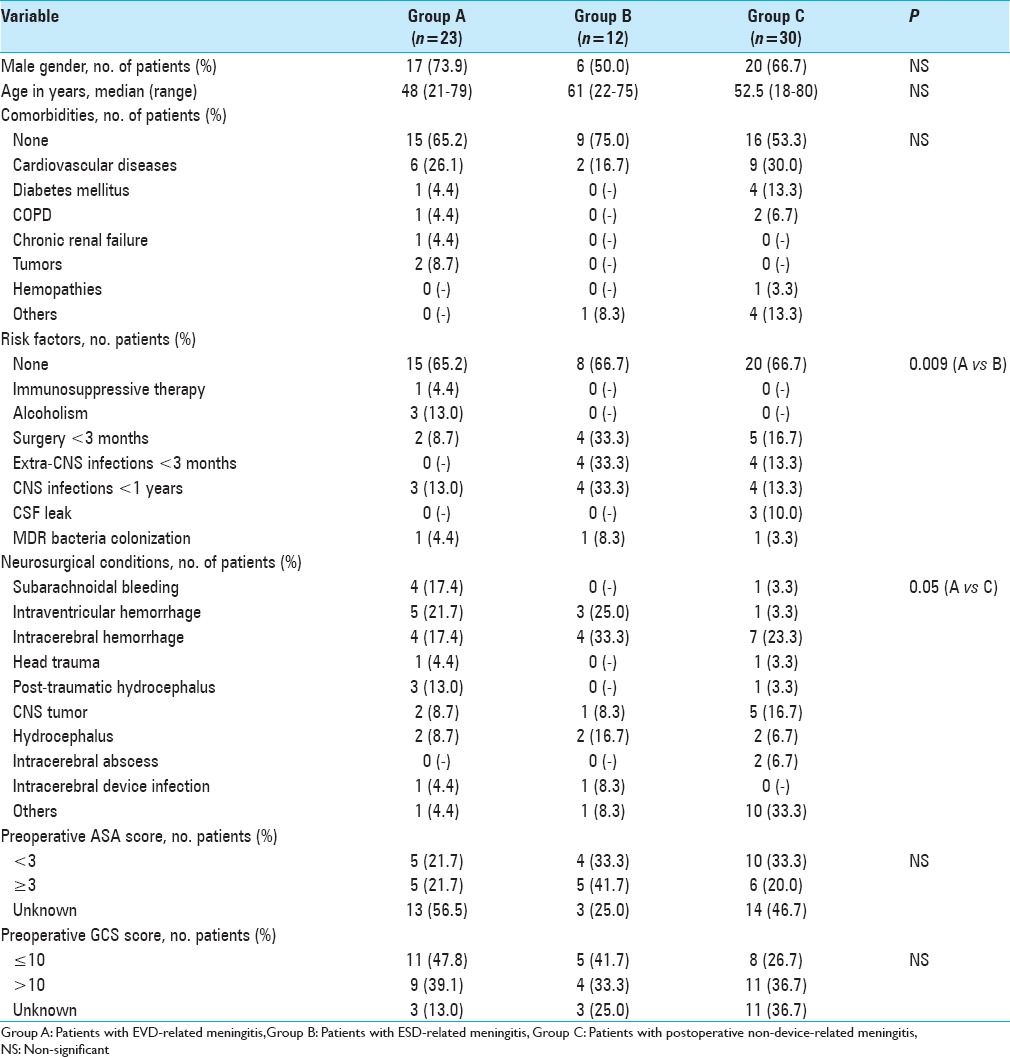
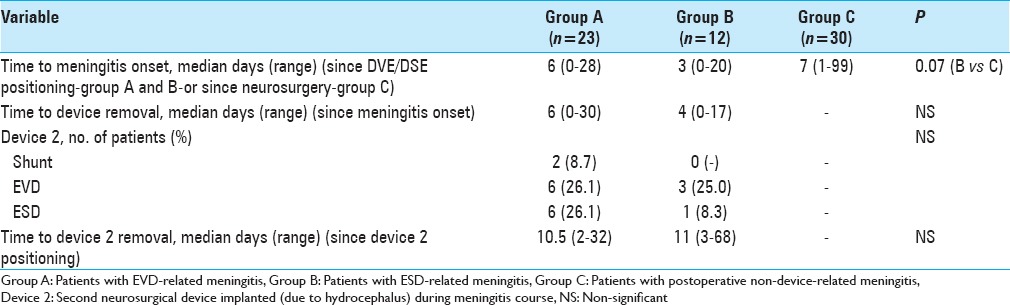
All 77 consecutive post-neurosurgical meningitis events in 65 patients were included in the analysis. Most were classified as external ventricular drainage (EVD)-related meningitis (23 cases, group A), external spinal drainage (ESD)-related meningitis (12 cases, group B), and non-device-related post-neurosurgical meningitis (30 cases, group C). Proven meningitis was identified in 78.3%, 91.7% and 56.7% of the events, respectively. ESD-related meningitis had a shorter onset time vs EVD and non-device-associated meningitis (3 days versus 6 and 7 days, respectively). Median antibiotic treatment duration was 20, 17, and 22.5 days in groups A, B, and C, respectively. Overall, 8 patients (34.8%) in group A, 3 (25.0%) in group B, and 3 (10.0%) in group C died. Median time to become afebrile was shorter in group C than in group A (10 days versus 12 days, P = 0.04). Removal of the device later than 48 hours after meningitis onset, as well as implantation of a second device were associated with a slower time of meningitis resolution.
Conclusions:
Early device removal and avoiding implantation of a second device were associated with short illness duration. Larger studies are warranted to confirm the conclusions of this study.
Keywords: Cerebrospinal fluid drainage catheters, meningitis, neurosurgery
INTRODUCTION
Postoperative meningitis is a rare but serious complication of neurosurgery. In clean neurosurgery, the rate of postoperative bacterial meningitis is low (1–2%).[9] The use of devices for therapeutic drainage of cerebrospinal fluid (CSF) or for intracranial pressure monitoring, such as external ventricular drains (EVD), external spinal drains (ESD), and shunts, is associated with an increase in the rate of postoperative meningitis of up to 22%.[3,9] Meningitis may lead to worsened outcome, including increased mortality, prolonged hospital stay, repeat surgery, and increased hospital costs.[2,10,11,14]
There is a large body of evidence regarding postoperative meningitis in terms of risk factors, pathogenesis, and microbiological aspects.[1,2,3,7,8,9,10,11,14] In contrast, little is known about the clinical course of postoperative meningitis; data concerning the time of symptom resolution and CSF parameters normalization are limited. Furthermore, there are no set guidelines on how to manage post-neurosurgical infections, especially in the presence of a device.
We performed an observational study of all consecutive cases of postoperative meningitis observed in adults at a tertiary hospital in Northern Italy to compare outcome and clinical evolution, including laboratory and microbiological parameters, of device (EVD and ESD-related) and non-device-related meningitis.
MATERIALS AND METHODS
Hospital setting
The study was conducted at the Department of Neurosurgery and Neurointensive care of Spedali Civili, Brescia, Italy. An intensive infectious diseases consultation program, with ≥3 consultations per week, has been ongoing since 1999.
Study population and data collection
We included all consecutive cases of postoperative meningitis observed between January 1999 and August 2007 in patients over 18 years of age. Data were collected retrospectively until June 2004 and prospectively thereafter. During the retrospective phase, episodes of meningitis were identified using the electronic register, identifying cases of meningitis from the discharge diagnosis and/or from the infectious diseases consultation records, and relevant medical charts were reviewed. The protocol for perioperative antibiotic prophylaxis consisted of cephazoline 2 g intravenously half an hour before surgery followed by 1 g after 3 hours, if the duration of the intervention exceeded 3 hours.
During the prospective phase, each episode was evaluated by infectious diseases consultants according to the predefined criteria (see below), and data was actively collected on ad-hoc charts. To evaluate the influence of neurosurgical devices on meningitis evolution and outcome, statistical analysis was performed to compare patients with EVD, with ESD, and without any device.
Case definitions
Proven meningitis was defined as a positive CSF culture and laboratory CSF findings of a neutrophilic CSF pleocytosis (>5 cells/µL) and a CSF/blood glucose ratio of <0.5 along with meningitis signs and/or symptoms [fever, headache, neck stiffness, cranial nerve signs, or Glasgow coma scale (GCS) worsening].
Possible meningitis was defined by laboratory CSF findings of a neutrophilic CSF pleocytosis (>5 cells/µL) and a CSF/blood glucose ratio of <0.5 along with meningitis signs and/or symptoms with a negative CSF culture.
Patients with postoperative, non-device-related meningitis were included if neurosurgery had been performed 3 months or less prior to meningitis onset. Patients with postoperative device-related meningitis were included if the neurosurgical device was in place at meningitis onset.
Fever clearance (T ≤ 37°C) was utilized to evaluate clinical response to therapy. Blood and CSF samples were periodically collected to evaluate improvement time (time from onset to the second consecutive improved measurement) and normalization of the following parameters: Blood/CSF leucocyte count and CSF glucose level.
Statistical analysis
Student's t-test was used to compare means and a Chi-square test was used to compare proportions. Continuous variables were compared using the Wilcoxon rank-sum test. A P value of 0.05 was considered to be statistically significant. A multivariate logistic regression model was fitted to the data to identify potential factors related to the outcome. All calculations were performed using the Stata version 13 software (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP)..
RESULTS
During the study period, 77 events of postoperative meningitis were identified among 65 patients. Description of the study population is shown in Table 1. There were 47 events of device-related meningitis (23 EVD, 12 ESD, 7 ventriculoperitoneal shunts, 2 intracranial pressure devices, and 3 subarachnoid catheters), and 30 non-device-associated events. Patients with EVD-related meningitis were defined as group A, those with ESD-related meningitis as group B, and those with postoperative, non-device-related meningitis as group C.
Table 1.
Demographic and clinical characteristics of the study population
Emergency surgery was performed in 14 patients (60.9%) in group A, 3 (25.0%) in group B, and 8 (26.7%) in group C (P = 0.03 for A vs C). Median duration of surgery in groups A, B, and C was, respectively, 85 (range: 15–360), 180 (range: 20–300), and 180 minutes (range: 60–990). Among patients who were not receiving antibiotic therapy at the time of neurosurgery, antibiotic prophylaxis was appropriately prescribed in, respectively, 6/16 (37.5%), 3/9 (33.3%), and 10/27 patients (37.0%). Eleven patients (47.8%) in group A, 5 (41.7%) in group B, and 12 (40%) in group C experienced postoperative complications (mainly CSF leakage and relapse of intraventricular hemorrhage). No statistically significant differences were found in length of surgery, appropriateness of prophylaxis, and frequency of postoperative complications among the three groups.
Table 2 summarizes the major features of neurosurgical devices. Among 465 EVDs implanted during the study period, the overall EVD-related meningitis rate was 4.9%, with large variability per year (0–14%).
Table 2.
Main characteristics of the neurosurgical devices
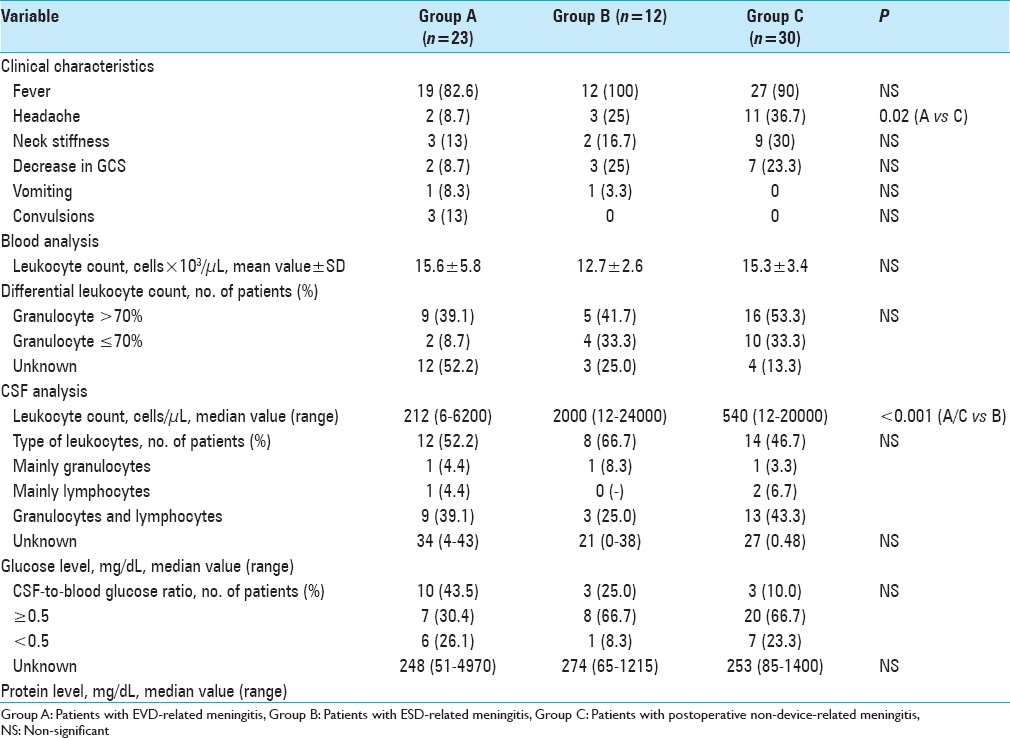
Variables related to meningitis clinical presentation and blood/CSF chemistry at meningitis onset are summarized in Table 3.
Table 3.
Clinical characteristics and blood/CSF chemistry at meningitis onset
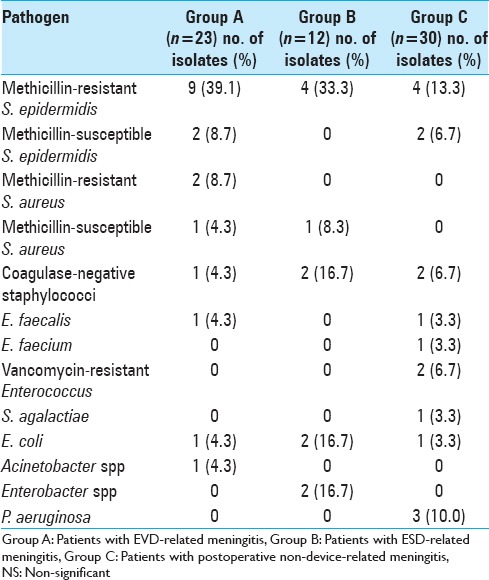
Meningitis was proven in 18 (78.3%), 11 (91.7%), and 17 (56.7%) in groups A, B, and C, respectively. Among patients for whom CSF culture yielded negative results (5 patients in group A, 1 in group B, and 13 in group C), 2 patients in group A and 4 in group C were on antibiotic therapy when the CSF culture was performed. Microorganisms responsible for meningitis are presented in Table 4.
Table 4.
Microbiological findings of CSF culture
All patients received intravenous antibiotic therapy. In addition, 3 patients (13%) in group A, 2 (16.7%) in group B, and 1 (3.3%) in group C received intrathecal antibiotics. Median intravenous antibiotic treatment duration was 20 days (range: 5–57 days) in group A, 17 days (range: 4–60 days) in group B, and 22.5 days (range: 3–41 days) in group C. There was no significant difference in the length of therapy between the three groups.
Clinical, biochemical, and microbiological course of meningitis
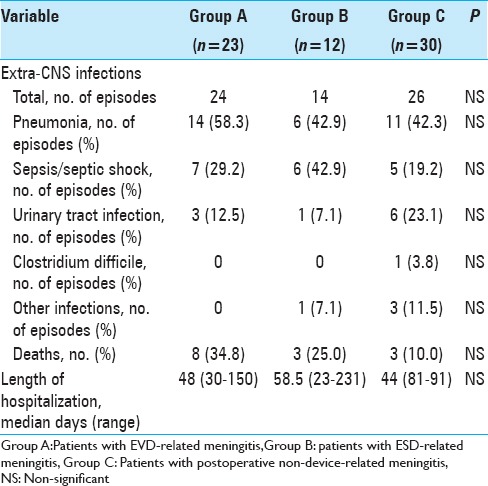
Description of the clinical, biochemical, and microbiological course of meningitis is shown in Table 5. Overall, 8 patients (34.8%) in group A, 3 (25.0%) in group B, and 3 (10.0%) in group C died. Among patients in group A, 1 patient (12.5%) experienced meningitis treatment failure and died because of methicillin-resistant Staphylococcus aureus meningitis, 6 patients (75.0%) extra-central nervous system (CNS) infections, and 1 patient (12.5%) died because of cardiovascular complications. All patients in groups B and C (100.0%) died due to severe, CNS infections [Table 6].
Table 5.
Clinical characteristics, blood/CSF chemistry and CSF bacterial clearance at the end of treatment
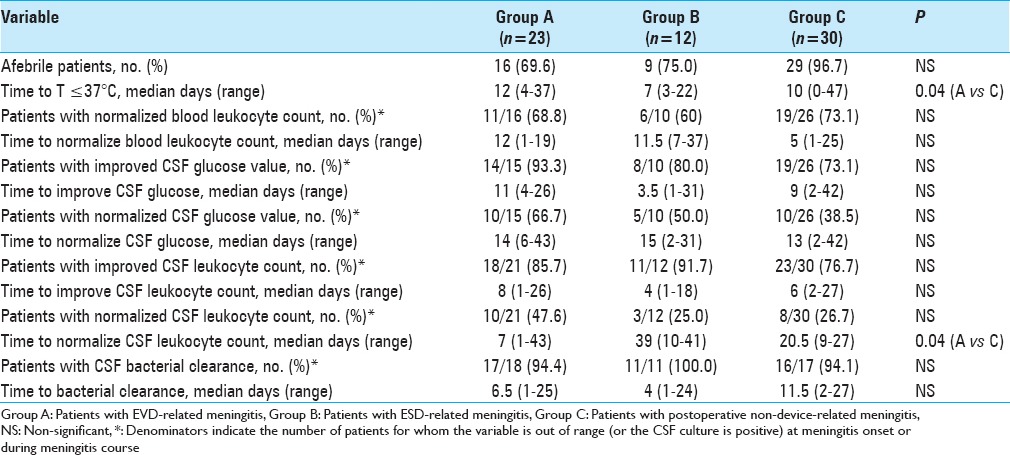
Table 6.
Concomitant extra-CNS infections, patients’ outcome and length of hospitalization
Removing devices (both EVD and ESD) in the first 48 hours after meningitis onset was associated with shorter time to CSF leukocyte reduction (median 5 days versus 8 days, P = 0.05), and shorter time to CSF bacterial clearance (median of 5 days versus 8 days, P = 0.05) [Table 7].
Table 7.
Effect of the device removal and repositioning on meningitis
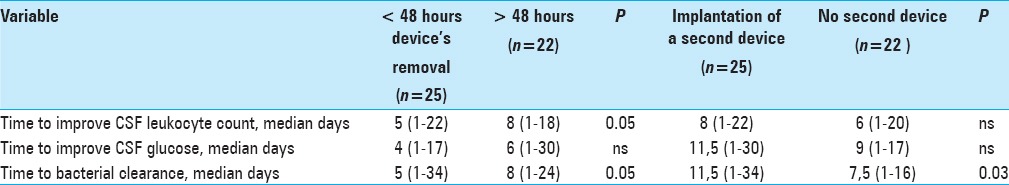
In patients with EVD and ESD-related meningitis, positioning of a second device was associated with longer median time to bacterial clearance (11.5 days versus 7.5 days, P = 0.03).
DISCUSSION
Our single-center study of 77 consecutive events of postoperative meningitis is, to our knowledge, the first study aimed to compare the clinical, biochemical, and microbiological course, as well as the outcome of device and non-device-related meningitis.
Our results show an overall post-neurosurgical meningitis rate of 4.9%, which is well within the range observe in other studies,[3,9] but with great variability per year (0-14%). Similar to the findings of other studies,[1,2,4,12,13] EVD-related meningitis in our population occurred within the first week (median of 6 days, range of 0–28) after EVD placement. ESD-associated meningitis occurred even earlier (median of 3 days, range of 0–20), probably due to a higher load of bacteria caused by the proximity between the drainage and the perineal area. As expected, bacteria belonging to the fecal flora were isolated more frequently in ESD patients (4 out of 12, 33.3%) compared to non-ESD patients (7 out of 46, 13.2%). The difference was not statistically significant (P = 0.1) probably due to a low number effect.
Of note, the presence of a ventricular drainage influenced the time to meningitis resolution. Time to bacterial clearance and improved CSF leukocyte count were significantly shorter in patients with early device removal; moreover, implantation of a second device was associated with longer time to bacterial clearance in CSF. This observation is consistent with the knowledge that any device and related biofilm facilitates bacterial survival and hampers the effect of antibiotic treatment. These findings support attempts to remove devices as early as possible and to avoid positioning of a second CSF drainage catheter whenever possible.
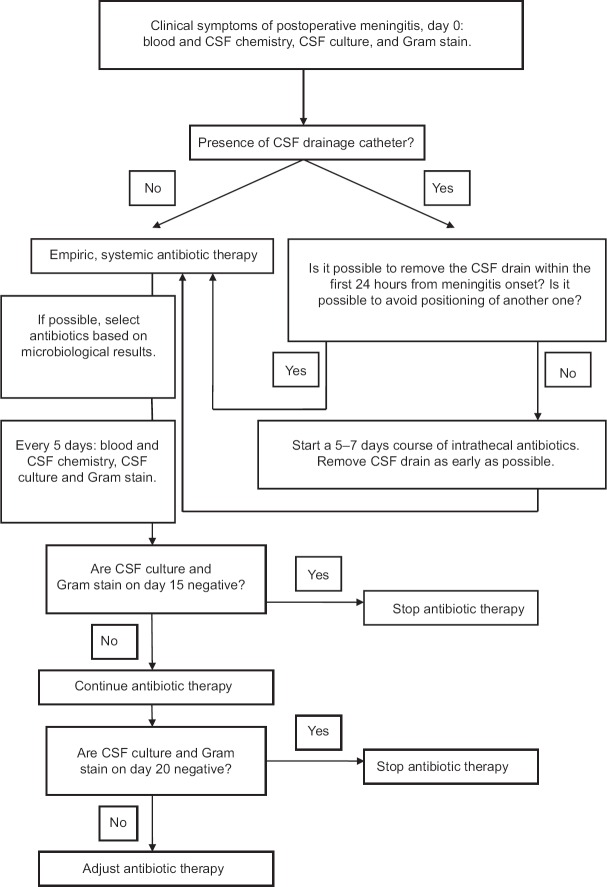
Based on the study results, we updated the algorithm for the management of patients with proven or possible postoperative meningitis [Figure 1].
Figure 1.
Diagnostic and therapeutic algorithm for suspicion of postoperative or drain-related meningitis
Our study has several limitations. Due to its partially retrospective design, diagnostic and therapeutic procedures were not systematically standardized. Furthermore, patients’ subgroups were small and with limited ability to identify significant associations of one or a group of variables. However, we analyzed a large number of ESD-related meningitis, which have rarely been investigated to date.[5,6] To our knowledge, this is the first study that takes into account the clinical, biochemical, and microbiological course of meningitis and its outcome with particular attention to the influence of CSF drainage catheters.
CONCLUSION
In summary, early device removal was associated with shorter illness duration, whereas implantation of a second device during the course of meningitis was associated with a longer resolution time. These observations will need to be validated in a prospective study in order to better define the timing of device removal, its eventual repositioning, as well as the best antibiotic regimen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Footnotes
Contributor Information
Laura Soavi, Email: laura.soavi@gmail.com.
Manuela Rosina, Email: manuelarosina@hotmail.com.
Roberto Stefini, Email: roberto.stefini@gmail.com.
Alessia Fratianni, Email: alessia.fratianni@yahoo.com.
Barbara Cadeo, Email: barbara.bs@libero.it.
Silvia Magri, Email: silviamagri@bresciaonline.it.
Nicola Latronico, Email: nicola.latronico@unibs.it.
Marco Fontanella, Email: marco.fontanella@unibs.it.
Liana Signorini, Email: l.signorini@infettivibrescia.it.
REFERENCES
- 1.Arabi Y, Memish ZA, Balkhy HH, Francis C, Ferayan A, Al Shimemeri A, et al. Ventriculostomy-associated infections: Incidence and risk factors. AJIC. 2005;33:137–43. doi: 10.1016/j.ajic.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Bogdahn U, Lau W, Hassel W, Gunreben G, Mertens HG, Brawanski A. Continuous-pressure controlled, external ventricular drainage for treatment of acute hydrocephalus – Evaluation of risk factors. Neurosurgery. 1992;31:898–904. doi: 10.1227/00006123-199211000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Citerio G, Signorini L, Bronco A, Vargiolu A, Rota M, Latronico N. Infezioni LIquorali Catetere Correlate Study Investigators. External Ventricular and Lumbar Drain Device Infections in ICU Patients: A Prospective Multicenter Italian Study. Crit Care Med. 2015;43:1630–7. doi: 10.1097/CCM.0000000000001019. [DOI] [PubMed] [Google Scholar]
- 4.Conen A, Walti LN, Merlo A, Fluckiger U, Battegay M, Trampuz A. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: A retrospective analysis over an 11-year period. Clin Infect Dis. 2008;47:73–82. doi: 10.1086/588298. [DOI] [PubMed] [Google Scholar]
- 5.Coplin WM, Avellino AM, Kim DK, Winn HR, Grady MS. Bacterial meningitis associated with lumbar drains: A retrospective cohort study. J Neurol Neurosurg Psychiatry. 1996;67:468–73. doi: 10.1136/jnnp.67.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Governale LS, Fein N, Logsdon J, Black PM. Techniques and complications of external lumbar drainage for normal pressure hydrocephalus. Neurosurgery. 2008;63(Suppl 2):379–84. doi: 10.1227/01.NEU.0000327023.18220.88. [DOI] [PubMed] [Google Scholar]
- 7.Holloway KL, Barnes T, Choi S, Bullock R, Marshall LF, Eisenberg HM, et al. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J Neurosurg. 1996;85:419–24. doi: 10.3171/jns.1996.85.3.0419. [DOI] [PubMed] [Google Scholar]
- 8.Korinek AM, Reina M, Boch AL, Rivera AO, De Bels D, Puybasset L. Prevention of external ventricular drain-related ventriculitis. Acta Neurochir. 2005;147:39–46. doi: 10.1007/s00701-004-0416-z. [DOI] [PubMed] [Google Scholar]
- 9.Lozier AP, Sciacca RR, Romagnoli MF, Sander Connolly E. Ventriculostomy-related infections: A critical review of the literature. Neurosurgery. 2002;51:170–82. doi: 10.1097/00006123-200207000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Lyke KE, Obasanjo OO, Williams MA, O’Brien M, Chotani R, Perl TM. Ventriculitis complicating use of intraventricular catheters in adult neurosurgical patients. Clin Infect Dis. 2001;33:2028–33. doi: 10.1086/324492. [DOI] [PubMed] [Google Scholar]
- 11.Mayhall CG, Archer NH, Lamb VA, Spadora AC, Baggett JW, Ward JD, et al. Ventriculostomy-related infections. A prospective epidemiologic study. N Engl J Med. 1984;310:553–9. doi: 10.1056/NEJM198403013100903. [DOI] [PubMed] [Google Scholar]
- 12.Park P, Garton HJ, Kocan MJ, Thompson BG. Risk of infection with prolonged ventricular catheterization. Neurosurgery. 2004;55:594–601. doi: 10.1227/01.neu.0000134289.04500.ee. [DOI] [PubMed] [Google Scholar]
- 13.Pfisterer W, Muehlbauer M, Czech T, Reinprecht A. Early diagnosis of external ventricular drainage infection: Results of a prospective study. J Neurol Neurosurg Psychiatry. 2003;74:929–32. doi: 10.1136/jnnp.74.7.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van de Beek D, Drake JM, Tunkel AR. Nosocomial bacterial meningitis. N Engl J Med. 2010;362:146–54. doi: 10.1056/NEJMra0804573. [DOI] [PubMed] [Google Scholar]