Abstract
Purpose of review
The purpose of this review is to highlight what the Chronic Renal Insufficiency Cohort (CRIC) study has taught us regarding arterial stiffness in chronic kidney disease. The CRIC study began in mid-2003 and enrolled more than 3900 people with chronic kidney disease.
Recent findings
The recent findings from the CRIC study are covered in 10 lessons. Within the CRIC study, we enrolled about 2800 participants who underwent a pulse wave velocity measurement. At the time of initial funding, very little was known about the role of arterial stiffness in chronic, nondialyzed, kidney disease. The lessons span the gamut from simple correlations to measures such as central arterial pressure profiles and reproducibility of pulse wave velocity measurements between operators, to relationships of pulse wave velocity to kidney function, protein excretion, cardiovascular disease prevalence, and incident cardiovascular events such as heart failure.
Summary
The implications from these lessons are that pulse wave velocity is a robust, reproducible measure of arterial stiffness which adds important information to standard clinical assessments such as SBP and DBP in a population with chronic kidney disease, a disorder with high likelihood of progressive kidney function loss, and a substantial predisposition to cardiovascular disease.
Keywords: arterial stiffness, chronic kidney disease, pulse wave velocity
INTRODUCTION
Chronic kidney disease (CKD) is an important condition because it leads to end-stage renal disease (ESRD) in many people, and is associated with an increased occurrence of cardiovascular and cerebrovascular disease and death [1,2]. Depending on how CKD is defined, between 10 and 15% of the adult US population have dipstick positive proteinuria or an estimated glomerular filtration rate (eGFR) of less than 60 ml/min/1.73 m2 [3].
Major factors which predict the rate at which kidney function declines in patients with CKD are diabetes, the level of blood pressure, and the magnitude of urine protein excretion. In more recent times, African descent [4], particularly when both the G1 and G2 risk alleles of the apolipoprotein L-1 (APOL1) gene are present [5▪▪], and the blood levels of fibroblast growth factor 23 (FGF23) [6] are related to the rate of loss-of-kidney function in patients with established CKD.
Over the past decade, societies such as the National Kidney Foundation (NKF) and the American Diabetes Association (ADA) have consistently encouraged the pursuit of goal blood pressure and glucose targets in an effort to stem the tide of CKD progression [7]. Improvements in the achievement of blood pressure control, in cardiovascular interventions in those patients with atherosclerotic manifestations, and in the management of patients with CKD and comorbidities such as heart failure have increased the survival of patients with CKD. These efforts may have changed the contemporary natural history of CKD since improvements in survival may be outstripping our ability to halt the progressive loss of kidney function, thereby allowing more patients to survive and develop ESRD, who, in the past years, might have succumbed to a cardiovascular or cerebrovascular event. To illustrate this point further, the 5% random Medicare sample of people enrolled in Medicare from 1996 to 2000, and who were followed for 2 years, indicated that a patient with CKD was at least five times more likely to die than to reach ESRD [8]. At the time of enrollment into the Chronic Renal Insufficiency Cohort (CRIC) study, about 34% of our participants had already experienced at least one cardiovascular event, including a myocardial infarction (MI), New York Heart Association (NYHA) heart failure stages I and II (stages III and IV were excluded), a stroke or transient ischemic attack, or a peripheral vascular intervention (angioplasty or lower-extremity amputation) [9].
KEY POINTS.
Carotid–femoral pulse wave velocity provides insight into vascular health that is independent of the standard brachial blood pressure.
Carotid–femoral pulse wave velocity is a potent predictor of cardiovascular outcomes, particularly incident heart failure, in patients with chronic kidney disease.
Carotid–femoral pulse wave velocity is a valuable measure to include in longitudinal cohort studies, but remains an ‘as-yet-untested’ candidate for an intervention trial.
The main purpose in assembling the CRIC study was to evaluate the influence of traditional risk factors on CKD progression and the incidence, or worsening, of cardiovascular disease in a population with established CKD [10]. As such, it also represented the ideal circumstance to pursue the relationships of less traditional risk factors on the same endpoints. Arterial stiffness represents one such nontraditional risk factor, and a measure of arterial stiffness was incorporated into the CRIC protocol in 2005. Moreover, when compared with arm (carotid–radial) or leg (femoral–posterior tibial) vascular segments, carotid–femoral pulse wave velocity (PWV) predicts outcomes clearly and robustly, whereas PWV measured in the arm or leg (in the same individuals as measured in the carotid–femoral segment) does not [11]. Measuring arterial stiffness using PWV is espoused by the European Society of Hypertension which recommendeds that the assessment of arterial stiffness be accomplished by measuring the PWV using waveform detection at a carotid and a femoral site, i.e., carotid–femoral PWV [12]. This can be done by tonometry, mechano-transduction, oscillometry, or ultrasound [13]. In the CRIC study, we measured carotid–femoral PWV using applanation tonometry [14].
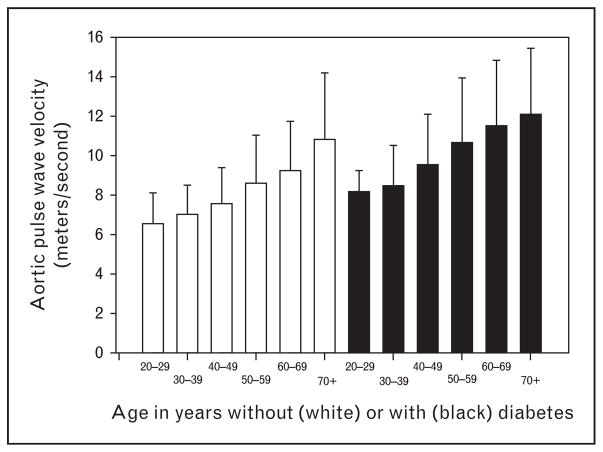
Lesson 1: the pulse wave velocity in patients with chronic kidney disease is much higher in patients with diabetes than those in the same age range without diabetes
As shown in Fig. 1, the PWV increased with age in CKD [14]. However, within any decade, the PWV was about 2 m/s higher in diabetic patients compared with the nondiabetic patients. Since the carotid–femoral PWV increases by roughly 1 m/s in a decade, this translates into about 20 years of ‘aging’ in the aorta of a diabetic CKD patient compared with an approximately age-matched nondiabetic CKD patient.
FIGURE 1.
Pulse wave velocity in meters/second in participants separated by decades of age, enrolled in the CRIC study, who did not have diabetes (left side, open bars) or did have diabetes (right side, black bars) with SD (error bars). On an average, pulse wave velocity is about 2 m/s faster, within any decade, when diabetes is present. Graphic re-drawn from published data in reference [14]. CRIC, Chronic Renal Insufficiency Cohort.
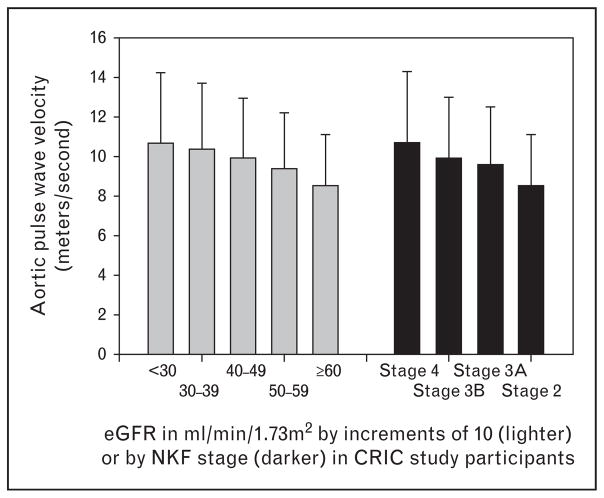
Lesson 2: as estimated glomerular filtration rate declines, pulse wave velocity increases
This has been a topic of discussion in other CKD cohorts. Generally, reduced eGFR is associated with greater arterial stiffness [15▪], but some studies were not able to show an independent contribution of decreased eGFR to increased PWV [16,17]. In the CRIC study, we observed that each 10 ml/min/1.73 m2 decline in eGFR was associated independently with a 0.23 m/s increase in PWV, as shown in Fig. 2 [14].
FIGURE 2.
Pulse wave velocity in meters/second in participants separated by 10 ml/min/1.73 m2 decrements in eGFR (left side, open bars) or by NKF stage (right side, black bars) with SD (error bars). Graphic re-drawn from published data in reference [14]. eGFR, estimated glomerular filtration rate; NKF, National Kidney Foundation.
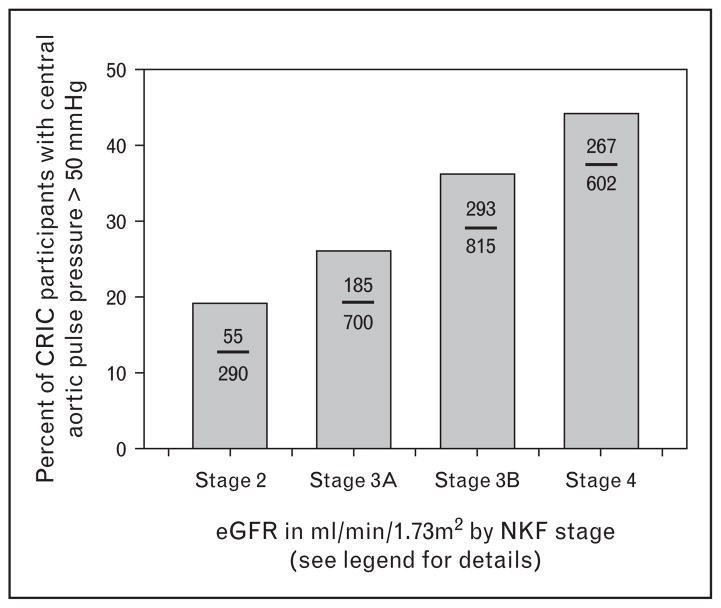
Lesson 3: declining stages of kidney function have higher central aortic pressures
One consequence of increased arterial stiffness is greater reflection of the pulse wave back to the central aorta [18]. This happens because the PWV, like all waves, experiences reflection back upon its travel path when it meets branch points, plaque, and changes in the visco-elastic components in the wall of the conduit circulation vessels. As shown in Fig. 3, the likelihood of a central pulse pressure of more than 50 mmHg – a value found to be a useful cut point in the Strong Heart Study [19] – was increasingly more prevalent as NKF kidney function stage worsened [20]. An important consequence of greater wave reflection is that it adds extra work to each left ventricular contraction, and may be important in the development of left ventricular hypertrophy and heart failure (see the section ‘Lesson 4: increased pulse wave velocity predicts incident heart failure in chronic kidney disease’).
FIGURE 3.
Percentage of CRIC participants with a central pulse pressure above 50 mmHg separated by NKF stage, in which case stage 2 is an eGFR of 60 ml/min/1.73 m2 and above, stage 3A is an eGFR of 45–59 ml/min/1.73 m2, stage 3B is an eGFR of 30–44 ml/min/1.73 m2, and stage 4 is an eGFR of below 30 ml/min/1.73 m2. Values within bars show number of participants above central pulse pressure of 50 mmHg (numerator) versus total number of CRIC participants within that NKF stage (denominator). Graphic re-drawn from published data in reference [20]. CRIC, Chronic Renal Insufficiency Cohort; eGFR, estimated glomerular filtration rate; NKF, National Kidney Foundation.
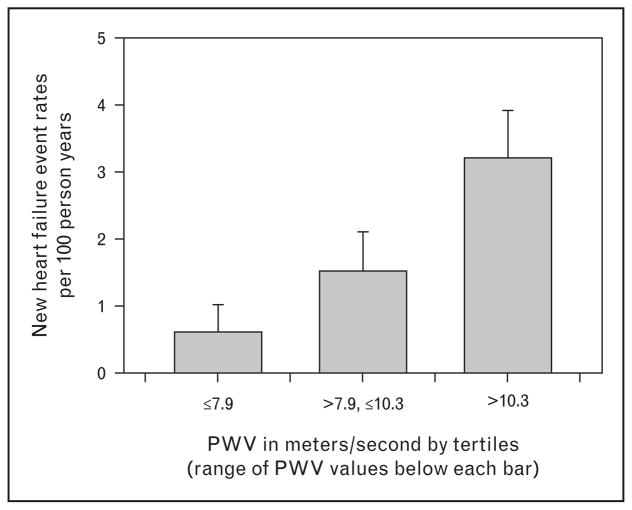
Lesson 4: increased pulse wave velocity predicts incident heart failure in chronic kidney disease
As noted in the section ‘Lesson 3: declining stages of kidney function have higher central aortic pressures’, a consequence of greater arterial stiffness is a predisposition to heart failure. Heart failure is the most common reason people over the age of 60 years are admitted to the hospital in the United States [21]. In the CRIC PWV heart failure analyses, we specifically excluded participants with a history of NYHA stage I and II at enrollment and observed that increased PWV was associated with a greater likelihood of developing heart failure [22▪▪]. This association was independent of blood pressure levels, sex, and the level of kidney function. Figure 4 compares the heart failure free survival of CRIC participants by tertile of PWV. These data show PWV to be an important and potent predictor of incident heart failure in CKD.
FIGURE 4.
New incident heart failure free event among CRIC participants initially free of heart failure by self-report. Heart failure event rates are per 100 person-years and separated by tertiles of PWV with the range of PWV for each tertile shown underneath the bar. Re-drawn from reference [22▪▪]. CRIC, Chronic Renal Insufficiency Cohort; PWV, pulse wave velocity.
Lesson 5: increased arterial stiffness is associated with poorer cognitive function in chronic kidney disease
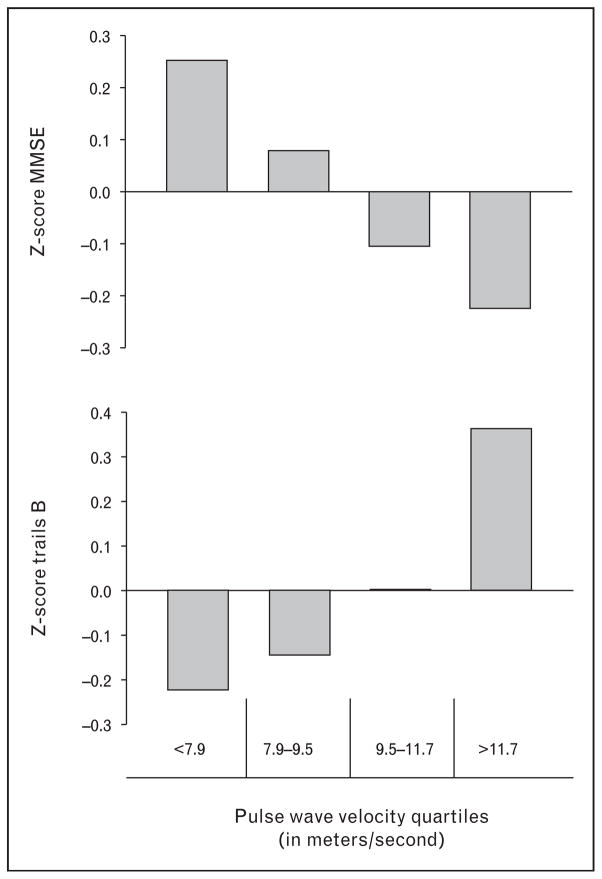
The brain and the kidney share one thing in common – they are low vascular resistance circulatory beds, and as a result, the pulse wave penetrates deeply into the microvascular level in these two organs [23]. We evaluated whether the PWV was associated, cross-sectionally, with cognitive function tests in patients with CKD. Cognitive function testing was undertaken in the CRIC study, through an ancillary study, which allowed an extensive battery of tests to be administered to a subset of approximately 800 patients in the CRIC study [24]. Among these participants, 664 had a PWV measure either at the time of, or within 1 year of, cognitive function testing. Figure 5 shows examples of the relationship of PWV to two cognitive function measures as presented at the 2012 American Society of Nephrology (ASN) meeting in San Diego [25]. In the case of the Mini-Mental Status Exam (MMSE) test, a lower score was worse. In the case of trails B, a lower score in this test indicated better ability to connect numbers and letters during a timed testing period, so lower is better. As shown in the figure, quartiles of PWV were clearly, and independently of SBP, associated with Z-score-averaged values for these cognitive function tests. Increasing PWV was an independent predictor of cognitive function impairment in CKD.
FIGURE 5.
Representative cognitive function tests and quartiles of pulse wave velocity. Upper panel compares PWV quartiles with Z-scores in participants performing the Mini-Mental Status Exam (MMSE). Lower panel compares PWV quartiles with Z-scores in participants performing the trails B test. Graphic re-drawn from published abstract cited as reference [38]. PWV, pulse wave velocity.
Lesson 6: increased chronic kidney disease predicts further kidney function loss in patients with established chronic kidney disease
As noted in the section ‘Lesson 5: increased arterial stiffness is associated with poorer cognitive function in chronic kidney disease’, the low resistance in the kidney, as in the brain, may contribute to its vulnerability to trauma from the pressure wave. The level of blood pressure control in the CRIC study is surprisingly good, with average levels of blood pressure at enrollment of 128/71 mmHg and about 53% below NKF and ADA BP targets of less than 130/<80 mmHg [9]. Despite this reasonable level of blood pressure control, many CRIC participants still progress to ESRD. In the data we presented at the American Heart Association (AHA) Council for High Blood Pressure Research in September of 2014, we observed that CRIC participants with the highest tertiles of PWV (>10.8 m/s) and brachial pulse pressure (>62 mmHg) were about three-fold more likely to reach ESRD when compared with CRIC participants with the lowest tertile of PWV (<7.9 m/s) and the lowest tertile of pulse pressure (<46 mmHg).
Lesson 7: the SBP at enrollment in the CRIC study was not highly associated with prevalent cardiovascular disease, whereas the pulse wave velocity was highly associated with prevalent cardiovascular disease and this association was independent of blood pressure
In data presented only in abstract form so far, we evaluated whether arterial stiffness (obtained about 2 years after enrollment) was associated with prevalent cardiovascular disease at enrollment into the CRIC study. Figure 6 shows the relationships between tertiles of PWV, and the prevalence of cardiovascular disease (self-reported by CRIC participants), broken down by three clinical age groupings. In multivariable analyses, PWV had a strong relationship to cardiovascular disease, whereas SBP had no significant association with prevalent cardiovascular disease in a model where both SBP and PWV were present.
FIGURE 6.
Data presented at regional symposium (no published abstract available). Shown are percentages of CRIC participants with any cardiovascular disease (self-report) at baseline in CRIC separated by age categories of 21–44 years, 45–64 years, and above 64 years. The graphic is subdivided by tertiles of pulse wave velocity within each age category. CRIC, Chronic Renal Insufficiency Cohort; CVD, cardiovascular disease.
Lesson 8: in chronic kidney disease, pulse wave velocity is predicted by proteins important in vascular calcification
Patients with vascular calcification are at substantially higher risk for cardiovascular events [26]. In CKD, a large body of data attests to disordered bone mineral metabolism [27–29]. We conducted an analysis to see if a biomarker-associated vascular calcification was related to PWV. We tested, among a subset of 226 participants who underwent concurrent PWV measurements and evaluation at a single-center CRIC ancillary study on bone metabolism, whether increasing tertiles of serum osteoprotegerin (OPG) were associated with PWV after adjustment for demographics, and a host of vascular risk factors including albuminuria, serum phosphate, corrected serum calcium, and C-reactive protein. We observed a modest but independent relationship of increasing tertiles of OPG with increasing PWV. Across the three tertiles of OPG, PWV was about 1 m/s higher in the highest compared with the lowest OPG tertile [30].
Lesson 9: central aortic pressure profiles, thought to represent arterial stiffness, are not superimposable on pulse wave velocity measurements, but pulse wave velocity measures are reproducible
This may seem intuitive, but many investigators feel that measures of central aortic pressure profile, such as the augmentation index, are good surrogates of arterial stiffness. The augmentation index is derived from an analysis of the central aortic pressure waveform (generated by a transfer function from a calibrated radial artery waveform) in which the amount of systolic pressure profile in the aorta from wave reflection is divided by the aortic pulse pressure to yield a unitless ratio (depicted as a %) [31]. This measure – the augmentation index – is easier to obtain than PWV as it requires only a single radial artery measurement. In the CRIC study, we evaluated how well augmentation index correlated with the carotid–femoral PWV [32]. We had two purposes in mind when undertaking this study. One was to evaluate whether central pressure measures (augmentation index) were a good surrogate for PWV. Secondly, we sought to understand how reproducible, between operators, measures of central pressures and PWV were.
We observed a statistically significant but clinically modest correlation (ρ values of ~0.3) between augmentation index and PWV in men and women with CKD. We also observed a mean difference of about 0 m/s, with a SD of 1.1 m/s, between operators for measurement of PWV using Bland–Altman plots [33]. For augmentation index, we found a mean difference of 1% with a SD of 7% using Bland–Altman plots. We suggested that measures of central pressure profile are not a surrogate for an actual measure of central arterial stiffness, though they do reflect arterial stiffness to a degree. On the contrary, PWV, in particular, is a very reproducible measurement as noted by others [34,35].
Lesson 10: pulse wave velocity independently predicts 24-h urinary protein excretion in chronic kidney disease in diabetic patients
One difficult concept to disentangle is the relationship between blood pressure and arterial stiffness. This is partly due to the influence that blood pressure has on PWV because blood pressure is a ‘loading’ characteristic on the vessels, particularly elastic vessels such as the aorta [36]. However, within a cohort, even at the same level of mean arterial pressure, there is a spectrum of PWV values; thus, PWV measures seem to provide information not available from the blood pressure alone. We evaluated this aspect of blood pressure and PWV as factors associated with urinary protein excretion in the CRIC study.
In a sample of 2144 participants from the CRIC study, we observed 24-h urinary excretion of protein to be 790 mg in a population in which approximately 70% were on an angiotensin-converting enzyme (ACE)-inhibitor, or an angiotensin receptor-blocking drug [37]. In these participants, the average blood pressure was 126/71 mmHg, higher in those with diabetes (130/68 mmHg) compared to those without diabetes (120/72 mmHg). The average PWV was 9.4 m/s in these participants, with values averaging 10.6 m/s in those with diabetes compared to 8.6 m/s in those without diabetes.
Each 1 m/s increase in PWV was associated with an increase of 114 mg/day of urine protein loss, and this relationship was significant in the diabetic patients (P <0.01), but not in those without diabetes (P = 0.11). Each 10 mmHg of SBP increase was associated with 228 mg of urine protein loss and was significant in the diabetic (P <0.01) but not in the nondiabetic (P = 0.14) groups. In multivariable models adjusted for age, sex, race, eGFR, heart rate, and usage of an ACE-inhibitor or an ARB, both SBP and PWV remained significant and independent predictors of urine protein excretion in the diabetic participants with CKD. Although speculative, greater arterial stiffness may be part of the reason for the more rapid loss of kidney function in diabetic compared with nondiabetic forms of CKD.
CONCLUSION
Arterial stiffness is a strong, independent predictor for a variety of vascular outcomes in a variety of longitudinal population cohorts and in this instance represents an exposure variable. However, arterial stiffness is also an outcome, with blood pressure and age representing the strongest determinants of PWV. Consequently, the ability of PWV to predict outcomes independently of age and blood pressure – two very potent vascular outcome predictors – is further testimony to its clinical utility. The incorporation of measures of PWV into a number of cohorts around the world further supports its value as a cardiovascular risk marker. Attention is turning to ways to de-stiffen the aorta, with at least one randomized clinical trial undertaken in this area – the Statégie de Prévention Cardiovasculaire Basée sur la Rigidité Arterielle Study (SPARTE) in France [38].
Acknowledgments
Financial support and sponsorship
Funding for the CRIC study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131.
Footnotes
Conflicts of interest
The author acknowledges grant support from the NIH, as outlined above.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
- 1.Shulman NB, Ford CE, Hall WD, et al. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13:I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Coresh J, Selvin E, Stevens LA, Levey AS. Prevalence of chronic kidney disease in the United States. J Am Med Assoc. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4.Rostand SG, Brown G, Kirk KA, et al. Renal insufficiency in treated essential hypertension. Clin Nephrol. 1989;320:684–688. doi: 10.1056/NEJM198903163201102. [DOI] [PubMed] [Google Scholar]
- 5▪▪.Parsa A, Kao WH, Xie D, et al. APOL1 risk variants, race, and progression of chronic kidney disease. N Engl J Med. 2013;369:2183–2196. doi: 10.1056/NEJMoa1310345. This study shows a significant role for the presence of the G1 and G2 risk alleles in patients of African descent with CKD. They progress to worsened levels of renal function, whether or not diabetes is present, more rapidly than African descent patients without the risk alleles, or whites. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. J A Med Assoc. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis. 2000;36:646–661. doi: 10.1053/ajkd.2000.16225. [DOI] [PubMed] [Google Scholar]
- 8.Collins AJ, Li S, Gilbertson DT, et al. Chronic kidney disease and cardiovascular disease in the Medicare population. Kidney Int Suppl. 2003;87:S24–S31. doi: 10.1046/j.1523-1755.64.s87.5.x. [DOI] [PubMed] [Google Scholar]
- 9.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: design and methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 11.Pannier B, Guerin AP, Marchais SJ, et al. Stiffness of capacitive and conduit arteries: prognostic significance for end-stage renal disease patients. Hypertension. 2005;45:592–596. doi: 10.1161/01.HYP.0000159190.71253.c3. [DOI] [PubMed] [Google Scholar]
- 12.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 13.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 14.Townsend RR, Wimmer NJ, Chirinos JA, et al. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Tomiyama H, Townsend RR, Matsumoto C, et al. Arterial stiffness/central hemodynamics, renal function, and development of hypertension over the short term. J Hypertens. 2014;32:90–99. doi: 10.1097/HJH.0b013e3283658e7d. This study from Japan provides independent confirmation of the importance of arterial stiffness both in the development of CKD and the development of high blood pressure. [DOI] [PubMed] [Google Scholar]
- 16.Lilitkarntakul P, Dhaun N, Melville V, et al. Blood pressure and not uraemia is the major determinant of arterial stiffness and endothelial dysfunction in patients with chronic kidney disease and minimal co-morbidity. Atherosclerosis. 2011;216:217–225. doi: 10.1016/j.atherosclerosis.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 17.Briet M, Collin C, Karras A, et al. Arterial remodeling associates with CKD progression. J Am Soc Nephrol. 2011;22:967–974. doi: 10.1681/ASN.2010080863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katakam R, Townsend RR. What’s in a pulse? J Clin Hypertens (Greenwich) 2006;8:140–141. doi: 10.1111/j.1524-6175.2006.05173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roman MJ, Devereux RB, Kizer JR, et al. High central pulse pressure is independently associated with adverse cardiovascular outcome: The Strong Heart Study. J Am Coll Cardiol. 2009;54:1730–1734. doi: 10.1016/j.jacc.2009.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend RR, Chirinos JA, Parsa A, et al. Central pulse pressure in chronic kidney disease. A Chronic Renal Insufficiency Cohort Ancillary Study. Hypertension. 2010;56:518–524. doi: 10.1161/HYPERTENSIONAHA.110.153924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hunt SA, Abraham WT, Chin MH, et al. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 22▪▪.Chirinos JA, Khan AM, Bansal N, et al. Arterial stiffness, central pressures and incident hospitalized heart failure in the Chronic Renal Insufficiency Cohort (CRIC) Study. Circ Heart Fail. 2014;7:709–716. doi: 10.1161/CIRCHEARTFAILURE.113.001041. Epub ahead of print This observation from the CRIC study shows the importance of arterial stiffness independent of several risk factors (sex, age, eGFR, and blood pressure) in the development of hospitalized heart failure in CKD patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 24.Yaffe K, Ackerson L, Tamura MK, et al. Chronic kidney disease and cognitive function in older adults: findings from the chronic renal insufficiency cohort cognitive study. J Am Geriatr Soc. 2010;58:338–345. doi: 10.1111/j.1532-5415.2009.02670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Townsend RR, Kling M, Seliger SL, et al. Relationship of aortic pulse wave velocity to cognitive function in chronic kidney disease: the Chronic Renal Insufficiency Cohort Study. J Am Soc Nephrol. 2012;23:667A–668A. [Google Scholar]
- 26.Verbeke F, Van BW, Honkanen E, et al. Prognostic value of aortic stiffness and calcification for cardiovascular events and mortality in dialysis patients: outcome of the calcification outcome in renal disease (CORD) study. Clin J Am Soc Nephrol. 2011;6:153–159. doi: 10.2215/CJN.05120610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jono S, Shioi A, Ikari Y, Nishizawa Y. Vascular calcification in chronic kidney disease. J Bone Miner Metab. 2006;24:176–181. doi: 10.1007/s00774-005-0668-6. [DOI] [PubMed] [Google Scholar]
- 28.Kerr PG, Guerin AP. Arterial calcification and stiffness in chronic kidney disease. Clin Exp Pharmacol Physiol. 2007;34:683–687. doi: 10.1111/j.1440-1681.2007.04660.x. [DOI] [PubMed] [Google Scholar]
- 29.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36(Suppl 2):51–62. doi: 10.1111/j.1365-2362.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 30.Scialla JJ, Leonard MB, Townsend RR, et al. Correlates of osteoprotegerin and association with aortic pulse wave velocity in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2612–2619. doi: 10.2215/CJN.03910411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Rourke MF, Pauca AL. Augmentation of the aortic and central arterial pressure waveform. Blood Press Monit. 2004;9:179–185. doi: 10.1097/00126097-200408000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Wimmer NJ, Townsend RR, Joffee MM, et al. CRIC Study Investigators: Correlation between pulse wave velocity and other measures of arterial stiffness in chronic kidney disease. Clin Nephrol. 2007;68:133–143. [PubMed] [Google Scholar]
- 33.Bland JM, Altman DG. Measuring agreement in method comparison studies. Stat Methods Med Res. 1999;8:135–160. doi: 10.1177/096228029900800204. [DOI] [PubMed] [Google Scholar]
- 34.Frimodt-Moller M, Nielsen AH, Kamper AL, Strandgaard S. Reproducibility of pulse-wave analysis and pulse-wave velocity determination in chronic kidney disease. Nephrol Dial Transplant. 2008;23:594–600. doi: 10.1093/ndt/gfm470. [DOI] [PubMed] [Google Scholar]
- 35.Naidu MU, Reddy BM, Yashmaina S, et al. Validity and reproducibility of arterial pulse wave velocity measurement using new device with oscillometric technique: a pilot study. Biomed Eng Online. 2005;4:49. doi: 10.1186/1475-925X-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 37.Weir MR, Townsend RR, Fink JC, et al. Hemodynamic correlates of proteinuria in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:2403–2410. doi: 10.2215/CJN.01670211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: needed clinical trials. Hypertension. 2012;60:518–522. doi: 10.1161/HYPERTENSIONAHA.112.194456. [DOI] [PubMed] [Google Scholar]