Abstract
OBJECTIVE
Little is known about arsenic and diabetes in youth. We examined the association of arsenic with type 1 and type 2 diabetes in the SEARCH for Diabetes in Youth Case-Control (SEARCH-CC) study. Because one-carbon metabolism can influence arsenic metabolism, we also evaluated the potential interaction of folate and vitamin B12 with arsenic metabolism on the odds of diabetes.
RESEARCH DESIGN AND METHODS
Six hundred eighty-eight participants <22 years of age (429 with type 1 diabetes, 85 with type 2 diabetes, and 174 control participants) were evaluated. Arsenic species (inorganic arsenic [iAs], monomethylated arsenic [MMA], dimethylated arsenic [DMA]), and one-carbon metabolism biomarkers (folate and vitamin B12) were measured in plasma. We used the sum of iAs, MMA, and DMA (∑As) and the individual species as biomarkers of arsenic concentrations and the relative proportions of the species over their sum (iAs%, MMA%, DMA%) as biomarkers of arsenic metabolism.
RESULTS
Median ∑As, iAs%, MMA%, and DMA% were 83.1 ng/L, 63.4%, 10.3%, and 25.2%, respectively. ∑As was not associated with either type of diabetes. The fully adjusted odds ratios (95% CI), rescaled to compare a difference in levels corresponding to the interquartile range of iAs%, MMA%, and DMA%, were 0.68 (0.50–0.91), 1.33 (1.02–1.74), and 1.28 (1.01–1.63), respectively, for type 1 diabetes and 0.82 (0.48–1.39), 1.09 (0.65–1.82), and 1.17 (0.77–1.77), respectively, for type 2 diabetes. In interaction analysis, the odds ratio of type 1 diabetes by MMA% was 1.80 (1.25–2.58) and 0.98 (0.70–1.38) for participants with plasma folate levels above and below the median (P for interaction = 0.02), respectively.
CONCLUSIONS
Low iAs% versus high MMA% and DMA% was associated with a higher odds of type 1 diabetes, with a potential interaction by folate levels. These data support further research on the role of arsenic metabolism in type 1 diabetes, including the interplay with one-carbon metabolism biomarkers.
Introduction
Increasing evidence supports the role of environmental chemicals in diabetes development, although most studies have focused on adult populations, and little information is available for type 1 diabetes (1). Inorganic arsenic (iAs) is a toxicant and carcinogen that occurs naturally in the environment, especially in groundwater. Dietary sources are increasingly recognized as a concern for general populations because arsenic is found in rice, grains, and certain juices (2). Studies in adult populations support the association of chronic exposure to iAs in drinking water with diabetes, specifically type 2 diabetes (3,4). Research in children and adolescents and in populations exposed to arsenic mostly from food is needed.
No epidemiological studies to our knowledge have evaluated the association between arsenic and type 1 diabetes, which is primarily characterized by impaired insulin secretion as a result of an autoimmune assault on pancreatic β-cells. No experimental animal studies have been specifically designed to evaluate the role of arsenic in type 1 diabetes. Several lines of evidence, however, support the consideration of arsenic in the pathogenesis of type 1 diabetes. First, arsenic may impair the immune system (5). Second, prenatal arsenic exposure may alter the microbiome composition in early childhood, potentially influencing arsenic metabolism and susceptibility to type 1 diabetes (6). Third, pancreatic β-cells are recognized as a target tissue for various arsenic species in humans, animals, and in vitro model systems (4,7). After exposure, iAs is methylated in the body into monomethylated arsenic (MMA) and dimethylated arsenic (DMA) compounds that are excreted in the urine together with iAs, with a half-life of 4 days (8). Arsenic metabolism, in particular higher relative concentrations of DMA together with lower concentrations of MMA in the urine, has been related to increased prevalence and incidence of diabetes in adults (9–11).
SEARCH for Diabetes in Youth Case-Control (SEARCH-CC) is a two-center study designed to characterize type 1 and type 2 diabetes and assess selected risk factors for both diabetes types in youth (12). To provide novel information on the potential role of arsenic in diabetes in children, we used biospecimens collected in SEARCH-CC to measure arsenic species in plasma and investigate their association with type 1 and type 2 diabetes. By taking together the epidemiological and experimental studies and potential mechanisms involved, we hypothesized that arsenic exposure and metabolism is associated with an increased odds of both type 1 and type 2 diabetes. Because one-carbon metabolism nutrients, such as folate and vitamin B12, are related to arsenic metabolism (13,14) and diabetes-related outcomes (15), we adjusted for and conducted an interaction analysis of plasma folate and vitamin B12 in regression models of the association between arsenic and diabetes.
Research Design and Methods
Study Population and Case-Control Recruitment
SEARCH is a population-based study initiated in 2000 to better understand how diabetes develops in children and youth. SEARCH is being conducted in five sites across the U.S. (South Carolina, Ohio, Colorado, California, and Washington) and comprises >20,000 participants age <20 years, including a wide range of racial and ethnic groups and socioeconomic backgrounds (16).
For the current study, we used data from SEARCH-CC, an ancillary study conducted in two of the five SEARCH sites (four counties around Columbia, SC, and six counties around Denver, CO). Recruitment of SEARCH-CC participants between 10 and 22 years of age occurred between July 2003 and March 2006 in primary care offices. All patients with a clinical diagnosis of type 1 and type 2 diabetes, potential control participants, and their parents/guardians were provided an informational brochure and invited to complete a one-page permission form for further contact. Control participants were concurrently recruited from the same primary care offices, following the rationale that all SEARCH cases arose from health care provider offices.
The clinical diabetes type assigned by the health care provider was recorded as part of the case validation process and then categorized as type 1 (combining type 1, type 1a, and type 1b) and type 2. Other types (including gestational diabetes mellitus, hybrid type, maturity onset of diabetes in youth, secondary diabetes, type unknown by the reporting source, type designated as other, and missing type) were excluded. In a study of the full SEARCH cohort, the provider’s diagnosis was shown to be consistent with a classification based on diabetes autoantibodies and estimated insulin sensitivity (17). Control participants were confirmed as not having diabetes by fasting glucose levels obtained at the SEARCH-CC study visit (12,18).
The response rate was 53% in case participants and 49% in control participants (12) for a total sample size of 722 participants (445 with type 1 diabetes, 91 with type 2 diabetes, and 186 control participants). Participants were similar in age, sex, and race/ethnicity to nonparticipants (12,18). We further excluded participants with missing data on plasma arsenic species concentrations (n = 7), BMI (n = 8), parental educational level (n = 2), and vitamin B12 (n = 17), resulting in a total of 688 participants for the current study (429 with type 1 diabetes, 85 with type 2 diabetes, and 174 control participants). Participants ≥18 years of age and parents of participants <18 years of age provided written informed consent.
Data and Sample Collection
Trained staff used a standardized protocol to collect sociodemographic data (sex, age, race/ethnicity, parental educational level), conduct a physical examination (blood pressure, height and weight to assess BMI, waist circumference), and collect fasting blood and urine samples (12). Waist circumference was measured just above the uppermost lateral border of the right ilium based on National Health and Nutrition Examination Study protocol (19). Height was measured with a stadiometer. Weight was measured with an electronic scale. Age- and sex-specific BMI z scores were derived on the basis of Centers for Disease Control and Prevention national standards (20). Obesity was defined as having a BMI ≥95th percentile for age and sex.
Blood samples were obtained under conditions of metabolic stability after at least 8 h of fasting. Specimens were processed at the sites and shipped within 24 h to the central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratories, University of Washington, Seattle, WA) and stored at <−70°C. Total folate and vitamin B12 concentrations in plasma were analyzed by using an AccuBind ELISA kit (Monobind, Lake Forest, CA) while following the manufacturer’s protocol. A reference analysis of folate was performed in 50 randomly selected samples by microbial assay (21) in the Nutrition Obesity Core of the Nutrition Research Institute (Kannapolis, NC).
Plasma Arsenic Determinations
In 2014, stored aliquots of 0.5 mL plasma per participant were shipped to the laboratory of M.St. at the University of North Carolina where they were analyzed for arsenic species. The iAs, MMA, and DMA concentrations were measured in 25 μL of plasma by using hydride generation–cryotrapping inductively coupled mass spectrometry (22). The limits of detection were 0.63 ng arsenic/L for iAs and 0.05 ng arsenic/L for MMA and DMA. The intraassay coefficients of variation were 3.6% for iAs, 3.8% for MMA, and 3.3% for DMA. To ensure accuracy of the analysis, a standard reference material, Arsenic Species in Frozen Human Urine (SRM 2669; National Institute of Standards and Technology) diluted in deionized water was analyzed with approximately every other group of samples for a total of 34 times. The average concentrations of iAs, MMA, and DMA in SRM 2669 determined by hydride generation–cryotrapping inductively coupled mass spectrometry represented 90.4%, 94.5%, and 79.6% of the certified values, respectively (Supplementary Table 1). For additional quality control, SRM 2669 was diluted in human plasma. The averaged concentrations determined by 12 independent analyses represented 97.1%, 98.6%, and 89.0% of the certified values for iAs, MMA, and DMA, respectively (Supplementary Table 2).
We used the sum of iAs, MMA, and DMA concentrations (∑As) to assess total arsenic exposure. The proportions of iAs, MMA, and DMA over their sum and multiplied by 100 (expressed as iAs%, MMA%, and DMA%) were used as arsenic metabolism biomarkers.
Statistical Analysis
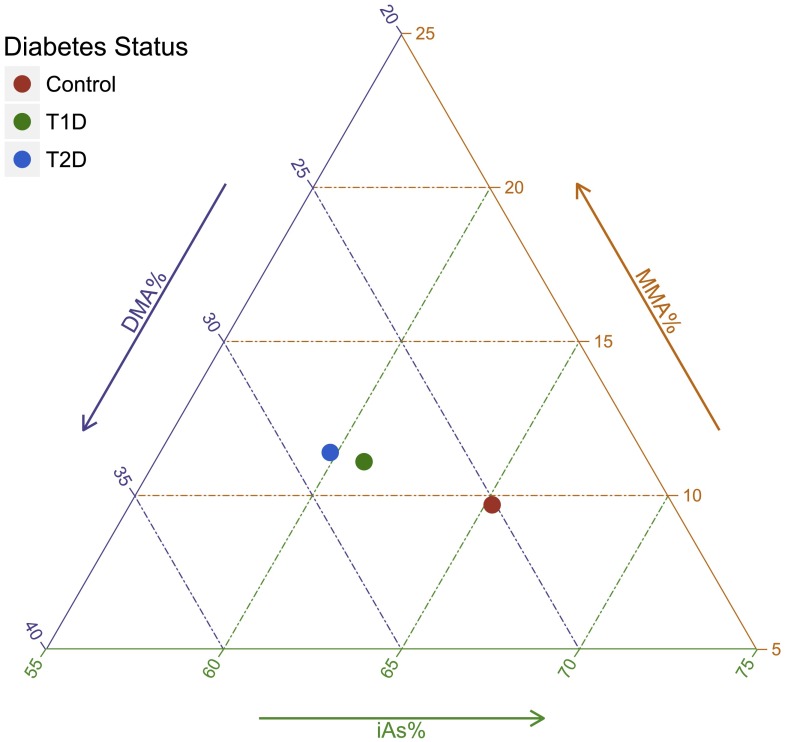
Statistical analyses were performed using R version 3.1.2 software (23). Differences in characteristics between participants with type 1 and type 2 diabetes and control participants were determined with Mann-Whitney U test for continuous variables and χ2 test for categorical variables (Table 1). Because arsenic metabolism biomarkers add up to 100%, we graphically described the distribution of arsenic metabolism by case-control status by using a triplot (diagram with three axes) and compared the compositional means of iAs%, MMA%, and DMA% in participants with type 1 and type 2 diabetes compared with control participants (Fig. 1). We also reported the pairwise Spearman correlations between ∑As concentrations and each arsenic metabolism biomarker (Supplementary Fig. 1) and graphically described the median and interquartile range for ∑As, iAs, MMA, DMA, iAs%, MMA%, and DMA% across participant subgroups (Supplementary Fig. 2). Supplementary Figs. 1 and 2 represent analyses conducted only among participants without diabetes because control participants are more likely to represent the source population.
Table 1.
Participant characteristics by diabetes status
| Control (n = 174) | T1D (n = 429) | T2D (n = 85) | P value control vs. T1D | P value control vs. T2D | |
|---|---|---|---|---|---|
| Age (years) | 14.1 (11.9, 16.4) | 14.7 (12.2, 17.5) | 15.8 (13.5, 17.8) | 0.060 | <0.001 |
| Male sex | 66 (37.9) | 208 (48.5) | 28 (32.9) | 0.023 | 0.518 |
| Race | <0.001 | <0.001 | |||
| Non-Hispanic white | 97 (55.7) | 339 (79.0) | 21 (24.7) | ||
| African American | 48 (27.6) | 43 (10.0) | 52 (61.2) | ||
| Hispanic | 29 (16.7) | 47 (11.0) | 12 (14.1) | ||
| Parental educational level | 0.019 | <0.001 | |||
| ≤12 years | 87 (50.0) | 168 (39.2) | 67 (78.8) | ||
| >12 years | 87 (50.0) | 261 (60.8) | 18 (21.2) | ||
| Waist circumference (cm) | 76.4 (69.4, 87.6) | 78.0 (69.8, 86.0) | 108.8 (100.0, 120.2) | 0.978 | <0.001 |
| BMI (kg/m2) | 22.2 (19.1, 26.5) | 21.8 (19.1, 25.0) | 34.5 (29.8, 40.1) | 0.125 | <0.001 |
| Obesity | 40 (23.0) | 52 (12.1) | 74 (87.1) | 0.001 | <0.001 |
| Diabetes duration (years) | NA | 3.3 (0.9, 7.9) | 1.3 (0.6, 2.0) | <0.001* | |
| Total folate (μg/L) | 19.9 (14.5, 25.2) | 20.2 (15.2, 25.0) | 16.0 (11.6, 22.0) | 0.754 | 0.002 |
| Vitamin B12 (μg/L) | 555.3 (416.7, 688.7) | 599.8 (485.5, 728.0) | 479.6 (401.7, 607.5) | <0.001 | 0.019 |
| Arsenic exposure | |||||
| ∑As (ng/L) | 83.1 (63.8, 110.3) | 81.7 (64.3, 106.7) | 82.9 (62.3, 98.9) | 0.665 | 0.521 |
| iAs (ng/L) | 50.2 (40.7, 63.05) | 46.6 (36.4, 59.3) | 42.9 (31.9, 55.6) | 0.046 | 0.010 |
| MMA (ng/L) | 8.1 (4.2, 13.9) | 8.9 (5.5, 15.3) | 9.6 (5.2, 16.0) | 0.239 | 0.182 |
| DMA (ng/L) | 21.0 (13.0, 30.1) | 22.8 (15.4, 33.7) | 23.4 (17.7, 30.5) | 0.196 | 0.227 |
| Arsenic metabolism | |||||
| iAs% | 63.4 (51.2, 74.0) | 58.7 (47.7, 68.9) | 58.0 (46.3, 65.6) | 0.011 | 0.027 |
| MMA% | 10.3 (6.3, 14.9) | 11.2 (7.2, 16.6) | 12.7 (7.4, 17.9) | 0.072 | 0.036 |
| DMA% | 25.2 (18.6, 33.5) | 28.9 (21.0, 36.1) | 29.3 (21.4, 35.7) | 0.014 | 0.048 |
Data are median (interquartile range) and n (%). P values were obtained by Mann-Whitney U test for continuous variables and χ2 test for categorical variables. T1D, type 1 diabetes; T2D, type 2 diabetes.*P value for the comparison between T1D and T2D.
Figure 1.
Triplot representing the compositional means for arsenic metabolism biomarkers (iAs%, MMA%, and DMA%) in control participants and participants with type 1 diabetes (T1D) and type 2 diabetes (T2D). The P values comparing the arsenic metabolism biomarker compositional means were 0.02 for T1D vs. control, 0.12 for T2D vs. control, and 0.87 for T1D vs. T2D.
Odds ratios (ORs) and 95% CI comparing arsenic levels for each type of diabetes with control were computed by using progressively adjusted logistic regression models (Table 2). Arsenic was modeled as log-transformed for ∑As, iAs, MMA, and DMA concentrations and in the original scale for arsenic metabolism biomarkers (iAs%, MMA%, and DMA%). Instead of reporting the results for a change in 1 log-unit (arsenic concentrations) or in 1 unit (arsenic metabolism biomarkers), the ORs (95% CI) for type 1 or type 2 diabetes were rescaled for an interquartile range difference in arsenic levels (i.e., comparing participants in the 75th vs. the 25th percentiles of each arsenic variable based on the distribution among control participants). The goal of rescaling was to report the magnitude of the association for a difference in exposure levels that is relevant for the study population and to facilitate the comparison across variables (24). Model 1 was unadjusted. Model 2 was adjusted for age (continuous), sex (male, female), BMI (continuous), parental educational level (≤12 years, >12 years), and race/ethnicity (non-Hispanic white, African American, and Hispanic). Model 3 was further adjusted for total folate (continuous) and vitamin B12 (continuous). We ran additional models to assess possible nonlinear relationships. First, each arsenic variable was introduced as tertile categories, and the ORs of each type of diabetes were compared with arsenic tertiles 2 and 3 to the lowest tertile (Supplementary Table 3). The cutoffs for the tertiles were based on the percentiles of probabilities tertiles 1/3 and 2/3 of each arsenic variable in control participants. Second, we graphically estimated the ORs of each type of diabetes based on restricted quadratic splines with knots at the 10th, 50th, and 90th percentiles of each arsenic variable distribution (Supplementary Figs. 3–6).
Table 2.
ORs (95% CI) for type 1 and type 2 diabetes and interquartile range increase (i.e., participants in the 75th vs. 25th percentiles) in measures of arsenic species in plasma
| 75th vs. 25th percentile* | Type 1 diabetes |
Type 2 diabetes |
|||||
|---|---|---|---|---|---|---|---|
| Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | ||
| Concentrations of arsenic species | |||||||
| ∑As (ng/L) | 110.3 vs. 63.8 | 0.89 (0.72, 1.11) | 0.90 (0.71, 1.13) | 0.88 (0.69, 1.12) | 0.93 (0.68, 1.27) | 1.04 (0.66, 1.64) | 1.03 (0.66, 1.62) |
| iAs (ng/L) | 63.1 vs. 40.7 | 0.87 (0.75, 1.01) | 0.85 (0.73, 1.00) | 0.84 (0.71, 0.99) | 0.84 (0.67, 1.06) | 0.94 (0.69, 1.28) | 0.93 (0.69, 1.27) |
| MMA (ng/L) | 13.9 vs. 4.2 | 1.21 (0.94, 1.54) | 1.21 (0.93, 1.57) | 1.22 (0.93, 1.60) | 1.23 (0.87, 1.75) | 1.06 (0.63, 1.77) | 1.03 (0.62, 1.73) |
| DMA (ng/L) | 30.1 vs. 13 | 1.14 (0.93, 1.39) | 1.15 (0.94, 1.42) | 1.15 (0.92, 1.43) | 1.17 (0.88, 1.55) | 1.14 (0.74, 1.75) | 1.13 (0.73, 1.73) |
| Arsenic metabolism biomarkers | |||||||
| iAs% | 74 vs. 51.2 | 0.74 (0.57, 0.96) | 0.70 (0.53, 0.92) | 0.68 (0.50, 0.91) | 0.68 (0.47, 0.98) | 0.81 (0.48, 1.36) | 0.82 (0.48, 1.39) |
| MMA% | 14.9 vs. 6.3 | 1.27 (1.00, 1.60) | 1.29 (1.01, 1.65) | 1.33 (1.02, 1.74) | 1.42 (1.01, 2.00) | 1.11 (0.67, 1.85) | 1.09 (0.65, 1.82) |
| DMA% | 33.5 vs. 18.6 | 1.21 (0.97, 1.49) | 1.27 (1.01, 1.58) | 1.28 (1.01, 1.63) | 1.25 (0.94, 1.66) | 1.17 (0.78, 1.76) | 1.17 (0.77, 1.77) |
Model 1 is unadjusted; model 2 adjusted for age at visit (years), sex, BMI, parental educational level, and race; model 3 further adjusted for total folate and vitamin B12 levels.
*75th and 25th percentiles of each arsenic variable distribution were among control participants.
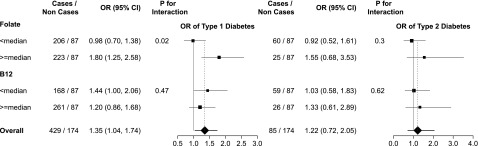
One-carbon metabolism plays a role in arsenic metabolism. In an exploratory analysis, we evaluated the potential interaction of folate and vitamin B12 levels with arsenic metabolism on diabetes by including the product regression term of each arsenic variable with categorical folate and vitamin B12 variables in the fully adjusted model (Fig. 2).
Figure 2.
ORs and 95% CI for type 1 and type 2 diabetes by MMA% in plasma and by folate and vitamin B12 levels. ORs were obtained by comparing the 75th vs. 25th percentiles of MMA% distribution among control participants. The 75th and 25th percentiles of MMA% distribution among control participants were 14.9% and 6.3%, respectively. ORs were also adjusted for age (continuous), sex, BMI (continuous), parental educational level (≤12, >12 years), race/ethnicity (non-Hispanic white, African American, Hispanic), total folate levels (continuous), and/or vitamin B12 levels (continuous). The area of each data marker is proportional to each subsample size.
Several sensitivity analyses were performed to evaluate the consistency of the findings. First, we adjusted the models for geographical area (South Carolina and Colorado) (Supplementary Table 4) with similar findings. Second, we repeated the analyses with the exclusion of participants with extreme values in arsenic variables (Supplementary Table 5). Third, because of the unbalanced number of participants with type 1 diabetes and control participants, we repeated the analyses for a 1:1 ratio for a total of 174 control participants and 174 participants with type 1 diabetes (selected at random among the 429), with similar findings (Supplementary Table 6). Finally, given the possibility that arsenic could contribute to type 1 and type 2 diabetes in similar ways, we also analyzed both types of diabetes jointly (Supplementary Table 7).
Results
Participant Characteristics
Compared with control participants, participants with type 1 diabetes were older, more likely to be male and non-Hispanic white with higher parental educational level and to be leaner with higher vitamin B12 levels. Participants with type 2 diabetes were older, more likely to belong to racial/ethnic minority groups, and more likely to be obese with lower parental educational level and lower plasma folate and vitamin B12 levels (Table 1). Participants with both type 1 and type 2 diabetes had similar concentrations for ∑As, MMA, and DMA and lower concentrations for iAs as well as lower iAs%, higher MMA%, and higher DMA% than control participants. When considering the three arsenic metabolism biomarkers simultaneously, controls had higher iAs% and lower MMA% and DMA% compared with participants with type 1 or type 2 diabetes (Fig. 1).
The iAs% was strongly and negatively correlated with MMA% and DMA% (Spearman correlations −0.65 and −0.92, respectively), whereas the correlation between MMA% and DMA% was weak and positive (0.36) (Supplementary Fig. 1). ∑As concentrations were similar across participant subgroups (Supplementary Fig. 2). For arsenic metabolism biomarkers, we found some differences by race/ethnicity, parental educational level, and obesity status (Supplementary Fig. 2).
Plasma Arsenic and Odds of Diabetes
Plasma ∑As concentrations were not associated with type 1 diabetes compared with control (fully adjusted OR [95% CI] for an interquartile range difference in log-transformed ∑As concentrations 0.88 [0.69, 1.12]) (Table 2). The direction of the association for the corresponding interquartile range difference, however, was different for the individual arsenic species, with a significant inverse association for iAs (0.84 [0.71, 0.99]) and nonsignificant positive associations for MMA (1.22 [0.93, 1.60]) and DMA (1.15 [0.92, 1.43]), although these associations were significant in sensitivity analysis that excluded participants with extreme arsenic concentrations (1.42 [1.03, 1.96] for MMA and 1.33 [1.03, 1.72] for DMA) (Supplementary Table 5).
For arsenic metabolism, the ORs (95% CI) of type 1 diabetes were 0.68 (0.50, 0.91), 1.33 (1.02, 1.74), and 1.28 (1.01, 1.63) for an interquartile range difference in iAs%, MMA%, and DMA%, respectively (Table 2, model 3). Flexible models based on tertiles (Supplementary Table 3) and quadratic splines (Supplementary Figs. 3 and 4) showed similar findings. For type 2 diabetes, the associations with plasma arsenic concentrations and arsenic metabolism biomarkers were not statistically significant but mostly in the same direction as for type 1 diabetes (Table 2 and Supplementary Figs. 5 and 6). In analyses for both types of diabetes combined, we found no interaction between any of the arsenic variables and type of diabetes (P = 0.99 for ∑As, iAs%, MMA%, and DMA%). The fully adjusted OR (95% CI) of diabetes combined compared with control for an interquartile range difference in ∑As, iAs%, MMA%, and DMA% distributions were 0.92 (0.74, 1.15), 0.71 (0.54, 0.93), 1.33 (1.04, 1.70), and 1.23 (0.99, 1.53), respectively.
Interaction of Arsenic With Folate and Vitamin B12
In interaction analysis, the fully adjusted ORs (95% CI) of type 1 diabetes for an interquartile range difference in MMA% were 0.98 (0.70, 1.38) and 1.80 (1.25, 2.58) among participants with folate levels below and above the median, respectively (P for interaction = 0.02) (Fig. 2). We also found an interaction between MMA% and folate for both types of diabetes combined (P for interaction = 0.01) (data not shown) but not for type 2 diabetes (Fig. 2). We found no other statistically or borderline interactions between folate categories and other arsenic measures (data not shown). No effect modification by vitamin B12 was observed (data not shown).
Conclusions
In children and adolescents who participated in the SEARCH-CC study, type 1 diabetes was associated with lower plasma iAs% and higher MMA% and DMA%. For type 2 diabetes, the associations were in the same direction but not statistically significant possibly because of the smaller number of cases. In analyses with type 1 and type 2 diabetes combined, we found no effect modification by type, suggesting that the association between arsenic metabolism biomarkers and diabetes may be independent of diabetes type, although this interaction analysis may lack power as a result of the small number of type 2 diabetes cases. The associations of arsenic metabolism biomarkers and diabetes remained after adjustment for sociodemographic factors, BMI, and biomarkers of one-carbon metabolism nutrients. In interaction analyses, the association between MMA% and type 1 diabetes was stronger for participants with higher folate levels. Similar but nonsignificant interaction between MMA% and folate was found for type 2 diabetes.
Plasma ∑As was not associated with either type of diabetes, although type 1 diabetes was associated with lower iAs concentrations in multiadjusted analyses and with higher MMA and DMA in analyses that excluded arsenic outliers. Because concentrations of ∑As or individual arsenic species in plasma are not established biomarkers of arsenic exposure, the lack of association between ∑As and diabetes in this study does not necessarily exclude a possible association for arsenic exposure assessed with more established biomarkers, such as arsenic species in urine. A possible explanation for lower plasma iAs but higher MMA and DMA concentrations in participants with type 1 and maybe type 2 diabetes compared with control participants is that plasma arsenic concentrations may reflect the impact of arsenic metabolism, with faster methylation processes (higher MMA% and DMA%) resulting in lower iAs concentrations.
Participants came from areas of Colorado and South Carolina that surrounded large cities, and they were most likely exposed to low levels of iAs in drinking water compared with other rural areas of the U.S. (25). The major source of iAs exposure in the study population was probably through diet [rice, grains, and juices (2,26)]. Information on the exact sources, however, is not available.
Few studies have evaluated the health effects of arsenic exposure in children beyond studies of birth outcomes, infectious diseases, and neurocognitive outcomes (27–29). In adults, arsenic exposure has been associated with diabetes in populations exposed to drinking water arsenic levels >100 μg/L (30) and <100 μg/L (3,11,31). Several prospective studies are available. Baseline urinary concentrations of ∑As were associated with incident diabetes in rural Arizona (32) and Colorado (33) but not in rural areas of the southwestern and midwestern U.S. (9). These studies reported no association with the individual arsenic species.
For arsenic metabolism, increasing evidence suggests an association with diabetes, although the nature and implications of this relationship are not clearly understood. For cancer and cardiovascular disease, higher MMA% and lower DMA% in urine have generally been associated with a higher risk of disease (34,35). For diabetes, however, studies have shown that the relationship with arsenic metabolism is in the opposite direction, with a positive association between higher DMA% and diabetes in urine, including cross-sectional evidence from Bangladesh (36) and Mexico (11) and prospective evidence from the U.S. (9). Experimental findings support that increased DMA% could be related to diabetes development because the toxic trivalent form of DMA, DMA(III), has been shown to inhibit insulin-stimulated glucose uptake in cultured adipocytes (37) and as a potent inhibitor of glucose-stimulated insulin secretion by isolated pancreatic islets (7).
We included folate and vitamin B12 in model adjustments and interaction analyses based on evidence that suggests a role for vitamins involved in regulating one-carbon metabolism with both arsenic metabolism and metabolic-related outcomes (13–15). Although the relationship with vitamin B12 is less clear, the association between plasma folate and enhanced arsenic metabolism is well established (13,14). Clinical trials in highly arsenic-exposed populations in Bangladesh showed that folate supplementation reduced iAs% and MMA% in urine, reduced total arsenic and MMA concentrations in blood, and increased DMA% in urine (13,14). The one-carbon metabolism cycle generates methyl groups, which are necessary for the oxidative methylation of arsenite to MMA(V) and MMA(III) to DMA(V). Data on the relationship between folate and vitamin B12 with diabetes are limited. Current experimental and epidemiological evidence suggests that both excesses and deficiencies in folate may be associated with type 2 diabetes–related outcomes (15). Less is known about the association between one-carbon metabolism and type 1 diabetes. The current findings suggest a potentially complex interconnection among one-carbon metabolism, arsenic metabolism, and diabetes, which warrants additional research, including experimental studies.
Strengths of this study include the large number of type 1 diabetes cases available, the similarity of characteristics of study cases to all cases identified in South Carolina and Colorado, the accuracy of provider type using autoantibodies and insulin sensitivity in the main SEARCH study (17), and the ability to adjust for relevant confounders, including one-carbon metabolism nutrients. The study has several limitations. The number of type 2 diabetes cases was small, and we could not evaluate the association between arsenic and type 2 diabetes with sufficient power. Most studies of arsenic exposure and metabolism measured iAs and methylated arsenic species in urine. Because urine samples were not collected, we could not compare the current findings in plasma with those traditional measures. Studies that measured arsenic in whole blood found a good correlation with arsenic in urine (38). Arsenic measured in blood and plasma could potentially reflect biologically effective dose better than urine because it is in more direct contact with relevant tissues (10). Another advantage is that concentrations in plasma do not need to be adjusted for urine dilution (4). Data on the repeatability of arsenic concentrations in plasma or in other biomarkers in children exposed to low arsenic through the diet are missing. Similar to most epidemiologic studies, we could not differentiate between trivalent and pentavalent forms of arsenic species. The ability to make this distinction would be useful given the inverse association between iAs concentrations and iAs% with diabetes in the current study and the potential role of MMA(III) and DMA(III) to explain those findings. Because of the cross-sectional design, we cannot evaluate the direction of the relationships between arsenic measures in plasma and diabetes development. The findings, however, would be important even if diabetes changed arsenic metabolism and internal dose because this would imply that diabetes can affect susceptibility to arsenic-related health effects, including cancer risk.
In conclusion, we found that arsenic metabolism, characterized by the relative proportions of iAs and methylated arsenic species in plasma, was associated with type 1 diabetes in children and adolescents who participated in SEARCH-CC. In particular, low iAs% versus high MMA% and DMA% were associated with a higher odds of type 1 diabetes. Low iAs concentrations were also associated with a higher prevalence of type 1 diabetes. In post hoc analysis, we found an interaction between MMA% and folate levels, with stronger associations between MMA% and type 1 diabetes among participants with higher folate levels in plasma. These findings provide novel evidence that links arsenic and diabetes in youth and support the need for additional research that focuses specifically on the relationship with arsenic metabolism, including a careful evaluation of the interplay between arsenic metabolism and one-carbon metabolism on type 1 and type 2 diabetes development.
Supplementary Material
Article Information
Funding. This study was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (DK059184, DK056350, and Intramural Research Program of NIDDK) and the National Institute of Environmental Health Sciences (NIEHS) (R01-ES-021367, R01-ES-025216, P30-ES-010126, 5P42-ES-10349, and Intramural Research Program of NIEHS).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. M.G.-P. conducted the statistical analyses and contributed to the discussion and writing of the manuscript. C.-C.K. contributed to the discussion and writing of the manuscript. M.Sp. contributed to the discussion and writing of the manuscript. K.A.T. contributed to the study design, discussion, and writing of the manuscript. M.A.M. contributed to the discussion and writing of the manuscript. R.F.H. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. D.D. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. J.L.A. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. W.C.K. contributed to the discussion and review and editing of the manuscript. R.A.B. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. F.W.M. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. A.D.L. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. C.Z. conducted the laboratory measures of arsenic and one-carbon metabolism nutrient biomarkers. C.D. conducted the laboratory measures of arsenic and one-carbon metabolism nutrient biomarkers. Z.D. conducted the laboratory measures of arsenic and one-carbon metabolism nutrient biomarkers. E.M.-D. contributed to the planning and conduct of the SEARCH-CC, discussion, and review and editing of the manuscript. M.St. contributed to the study design, conduct of the laboratory measures of arsenic and one-carbon metabolism nutrient biomarkers, discussion, and writing of the manuscript. A.N.-A. contributed to the study design, statistical analyses, discussion, and writing of the manuscript. M.G.-P. and A.N.-A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract and oral form at the International Society for Environmental Epidemiology 28th Annual Conference, Rome, Italy, 1–4 September 2016.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc16-0810/-/DC1.
References
- 1.Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep 2013;13:831–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.European Food Safety Authority. Scientific opinion on arsenic in food: Panel on Contaminants in the Food Chain. EFSA J 2009;7:1351. Available from http://www.efsa.europa.eu/en/scdocs/scdoc/1351.htm. Accessed 12 December 2011
- 3.Navas-Acien A, Silbergeld EK, Pastor-Barriuso R, Guallar E. Arsenic exposure and prevalence of type 2 diabetes in US adults. JAMA 2008;300:814–822 [DOI] [PubMed] [Google Scholar]
- 4.Maull EA, Ahsan H, Edwards J, et al. . Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 2012;120:1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dangleben NL, Skibola CF, Smith MT. Arsenic immunotoxicity: a review. Environ Health 2013;12:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu K, Abo RP, Schlieper KA, et al. . Arsenic exposure perturbs the gut microbiome and its metabolic profile in mice: an integrated metagenomics and metabolomics analysis. Environ Health Perspect 2014;122:284–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douillet C, Currier J, Saunders J, Bodnar WM, Matoušek T, Stýblo M. Methylated trivalent arsenicals are potent inhibitors of glucose stimulated insulin secretion by murine pancreatic islets. Toxicol Appl Pharmacol 2013;267:11–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Research Council Arsenic in Drinking Water. Washington, DC, National Academies Press, 1999 [Google Scholar]
- 9.Kuo CC, Howard BV, Umans JG, et al. . Arsenic exposure, arsenic metabolism, and incident diabetes in the Strong Heart Study. Diabetes Care 2015;38:620–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currier JM, Ishida MC, González-Horta C, et al. . Associations between arsenic species in exfoliated urothelial cells and prevalence of diabetes among residents of Chihuahua, Mexico. Environ Health Perspect 2014;122:1088–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendez MA, González-Horta C, Sánchez-Ramírez B, et al. . Chronic exposure to arsenic and markers of cardiometabolic risk: a cross-sectional study in Chihuahua, Mexico. Environ Health Perspect 2016;124:104–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dabelea D, Mayer-Davis EJ, Lamichhane AP, et al. . Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth: the SEARCH Case-Control Study. Diabetes Care 2008;31:1422–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamble MV, Liu X, Ahsan H, et al. . Folate and arsenic metabolism: a double-blind, placebo-controlled folic acid-supplementation trial in Bangladesh. Am J Clin Nutr 2006;84:1093–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters BA, Hall MN, Liu X, et al. . Folic acid and creatine as therapeutic approaches to lower blood arsenic: a randomized controlled trial. Environ Health Perspect 2015;123:1294–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finer S, Saravanan P, Hitman G, Yajnik C. The role of the one-carbon cycle in the developmental origins of type 2 diabetes and obesity. Diabet Med 2014;31:263–272 [DOI] [PubMed] [Google Scholar]
- 16.Hamman RF, Bell RA, Dabelea D, et al.; SEARCH for Diabetes in Youth Study Group . The SEARCH for Diabetes in Youth study: rationale, findings, and future directions. Diabetes Care 2014;37:3336–3344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabelea D, Pihoker C, Talton JW, et al.; SEARCH for Diabetes in Youth Study . Etiological approach to characterization of diabetes type: the SEARCH for Diabetes in Youth Study. Diabetes Care 2011;34:1628–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liese AD, Puett RC, Lamichhane AP, et al. . Neighborhood level risk factors for type 1 diabetes in youth: the SEARCH Case-Control Study. Int J Health Geogr 2012;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J Pediatr 2004;145:439–444 [DOI] [PubMed] [Google Scholar]
- 20.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. . CDC growth charts: United States. Adv Data 2000. (314):1–27 [PubMed] [Google Scholar]
- 21.Tamura T. Determination of food folate. J Nutr Biochem 1998;9:285–293 [Google Scholar]
- 22.Matoušek T, Currier JM, Trojánková N, et al. . Selective hydride generation- cryotrapping-ICP-MS for arsenic speciation analysis at picogram levels: analysis of river and sea water reference materials and human bladder epithelial cells. J Anal At Spectrom 2013;28:1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.R Core Team. R: a language and environment for statistical computing [Internet], 2014. Vienna, Austria, R Foundation for Statistical Computing. Available from http://R-project.org. Accessed 27 October 2016
- 24.Babyak MA. Rescaling continuous predictors in regression models [article online]. Psychosom Med 24 September 2009. Available from http://stattips.blogspot.com/2009/08/rescaling-continuous-predictors-in.html. Accessed 27 October 2016 [Google Scholar]
- 25.Ryker SJ. Mapping arsenic in ground water [article online]. Geotimes 2001;46:34–36. Available from http://water.usgs.gov/nawqa/trace/pubs/geo_v46n11/fig2.html. Accessed 27 October 2016
- 26.Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in U.S. children. Environ Health Perspect 2012;120:1418–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laine JE, Bailey KA, Rubio-Andrade M, et al. . Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the Biomarkers of Exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect 2015;123:186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rager JE, Yosim A, Fry RC. Prenatal exposure to arsenic and cadmium impacts infectious disease-related genes within the glucocorticoid receptor signal transduction pathway. Int J Mol Sci 2014;15:22374–22391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tolins M, Ruchirawat M, Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann Glob Health 2014;80:303–314 [DOI] [PubMed] [Google Scholar]
- 30.Tseng CH, Tai TY, Chong CK, et al. . Long-term arsenic exposure and incidence of non-insulin-dependent diabetes mellitus: a cohort study in arseniasis-hyperendemic villages in Taiwan. Environ Health Perspect 2000;108:847–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maull EA, Ahsan H, Edwards J, et al. Evaluation of the association between arsenic and diabetes: a National Toxicology Program workshop review. Environ Health Perspect 2012;120:1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim NH, Mason CC, Nelson RG, et al. . Arsenic exposure and incidence of type 2 diabetes in southwestern American Indians. Am J Epidemiol 2013;177:962–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James KA, Marshall JA, Hokanson JE, Meliker JR, Zerbe GO, Byers TE. A case-cohort study examining lifetime exposure to inorganic arsenic in drinking water and diabetes mellitus. Environ Res 2013;123:33–38 [DOI] [PubMed] [Google Scholar]
- 34.Melak D, Ferreccio C, Kalman D, et al. . Arsenic methylation and lung and bladder cancer in a case-control study in northern Chile. Toxicol Appl Pharmacol 2014;274:225–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Wu F, Liu M, et al. . A prospective study of arsenic exposure, arsenic methylation capacity, and risk of cardiovascular disease in Bangladesh. Environ Health Perspect 2013;121:832–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nizam S, Kato M, Yatsuya H, et al. . Differences in urinary arsenic metabolites between diabetic and non-diabetic subjects in Bangladesh. Int J Environ Res Public Health 2013;10:1006–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walton FS, Harmon AW, Paul DS, Drobná Z, Patel YM, Styblo M. Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: possible mechanism of arsenic-induced diabetes. Toxicol Appl Pharmacol 2004;198:424–433 [DOI] [PubMed] [Google Scholar]
- 38.Peters BA, Hall MN, Liu X, et al. Renal function is associated with indicators of arsenic methylation capacity in Bangladeshi adults. Environ Res 2015;143:123–130 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.