Refractory angina pectoris is a chronic disabling condition affecting approximately 850,000 patients in the United States.1 It is characterized by frequent angina attacks unresponsive to maximal medical therapy and obstructive coronary artery disease not amenable to coronary revascularization.2 Although major progress has been made in medical therapy and cardiovascular interventions,1 up to 43% of patients continue to experience symptoms and 33% have positive exercise tests after angioplasty.3 It is now well recognized that these patients have concomitant microvascular disease, with reduced coronary and systemic flow reserve at a microvascular level and impaired endothelium-mediated vasorelaxation, i.e., endothelial dysfunction.4 Currently, the treatment of these patients remains a major clinical challenge.
To address this large unmet therapeutic need, research has focused on biological strategies for refractory angina. A key effort is the use of cell therapy, which has the potential to restore the microcirculation and improve myocardial tissue perfusion by stimulating neoangiogenesis.5 In this regard, accumulating evidence supports the idea that cell-based therapy can improve clinical outcomes, including frequency of angina episodes, myocardial infarction (MI) rate, and exercise tolerance, in patients with refractory angina,5, 6 and thus should be subject to further trials to evaluate this treatment option for this patient population.
In this issue of Circulation Research, Khan and colleagues present a comprehensive meta-analysis of cell-based therapy for refractory angina. Importantly, their analysis addresses the heterogeneity of the included trials, the problem of missing data, and limitations of the study.5 Six single- and double-blinded, randomized clinical trials were included in this meta-analysis, comprising a patient population that had class III–IV Canadian Cardiovascular Society (CCS) angina, were refractory to medical therapy, and were not coronary revascularization candidates (Table 1). The study included 192 patients that received cell therapy plus standard medical treatment and 161 patients who received only standard medical therapy. All six trials exhibited safety and efficacy. Three different cell types were examined: peripheral blood- or bone marrow-derived CD34+ cells in three trials, bone marrow-derived mononuclear cells (BM-MNCs) in two trials, and peripheral blood derived CD133+ cells in one trial. Only the PROGENITOR7 trial was a negative trial, as it did not meet its primary endpoints of cardiovascular death, non-fatal MI, ischemic stroke, need for revascularization, and procedure-related complications. Five studies used the NOGA mapping navigation system for intramyocardial cell injections, and the remaining study used intracoronary delivery of CD34+ cells during cardiac catheterization.5
Table 1.
Cell therapy trials for refractory angina included in the meta-analysis by Khan et al 2016
| Trial | Cell type | Number of patients Total/Treated | Improved Endpoints | Unchanged Endpoints |
|---|---|---|---|---|
| Jimenez-Quevedo et al 2014; Spain | Autologous peripheral blood-derived CD 133+ cells |
28/19 | myocardial perfusion CCS angina class angina episodes/month nitroglycerin use/month |
cardiovascular death non-fatal MI ischemic stroke need for revascularization procedure-related complications |
| Losordo et al 2011; USA | autologous peripheral blood-derived CD 34+ cells |
167/111 | angina frequency/week (low cell dose) exercise tolerance test (low cell dose) myocardial perfusion (low cell dose) |
nitroglycerin use/day CCS angina class MACE |
| Wang et al 2010; China | autologous bone marrow-derived CD 34+ cells |
112/56 | arrhythmia monitoring – no risk angina frequency nitroglicerine use/week exercise tolerance time CCS angina class | myocardial perfusion |
| Jan van Ramshorts et al 2009; Netherlands | autologous BM-MNCs |
50/25 | arrhythmia monitoring – no risk myocardial perfusion left ventricle ejection fraction CCS angina class quality of life score |
end systolic volume end diastolic volume |
| Losordo et al 2007; USA | autologous peripheral blood-derived CD 34+ cells |
24/18 | arrhythmia monitoring – no risk angina frequency* nitroglycerine use* exercise tolerance* CCS angina class* myocardial perfusion* quality-of-life testing* |
|
| Hung-Fat Tse et al 2007; Hong Kong Australia |
Autologous BM-MNCs |
28/19 | arrhythmia monitoring – no risk intramyocardial tumor or calcification – absent total exercise time myocardial perfusion left ventricle ejection fraction % of regional wall thickening NYHA functional class |
LV end-systolic volume LV end-diastolic volume CCS angina class |
probability values were not shown, because no power calculations to determine sample size were done
CCS = Canadian Cardiovascular Society; MACE = major adverse cardiac events; LV = left ventricular.
The efficacy outcomes were frequency of angina episodes, CCS angina class, exercise tolerance, left ventricular function, change in anti-anginal medications, and quality of life. The effect of stem cell treatment on myocardial perfusion was assessed by single photon emission computed tomography (SPECT). Clinical end-points were combined into major adverse cardiac events (MACE) and included MI, cardiac-related hospitalization, and mortality.5
Notably, the investigators found that cell-based therapy led to an improvement in myocardial perfusion (Figure 1). Pooled analysis from clinical trials demonstrated notable improvement in CCS angina class, left ventricular ejection fraction (LVEF), use of anti-anginal medications, and a decreased risk of MACE. Finally, the occurrence of atrial and ventricular arrhythmias was also significantly decreased in the cell therapy group.5 Previous meta-analyses6,8 reported similar results of decreased angina frequency and MI rate and improved exercise tolerance. However, the meta-analysis by Khan et al. advances the field by expanding the clinical parameters of the study and including results of myocardial perfusion as assessed by SPECT.
Figure 1.
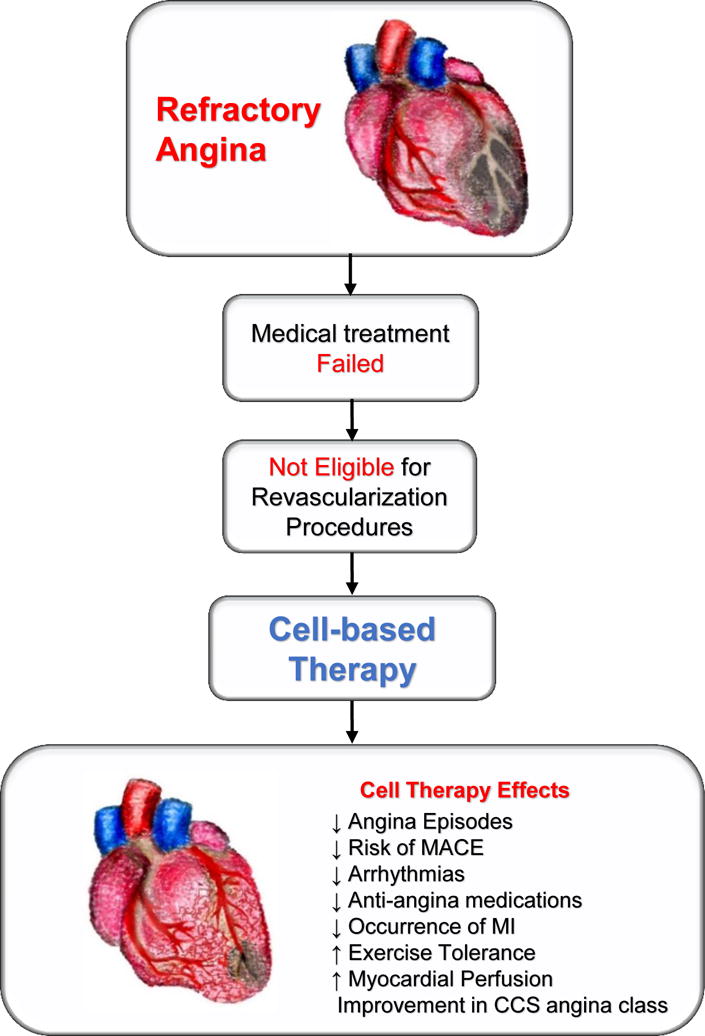
Schematic illustration of the benefits of cell-based therapy in patients with refractory angina
Mechanisms of cell therapy for refractory angina
Cell-based therapy represents a potent biological drug that promotes tissue regeneration through mechanisms including direct tissue transdifferentiation, cell-cell interaction with host tissue, and paracrine signaling.9–12 Because cell therapy promotes neoangiogenesis, therein lies the potential to restore the microvasculature and ameliorate refractory anginal symptoms. Mechanisms underlying promotion of neoangiogenesis with cell therapy involve release of paracrine factors, such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), hepatocyte growth factor (HGF), angiopoietin-1, and others. Many of these angiogenic factors are also expressed by stem cells, and various studies have shown direct involvement of stem cells in neoangiogenesis in ischemic tissues, by increasing capillary density and creating anastomoses with the host circulation.13, 14
Bone marrow-derived mononuclear cells (BM-MNCs) represent a heterogeneous population composed of hematopoietic stem cells, mesenchymal stem cells (MSCs), and endothelial progenitor cells (EPCs). In preclinical studies, BM-MNCs augmented neoangiogenesis in a rabbit ischemic limb model by inducing collateral vessel formation and blood perfusion.15 Consistent with these findings, BM-MNCs injected into swine ischemic hearts produced a significant increase of regional blood flow and capillary density. The cardiac levels of bFGF, VEGF, and angiopoietin were significantly increased after stem cell injection compared with control.13 Moreover, angiogenic cytokines, like cardiac interleukin-1β and tumor necrosis factor-α, were significantly increased after BM-MNC administration and contributed to angiogenesis.14
Asahara and coworkers first isolated EPCs from peripheral blood in 1997. EPCs are a specific population of progenitor cells that home to sites of tissue injury and participate in angiogenesis, by integrating with the host capillary vessels and forming capillaries.16 These cells are characterized by expression of surface markers, including FLK1 (VEGFR2), CD133, and CD34, and within a few weeks in tissue culture they begin expressing endothelial markers such as von Willebrand Factor and VE-cadherin.14 Their implantation has resulted in favorable effects on myocardial capillary density, perfusion, collagen deposition, and contractile function in a rodent MI model. Even in the setting of compromised macrovascular supply, improvement in microvascular and collateral perfusion can augment contractile function.17 A key mechanism for cell therapy is the stimulation of endogenous precursor cells,10, 11 and in this regard our group has shown that allogeneic MSCs injected into cardiac tissue stimulate EPC bioactivity and restore endothelial function in patients with idiopathic dilated and ischemic cardiomyopathy.12 Accordingly, cell therapy may directly activate neoangiogenic pathways by direct activation of endogenous precursor cells.
Other therapeutic stem cells for patients with refractory angina
What is the best cell-based treatment for the patients with refractory angina? Despite the positive results of the meta-analysis by Khan et al, previous clinical trials with BM-MNC treatment failed to show efficacy in patients with acute MI18, 19 and chronic ischemic cardiomyopathy,20 therefore other cell types should be investigated in future clinical trials. Numerous clinical and pre-clinical studies demonstrated the therapeutic efficacy of MSCs. For instance, in the TAC-HFT trial, MSCs improved cardiac function and structure in patients with chronic ischemic cardiomyopathy as compared to BM-MNCs and placebo.21 There is accumulating data from previous studies supporting a multifactorial mechanism of action by MSCs.11 These cells, when applied into a region of myocardial ischemia, can differentiate into smooth muscle cells and endothelial cells leading to increased vessel density and improved cardiac function.22 While preclinical data support substantial trilineage transdifferentiation (cardiomyocytes, vascular smooth muscle cells, endothelial cells) of MSCs in the porcine infarcted heart,23 the angiogenic effects of MSCs are enhanced by their paracrine actions involving secretion of VEGF, bFGF, and platelet-derived growth factor that influence adjacent cells and result in improvement of left ventricle remodeling, neovascularization, tissue repair, and decreased cell apoptosis, mitochondrial dysfunction, and microvascular dysfunction.11 The results of the PROMETHEUS clinical trial showed that MSCs injected into scarred myocardial segments that were not surgically revascularized produce significant improvement in myocardial perfusion, contractility, and reduction in scar size at 18 months after treatment.24 Additionally, MSCs can be important regulators of neo-vascularization by acting as pericytes, cells that stabilize the newly formed vasculature. Moreover, the paracrine mediators may also elicit autocrine effects on the biology of stem cells themselves. Therefore, the paracrine/autocrine mechanism extends the concept of the stem cell niche and includes the factors released by stem cells into the microenvironment controlling stem cell biology and tissue regeneration.11
Cardiac stem cells (CSCs) are a cell population that reside in the heart and are characterized by the expression of c-kit (CD117), stem cell antigen 1 (Sca-1), and Islet-1. In response to cardiac injury, CSCs promote increased vessel density, a mechanism associated with improved cardiac function.25 Interestingly, transplanted bone marrow-derived MSCs establish cell-cell interactions with host myocardium and stimulate endogenous c-kit+ CSC differentiation and cardiomyocyte cell cycling.10 In preclinical studies, interactions between MSCs and CSCs enhance cardiac performance to a greater extent than MSCs alone and are associated with increased cardiac perfusion assessed by CMR.9, 26 These findings support the novel hypothesis that cell interactions activate stem cell niches and modulate the microenvironment toward regeneration.9, 26
Induced pluripotent stem cells (iPSCs) are a novel cell type, derived by reprogramming somatic cells via expression of exogenous transcription factors. iPSCs can differentiate into mature cell types, including vascular endothelial cells, which can be used for treatment of myocardial and limb ischemia, increasing capillary density through activation of paracrine mechanisms.27 Thus this cell population is a potential candidate to treat refractory angina.
Allogeneic cell therapy
To date, clinical studies have used autologous cells to treat patients with refractory angina. There is intriguing evidence that allogeneic cell-based therapy produces similar safety and potentially greater efficacy in patients with ischemic and non-ischemic heart disease.11 Indeed, the POSEIDON clinical trial compared transendocardial injection of autologous vs. allogeneic bone marrow-derived MSCs, and reported similar safety profiles in the two groups and a significant reduction in left ventricular end diastolic volume in the allogeneic MSC treatment.28 Moreover, patients from the POSEIDON-DCM (NCT01392625) and TRIDENT (NCT02013674) trials showed improved endothelial function 3 months after transendocardial administration of allogeneic, but not autologous, MSCs.12 The advantages of allogeneic cell products also include the relative ease of accessibility from young healthy donors, ability to expand in high volumes, and availability for infusion. Therefore, allogeneic cell therapy may be a superior alternative that can be used as an “off-the shelf” product for patients with cardiovascular and other diseases.
Conclusion and future perspectives
Future studies should be designed to define the optimal cell type(s) to treat refractory angina, including combination cell therapy. One of the challenges in clinical trials has been the selection of appropriate time points and patient population. Most cell therapy studies have not examined the most important clinical end points, i.e., recurrent MI, cardiac related hospitalizations, and mortality, and have only followed-up the patients for 6 and/or 12 months. Longer follow-up would allow for a better understanding of the long-term effects of cell therapy and mechanisms of neoangiogenesis activated by stem cells.
In summary, the meta-analysis by Khan et al. showed promise for cell-based therapy for patients with refractory angina who are not candidates for revascularization. While cell therapy is not yet a cure, it may provide benefits in terms of quality of life and longevity. The time for Phase 3 clinical studies has arrived in order to determine the most effective stem cell treatment for patients with refractory angina and also to understand the mechanisms by which the cells exert their therapeutic effects. Well-designed human studies with meaningful endpoints will help supplement the unanswered questions and provide support to keep this promising innovative field of research moving forward.
Acknowledgments
Disclosures
This work was supported by the National Institutes of Health grant R01HL084275 awarded to Dr. Hare. Dr. Hare is also supported by the National Institutes of Health grants R01HL107110, UM1HL113460, and R01HL110737; and grants from the Starr Foundation and the Soffer Family Foundation. Dr. Hare has a patent for cardiac cell-based therapy; he holds equity in Vestion Inc.; and maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion did not play any role in the design or conduct of the study. Dr. Florea is supported by an award from the American Heart Association.
Nonstandard Abbreviations and Acronyms
- BM-MNC
Bone marrow mononuclear cells
- CCS
Canadian Cardiovascular Society
- CMR
Cardiac magnetic resonance imaging
- CSC
Cardiac stem cell
- EPC
Endothelial progenitor cell
- bFGF
Basic fibroblast growth factor
- HGF
Hepatocyte growth factor
- LVEF
Left Ventricular Ejection Fraction
- MACE
Major adverse cardiac events
- MI
Myocardial Infraction
- MSC
Mesenchymal stem cell
- SPECT
Single photon emission computed tomography
- VEGF
Vascular endothelial growth factor
Literature Cited
- 1.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA, Investigators AC Intramyocardial, autologous cd34+ cell therapy for refractory angina. Circulation research. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim MC, Kini A, Sharma SK. Refractory angina pectoris: Mechanism and therapeutic options. Journal of the American College of Cardiology. 2002;39:923–934. doi: 10.1016/s0735-1097(02)01716-3. [DOI] [PubMed] [Google Scholar]
- 3.Finci L, Meier B, Favre J, Righetti A, Rutishauser W. Long-term results of successful and failed angioplasty for chronic total coronary arterial occlusion. The American journal of cardiology. 1990;66:660–662. doi: 10.1016/0002-9149(90)91125-p. [DOI] [PubMed] [Google Scholar]
- 4.Gutterman DD, Chabowski DS, Kadlec AO, Durand MJ, Freed JK, Ait-Aissa K, Beyer AM. The human microcirculation: Regulation of flow and beyond. Circulation research. 2016;118:157–172. doi: 10.1161/CIRCRESAHA.115.305364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AR, Farid TA, Pathan A, Tripathi A, Ghafghazi S, Wysoczynski M, Bolli R. Impact of cell therapy on myocardial perfusion and cardiovascular outcomes in patients with angina refractory to medical therapy: A systematic review and meta-analysis. Circulation research. 2016 doi: 10.1161/CIRCRESAHA.115.308056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Yang YJ, Zhang Q, Jin C, Wang H, Qian HY. Stem cell therapy is a promising tool for refractory angina: A meta-analysis of randomized controlled trials. The Canadian journal of cardiology. 2013;29:908–914. doi: 10.1016/j.cjca.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Jimenez-Quevedo P, Gonzalez-Ferrer JJ, Sabate M, Garcia-Moll X, Delgado-Bolton R, Llorente L, Bernardo E, Ortega-Pozzi A, Hernandez-Antolin R, Alfonso F, Gonzalo N, Escaned J, Banuelos C, Regueiro A, Marin P, Fernandez-Ortiz A, Neves BD, Del Trigo M, Fernandez C, Tejerina T, Redondo S, Garcia E, Macaya C. Selected cd133(+) progenitor cells to promote angiogenesis in patients with refractory angina: Final results of the progenitor randomized trial. Circulation research. 2014;115:950–960. doi: 10.1161/CIRCRESAHA.115.303463. [DOI] [PubMed] [Google Scholar]
- 8.Fisher SA, Doree C, Brunskill SJ, Mathur A, Martin-Rendon E. Bone marrow stem cell treatment for ischemic heart disease in patients with no option of revascularization: A systematic review and meta-analysis. PloS one. 2013;8:e64669. doi: 10.1371/journal.pone.0064669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–223. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circulation research. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circulation research. 2015;116:1413–1430. doi: 10.1161/CIRCRESAHA.116.303614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine. 2015;2:467–475. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circulation research. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hou L, Kim JJ, Woo YJ, Huang NF. Stem cell-based therapies to promote angiogenesis in ischemic cardiovascular disease. American journal of physiology. Heart and circulatory physiology. 2015 doi: 10.1152/ajpheart.00726.2015. ajpheart 00726 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shintani S, Murohara T, Ikeda H, Ueno T, Sasaki K, Duan J, Imaizumi T. Augmentation of postnatal neovascularization with autologous bone marrow transplantation. Circulation. 2001;103:897–903. doi: 10.1161/01.cir.103.6.897. [DOI] [PubMed] [Google Scholar]
- 16.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 17.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 18.Traverse JH, Henry TD, Pepine CJ, Willerson JT, Zhao DX, Ellis SG, Forder JR, Anderson RD, Hatzopoulos AK, Penn MS, Perin EC, Chambers J, Baran KW, Raveendran G, Lambert C, Lerman A, Simon DI, Vaughan DE, Lai D, Gee AP, Taylor DA, Cogle CR, Thomas JD, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Kappenman C, Westbrook L, Piller LB, Simpson LM, Baraniuk S, Loghin C, Aguilar D, Richman S, Zierold C, Spoon DB, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The time randomized trial. JAMA: the journal of the American Medical Association. 2012;308:2380–2389. doi: 10.1001/jama.2012.28726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Traverse JH, Henry TD, Ellis SG, Pepine CJ, Willerson JT, Zhao DX, Forder JR, Byrne BJ, Hatzopoulos AK, Penn MS, Perin EC, Baran KW, Chambers J, Lambert C, Raveendran G, Simon DI, Vaughan DE, Simpson LM, Gee AP, Taylor DA, Cogle CR, Thomas JD, Silva GV, Jorgenson BC, Olson RE, Bowman S, Francescon J, Geither C, Handberg E, Smith DX, Baraniuk S, Piller LB, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy R Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: The latetime randomized trial. JAMA: the journal of the American Medical Association. 2011;306:2110–2119. doi: 10.1001/jama.2011.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perin EC, Willerson JT, Pepine CJ, Henry TD, Ellis SG, Zhao DX, Silva GV, Lai D, Thomas JD, Kronenberg MW, Martin AD, Anderson RD, Traverse JH, Penn MS, Anwaruddin S, Hatzopoulos AK, Gee AP, Taylor DA, Cogle CR, Smith D, Westbrook L, Chen J, Handberg E, Olson RE, Geither C, Bowman S, Francescon J, Baraniuk S, Piller LB, Simpson LM, Loghin C, Aguilar D, Richman S, Zierold C, Bettencourt J, Sayre SL, Vojvodic RW, Skarlatos SI, Gordon DJ, Ebert RF, Kwak M, Moye LA, Simari RD, Cardiovascular Cell Therapy Research N Effect of transendocardial delivery of autologous bone marrow mononuclear cells on functional capacity, left ventricular function, and perfusion in chronic heart failure: The focus-cctrn trial. JAMA: the journal of the American Medical Association. 2012;307:1717–1726. doi: 10.1001/jama.2012.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: The tac-hft randomized trial. JAMA: the journal of the American Medical Association. 2014;311:62–73. doi: 10.1001/jama.2013.282909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silva GV, Litovsky S, Assad JA, Sousa AL, Martin BJ, Vela D, Coulter SC, Lin J, Ober J, Vaughn WK, Branco RV, Oliveira EM, He R, Geng YJ, Willerson JT, Perin EC. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111:150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 23.Quevedo HC, Hatzistergos KE, Oskouei BN, Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu Q, McNiece I, Heldman AW, Hare JM. Allogeneic mesenchymal stem cells restore cardiac function in chronic ischemic cardiomyopathy via trilineage differentiating capacity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:14022–14027. doi: 10.1073/pnas.0903201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karantalis V, DiFede DL, Gerstenblith G, Pham S, Symes J, Zambrano JP, Fishman J, Pattany P, McNiece I, Conte J, Schulman S, Wu K, Shah A, Breton E, Davis-Sproul J, Schwarz R, Feigenbaum G, Mushtaq M, Suncion VY, Lardo AC, Borrello I, Mendizabal A, Karas TZ, Byrnes J, Lowery M, Heldman AW, Hare JM. Autologous mesenchymal stem cells produce concordant improvements in regional function, tissue perfusion, and fibrotic burden when administered to patients undergoing coronary artery bypass grafting: The prospective randomized study of mesenchymal stem cell therapy in patients undergoing cardiac surgery (prometheus) trial. Circulation research. 2014;114:1302–1310. doi: 10.1161/CIRCRESAHA.114.303180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hosoda T. C-kit-positive cardiac stem cells and myocardial regeneration. American journal of cardiovascular disease. 2012;2:58–67. [PMC free article] [PubMed] [Google Scholar]
- 26.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic effects of combined cell therapy for chronic ischemic cardiomyopathy. Journal of the American College of Cardiology. 2015;66:1990–1999. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clayton ZE, Sadeghipour S, Patel S. Generating induced pluripotent stem cell derived endothelial cells and induced endothelial cells for cardiovascular disease modelling and therapeutic angiogenesis. International journal of cardiology. 2015;197:116–122. doi: 10.1016/j.ijcard.2015.06.038. [DOI] [PubMed] [Google Scholar]
- 28.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: The poseidon randomized trial. JAMA: the journal of the American Medical Association. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]


