Abstract
Rationale
Culture expanded cells originating from cardiac tissue that express the cell surface receptor cKit are undergoing clinical testing as a cell source for heart failure and congenital heart disease. While accumulating data support that mesenchymal stem cells (MSCs) enhance the efficacy of cardiac cKit+ cells (CSCs), the underlying mechanism for this synergistic effect remain incompletely understood.
Objective
To test the hypothesis that MSCs stimulate endogenous CSCs to proliferate, migrate, and differentiate via the SDF1/CXCR4 and SCF/cKit pathways.
Methods and Results
Using genetic lineage-tracing approaches we show that in the postnatal murine heart, cKit+ cells proliferate, migrate, and form cardiomyocytes, but not endothelial cells. CSCs exhibit marked chemotactic and proliferative responses when co-cultured with MSCs but not cardiac stromal cells. Antagonism of the CXCR4 pathway with AMD3100 inhibited MSC-induced CSC chemotaxis but stimulated CSC cardiomyogenesis (p<0.0001). Furthermore, MSCs enhanced CSC proliferation via the SCF/cKit and SDF1/CXCR4 pathways (p<0.0001).
Conclusions
Together these findings show that MSCs exhibit profound, yet differential, effects upon CSC migration, proliferation and differentiation, and suggest a mechanism underlying the improved cardiac regeneration associated with combination therapy using CSCs and MSCs. These findings have important therapeutic implications for cell-based therapy strategies that employ mixtures of CSCs and MSCs.
Keywords: Cardiac Stem Cells, Mesenchymal Stem Cells, Myocardial Regeneration, SDF1, CXCR4
INTRODUCTION
The optimal source of cells for tissue regeneration of the human heart remains controversial1, 2. The capacity of the cardiac cKit+ cell to form cardiomyocytes in several murine models has recently intensified this controversy by suggesting a relatively low contribution of this cell type to endogenous cardiomyogenesis3-6. Several findings suggest that the cardiomyogenic capacity of this cell may be endogenously suppressed on the one hand and may be augmented in a therapeutic situation on the other4, 7-9. In particular, an increasing number of experimental7, 8, 10, 11 and clinical studies (NCT02501811) support the idea that therapeutic responses may be substantially augmented by the interaction of two cell types – MSCs and cardiac-derived CSCs.
Here we hypothesized that MSCs employ the SDF1/CXCR4 and SCF/ckit signaling pathways to regulate the proliferation, migration, and differentiation of endogenous CSCs. Using a previously described cKitCreERT2/+ knock-in allele4, 12, we show that cKit marks postnatal CSCs in the mammalian heart from which a relatively small number of cardiomyocytes are generated after birth. The degree to which the postnatal heart activates endogenous CSCsmay be significantly enhanced via cell-cell interactions with MSCs. These interactions are co-operatively regulated via the SDF1/CXCR4 and SCF/cKit signaling pathways [Online. Fig. I]. Thus, MSC-CSC interactions offer a novel therapeutic target for enhancing cardiomyogenesis from endogenous CSCs in the postnatal heart.
METHODS
An expanded Methods section describing all procedures and protocols is available in the Online Data Supplement.
This study was reviewed and approved by the University of Miami Institutional Animal Care and Use Committee and complies with all Federal and State guidelines concerning the use of animals in research and teaching as defined by The Guide for the Care and use of Laboratory Animals (National Institutes of Health, revised 2011).
The cKitCreERT2/+ and IRG mice have been described elsewhere4. iPSCkit were generated from adult cKitCreERT2/IRG tail-tip fibroblasts as previously described4. Genotyping, TAM injections, gene-expression analysis, lineage-tracing and histological analysis were performed as previously described4. Manufacturing of human and porcine MSCs and CSCs was performed as previously described7-9, 13.
RESULTS
MSCs stimulate chemotaxis in postnatal CSCs
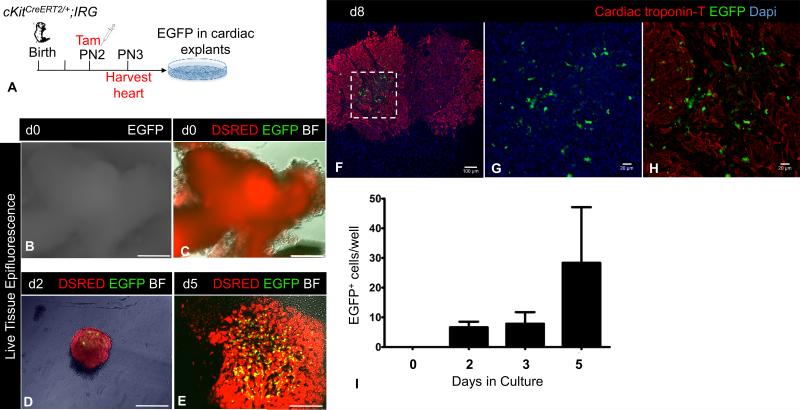
To characterize the properties of postnatal CSCs, we pulsed postnatal day 2 (PN2) cKitCreERT2;IRG neonates with a single subcutaneous injection of tamoxifen (n=9) and collected their hearts 24h later in order to analyze EGFP expression in culture [Fig. 1A]. Live tissue imaging and confocal immunofluorescence showed that EGFP marked a non-contractile, cardiac troponin T-negative, proliferative cell type [Online Video I, Fig. 1B-I]. Importantly, EGFP+ CSCs were consistently present within the myocardial explants and did not migrate along with other explant-derived cells [Figure 1D-H and Online Fig. II-III].
Figure 1. Neonatal heart cells originally marked by cKitCreERT2/+ are non-contractile and proliferate.
A, Schematic of the ex-vivo genetic fate-mapping strategy to assess the original identity of cKitCreERT2/+ -recombined heart cells. B-C, Live-tissue fluorescence imaging of tamoxifen-pulsed neonatal cardiac explants. At the time of harvest [Day (d)0], explants are DSRED+ and do not express EGFP epifluorescence. D-E, Live-tissue fluorescence imaging of tamoxifen-pulsed neonatal cardiac explants on d2 (D) and d5 (E) of culture. Expression of EGFP is restricted in a minor population of non-contractile cardiac cells, which proliferate with time. F-H, Confocal immunofluorescence against cardiac troponin T and EGFP of PN1 explants, after 8 days in culture. Panels G-H are a higher magnification of the area in inset of panel F. EGFP does not co-localize with cyanine-5 labeled cardiac troponin T. I, Quantification of EGFP+ cells during a 5-day culture period. BF, Brightfield. Scale bars, B-E, 200μm; F, 100μm; G-H, 20μM.
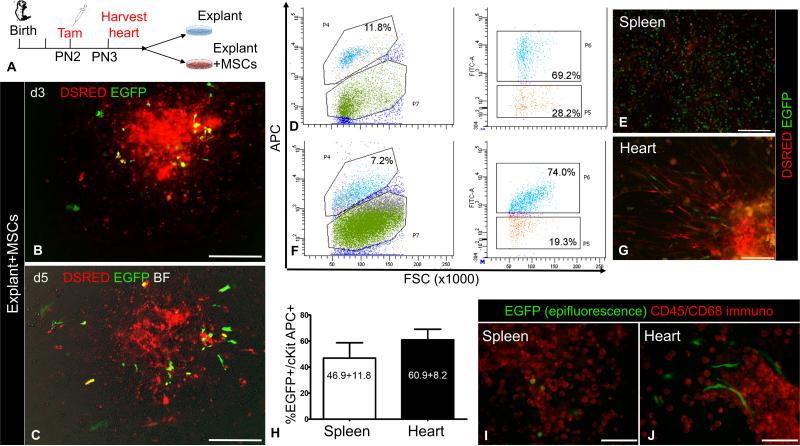
MSCs comprise a heterogeneous cell mixture of neural crest- and non-neural crest-derived cells14, 15 that exert stimulatory effects on CSCs7, 9, 10, 16, 17. To address if MSCs stimulate cKit+ cell migration, myocardial explants from the same PN3 cKitCreERT2;IRG tamoxifen-pulsed hearts were plated either with mitotically-arrested MSCs or on gelatin (control), and cell migration assessed using EGFP epifluorescence [Fig. 2A]. Co-culture with MSCs (n=6 neonates) promoted the outgrowth of both EGFP+ and DSRED+ cells from myocardial explants [Fig. 2B-C]
Figure 2. MSCs stimulate outgrowth of CSCs from cardiac explants.
A, Schematic of the ex-vivo lineage-tracing experiments to assess the effect of MSCs on cKit+ cardiac cells. B-C, Ex-vivo culture of a cKitCreERT2/+;IRG myocardial explant on days (d)3 (B) and d5 (C), after co-culture with MSCs.. D, Representative flow cytometric analysis of EGFP and cKit-APC co-localization in the spleen. E, Live epifluorescence imaging of EGFP and DSRED in spleen cells of panel A, prior to FACS analysis. F, Representative flow cytometric analysis of EGFP and cKit-APC co-localization in the heart. G, Live epifluorescence imaging of EGFP and DSRED in heart cells of panel C, prior to FACS analysis. H, summary of Cre-mediated recombination efficiency in cKit+ cardiac and spleen cells (n=3 per group). I, Immunostaining of neonatal spleen explant-derived cells with a cyanine-5 CD45/CD68 antibody cocktail (pseudocolored red). All spleen EGFP+ cells have a blast-like morphology and exhibit strong CD45/CD68 immunoreactivity. J, Immunostaining of neonatal cardiac explant-derived cells from the same mouse, with a cyanine-5 CD45/CD68 antibody cocktail. All cardiac EGFP+ cells have a spindle cell-like morphology and are negative for CD45/CD68. Scale bars, 200μm.
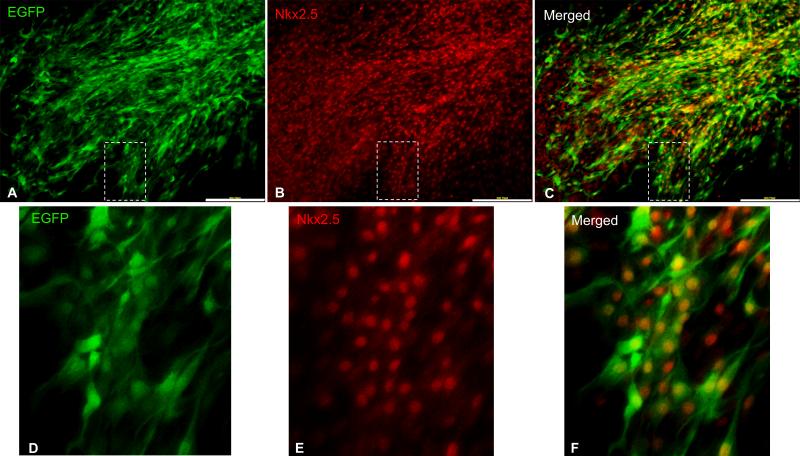
Flow cytometric analysis indicated that 60.9%±8.2% of cardiac explant-derived cKit+ cells were marked by EGFP [Fig. 2D-H]. Unlike splenic EGFP+ cells, which homogenously expressed CD45+ and/or CD68+ and exhibited a blast-like phenotype [Fig. 2E, I], the cardiac EGFP+ cardiac explant-derived cells did not express CD45 or CD68 and had a spindle-like morphology [Fig. 2G, J], indicating that the EGFP+ cardiac explant-derived cells have a distinct non-hematopoietic lineage. Co-expression of Nkx2.5 corroborated the CSC lineage of EGFP+ cardiac explant-derived cells [Fig. 3]. Notably, the spindle-like, Nkx2.5+ in-vitro phenotype of postnatal CSCs closely matches the phenotype of the primordial neural crest-derived cKit+ cardiac stem cells that we recently described4. Collectively, these findings support that the EGFP+ cells in the postnatal heart are CSCs. Their migratory capacity is limited in chemotactic responsiveness to the postnatal cardiac stroma but is substantially augmented by MSCs.
Figure 3. Co-localization of EGFP and Nkx2.5 in cardiac explant derived cells from cKitCreERT2;IRG mice.
A-C, Fluorescent immunocolocalization of EGFP (A) and Nkx2.5 (B) in cardiac explant-derived CSCs. Panels D-F are blown-up images of the areas delineated with insets in panels A-C, respectively. Scale bars, 200μm.
Dichotomous effects of the SDF1/CXCR4 pathway on CSC migration and differentiation
SDF1/CXCR4 signaling participates in the mobilization of CSCs in the heart18-23, and plays an important role in cardiac neural crest cell migration24. It has also been suggested that the therapeutic effect of MSCs is partly attributed to their rich SDF1α secretion18, 25. We therefore tested the hypothesis that the SDF1/CXCR4 pathway underlies the migratory and differentiation effects of MSCs on CSCs.
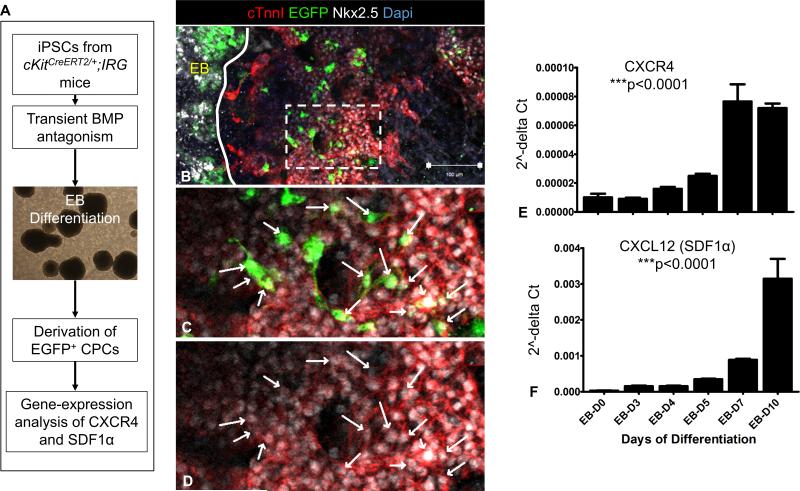
We first explored whether the SDF1/CXCR4 signaling pathway is normally active in CSCs, during cardiomyogenesis. Accordingly, we utilized a previously described in-vitro model of mouse cardiogenesis4, whereby embryoid bodies (EBs) from induced pluripotent stem cells carrying the cKitCreERT2/+;IRG alleles (iPSCkit) are directed to undergo stage-specific differentiation into CSCs via transient BMP antagonism [Online Fig. IV A]. Similar to postnatal CSCs, iPSCkit-derived CSCs were migratory EGFP+/Nkx2.5+ cells and were exclusively detected as cells outgrowing from – but not within – the EBs [Online Fig. IV B-D, Online Video II]. Differentiation of iPSCs to CSCs4 resulted in stage-specific induction of SDF1α and CXCR4 gene expression [Online Fig. IV E-F], suggesting that activation of the SDF1/CXCR4 signaling pathway plays an important role in the development of cardiomyogenic CSCs.
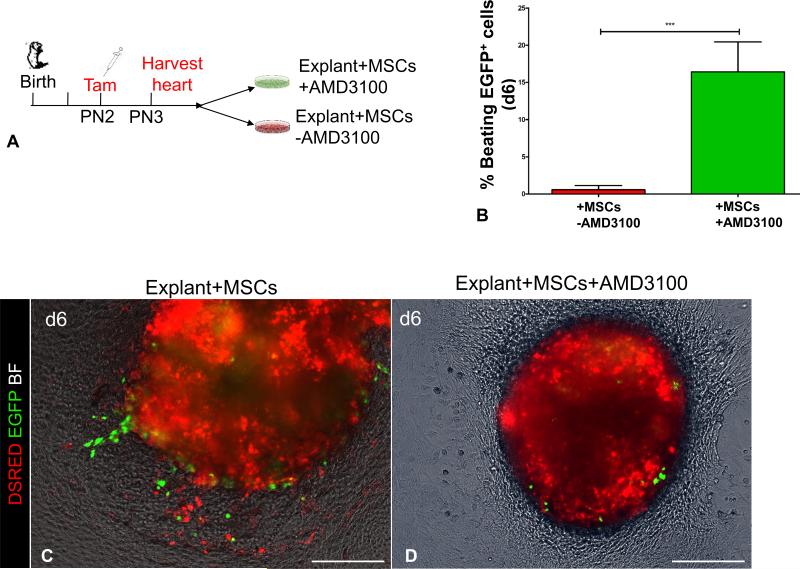
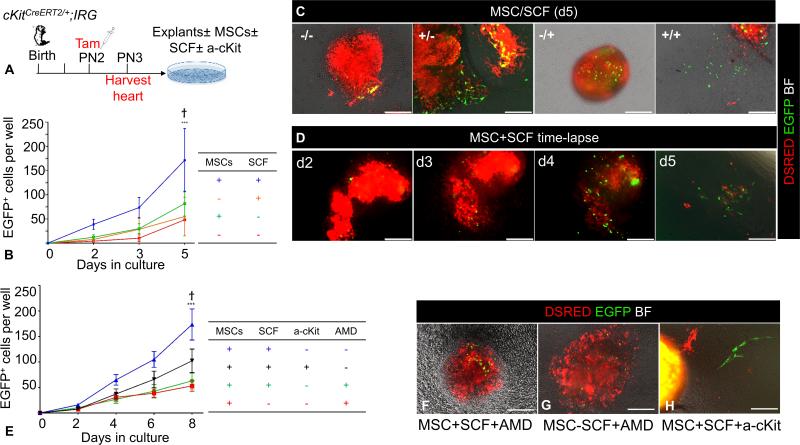
Next, we tested the role of SDF1/CXCR4 in postnatal CSCs. Myocardial explant culture assays (n=2) were generated as described above, in order to analyze the emergence of EGFP+ cells in the presence or absence of AMD3100, an inhibitor of the SDF1/CXCR4 axis18, 26 [Fig. 5A]. Consistent with previous findings18, exposure to AMD3100 did not block outgrowth of DSRED+ cells, but prevented EGFP+ CSCs from migrating from the cultured explants in the presence of MSCs, strongly suggesting that the chemotactic effect of MSCs on CSCs is regulated via the SDF1/CXCR4 signaling pathway [Fig. 5B-D]. Moreover, exposure to AMD3100 resulted in a ~29-fold increase in the rate of CSCs differentiation into spontaneously contracting cardiomyocytes [from 0.57%±0.57% to 16.41±4.03% EGFP+ beating cells, p=0.0001; Fig. 5B, Online Video III].
Figure 5. AMD3100 disables chemotactic responsiveness of CSCs to MSCs and promotes cardiac differentiation.
A, Schematic of the ex-vivo genetic fate-mapping strategy to assess the role of SDF1/CXCR4 signaling pathway in MSCs-CSCs interactions. cKitCreERT2/+;IRG neonates (n=2 neonates; 3 myocardial explants/neonate/time point) were pulsed with tamoxifen at PN2; on PN3 heart tissue was explanted and cultured ex-vivo on MSCs-coated vessels in the presence or absence of AMD3100. The migratory activity of CSCs was assessed as the outgrowth of EGFP+ myocardial cells from within the explanted tissue into the coated surface of the culture vessel. B, Inhibition of the chemotactic responsiveness to MSCs by AMD3100, dramatically enhances differentiation of EGFP+ cells into spontaneously beating derivatives.. C, A myocardial explant cultured on MSCs feeders for five 6 days. D, Supplementing the growth medium with AMD3100 inhibits migration of EGFP+ cells. AMD, AMD3100. Data are presented as mean±SEM. ***p≤0.0001. Scale bars, 150μm.
MSCs promote expansion of CSCs via the SCF/cKit signaling pathway
We investigated whether the increased abundance of EGFP+ cells reflects increased mobilization due to the chemotactic responsiveness of CSCs to MSCs, or to an increase in CSC proliferation. Tamoxifen-pulsed PN3 cKitCreERT2;IRG myocardial explant culture assays (n=6) were performed as described above with or without MSCs, and the growth of EGFP+ CSCs was continuously quantified using live-tissue epifluorescence imaging every other day for 5 days [Fig. 6A]. Although EGFP-negative cells grew out from myocardial explants, reaching >50% confluency within 5 days of culture, growth of EGFP+ CSCs was less robust, although their number increased significantly over time [Fig. 6B]. Intriguingly, in the absence of MSCs, EGFP+ cell expansion occurred primarily within the explanted tissue, whereas in the presence of MSCs, CSCs proliferated within the explant but also migrated out and expanded freely from the myocardial explants within the MSC feeder layers [Figs. 2, 5]. However, the abundance of CSCs was not significantly affected by the presence of MSCs compared to controls [Fig. 6B].
Figure 6. Regulation of CSCs self-renewal by MSCs.
A. Schematic of the ex-vivo genetic fate-mapping strategy to assess the role of MSCs in modulating self-renewal of CSCs (n=6 cKitCreERT2/+;IRG neonates). B, Comparison of the impact of MSC feeders and/or SCF, in the growth of EGFP+ cells after 5 days of cardiac explant culture. C, Representative photomicrographs of day-5 myocardial explant cultures in the presence or absence of MSCs and SCF. Note that, establishment of primary, explant-free, proliferating EGFP+ colonies, requires the presence of both an MSCs feeder layer and exogenous administration of SCF. D, Time-lapse live tissue epifluorescent image of a myocardial explant cultured on an MSC feeder layer, in the presence of exogenously administered SCF. Note the time-dependent proliferative expansion of EGFP+ cells, and eventually their outgrowth and establishment of explant-free colonies within 5 days of tissue culture. E, Impact of SCF/cKit and SDF1/CXCR4 signaling neutralization with an anti-cKit (a-cKit) antibody and AMD3100 respectively (n=2 neonates; 3 myocardial explants/neonate/time point). F-H, Representative photomicrographs of day-6 myocardial explants, cultured on MSCs feeders, in the presence or absence of SCF. Modulating the activity of the SCF/cKit signaling pathway by adding (F) or withdrawing (G) SCF does not rescue the migratory activity of CSCs, following AMD3100 antagonism (n=2 neonates; 3 myocardial explants/neonate/time point). H, Neutralization of the SCF/cKit signaling pathway does not affect EGFP+ cell migration (n=2 neonates; 3 myocardial explants/neonate/time point). AMD, AMD3100. Data are presented as mean±SEM. ***p≤0.0001 within groups. Ϯp=0.001 between groups. Scale bars, 150μm.
We previously showed that the combination of MSC feeders and exogenous Stem Cell Factor (SCF), the ligand of cKit, significantly improves the ex-vivo propagation of CSCs9. Consequently, we investigated whether the SCF/cKit signaling pathway is important for CSC migration and proliferation. Accordingly, tamoxifen-pulsed PN3 cKitCreERT2;IRG myocardial explant culture assays were carried out as described above with or without MSCs (n=6), and the growth of EGFP+ cells was quantified using live-tissue epifluorescence imaging in the presence or absence of recombinant murine SCF [Fig. 6A]. Surprisingly, the presence of both MSCs and exogenous SCF produced a 3.5-fold increase in the abundance of EGFP+ cells compared to MSCs alone [Fig. 6B-D] (p=0.001). The effects of SCF and MSCs on the abundance of EGFP+ cells were abrogated when we neutralized SCF/cKit signaling with an anti-murine cKit antibody [Fig. 6E]. Similarly, EGFP+ cell abundance was significantly reduced (p≤0.0001) in the presence of the SDF1/CXCR4 pathway inhibitor, AMD3100 [Fig. 6E-G]. Interestingly, modulation of the SCF/cKit signaling pathway, either through its exogenous activation with recombinant murine SCF or its neutralization with an anti-murine cKit antibody, did not significantly alter EGFP+ cell migration and differentiation [Figure 6E, H].
A proportion of myocardium is generated after birth by neonatal cKit+ cells
We next tested whether postnatal CSCs generate new cardiomyocytes in-vivo27-29. cKitCreERT2/+;IRG neonates (n=6) were pulsed with tamoxifen on PN3 and PN4, and genetic fate-mapping analysis was performed at PN7 [Fig. 7A]. Expression of EGFP was conditional on the expression of both the cKitCreERT2/+ and IRG alleles and did not occur without tamoxifen induction [Fig. 7B].
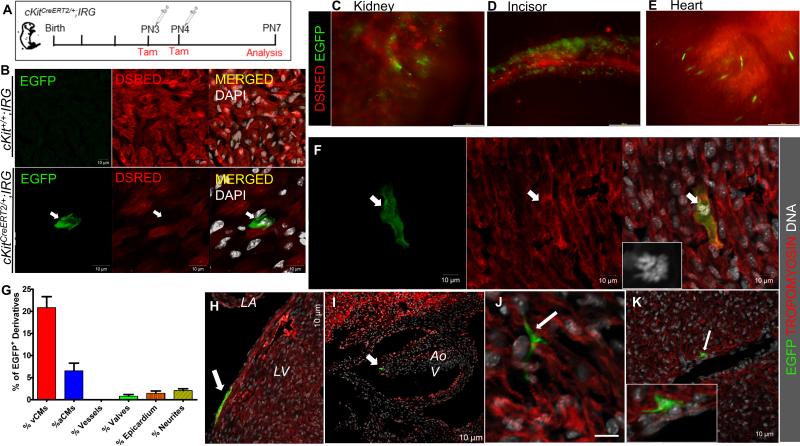
Figure 7. Genetic fate-map of postnatal cKit-expressing cells in the neonatal mouse.
A, Schematic of the genetic fate-mapping strategy. cKitCreERT2/+;IRG neonates (n=6) were pulsed with tamoxifen at PN3 and PN4 and expression of EGFP in cKit+ cells and their derivatives was analyzed on PN7. B. Representative confocal immunofluorescence of EGFP and DSRED in tamoxifen-treated cKit+/+;IRG (top) and cKitCreERT2/+;IRG (bottom) littermates. EGFP is exclusively expressed upon Cre-mediated recombination. C-E, Expression of EGFP was detected in all tissues in which contribution from cKit-expressing cells has been previously reported, including the kidney (C), the developing incisors (D) and the heart (E). F, confocal immunofluorescence of an EGFP+/Tropomyosin+ ventricular cardiomyocyte (arrow) in mitosis (inset, DNA). G, Quantification of EGFP+ derivatives in the postnatal heart (n=2). H-J, In addition to cardiomyocytes, EGFP+ derivatives of postnatal CSCs were also detected in the epicardium (H, arrow), cardiac valves (I, arrow) and in neuronal cardiac cells (J). K, Although EGFP cells were occasionally detected in close proximity to large coronary vessels (arrow), contribution of CSCs to coronary vascular cells was never detected. vCMs, ventricular cardiomyocytes; aCMs, atrial cardiomyocytes; LA, left atrium; LV, left ventricle; Aov, aortic valve. Data are presented as mean±SEM. Scale bars, 200μm (C-E); 10μm (B, F-K).
At PN7, EGFP epifluorescence was detected in all expected cKit-expressing tissues, including blood, skin, testes, intestine, lungs4, 30-32, kidney [Fig. 7C], and teeth [Fig. 7D]. Using live tissue imaging, EGFP+ cells were visualized throughout the heart, including spontaneously beating cardiomyocytes within the left and right ventricular walls and the ventricular apex, suggesting that a proportion of neonatal cardiac cells expressed cKit at the time of tamoxifen treatment [Fig. 7E and Online Videos III-VI].
A mean of 76.9.3±4.5 EGFP+ cells were detected per heart section analyzed, 20.9%±2.4% of which were tropomyosin+ ventricular cardiomyocytes [Fig. 7F,G, Online Video VII]. In addition, 6.5%±1.7% of atrial EGFP+ cells co-expressed tropomyosin, indicative of fully differentiated atrial cardiomyocytes [Fig. 7G]. Intriguingly, we detected only one EGFP+/Tropomyosin+ mononucleated ventricular cardiomyocyte undergoing mitosis in the two neonatal mouse hearts in which we quantified cKit-mediated cardiomyogenesis, suggesting that cKit-dependent cardiomyogenesis and cardiomyocyte proliferation represent two distinct mechanisms by which the neonatal mouse heart generates cardiomyocytes [Fig. 7F]. Additionally, and consistent with our previous findings4, expression of EGFP was observed in neuronal-like cardiac cells, ventricular epicardium, and cardiac valves, but not in coronary vascular cells [Fig. 7G-K].
Stimulation of dilated cardiomyopathy human CSCs by human MSCs
Last, we sought to investigate the clinical relevance of our neonatal mice and iPSCs findings and tested if isolated CSCs from chronically diseased human (h) hearts (hCSCs) are chemotactically responsive to hMSCs, under both normal and hypoxic conditions. We prepared hCSCs from three dilated cardiomyopathy patients admitted to our center, following percutaneous endomyocardial biopsy as previously described8. We used three hMSCs lines previously established in our lab from healthy donors [Online Fig. IV A]13. Flow cytometric analysis indicated that 98.85%±0.5% of human CSCs expressed cKit [Online Fig. IV B-D]. In addition, similar to mouse CSCs, hCSCs expressed Nkx2.5 and the neural crest-specific marker Pax3 [Online Fig. IV E-F]. A Fluo-8 intracellular Ca2+ mobilization-based assay of CXCR4 activity, with a Chem-1 cell line transduced to overexpress human CXCR4 the and the G protein Gα15, illustrated activation of the human SDF1/CXCR4 pathway by hMSCs; and that this effect is blocked in the presence of 1μM AMD3100 (data not shown).
The migratory capacity of hCSCs was tested in a transwell migration assay. Accordingly, 1×105 hCSCs were suspended in serum-free medium and loaded into the upper wells of Boyden chambers. To test hCSC chemotactic responsiveness to hMSCs, the lower wells of the Boyden chambers were coated 24h earlier with 1×105 mitotically inactivated hMSCs in serum-free medium. hCSCs were allowed to migrate for 24h, in either 20% O2 or 0.5% O2 [Online Fig. V A]. Quantification of the migrated cells corroborated a marked increase in hCSCs mobilization by hMSCs. The chemotactic effect of hMSCs was abrogated in the presence of AMD3100 [Online Fig. V B-E]. Interestingly, exposure of the cell cultures to 0.5% O2, resulted in acute loss of the migratory activity of hCSCs, which could be partially rescued in the presence of hMSC [Online Fig. V E]. Gene-expression analysis illustrated that exposure of hCSCs and hMSCs to hypoxia did not affect expression of CXCR4, which was highly expressed in hCSCs but not in hMSCs [Online Fig. V F], but resulted in a significant downregulation of SDF1α expression, both in hCSCs and hMSCs [Online Fig. V G].
DISCUSSION
A major hypothesis in the field of cell-based therapy is that different cell types from different tissue origins can have additive effects on tissue repair. One particular promising example is that of cKit+ CSCs and MSCs, a concept that has formed the basis for several clinical trials, including the CONCERT-HF7-10, 16, 17, 33-35. Here, we have uncovered the signaling mechanisms responsible for these interactions. We have shown that SDF1α and SCF secreted by bone marrow-derived MSCs have important effects on CSCs. SDF1α promotes migration of cardiac derived CSCs and enhances their lineage commitment toward contractile cardiac myocytes, whereas the cKit receptor activation by SCF, plays an important role in CSC proliferation [Online Fig I]. These data provide the mechanistic basis by which MSCs activate endogenous CSCs to migrate, proliferate, differentiate and stimulate repair in the diseased heart.
Our data are in agreement with earlier studies documenting that cell-cell interactions between MSCs and CSCs are associated with enhanced regeneration and therapeutic benefit9, 10, 16-18, 25, 36. We previously showed in a large animal model of myocardial infarction, that transplantation of MSCs produces significant improvements in heart function and scar size reduction, and that this effect is partly attributed to a transient mobilization of CSCs at the sites of MSCs engraftment9. Further, we7, 8 and others10 recently showed that when MSCs and CSCs are co-transplanted as a cell mixture, the therapeutic benefit may be further enhanced.
Here, we have used several in-vivo and ex-vivo sophisticated experimental models, to test these cellular interactions. Our ex-vivo genetic lineage-tracing studies, and our experiments with human CSCs and MSCs, document a chemotactic effect of MSCs on CSCs that relies on the SDF1/CXCR4 signaling pathway. Although our transwell experiments with human CSCs and MSCs indicate that direct cell-cell interactions between MSCs and CSCs are not necessary for in-vitro chemotaxis, experiments in large animal-models of ischemic heart disease show that administering the MSC secretome is not a therapeutically sufficient substitute for MSC transplantation9. Accordingly, these data do not exclude cell-cell effects, in addition to paracrine effects, as the underlying mechanisms of cell-based therapies.
We observed that MSCs significantly enhance CSC growth rates in the presence of SCF. Based on previous reports23, 37, but also due to the white-spotting phenotype that is often produced from mutations in the cKit and SCF loci, we expected that the SCF/cKit signaling pathway would affect CSCs mobilization by MSCs. However, we did not find evidence that modulation of SCF/cKit is sufficient to control CSC mobilization. Importantly, cKitCreERT2/+-labeled cells in other postnatal tissues exhibited full ex-vivo migratory capacity (Online Fig. III), suggesting that the observed effect is unlikely due to an underlying cKit haploinsufficiency. Notably, consistent with our findings, a recent study reported that activation of SCF/cKit signaling alone is not sufficient to regulate CSC migration, and requires transactivation of SDF1/CXCR423.
A recent report showed that Ephrin signaling also regulates CSC traffickingCSC38, 39. MSCs express Ephrins40, therefore, it is possible that part of the chemotactic effects of MSCs on CSCs that were documented in this study, are Ephrin-mediated. Interestingly, developmental studies indicate that the two signaling pathways may control different aspects of cardiac neural crest cell migration41. Signaling through Ephrins has been suggested to provide directional guidance, whereas SDF1/CXCR4 is thought to regulate motility of cardiac neural crest cells. Our findings suggest that, SDF1/CXCR4-mediated inhibition of CSC migration may be required for triggering differentiation into cardiomyocytes . It would be interesting to explore whether MSCs could also enhance directional guidance of CSCs into the damaged myocardium via Ephrin-mediated signaling.
Using the cKitCreERT2/+;IRG reporter mouse line we were able to track the fate of cKit+ cells in the heart (~60% EGFP+). Using the same knock-in mouse line, we recently delineated a neural crest-derived cardiomyogenic CSC lineage as a source of a relatively small proportion of embryonic cardiomyocytes4. Our findings strongly suggest that, as with its embryonic cardiomyogenic cell program3-5, the mammalian heart differentiates a relatively small proportion of postnatal CSCs, to establish its full complement of cardiomyocytes after birth 27, 42. The postnatal CSCs represent a migratory population of Nkx2.5+ cells whose migration and differentiation are modulated by the SDF1/CXCR4 signaling pathway and can be significantly stimulated by MSCs for therapeutic purposes [Online Fig. I].
Consistent with the fate of the embryonic CSC lineage4, 12, our postnatal studies found no evidence for coronary vascular cell labeling under the cKitCreERT2/+ allele. Although this finding appears to contrast with other recent cKit lineage-tracing studies3, 5, 6, we believe this discrepancy reflects an apparent lower fate-mapping sensitivity of the cKitCreERT2/+ mouse compared to the other 6 cKit knock-in models12. Apparently, although all 7 cKit knock-in alleles3-6 exhibit similar sensitivity in tracing cKit-derived cardiomyocytes12, 43, the cKitCreERT2/+ allele specifically marks the cardiomyogenic over the vasculogenic postnatal cKit+ lineages since if they were of same ancestry, we should have detected at least a few vascular derivatives with the cKitCreERT2/+ allele.
We also examined whether the expression of reporter genes in cardiomyocytes indicates CSC differentiation3, 4, 44, 45 or in-situ expression of cKit in cardiomyocytes6. Our in-vitro experiments with cells derived from mice with the cKitCreERT2/+ allele show that expression of reporter genes marks non-contractile, proliferative and migratory cKit+ cells, and not differentiated cardiomyocytes. These findings are consistent with previous reports3, 4, 44, 46. Nonetheless, because of the apparently lower cardiac sensitivity of the cKitCreERT2/+ allele compared to other cKit knock-ins5, 6, we do not exclude the possibility that a population of cardiomyocytes, undetectable by our reagents, express cKit.
To this end, we would like to acknowledge a number of limitations of the knock-in models compared to transgenic approaches, which may have influenced the cKit lineage tracing findings reported by us and others3, 5, 6. For example, coat color differences between the wild-type and cKit knock-in mice likely suggests improper function of neural crest cells due to cKit haploinsufficiency. It is possible that this deficit also affected cardiac cKit+ cells to some extent, and therefore our findings may have somewhat underestimated the role of cKit+ cells in the heart.
In summary, our findings indicate that postnatal CSCs are bona fide cardiomyogenic stem cells from which new cardiomyocytes are generated after birth. We further show that a biologically important relationship exists between MSCs and CSCs, which involves the SCF/cKit and the SDF1/CXCR4 pathways, and may be harnessed for therapeutically enhancing the degree of cardiomyogenesis from endogenous CSCs. Together these findings support the development of novel cell combination-based therapies for the prevention and treatment of heart disease.
Supplementary Material
Figure 4. Expression of SDF1α and CXCR4 during derivation of CSCs from iPSCkit.
A, Schematic of the experimental design. B, Confocal immunofluorescence illustrates that the generation of CSCs from iPSCkit involves migration of CSCs from the embryoid bodies (EBs, solid line). C-D, high magnification of the area delineated by the inset in panel B, illustrates co-localization of EGFP with Nkx2.5 in the migrated CSC derivatives. E-F Gene-expression analysis during differentiation of iPSCkit to CSCs shows a dramatic upregulation in SDF1α and CXCR4. n=3; Scale bars, 100μm.
Novelty and Significance.
- What is Known?
- Because bone marrow mesenchymal stem cells (MSCs) stimulate endogenous c-Kit+ cardiac stem cells (CSCs) the idea of mixing MSCs and CSCs to create a novel and potent cell-based therapeutic has arisen.
- While mixtures of MSCs and CSCs have enhanced therapeutic effects in porcine models, the precise molecular and cellular mechanisms for this interactive effect are not fully known.
- What new information does this article contribute?
- Using organotypic co-culturing and genetic labeling of CSCs this study shows that MSCs stimulate profoundly the abundance and migration of CSCs.
- MSCs promote CSC proliferation via the stem cell factor (SCF)/c-Kit signaling axis.
- MSCs induce CSC migration and differentiation into de-novo cardiomyocytes via regulation of the SDF1/CXCR4 signaling axis.
Summary of the Novelty and Significance Section.
Combining MSC and CSCs is a potential means to augment therapeutic cell based repair of the injured heart. While animal models support the idea that these mixtures have enhanced efficacy to reduce infarct scar in acute myocardial infarction and enhance LV performance in chronic heart failure, the mechanistic basis for MSC-CSC interactions action are incompletely understood. Here we show that MSCs stimulate CSCs to proliferate, migrate and differentiate in de-novo cardiomyocytes, by regulating the SCF/cKit and SDF1/CXCR4 signaling pathways. Using iPSC modeling, we demonstrate that developmental activation of SDF1/CXCR4 signaling is involved in CSC specification, migration and differentiation into Nkx2.5+ cardiomyocytes. Paradoxically, genetic lineage-tracing in neonatal mice reveals loss of migratory and proliferative activity of postnatal CSCs, which may be recovered via MSC-dependent activation of SDF1/CXCR4. Antagonism of MSC-mediated SDF1/CXCR4 signaling by AMD3100 inhibits postnatal CSC migration and proliferation but promotes differentiation into beating cardiomyocytes. On the other hand, SCF/cKit signaling enhances CSC proliferation but not migration. Human MSCs elicit similar effects in culture-expanded adult human CSCs. Together these findings offer novel insights into the mechanisms of cell-based cardiac repair, and support the concept that MSC-CSC interactions may be deployed therapeutically to enhance cardiac regenerative repair.
Acknowledgments
We thank Irene S. Margitich, Courtney Premer, Michael A. Belio, Dr. Ketty Bacallao, and Rejane Lamazares, for technical assistance.
Sources of Funding. This study was funded by the National Institutes of Health grants (awarded to J.M.H.): R01 HL107110, R01 HL094849. JMH is also supported by the NIH grants R01 HL110737, R01 HL084275 and 5UM HL113460; and grants from the Starr Foundation and the Soffer Family Foundation. D.S is supported by a DFG SA 1374/1-3 grant.
Non Standard Abbreviations
- MSCs
Mesenchymal Stem Cells
- CSCs
cKit+ cardiac stem cells
- PN
Days of postnatal life
- TAM
Tamoxifen
- EBs
Embryoid bodies from induced pluripotent stem cells
- DSRED
the red fluorescent protein variant DsRed-Express
- SCF
Stem cell factor
- AMD3100
An SDF1/CXCR4 anatgonist
- BF
Brightfield
- APC
Allophycocyanin
- SEM
Standard error of means
Footnotes
Disclosures: Drs Hare and Hatzistergos report having a patent for cardiac cell-based therapy; owning equity in Vestion Inc; members of the scientific advisory board and consultants of Vestion, Inc; Dr Hare reports being a board member of Vestion Inc. Vestion Inc did not participate in funding this work. The other authors report no conflicts.
REFERENCES
- 1.Anversa P, Kajstura J, Rota M, Leri A. Regenerating new heart with stem cells. The Journal of clinical investigation. 2013;123:62–70. doi: 10.1172/JCI63068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.van Berlo JH, Molkentin JD. An emerging consensus on cardiac regeneration. Nature medicine. 2014;20:1386–93. doi: 10.1038/nm.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, Middleton RC, Marban E, Molkentin JD. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509:337–41. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatzistergos KE, Takeuchi LM, Saur D, Seidler B, Dymecki SM, Mai JJ, White IA, Balkan W, Kanashiro-Takeuchi RM, Schally AV, Hare JM. cKit+ cardiac progenitors of neural crest origin. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:13051–6. doi: 10.1073/pnas.1517201112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, Jeong D, Sheng W, Bu L, Xu M, Huang GY, Hajjar RJ, Zhou B, Moon A, Cai CL. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nature communications. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, Tian X, Nie Y, Hu S, Yan Y, Zhang L, Qiao Z, Wang QD, Lui KO, Zhou B. Genetic lineage tracing identifies in situ Kit-expressing cardiomyocytes. Cell research. 2015 doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karantalis V, Suncion-Loescher VY, Bagno L, Golpanian S, Wolf A, Sanina C, Premer C, Kanelidis AJ, McCall F, Wang B, Balkan W, Rodriguez J, Rosado M, Morales A, Hatzistergos K, Natsumeda M, Margitich I, Schulman IH, Gomes SA, Mushtaq M, DiFede DL, Fishman JE, Pattany P, Zambrano JP, Heldman AW, Hare JM. Synergistic Effects of Combined Cell Therapy for Chronic Ischemic Cardiomyopathy. J Am Coll Cardiol. 2015;66:1990–9. doi: 10.1016/j.jacc.2015.08.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation. 2013;127:213–23. doi: 10.1161/CIRCULATIONAHA.112.131110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE. Bone Marrow Mesenchymal Stem Cells Stimulate Cardiac Stem Cell Proliferation and DifferentiationNovelty and Significance. Circulation research. 2010;107:913–922. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quijada P, Salunga HT, Hariharan N, Cubillo J, El-Sayed F, Moshref M, Bala KM, Emathinger JM, De La Torre A, Ormachea L, Alvarez R, Gude NA, Sussman MA. Cardiac Stem Cell Hybrids Enhance Myocardial Repair. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.306838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avolio E, Meloni M, Spencer HL, Riu F, Katare R, Mangialardi G, Oikawa A, Rodriguez-Arabaolaza I, Dang Z, Mitchell K, Reni C, Alvino VV, Rowlinson J, Livi U, Cesselli D, Angelini G, Emanueli C, Beltrami AP, Madeddu P. Combined intramyocardial delivery of human pericytes and cardiac stem cells additively improves the healing of mouse infarcted hearts through stimulation of vascular and muscular repair. Circ Res. 2015;116:e81–94. doi: 10.1161/CIRCRESAHA.115.306146. [DOI] [PubMed] [Google Scholar]
- 12.Hatzistergos KE, Hare JM. Murine Models Demonstrate Distinct Vasculogenic and Cardiomyogenic cKit+ Lineages in the Heart. Circulation Research. 2016;118:382–387. doi: 10.1161/CIRCRESAHA.115.308061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic Mesenchymal Stem Cells Restore Endothelial Function in Heart Failure by Stimulating Endothelial Progenitor Cells. EBioMedicine. 2015;2:467–75. doi: 10.1016/j.ebiom.2015.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isern J, Garcia-Garcia A, Martin AM, Arranz L, Martin-Perez D, Torroja C, Sanchez-Cabo F, Mendez-Ferrer S. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife. 2014;3:e03696. doi: 10.7554/eLife.03696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takashima Y, Era T, Nakao K, Kondo S, Kasuga M, Smith AG, Nishikawa S. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–88. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki G, Iyer V, Lee TC, Canty JM., Jr. Autologous mesenchymal stem cells mobilize cKit+ and CD133+ bone marrow progenitor cells and improve regional function in hibernating myocardium. Circ Res. 2011;109:1044–54. doi: 10.1161/CIRCRESAHA.111.245969. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z, Yang J, Yan W, Li Y, Shen Z, Asahara T. Pretreatment of Cardiac Stem Cells With Exosomes Derived From Mesenchymal Stem Cells Enhances Myocardial Repair. Journal of the American Heart Association. 2016:5. doi: 10.1161/JAHA.115.002856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang JM, Wang JN, Zhang L, Zheng F, Yang JY, Kong X, Guo LY, Chen L, Huang YZ, Wan Y, Chen SY. VEGF/SDF-1 promotes cardiac stem cell mobilization and myocardial repair in the infarcted heart. Cardiovascular research. 2011;91:402–11. doi: 10.1093/cvr/cvr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007;13:2063–71. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 20.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 21.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 22.Elmadbouh I, Haider H, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. Journal of molecular and cellular cardiology. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo K, Kuang D, Wang Y, Xia Y, Tong W, Wang X, Chen Y, Duan Y, Wang G. SCF/c-kit transactivates CXCR4-serine 339 phosphorylation through G protein-coupled receptor kinase 6 and regulates cardiac stem cell migration. Sci Rep. 2016;6:26812. doi: 10.1038/srep26812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escot S, Blavet C, Hartle S, Duband JL, Fournier-Thibault C. Mis-Regulation of SDF1-CXCR4 Signalling Impairs Early Cardiac Neural Crest Cell Migration Leading to Conotruncal Defects. Circ Res. 2013 doi: 10.1161/CIRCRESAHA.113.301333. [DOI] [PubMed] [Google Scholar]
- 25.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–58. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Young KC, Torres E, Hatzistergos KE, Hehre D, Suguihara C, Hare JM. Inhibition of the SDF-1/CXCR4 axis attenuates neonatal hypoxia-induced pulmonary hypertension. Circ Res. 2009;104:1293–301. doi: 10.1161/CIRCRESAHA.109.197533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alkass K, Panula J, Westman M, Wu TD, Guerquin-Kern JL, Bergmann O. No Evidence for Cardiomyocyte Number Expansion in Preadolescent Mice. Cell. 2015;163:1026–36. doi: 10.1016/j.cell.2015.10.035. [DOI] [PubMed] [Google Scholar]
- 28.Ali SR, Hippenmeyer S, Saadat LV, Luo L, Weissman IL, Ardehali R. Existing cardiomyocytes generate cardiomyocytes at a low rate after birth in mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:8850–5. doi: 10.1073/pnas.1408233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature. 2013;493:433–6. doi: 10.1038/nature11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldstein BJ, Goss GM, Hatzistergos KE, Rangel EB, Seidler B, Saur D, Hare JM. Adult c-Kit(+) progenitor cells are necessary for maintenance and regeneration of olfactory neurons. J Comp Neurol. 2015;523:15–31. doi: 10.1002/cne.23653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heger K, Seidler B, Vahl JC, Schwartz C, Kober M, Klein S, Voehringer D, Saur D, Schmidt-Supprian M. CreER(T2) expression from within the c-Kit gene locus allows efficient inducible gene targeting in and ablation of mast cells. Eur J Immunol. 2014;44:296–306. doi: 10.1002/eji.201343731. [DOI] [PubMed] [Google Scholar]
- 32.Klein S, Seidler B, Kettenberger A, Sibaev A, Rohn M, Feil R, Allescher HD, Vanderwinden JM, Hofmann F, Schemann M, Rad R, Storr MA, Schmid RM, Schneider G, Saur D. Interstitial cells of Cajal integrate excitatory and inhibitory neurotransmission with intestinal slow-wave activity. Nature communications. 2013;4:1630. doi: 10.1038/ncomms2626. [DOI] [PubMed] [Google Scholar]
- 33.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–79. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the Damaged Heart: Mesenchymal Stem Cells, Cell-Based Therapy, and Engineered Heart Tissue. Physiol Rev. 2016;96:1127–68. doi: 10.1152/physrev.00019.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. American journal of physiology Heart and circulatory physiology. 2012;303:H256–70. doi: 10.1152/ajpheart.00221.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Z, Pu WT. Strategies for cardiac regeneration and repair. Sci Transl Med. 2014;6:239rv1. doi: 10.1126/scitranslmed.3006681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishikawa K, Fish K, Aguero J, Yaniz-Galende E, Jeong D, Kho C, Tilemann L, Fish L, Liang L, Eltoukhy AA, Anderson DG, Zsebo K, Costa KD, Hajjar RJ. Stem cell factor gene transfer improves cardiac function after myocardial infarction in swine. Circ Heart Fail. 2015;8:167–74. doi: 10.1161/CIRCHEARTFAILURE.114.001711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goichberg P, Bai Y, D'Amario D, Ferreira-Martins J, Fiorini C, Zheng H, Signore S, del Monte F, Ottolenghi S, D'Alessandro DA, Michler RE, Hosoda T, Anversa P, Kajstura J, Rota M, Leri A. The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction. Circ Res. 2011;108:1071–83. doi: 10.1161/CIRCRESAHA.110.239459. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Goichberg P, Kannappan R, Cimini M, Bai Y, Sanada F, Sorrentino A, Signore S, Kajstura J, Rota M, Anversa P, Leri A. Age-associated defects in EphA2 signaling impair the migration of human cardiac progenitor cells. Circulation. 2013;128:2211–23. doi: 10.1161/CIRCULATIONAHA.113.004698. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Nguyen TM, Arthur A, Gronthos S. The role of Eph/ephrin molecules in stromal hematopoietic interactions. Int J Hematol. 2016;103:145–54. doi: 10.1007/s12185-015-1886-x. [DOI] [PubMed] [Google Scholar]
- 41.Kirby ML, Hutson MR. Factors controlling cardiac neural crest cell migration. Cell adhesion & migration. 2010;4:609–21. doi: 10.4161/cam.4.4.13489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, Malm T, Andra M, Jashari R, Nyengaard JR, Possnert G, Jovinge S, Druid H, Frisen J. Dynamics of Cell Generation and Turnover in the Human Heart. Cell. 2015;161:1566–75. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 43.van Berlo JH, Molkentin JD. Most of the Dust Has Settled: cKit+ Progenitor Cells Are an Irrelevant Source of Cardiac Myocytes In Vivo. Circ Res. 2016;118:17–9. doi: 10.1161/CIRCRESAHA.115.307934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaruba MM, Soonpaa M, Reuter S, Field LJ. Cardiomyogenic potential of C-kit(+)-expressing cells derived from neonatal and adult mouse hearts. Circulation. 2010;121:1992–2000. doi: 10.1161/CIRCULATIONAHA.109.909093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tallini YN, Greene KS, Craven M, Spealman A, Breitbach M, Smith J, Fisher PJ, Steffey M, Hesse M, Doran RM, Woods A, Singh B, Yen A, Fleischmann BK, Kotlikoff MI. c-kit expression identifies cardiovascular precursors in the neonatal heart. ProcNatlAcadSciUSA. 2009;106:1808–1813. doi: 10.1073/pnas.0808920106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–1150. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.