Abstract
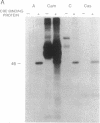
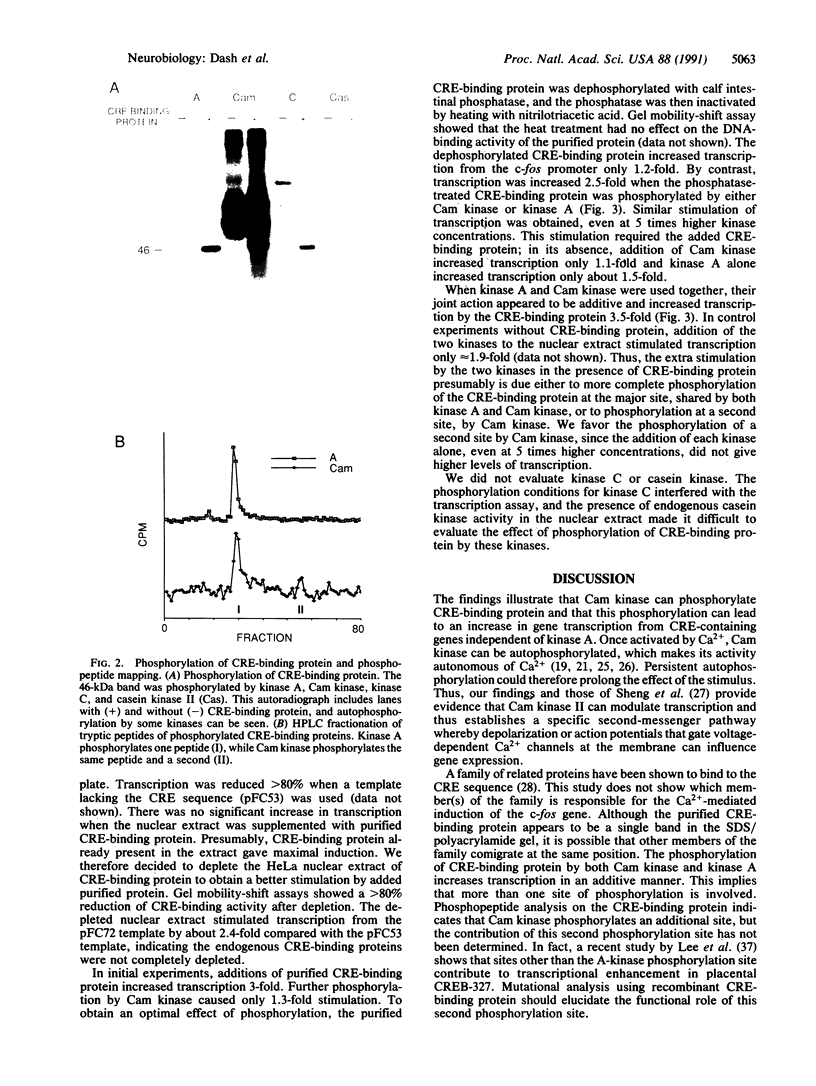
In a variety of nerve cells of the brain, action potentials activate gene expression by means of Ca2+ influx. To determine how Ca2+ influx alters gene expression, we have examined the pattern of phosphorylation of a protein that binds to the cAMP response element (CRE). We have found that purified bovine brain CRE-binding protein is a substrate for the Ca2+/calmodulin-dependent kinase II (Cam kinase) as it is for the cAMP-dependent protein kinase (kinase A). Tryptic peptide maps show that the same peptide is phosphorylated in vitro both by kinase A and by Cam kinase. Moreover, in vitro transcription assays using a CRE-containing c-fos promoter indicate that phosphorylation of CRE-binding protein by Cam kinase increases gene transcription. Thus, action potentials in nerve cells and the consequent influx of Ca2+ can activate CRE-binding proteins by means of Cam kinase. This kinase therefore provides a direct second-messenger pathway by which impulse activity at the membrane can influence gene transcription. This has been shown independently by Sheng et al. (Sheng, M., Thomson, M. A. & Greenberg, M. E. (1991) Science, in press), who found that depolarization and Ca2+ influx mediate induction of c-fos in PC12 rat pheochromocytoma cells through phosphorylation of CRE-binding protein. These several findings indicate that CRE-binding protein(s) is a convergence point for synaptic activity acting through kinase A and impulse activity acting through Cam kinase. Together the two kinases could activate transcription in a synergistic manner, which could allow CRE-binding protein to couple short-term to long-term associative forms of synaptic plasticity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Cole A. J., Saffen D. W., Baraban J. M., Worley P. F. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989 Aug 10;340(6233):474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Comb M., Birnberg N. C., Seasholtz A., Herbert E., Goodman H. M. A cyclic AMP- and phorbol ester-inducible DNA element. 1986 Sep 25-Oct 1Nature. 323(6086):353–356. doi: 10.1038/323353a0. [DOI] [PubMed] [Google Scholar]
- Dash P. K., Hochner B., Kandel E. R. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990 Jun 21;345(6277):718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y., Sher B., Segal D., Yovell Y. Defective responsiveness of adenylate cyclase to forskolin in the Drosophila memory mutant rutabaga. J Neurogenet. 1985 Dec;2(6):365–380. doi: 10.3109/01677068509101423. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goelet P., Castellucci V. F., Schacher S., Kandel E. R. The long and the short of long-term memory--a molecular framework. 1986 Jul 31-Aug 6Nature. 322(6078):419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- Gonzalez G. A., Menzel P., Leonard J., Fischer W. H., Montminy M. R. Characterization of motifs which are critical for activity of the cyclic AMP-responsive transcription factor CREB. Mol Cell Biol. 1991 Mar;11(3):1306–1312. doi: 10.1128/mcb.11.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez G. A., Yamamoto K. K., Fischer W. H., Karr D., Menzel P., Biggs W., 3rd, Vale W. W., Montminy M. R. A cluster of phosphorylation sites on the cyclic AMP-regulated nuclear factor CREB predicted by its sequence. Nature. 1989 Feb 23;337(6209):749–752. doi: 10.1038/337749a0. [DOI] [PubMed] [Google Scholar]
- Hawkins R. D., Abrams T. W., Carew T. J., Kandel E. R. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983 Jan 28;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Hoeffler J. P., Meyer T. E., Yun Y., Jameson J. L., Habener J. F. Cyclic AMP-responsive DNA-binding protein: structure based on a cloned placental cDNA. Science. 1988 Dec 9;242(4884):1430–1433. doi: 10.1126/science.2974179. [DOI] [PubMed] [Google Scholar]
- Hurst H. C., Masson N., Jones N. C., Lee K. A. The cellular transcription factor CREB corresponds to activating transcription factor 47 (ATF-47) and forms complexes with a group of polypeptides related to ATF-43. Mol Cell Biol. 1990 Dec;10(12):6192–6203. doi: 10.1128/mcb.10.12.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Lee C. Q., Yun Y. D., Hoeffler J. P., Habener J. F. Cyclic-AMP-responsive transcriptional activation of CREB-327 involves interdependent phosphorylated subdomains. EMBO J. 1990 Dec;9(13):4455–4465. doi: 10.1002/j.1460-2075.1990.tb07896.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lou L. L., Lloyd S. J., Schulman H. Activation of the multifunctional Ca2+/calmodulin-dependent protein kinase by autophosphorylation: ATP modulates production of an autonomous enzyme. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9497–9501. doi: 10.1073/pnas.83.24.9497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986 Aug 7;322(6079):552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- Prywes R., Fisch T. M., Roeder R. G. Transcriptional regulation of c-fos. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):739–748. doi: 10.1101/sqb.1988.053.01.084. [DOI] [PubMed] [Google Scholar]
- Saitoh T., Schwartz J. H. Phosphorylation-dependent subcellular translocation of a Ca2+/calmodulin-dependent protein kinase produces an autonomous enzyme in Aplysia neurons. J Cell Biol. 1985 Mar;100(3):835–842. doi: 10.1083/jcb.100.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M., McFadden G., Greenberg M. E. Membrane depolarization and calcium induce c-fos transcription via phosphorylation of transcription factor CREB. Neuron. 1990 Apr;4(4):571–582. doi: 10.1016/0896-6273(90)90115-v. [DOI] [PubMed] [Google Scholar]
- Singh H., Clerc R. G., LeBowitz J. H. Molecular cloning of sequence-specific DNA binding proteins using recognition site probes. Biotechniques. 1989 Mar;7(3):252–261. [PubMed] [Google Scholar]
- Stanton P. K., Sarvey J. M. Blockade of long-term potentiation in rat hippocampal CA1 region by inhibitors of protein synthesis. J Neurosci. 1984 Dec;4(12):3080–3088. doi: 10.1523/JNEUROSCI.04-12-03080.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., Czernik A. J., Gorelick F., Nairn A. C., Greengard P. Ca2+/calmodulin-dependent protein kinase II: identification of threonine-286 as the autophosphorylation site in the alpha subunit associated with the generation of Ca2+-independent activity. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6337–6341. doi: 10.1073/pnas.85.17.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters E. T., Byrne J. H. Associative conditioning of single sensory neurons suggests a cellular mechanism for learning. Science. 1983 Jan 28;219(4583):405–408. doi: 10.1126/science.6294834. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Westgate S. A., Kelly P. T. Mutagenesis of Thr-286 in monomeric Ca2+/calmodulin-dependent protein kinase II eliminates Ca2+/calmodulin-independent activity. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1273–1277. doi: 10.1073/pnas.87.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W., Errington M. L., Williams S., Dunnett S. B., Waters C., Hitchcock D., Evan G., Bliss T. V., Hunt S. P. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990 Apr;4(4):603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Biggs W. H., 3rd, Montminy M. R. Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature. 1988 Aug 11;334(6182):494–498. doi: 10.1038/334494a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. K., Gonzalez G. A., Menzel P., Rivier J., Montminy M. R. Characterization of a bipartite activator domain in transcription factor CREB. Cell. 1990 Feb 23;60(4):611–617. doi: 10.1016/0092-8674(90)90664-z. [DOI] [PubMed] [Google Scholar]