Abstract
Peripheral nerves contain large myelinated and small unmyelinated (Remak) fibers that perform different functions. The choice to myelinate or not is dictated to Schwann cells by the axon itself, based on the amount of Neuregulin I-type III exposed on its membrane. Peripheral axons are more important in determining the final myelination fate than central axons, and the implications for this difference in Schwann cells and oligodendrocytes are discussed. Interestingly, this choice is reversible during pathology, accounting for the remarkable plasticity of Schwann cells, and contributing to the regenerative potential of the peripheral nervous system. Radial sorting is the process by which Schwann cells choose larger axons to myelinate during development. This crucial morphogenetic step is a prerequisite for myelination and for differentiation of Remak fibers, and is arrested in human diseases due to mutations in genes coding for extracellular matrix and linkage molecules. In this review we will summarize progresses made in the last years by a flurry of reverse genetic experiments in mice and fish. This work revealed novel molecules that control radial sorting, and contributed unexpected ideas to our understanding of the cellular and molecular mechanisms that control radial sorting of axons.
RADIAL SORTING IS A MULTISTEP MORPHOGENETIC PROCESS
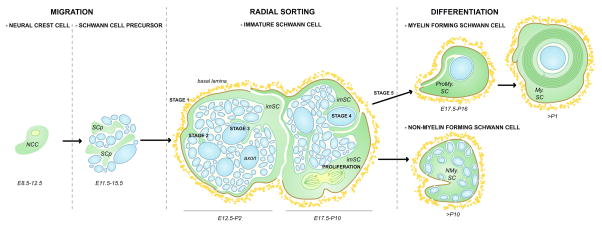
During peripheral nerve development, some neural crest cells give rise to Schwann cell precursors that migrate along axons extending to their final target tissues (Fig 1). Immature Schwann cells derive from Schwann cell precursors. They perform radial sorting of axons, a physiological process that starts perinatally and proceeds until ~ post/natal day 10 (P10) in the rodent peripheral nervous system (PNS) (Fig. 1). A similar process occurs in all vertebrates, including humans. At the beginning of radial sorting, axons are grouped in bundles by a “family” of 3–8 immature Schwann cells that organize a common basal lamina around them (Jessen and Mirsky, 2005; Webster and others, 1973) (Fig. 1). Ultrastructural reconstructions of developing nerves by Henry deF. Webster (Fig. 2) showed that these Schwann cell families surround axons of mixed caliber, and send cytoplasmic processes that resemble lamellipodia between axons, to progressively choose and segregate the larger axons at the periphery of the bundle. Through progressive Schwann cell proliferation and subdivision of the axon bundles, these large axons acquire a 1:1 relationship with a Schwann cell (pro-myelinating) and become myelinated, following the deposition of the Schwann cell own basal lamina (defasciculation). As Schwann cell proliferation and axonal segregation progresses axon bundles become smaller until they contain only small caliber axons, which will subsequently differentiate into Remak bundles.
Figure 1. Schematic representation of Schwann cell development and the morphogenic steps of radial sorting in rodents.
NCC= neural crest cells give rise to Schwann cell precursor (SCp). Radial sorting is performed by immature Schwann cells (imSC) and can be divided in stages. Stage 1, deposition of the basal lamina and formation of Schwann cells and axons ‘families’. Stage 2, insertion of Schwann cell processes into axonal bundles. Stage 3, recognition of large caliber axons. Stage 4, radial segregation of large axons to the periphery. Stage 5, defasciculation and establishment of 1:1 relationship between an axon and a promyelinating Schwann cells (ProMy.). The promyelinated SC will differentiate into a myelinating Schwann cell (My.SC), while the Schwann cells in contact with small caliber axons will surround them and differentiate into a non-myelinating Schwann cell (NMy.SC) to form a Remak bundle.
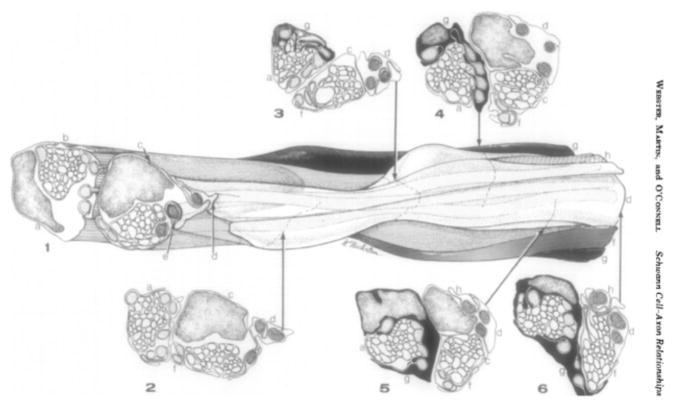
Figure 2.
From (Webster and others, 1973). Drawing of 3-dimensional reconstruction from electron microscopy sections of two families of immature Schwann cells in the newborn marginal bundle of the rat sciatic nerve (Webster and others, 1973). “Parts of 8 Schwann cells form two families proximally (level 1–3) and join at level 4. Four Schwann cells (a–d) participate in surrounding the two bundles. Three of these (a, c, d) also surround some of the segregated axons; the rest are enclosed by two other Schwann cells (f, g). The remaining two Schwann cells (e, h) and the proximal end of (d) at level 1 are in 1:1 relationship with large axons. Nine large axons (stippled) are at the margin of a bundle, are segregated in a furrow, or are in a 1:1 relationship at all levels; the tenth stippled axon lies between Schwann cell (c) and (b) from levels 2–5”. Note that immature SC form lamelipodia-like processes around axons.
Figure 2 was reprinted from Developmental Biology, 32, Henry DeF. Webster, John R. Martin and Maureen F. O’Connell, The Relationships between Interphase Schwann Cells and Axons before Myelination: A Quantitative Electron Microscopic Study, 401–416, Copyright (1973), with permission from Elsevier
Thus, radial sorting is a morphogenetic process that can be divided in various steps (Fig. 3. Upper panel), namely: formation of Schwann cell families and basal lamina deposition (stage 1), insertion of Schwann cell processes into axonal bundles (stage 2), recognition of large caliber axons (stage 3), radial segregation of large axons to the periphery (hence the name “radial sorting”; stage 4), matching of Schwann cell and axon number (proliferation), and finally defasciculation of promyelinating Schwann cells and establishment of 1:1 fibers (stage 5).
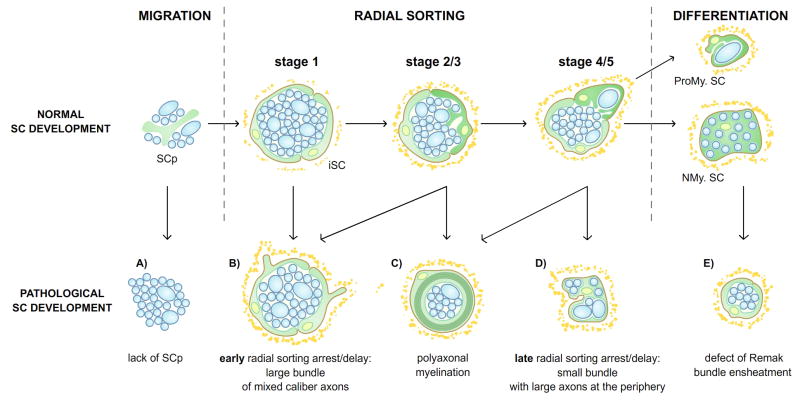
Figure 3. Representation of morphological defects of radial sorting.
The putative developmental-stage of origin (above) of the different abnormalities of radial sorting (below) is illustrated. A) Large bundles of unsorted axons that derive from the absence of Schwann cells. B) Large bundle of unsorted axons, surrounded by Schwann cells that sometimes have abnormal protrusions on the basal side of the Schwann cell, deriving from abnormalities in Steps 1, 2 or 3 of radial sorting. C) polyaxonal myelination, deriving from premature differentiation of Schwann cells before radial sorting is completed D) Small bundle of axons that include a few large diameter axons, deriving from defects in the late stages (4–5) of radial sorting. E) Defects in Remak Schwann cell differentiation and Remak bundle axon ensheathment. A and E are defects that occur before and after radial sorting, respectively. Although the defects have been represented in schematic way, large overlap between these abnormalities are often seen in pathological situations in which radial sorting is impaired.
ABNORMALITIES IN AXONAL SORTING: RADIAL SORTING ARREST, POLYAXONAL MYELINATION AND ABNORMAL ENSHEATMENT OF REMAK BUNDLES
The generation of mutant organisms together with spontaneous mutations in human, mice, fish, cats and dogs, has resulted in a variety of morphological abnormalities in peripheral nerves that are due to impairment in radial sorting. However, the morphology of these abnormalities is different, depending on the stage and mechanism of the impairment (Fig. 3, lower panel). These abnormalities can be grouped in three main categories: radial sorting arrest/delay, polyaxonal myelination and abnormal ensheatment of Remak bundles (Fig 3). The earliest possible abnormality is a failure of generating immature Schwann cells, due to insufficient proliferation, or to their death at the precursors or immature stage, as seen for example when Schwann cells die as a consequence of diphtheria toxin expression or of ErbB3 deletion. This causes a complete arrest of radial sorting even before stage 1, with the nerve being occupied by naked axons with few Schwann cells around them (Brinkmann and others, 2008; Messing and others, 1992) (Fig. 3A). In contrast, defect in the ability of Schwann cells to deposit a basal lamina or to interact with axons (stage 1, 2 or 3), as seen for example in integrin mutants, results in the presence of Schwann cell families containing large bundles of mixed-caliber axons that cannot be sorted (Fig. 3B). These bundles will contain a high number of axons, including many large axons, if the defect appears early (stages 2,3, Fig 3B), or a low number and smaller axons if the defect occurs at later stages (stage 4, Fig. 3D). A failure in regulating Schwann cell proliferation at this stage may also result in Schwann cell families containing bundles of mixed caliber axons. In contrast, mutants such as Jab1 that cause premature exit of Schwann cells from the cell cycle and premature differentiation, while radial sorting is still ongoing, generate polyaxonal myelination, where a smaller group of axons is myelinated by a single Schwann cell (Fig 3C). Finally, in some mutants such as Gab1 the defects are only observed in Remak bundles. If these Remak bundles also contain large axons (larger than 1 micron) they can be considered late axonal sorting defects (i.e. at or after stage 4, Fig. 3D). In contrast, if only small caliber axons remain abnormally ensheathed by Schwann cells in Remak bundles, the problem arises after radial sorting is completed, and is due to abnormal differentiation of the non-myelinating Schwann cells (Fig. 3E). Electron micrographs with examples of these differences, are categorized as “radial sorting abnormalities”, are shown in figure 4. Table 1 lists the known mutants that cause radial sorting abnormalities, and the main stage at which they occur.
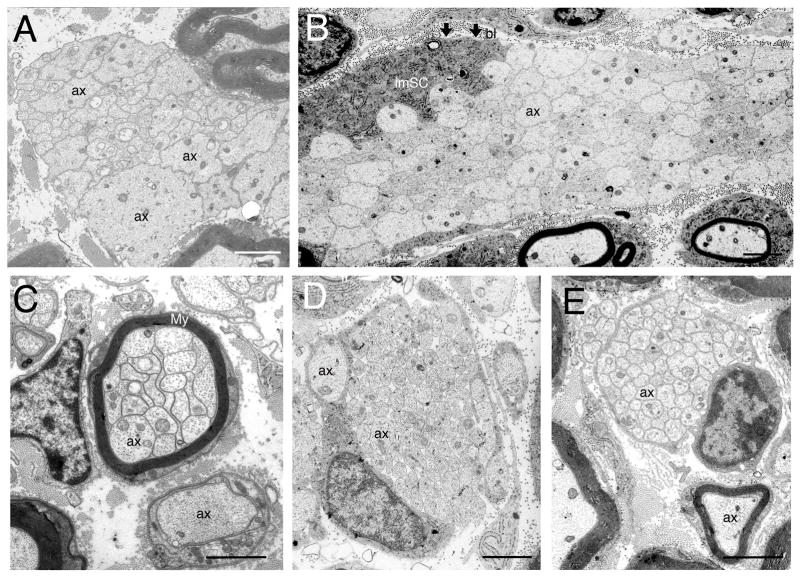
Figure 4. Electron micrographs examples of the morphological defects in radial sorting represented in Figure 3.
A. Electron micrograph of sciatic nerve from dy2J/2J mice, showing large bundles of unsorted, mixed caliber and naked axons (ax), not surrounded by any Schwann cells. This might be due to deficiency lack of Schwann cell precursors or immature Schwann cells. B. Large bundle of unsorted, mixed caliber, and naked axons in sciatic nerves of β1 integrin conditional null mice (a) surrounded by an immature Schwann cell (imSC) and basal lamina (arrows, bl). No cytoplasmic processes from the Schwann are interdigitating among axons, suggesting that the mutant Schwann cell is incapable of polarize, form these cytoplasmic processes, or interact with the axons (steps 2, 3). C. Polyaxonal myelination. Electron micrograph of sciatic nerve from Jab1 conditional null mice: several axons (ax) are surrounded by a common myelin sheath (My). D. Small bundles of axons, some of them larger than 1 μm indicating an arrest or delay in the final steps (4–5) of radial sorting, in electron micrographs of sciatic nerves from dystroglycan conditional null mice. E. Defects of Remak bundle in electron micrographs of sciatic nerve from dy3K/3K mice. Note than axons are not ensheathed by Schwann cell processes, but all the large axons have been sorted out of the bundle (no axons larger than 1 μm is present). Bars = 2 μm.
Table 1.
Mouse mutant assocaited with radial sorting defects
| Protein | Function | Mutant Model | Phenotype | Reference(s) |
|---|---|---|---|---|
| Ahnak | Scaffold protein | Ko | D: Late radial sorting arrest | von Boxberg 2014 |
| β-Catenin | Signaling molecule | cKo (Cnp-Cre) | D: Late radial sorting arrest | Lewallen 2011, Grigoryan 2013 |
| Cdc42 | Rho GTPase | cKo (Dhh-Cre) | D: Late radial sorting arrest | Benninger 2007, Guo 2013b |
| Collagen XV | ECM | Ko | C: Polyaxonal myelination D: Late radial sorting arrest E: Defect of Remak bundle B: Early radial sorting arrest when combined with Lama4−/− |
Rasi 2010 |
| Dicer | miRNA generation | cKo (P0-Cre, Dhh-Cre) | B: Early radial sorting delay | Pereira 2010, Yun 2010 |
| Dystroglycan | Adhesion molecule | cKo (P0-Cre) | B: Early radial sorting arrest in spinal roots D: Late radial sorting arrest in sciatic nerve |
Berti 2011 |
| Erbb2 | Surface receptor | cKo (Krox20-Cre), Zf (inhibitor) | B: Early radial sorting arrest in spinal roots | Garrat 2000, Raphael 2011 |
| Erbb3 | Surface receptor | cKo (CNP-Cre), Zf (inhibitor) | A: Lack of immature Schwann cells | Brinkmann 2008, Raphael 2011 |
| Fak | Signaling molecule | cKo (Cnp-Cre) | B: Early radial sorting arrest | Grove 2007 |
| Fukutin | Glycosyltransferase | Ko | B: Early radial sorting arrest | Saito 2007 |
| Gab1 | Signaling molecule | cKo (P0-Cre) | D: Late radial sorting arrest E: Defect of Remak bundle |
Shin 2014 |
| Gaba-B | Surface receptor | cKo (P0-Cre) | E: Defect of Remak bundle | Faroni 2014a |
| Gpr126 | Surface receptor | cKo (Dhh-Cre) | B: Early radial sorting arrest D: Late radial sorting arrest |
Mogha 2013 |
| Hdac1/Hdac2 | Histone deacetylases | cKo (Dhh-Cre), Ko | B: Early radial sorting delay | Jacob 2010 |
| Ilk | Signaling molecule | cKo (Dhh-Cre) | B: Early radial sorting arrest | Pereira 2008 |
| Integrin β1 | Adhesion molecule | cKo (P0-Cre) | B: Early radial sorting arrest | Feltri 2002 |
| Integrin α6 | Adhesion molecule | cKo (P0-Cre) | D: Late radial sorting arrest B: Early radial sorting arrest when combined with Itga7−/− |
Pellegatta 2013 |
| Jab1 | Signaling molecule, Cell cycle, Protein degradation | cKo (P0-Cre) | B: Early radial sorting arrest C: Polyaxonal myelination |
Porrello 2014 |
| L1 | Adhesion molecule | Ko Ki (L1-6D) |
B: Early radial sorting delay C: Polyaxonal myelination D: Late radial sorting arrest |
Dahme 1997, Itoh 2005 |
| Laminin α2 | ECM | Ko (dy3k), spt (dy,dy2J,dynmf417) | B: Early radial sorting arrest C: Polyaxonal myelination |
Yang 2005, Occhi 2005, Patton 2008 |
| Laminin α4 | ECM | Ko | B: Early radial sorting arrest C: Polyaxonal myelination |
Wallquist 2005, Yang 2005 |
| Laminin γ1 | ECM | cKo (P0-Cre), cKo (CaMKIIa-Cre) | B: Early radial sorting arrest | Chen 2003, Yu 2005 |
| Lck | Signaling molecule | Ko | B: Early radial sorting delay C: Polyaxonal myelination |
Ness 2013 |
| Lgi4 | Adhesion molecule | spt (clp) | B: Early radial sorting delay | Darbas 2004 |
| Lkb1 | Signaling molecule | cKo (P0-Cre) | B: Early radial sorting delay | Beirowski 2014 |
| N-Wasp | Actin polymerizaton | cKo (P0-Cre, Dhh-Cre) | B: Early radial sorting delay | Jin 2011, Novak 2011 |
| Nrg 1 type III | Signaling molecule | Ko | E: Defect of Remak bundle | Taveggia 2005 |
| P2X7 | Surface receptor | Ko | E: Defect of Remak bundle | Faroni 2014b |
| Plasmalogen | Plasmalogen synthesis | Ko | B: Early radial sorting delay | da Silva 2014 |
| Prkar1a | Signaling molecule | cKo (Dhh-Cre) | B: Early radial sorting arrest | Guo 2013a |
| Profilin 1 | Actin polymerizaton | cKo (Dhh-Cre,CNP-Cre) | B: Early radial sorting delay | Montani 2014 |
| Rac1 | Rho GTPase | cKo (P0-Cre, Dhh-Cre) | B: Early radial sorting delay | Nodari 2007, Benninger 2007 |
RADIAL SORTING AND HUMAN NEUROPATHIES
Defects in axonal sorting result in peripheral neuropathies. The prototype of this neuropathy was first described in the spontaneous dystrophic mutants dy/dy and dy2J/2J (Biscoe and others, 1974; Bradley and Jenkison, 1973), as the consequence of mutation of the Laminin α2 gene (Lama2) (Sunada and others, 1995; Xu and others, 1994). Lama2 encodes the α2 chain of Laminin 211 (previously called Merosin), the major component of the basal lamina of Schwann cells and muscles. Indeed, loss of function mutations of the Lama2 gene in rodents, and LAMA2 gene in humans, results in muscular dystrophy and a dysmyelinating neuropathy, which belongs to Merosin deficient Congenital Muscular Dystrophy (MDC1A, OMIM #607855). Mouse mutants have also been generated by homologous recombination that either completely lack (Miyagoe and others, 1997) (dy3K/3K) or have minimal (Kuang and others, 1998) (dyW/W) Laminin 211 expression. All of these mutants develop progressive motor impairment, ranging from more severe phenotype of dy3K/3K to less severe dy2J/2J, as the consequence of progressive wasting muscular dystrophy and dysmyelinating neuropathy.
The peripheral neuropathy is characterized by defective axonal sorting, with bundles of unsorted axons present in peripheral nerves and spinal roots, along with irregular myelination, short internodes, and abnormal nodes of Ranvier. Bundles of unsorted axons contain several axons, tightly packed and of mixed caliber (including larger and smaller than 1 μm), often completely un-ensheathed (“naked” axons) or sometimes partially or entirely surrounded by Schwann cells (Fig. 3 and 4). In many cases, bundles are largely devoid of Schwann cell processes, although occasionally Schwann cells extend partial processes between axons, and in rare cases may establish a promyelinating relationship with a solitary axon (Nakagawa and others, 2001; Stirling, 1975; Yang and others, 2005). Bundles are remnants of embryonic fascicles that do not last permanently. In less severe forms such as dy2J/2J only few and small axon bundles remain in one-year-old mice (Yang and others, 2005 and Previtali, personal observation). Less severe defects in radial sorting are described when Laminin 411, the other Laminin isoform expressed in Schwann cell basal lamina, is deleted. In contrast, a complete arrest in radial sorting in all portions of the PNS is observed when both Laminin 211 and 411 are deleted (Chen and Strickland, 2003; Yang and others, 2005). Interestingly, dysmyelination is more severe in spinal roots and cranial nerves of dy/dy and dy2J/2J mice than in distal peripheral nerves (Jaros and Jenkison, 1983). Conversely, other mutants, including those for Laminin 411 and Laminin receptors, showed more prominent involvement of sciatic nerves than roots. The reason for these differences is not known, it may be due to different compensatory effects by other Laminin subunits (Previtali and others, 2003; Yang and others, 2005). Alternatively, the different developmental origin of Schwann cells that myelinate spinal roots versus distal nerves may underlie a different requirement for laminins and other molecules during radial sorting (Coulpier and others, 2009; Kucenas and others, 2008). Not all fibers are un-sorted in laminin mutants, but even those fibers that are successfully myelinated show abnormalities, namely nodes of Ranvier wider than normal, and short internodes (Court and others, 2009; Jaros and Jenkison, 1983).
Neurophysiological studies confirm that a demyelinating motor neuropathy is present in the majority (80%) of MDC1A patients, and some morphological data is available from sensory sural nerve biopsies. These studies show reduced number of fibers, especially those of larger diameter (>6–7 μm), and variability of myelin thickness, including redundant myelin folds as well as thinner and uncompacted myelin. Moreover, these patients have shorter internodes and wider nodes of Ranvier, (> 5 μm) (Di Muzio and others, 2003; Mercuri and others, 1996; Quijano-Roy and others, 2004; Shorer and others, 1995) confirming a disorder in myelinogenesis that resembles the murine models.
MOLECULAR MECHANISMS OF RADIAL SORTING
Radial sorting relies on the deposition of extracellular matrix (ECM) components and their organization in the basal lamina, the establishment of Schwann cell polarity, the remodeling of the Schwann cell cytoskeleton, Schwann cell-axon interactions, Schwann cell proliferation and finally Schwann cell differentiation. As expected, many signaling pathways that control these processes in Schwann cells are important for radial sorting. For example, the ECM components Laminin 211, Laminin 411, and Collagen XV; their receptors Integrin α6β1, Integrin α7β1 and Dystroglycan, and the Dystroglycan glycosylation enzyme Fukutin are all involved (Berti and others, 2011; Feltri and others, 2002; Occhi and others, 2005; Patton and others, 2008; Pellegatta and others, 2013; Rasi and others, 2010; Saito and others, 2007; Saito and others, 2003; Wallquist and others, 2005; Yang and others, 2005), probably in relationship to the formation of the basal lamina and its control of Schwann cell polarization and signaling. Intracellular signaling molecules that regulate the cytoskeleton are also important, such as ILK, FAK, the RhoGTPases Rac1 and Cdc42, Profilin, Merlin/Nf2 and N-WASp (Benninger and others, 2007; Grove and others, 2007; Guo and others, 2012; Guo and others, 2013b; Jin and others, 2011; Montani and others, 2014; Nodari and others, 2007; Novak and others, 2011; Pereira and others, 2009). These molecules probably regulate actin polymerization to control the dynamics of the cytoplasmic processes that Schwann cells insert into axonal bundles, as these processes are defective or absent in many of these mutants (Benninger and others, 2007; Guo and others, 2012; Guo and others, 2013b; Jin and others, 2011; Montani and others, 2014; Pereira and others, 2009).
In addition, FAK and Cdc42 are also important in regulating Schwann cell proliferation and survival, vital for generating enough cells to engage with axons. One aspect to consider is that axonal molecules, such as Neuregulins, provide juxtacrine mitogenic and survival signals, and mutant Schwann cells that have impaired axonal sorting cannot come in contact with axonal juxtacrine factors. For this reason it is difficult to determine if the reduced number of Schwann cells in these mutants is the cause or the consequence of the radial sorting arrest.
Recent data indicated that lipids, such as plasmalogens, regulate radial sorting, possibly by regulating the Akt-Gsk3β axis in Schwann cell (da Silva and others, 2014). Interestingly, absence of non-coding RNAs due to deletion of dicer in Schwann cells delays radial sorting, (Pereira and others, 2010; Yun and others, 2010) but it is unclear which microRNAs are involved and which target they might regulate. Curiously, there is no information at the moment on transcription factors that influence radial sorting, but nuclear control of sorting is likely important, as deletion of Hdac1 and Jab1 (see also below) delays radial sorting (Jacob and others, 2011; Porrello and others, 2014).
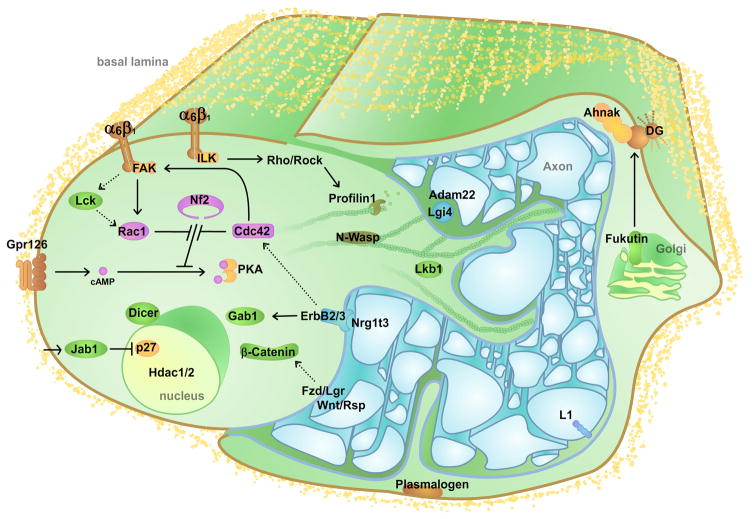
A comprehensive list of the molecules that have been shown to participate in-vivo is summarized in Table 1. Due to the fact that in the perinatal period Schwann cells are not synchronized, but mature at different moments, steps 1–5 of radial sorting occur simultaneously in a developing nerve and at any moment different Schwann cells are engaged in various stages of this process. For this reason, in most cases it is difficult to pinpoint which morphogenetic step is controlled by a certain pathway, or the location of the molecules involved in sorting. Figure 5 summarizes our current understanding of the location and role of some of the molecules involved in radial sorting.
Figure 5. Schematic representation of the localization and interaction of some of the molecules involved in radial sorting.
The figure depicts two immature Schwann cells surrounding a bundle of axons. The proteins have been divided arbitrarily between the two Schwann cells for readability. Dashed lines indicate signals with uncertain targets. Proteins involved in radial sorting and present in the basal lamina have not been depicted (Laminins211, 411, Collagen XV). Cyclic AMP (cAMP), Dystroglycan (DG), Focal adhesion kinase (FAK), Grb2-Associated Binder (Gab), Gpr (G protein-coupled receptor), Histone deacetylase (Hdac), Integrin (α6β1, α7β1), Integrin-linked kinase (ILK), Jun activation domain–binding (Jab), lymphoid cell kinase (Lck), Leucine-rich-glioma inactivated (Lgi), Liver Kinase B (Lkb), Neurofibromin (Nf), Neural Wiskott–Aldrich syndrome protein (N-Wasp) and Protein Kinase A (PKA).
CONTROL OF SCHWANN CELL PROLIFERATION, EXIT FROM THE CELL CYCLE AND DIFFERENTIATION
Proliferation and differentiation are closely linked in Schwann cells. Regulated exit from the cell cycle is a prerequisite for Schwann cell differentiation during nerve development (Morgan and others, 1991; Stevens and Fields, 2002). This process is tightly modulated by the cyclin-dependent kinase inhibitor 1b, also called p27, as axonal signals induce high p27 levels in differentiating Schwann cells, whereas inhibition of p27 abolishes the expression of pro-myelinating markers in Schwann cells (Li and others, 2011; Morgan and others, 1991). Premature Schwann cell exit from the cell cycle and differentiation is the likely cause of polyaxonal myelination, in which one bundle of unsorted axons is partially or entirely myelinated by one single Schwann cell (Fig. 3C, 4C). The myelin periodicity of polyaxonal bundles is comparable to that of normal myelinated fibers, and myelin thickness might be normal or reduced. Polyaxonal myelination is a rare feature in normal nerves, and usually limited to the early postnatal developmental period; instead it is observed quite often in mature nerves of many mouse mutants, such as those for Laminins 211, 411, where it is associated with reduced proliferation, and it was also recently described in mice with Schwann cell conditional deletion of the nuclear protein Jab1 (Brown and Radich, 1979; Porrello and others, 2014; Wallquist and others, 2005; Yang and others, 2005). Loss of Jab1 in Schwan cells is associated with abnormal p27 levels, arrest in cell cycle and aberrant differentiation. Strikingly, reducing p27 restores axonal sorting (Porrello and others, 2014). This observation supports the idea that immature Schwann cells that exit prematurely from the cell cycle will differentiate and myelinate a bundle of yet unsorted axons, creating polyaxonal myelination. This hypothesis is corroborated by the fact that polyaxonal myelination is also observed in nerves of mice that express a dominant-negative form of the pro-differentiation factor Pou3f-Oct6/SCIP (Jaegle and others, 1996; Weinstein and others, 1995), and should be validated by comparison with other mutants that exhibit polyaxonal myelination such as those for Lck, Collagen XV and L1-6D (Itoh and others, 2005; Ness and others, 2013; Rasi and others, 2010). On the other hand, one would expect that mutants having axonal sorting defects primarily as the consequence of abnormal cytoskeleton remodeling (Rac1, Ilk, Profilin1), rather than proliferation, do not develop substantial polyaxonal myelination. In accordance, Schwann cell conditional knockout for β1 integrin, Rac1, Ilk and Pfn1 did not show features of polyaxonal myelination (Angelo Quattrini and Joao Relvas, personal communication). Interestingly, abnormal proliferation of immature Schwann cells due to the neuronal overexpression of NRG1 type III-isoform β3 in transgenic mice also causes polyaxonal myelination, although restricted to Remak bundles (Gomez-Sanchez and others, 2009). It is possible that overexpression of NRG1 type III-β3 in small caliber axons impedes segregation of these axons in the Remak bundle, and reprograms non-myelin forming Schwann cells into a myelinating phenotype that results in the myelination of the whole Remak bundle (Gomez-Sanchez and others, 2009).
RADIAL SORTING: THE IMPORTANCE OF FORMING POLARIZED CELLULAR PROTRUSIONS
A common abnormality seen in mutant animals with impaired radial sorting is Schwann cells that fail to insert cytoplasmic processes into axon bundles (stage 2). In principle, this might be due to a failure of Schwann cells to generate the appropriate apico-basal polarity, to organize the cytoskeleton to form cellular protrusions, or to contact and recognize large diameter axons. So far, most of the molecular mechanisms that have been identified relate to the organization of the cytoskeleton, a few are clearly involved in polarity, and very little is known on how Schwann cells recognize large axons.
Signals from the basal lamina are fundamental for the first stages of radial sorting (stage 1–2) (Gomez-Sanchez and others, 2009). All evidences suggest that the basal lamina provides signals through different receptors (α6β1 Integrin, Gpr126, β-dystroglycan) (Berti and others, 2011; Feltri and others, 2002; Pellegatta and others, 2013; Petersen and others, 2015) and effectors (Ilk, Fak) (Grove and others, 2007; Pereira and others, 2009). These proteins polarize the basal side of Schwann cells, lead to the formation of cellular protrusions and may cause the redistribution of appropriate molecules to the apical plasma membrane to interact with axons. Cellular protrusions have been shown to resemble cellular lamellipodia of other cell types (fig. 2 and (Nodari and others, 2007; Webster and others, 1973), and deletion of components of the cellular machinery required for lamellipodia formation, such as Rac1 and N-WASP are required for radial sorting (Jin and others, 2011; Nodari and others, 2007; Novak and others, 2011).
Signaling from the basal lamina converge onto other signaling cascades that control the remodeling of actin cytoskeleton, and regulation of cyclic AMP and Protein kinase A (PKA) (Guo and others, 2013a; Guo and others, 2012; Montani and others, 2014). The remodeling of actin cytoskeleton and cell polarity is also regulated by Rho GTPase family members, and several Rho GTPases are required for radial sorting (Benninger and others, 2007; Guo and others, 2013b; Nodari and others, 2007). Rac1 and Cdc42 have been shown to regulate the activation of PKA through Nf2, as the ablation of Nf2 rescues the radial sorting phenotype of both Rac1 and Cdc42 mutant (Guo and others, 2012; Guo and others, 2013b). Recently, it was reported that LKB1/Par4 is phosphorylated by PKA to establish Schwann cell polarity and to promote radial sorting (Shen and others, 2014). Altogether, all these pathways allow Schwann cells to establish the correct apico-basal polarity and proper cytoskeleton reorganization to form polarized cytoplasmic protrusions that contact axons (stage 2).
Regarding the later stages of radial sorting (stage 4–5), so far only Schwann cells deficient for the Laminin receptor β-dystroglycan have shown a defect in axon defasciculation (stage 4/5) (Berti and others, 2011). Thus, signals originating from the basal lamina govern both the insertion of Schwann cell processes into axon bundles, (stage 2) and the segregation of the selected axons (stage 4), by regulating polarity and the actin cytoskeleton.
WHAT IS THE ROLE OF AXON SIGNALING IN RADIAL SORTING?
One gap in our knowledge of radial sorting concerns how Schwann cells recognize large diameter axons (stage 3) in order to segregate them. One possibility is that Schwann cell receptors bind to ligands on axons (or viceversa); however, the identity of such ligand/receptor pairs remains elusive. Candidates are ErbB2/3 receptors, N-cadherins, L1, LRP/Frizzled and Lgi4 on Schwann cells, although a precise role in this first recognition step has not been proven. β1 Integrin subunit has also been located at axoglial interface, but its role independent of Laminins during radial sorting remains to be established (Berti and others, 2011).
The amount of Neuregulin 1 (Nrg1) type III on axons determines the myelin fate of Schwann cells (Berti and others, 2011), strongly suggesting that Nrg1 type III or other axonal signals should be required for axonal recognition during radial sorting via ErbB2/3 receptor on Schwann cells (Nave and Salzer, 2006; Newbern and Birchmeier, 2010; Taveggia and others, 2005). The role of Nrg1 type III in axonal sorting could not be directly tested because knockout mice are not viable (Wolpowitz and others, 2000), however some Remak bundles contain partially ensheated axons, including axons larger than 1 μm, which, as shown in Fig. 3D, might represent mild radial sorting abnormalities (Taveggia and others, 2005). Inhibition of the ErbB pathway causes impaired axonal sorting in zebrafish nerves (Raphael and others, 2011), and conditional inactivation of ErbB2 or ErbB3 in Schwann cells prevents radial sorting in spinal roots. This occurs secondary to defects arising prior to radial sorting, presumably due to the death of Schwann cells (similar to situation in the fig 3A) that are absent in the roots (Brinkmann and others, 2008; Garratt and others, 2000). Finally, Schwann cell inactivation of Gab1 (Grb2 associated binder 1), a scaffolding molecule implicated in Erk/Akt signaling downstream of Nrg1, generates abnormal Remak bundles containing unsorted large caliber axons (Shin and others, 2014). Thus, although the evidences is incomplete, overall it is likely that Schwann cells adopt Nrg1-ErbB2/3 interaction as mechanism for axonal recognition and sorting.
Lgi4, secreted by Schwann cells, and Adam22 may also be implicated in axonal recognition. This is because mutants for Lgi4 show a transient delay in radial sorting, and Lgi4 secreted by Schwann cells binds axonal Adam22. However, targeted deletion of Adam22 seems to be compatible with radial sorting and causes arrest of myelination at the pro-myelinating stage (Bermingham and others, 2006; Ozkaynak and others, 2010; Sagane and others, 2005).
The role of Wnt/β-catenin signals in controlling axonal radial sorting has been reported recently (Grigoryan and others, 2013). The Wnt/β-catenin complex, involved in neural crest and sensory lineage of peripheral nerve specification, assembles with Respondin, Lgr receptors and Lrp/Frizzled to promote Wnt signaling (Hari and others, 2002; Lee and others, 2004). It has been shown that neurons express several Wnt and Respondin ligands that signal to Schwann cells in a paracrine manner. Conditional inactivation of β-catenin in Schwann cells results in mild radial sorting defects that are consistent with impaired process extension and lamellipodia formation (Grigoryan and others, 2013; Lewallen and others, 2011). Conversely, mutants with constitutive active β-catenin in Schwann cells accelerate axonal sorting resulting in smaller Remak bundles (Grigoryan and others, 2013).
In the future, it will be important to determine if these axonal ligands are required for Schwann cells to recognize axons for proper sorting.
ECM AND AXON COOPERATION DURING RADIAL SORTING
An interesting and yet partly unanswered question is how axonal and ECM-derived signals are coordinated during radial sorting. A possible mediator of this cross-talk is the G-protein-coupled receptor (Gpr) 126. This is a classic seven-membrane helix region receptor, putatively involved in cell-cell or cell-matrix adhesion, required for peripheral nerve myelination (Monk and others, 2009; Monk and others, 2011). Schwann cell conditional deletion of Gpr126 revealed problems in radial sorting in addition to myelination, with the presence of abnormal bundles of large unsorted axons, often without cytoplasmic interdigitations, and aberrant abaxonal cytoplasmic protrusions of mutant Schwann cells, suggesting a defect in cytoskeleton organization. This radial sorting defect is not due to reduced number of Schwann cells, since their number actually increased (Mogha and others, 2013). Because Gpr126 acts through the elevation of cAMP levels and protein kinase A (PKA) activation (Monk and others, 2009; Monk and others, 2011), and cAMP has been classically associated to axonal signals, (Arthur-Farraj and others, 2011; Mokuno and others, 1988; Montani and others, 2014; Morgan and others, 1991) it was immediately postulated that Gpr126 may be a ligand for an axonal molecule. Surprisingly, recent data shows that ligands of Gpr126 are ECM molecules, collagen IV and Laminin 211, abundant in basal lamina and possibly acting in contrasting ways during radial sorting and myelination (Glenn and Talbot, 2013; Paavola and others, 2014; Petersen and others, 2015). Thus, Gpr126 may provide a handle of how axons and the ECM cooperate to promote Schwann cell differentiation via regulation of cAMP.
A DIFFERENT KIND OF “AXONAL SORTING” OCCURS IN THE CENTRAL NERVOUS SYSTEM
Similarly to the PNS, not all axons in the central nervous system (CNS) are myelinated. Oligodendrocytes must also “choose” only certain axons for myelination. Can the process of radial sorting in the PNS offer insights on how oligodendrocytes select axons for myelination in the CNS? Several studies indicate that axonal properties are less important in the CNS than in the PNS in determining myelination fate (Brinkmann and others, 2008; Lee and others, 2013). There is the emerging idea that myelination contributes to neuronal plasticity (Gibson and others, 2014; Liu and others, 2012; Makinodan and others, 2012). Oligodendrocyte precursors persist in the adult brain, providing a reservoir of cells that do not differentiate, but can myelinate when needed, observed during experience-based remodelling or after injury. Indeed, myelination in the CNS can continue well into adult life (Miller and others, 2012), and occurs efficiently after demyelination (Franklin and Ffrench-Constant, 2008). Recent data show that the same myelinated axons in the mouse cortex have long stretches of non-myelinated segments (Tomassy and others, 2014). Late de-novo myelination, long stretches of unmyelination and a reservoir of oligodendrocyte precursors could assure myelination as needed, and it is postulated to contribute to neuronal plasticity. All this indicates that, at least in certain regions, there cannot be strict axonal characteristics (i.e. axonal size, or axonal threshold of a certain growth factor) that dictate the myelination status of a CNS axon. Indeed, there is no strict correlation between the two known myelin-inducing signals in the PNS, namely axon size and amount of Neuregulins, with the extent of CNS myelination. In vitro studies suggest that biologically active axonal molecules might not even be strictly necessary for CNS myelination (Lee and others, 2013). In contrast with this view however, electrical activity (Mateu and Moran, 1986; Wake and others, 2011), axons size (Almeida and others, 2011; Lee and others, 2013), and levels of Neuregulins all influence the timing and degree of myelination in the CNS (Taveggia and others, 2005). Thus, although the correlation between axonal properties and myelination is less strict in the CNS, it is conceivable that similar mechanisms favor the “sorting” of axons for myelination in the PNS and CNS, but additional layers of control in the CNS are required to permit the plasticity that characterizes higher brain functions. In support of this idea, Laminins, which are strictly required for axonal sorting in the PNS (see below), also decrease the axon size threshold for myelination in the CNS (Camara and others, 2009). So, even if the “degree of freedom” is higher in the CNS than PNS, the molecular mechanisms at play may overlap.
CONCLUSIONS AND FUTURE PERSPECTIVES
Many gaps remain in our knowledge of the molecular control of radial sorting. We think that two areas will be particularly stimulating for future research.
One regards the molecules and mechanisms that Schwann cells use to recognize and segregate large diameter axons (stage 3). It is possible that some ligand/receptor couples between Schwann cell and axon have yet to be identified. It is also possible that the recognition and locking of large diameter axons is triggered by neurotransmitters, since Schwann cells appear to be very sensitive to neuronal activity (see (Samara and others, 2013) for a comprehensive analysis of Schwann cells neurotransmitter receptors and ion channels). The radial sorting of axons coincides with the onset of active spontaneous low frequency impulse activity in peripheral neurons (Fitzgerald, 1987) and this activity induces ionic imbalances and neurotransmitter secretion that affect immature Schwann cell proliferation and differentiation in-vitro (Stevens and Fields, 2000; Stevens and others, 2004). Indeed, two Schwann cell neurotransmitter receptors, the purinergic receptor P2X7, and the GABA-B1 G protein receptor, have shown to be necessary for the formation of Remak bundles (Faroni and others, 2014a; Faroni and others, 2014b). While the localization of these receptors at the axoglial interface remains to be proven, they might represent good candidates to regulate axonal recognition during radial sorting.
The second important area involves the negative regulators of radial sorting. We have known for a long time that the caliber of an axon determines the fate of the Schwann cell that ensheaths it (Voyvodic, 1989). Over the last decade we learned that the amount of Neuregulin I-type III present on axons instructs the Schwann cell whether or not to take on myelinating fate, with high levels of Neuregulin promoting the myelin fate and low levels instructing the non-myelinated fate (Michailov and others, 2004; Taveggia and others, 2005). However, we do not know if low levels of Neuregulins are enough to determine the non-myelination state, or if there are also specific inhibitory molecules expressed on small axons. Recent data indicate that several signaling pathways in Schwann cells, such as excessive PKA (Guo and others, 2013a), GSK3-beta activation (da Silva and others, 2014), and ILK-RhoA-ROCK signaling, (da Silva and others, 2014) all negatively regulate radial sorting. Are low levels of Neuregulin or other specific ligands activating these negative signals in Schwann cells? If it is the latter, do these putative ligands reside on axons, the ECM or are they soluble signals? What receptor do they bind to trigger the activation of these negative regulators in Schwann cells? These are all interesting questions for future work.
Acknowledgments
Work in the MLF and SCP laboratories was funded by the NINDS (NS045630 to MLF), NICHD (HD075363 to MLF), the European Community FP7-EU-NGIDD (MLF), and Telethon Italy (GPP10007 to SCP and MLF; GGP12024 and GUP13006 to SCP) and Italian Ministry of Health (96/RF-2011-02347127 to SCP).
We thank current and past members of the Feltri and Previtali’s laboratories, and our life-long collaborators Sophie Belin, Alessandra Bolino, Angelo Quattrini, Carla Taveggia and Lawrence Wrabetz. A special thank you to Elisabetta Babetto, Bogdan Beirowski and Carla Taveggia for critical reading of the manuscript.
References
- Almeida RG, Czopka T, Ffrench-Constant C, Lyons DA. Individual axons regulate the myelinating potential of single oligodendrocytes in vivo. Development. 2011;138:4443–4450. doi: 10.1242/dev.071001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur-Farraj P, Wanek K, Hantke J, Davis CM, Jayakar A, Parkinson DB, Mirsky R, Jessen KR. Mouse schwann cells need both NRG1 and cyclic AMP to myelinate. Glia. 2011;59:720–733. doi: 10.1002/glia.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. The Journal of cell biology. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermingham JR, Jr, Shearin H, Pennington J, O’Moore J, Jaegle M, Driegen S, van Zon A, Darbas A, Ozkaynak E, Ryu EJ, Milbrandt J, Meijer D. The claw paw mutation reveals a role for Lgi4 in peripheral nerve development. Nature neuroscience. 2006;9:76–84. doi: 10.1038/nn1598. [DOI] [PubMed] [Google Scholar]
- Berti C, Bartesaghi L, Ghidinelli M, Zambroni D, Figlia G, Chen ZL, Quattrini A, Wrabetz L, Feltri ML. Non-redundant function of dystroglycan and beta1 integrins in radial sorting of axons. Development. 2011;138:4025–4037. doi: 10.1242/dev.065490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biscoe TJ, Caddy KW, Pallot DJ, Pehrson UM, Stirling CA. The neurological lesion in the dystrophic mouse. Brain research. 1974;76:534–536. doi: 10.1016/0006-8993(74)90830-0. [DOI] [PubMed] [Google Scholar]
- Bradley WG, Jenkison M. Abnormalities of peripheral nerves in murine muscular dystrophy. Journal of the neurological sciences. 1973;18:227–247. doi: 10.1016/0022-510x(73)90009-9. [DOI] [PubMed] [Google Scholar]
- Brinkmann BG, Agarwal A, Sereda MW, Garratt AN, Muller T, Wende H, Stassart RM, Nawaz S, Humml C, Velanac V, Radyushkin K, Goebbels S, Fischer TM, Franklin RJ, Lai C, Ehrenreich H, Birchmeier C, Schwab MH, Nave KA. Neuregulin-1/ErbB signaling serves distinct functions in myelination of the peripheral and central nervous system. Neuron. 2008;59:581–595. doi: 10.1016/j.neuron.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Radich SJ. Polyaxonal myelination in developing dystrophic and normal mouse nerves. Muscle & nerve. 1979;2:217–222. doi: 10.1002/mus.880020310. [DOI] [PubMed] [Google Scholar]
- Camara J, Wang Z, Nunes-Fonseca C, Friedman HC, Grove M, Sherman DL, Komiyama NH, Grant SG, Brophy PJ, Peterson A, ffrench-Constant C. Integrin-mediated axoglial interactions initiate myelination in the central nervous system. The Journal of cell biology. 2009;185:699–712. doi: 10.1083/jcb.200807010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. The Journal of cell biology. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulpier F, Le Crom S, Maro GS, Manent J, Giovannini M, Maciorowski Z, Fischer A, Gessler M, Charnay P, Topilko P. Novel features of boundary cap cells revealed by the analysis of newly identified molecular markers. Glia. 2009;57:1450–1457. doi: 10.1002/glia.20862. [DOI] [PubMed] [Google Scholar]
- Court FA, Hewitt JE, Davies K, Patton BL, Uncini A, Wrabetz L, Feltri ML. A laminin-2, dystroglycan, utrophin axis is required for compartmentalization and elongation of myelin segments. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3908–3919. doi: 10.1523/JNEUROSCI.5672-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva TF, Eira J, Lopes AT, Malheiro AR, Sousa V, Luoma A, Avila RL, Wanders RJ, Just WW, Kirschner DA, Sousa MM, Brites P. Peripheral nervous system plasmalogens regulate Schwann cell differentiation and myelination. The Journal of clinical investigation. 2014;124:2560–2570. doi: 10.1172/JCI72063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Muzio A, De Angelis MV, Di Fulvio P, Ratti A, Pizzuti A, Stuppia L, Gambi D, Uncini A. Dysmyelinating sensory-motor neuropathy in merosin-deficient congenital muscular dystrophy. Muscle & nerve. 2003;27:500–506. doi: 10.1002/mus.10326. [DOI] [PubMed] [Google Scholar]
- Faroni A, Castelnovo LF, Procacci P, Caffino L, Fumagalli F, Melfi S, Gambarotta G, Bettler B, Wrabetz L, Magnaghi V. Deletion of GABA-B receptor in Schwann cells regulates remak bundles and small nociceptive C-fibers. Glia. 2014a;62:548–565. doi: 10.1002/glia.22625. [DOI] [PubMed] [Google Scholar]
- Faroni A, Smith RJ, Procacci P, Castelnovo LF, Puccianti E, Reid AJ, Magnaghi V, Verkhratsky A. Purinergic signaling mediated by P2X7 receptors controls myelination in sciatic nerves. Journal of neuroscience research. 2014b;92:1259–1269. doi: 10.1002/jnr.23417. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Graus Porta D, Previtali SC, Nodari A, Migliavacca B, Cassetti A, Littlewood-Evans A, Reichardt LF, Messing A, Quattrini A, Mueller U, Wrabetz L. Conditional disruption of beta 1 integrin in Schwann cells impedes interactions with axons. The Journal of cell biology. 2002;156:199–209. doi: 10.1083/jcb.200109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald M. Spontaneous and evoked activity of fetal primary afferents in vivo. Nature. 1987;326:603–605. doi: 10.1038/326603a0. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews Neuroscience. 2008;9:839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Garratt AN, Voiculescu O, Topilko P, Charnay P, Birchmeier C. A dual role of erbB2 in myelination and in expansion of the schwann cell precursor pool. The Journal of cell biology. 2000;148:1035–1046. doi: 10.1083/jcb.148.5.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, Barres BA, Woo PJ, Vogel H, Monje M. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:1252304. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn TD, Talbot WS. Analysis of Gpr126 function defines distinct mechanisms controlling the initiation and maturation of myelin. Development. 2013;140:3167–3175. doi: 10.1242/dev.093401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Lopez de Armentia M, Lujan R, Kessaris N, Richardson WD, Cabedo H. Sustained axon-glial signaling induces Schwann cell hyperproliferation, Remak bundle myelination, and tumorigenesis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11304–11315. doi: 10.1523/JNEUROSCI.1753-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan T, Stein S, Qi J, Wende H, Garratt AN, Nave KA, Birchmeier C, Birchmeier W. Wnt/Rspondin/beta-catenin signals control axonal sorting and lineage progression in Schwann cell development. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18174–18179. doi: 10.1073/pnas.1310490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove M, Komiyama NH, Nave KA, Grant SG, Sherman DL, Brophy PJ. FAK is required for axonal sorting by Schwann cells. The Journal of cell biology. 2007;176:277–282. doi: 10.1083/jcb.200609021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Lee AA, Rizvi TA, Ratner N, Kirschner LS. The protein kinase A regulatory subunit R1A (Prkar1a) plays critical roles in peripheral nerve development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013a;33:17967–17975. doi: 10.1523/JNEUROSCI.0766-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Niehaus K, Zheng Y, Ratner N. Rac1 controls Schwann cell myelination through cAMP and NF2/merlin. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:17251–17261. doi: 10.1523/JNEUROSCI.2461-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Moon C, Zheng Y, Ratner N. Cdc42 regulates Schwann cell radial sorting and myelin sheath folding through NF2/merlin-dependent and independent signaling. Glia. 2013b;61:1906–1921. doi: 10.1002/glia.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hari L, Brault V, Kleber M, Lee HY, Ille F, Leimeroth R, Paratore C, Suter U, Kemler R, Sommer L. Lineage-specific requirements of beta-catenin in neural crest development. The Journal of cell biology. 2002;159:867–880. doi: 10.1083/jcb.200209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh K, Fushiki S, Kamiguchi H, Arnold B, Altevogt P, Lemmon V. Disrupted Schwann cell-axon interactions in peripheral nerves of mice with altered L1-integrin interactions. Molecular and cellular neurosciences. 2005;30:131–136. doi: 10.1016/j.mcn.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Jacob C, Christen CN, Pereira JA, Somandin C, Baggiolini A, Lotscher P, Ozcelik M, Tricaud N, Meijer D, Yamaguchi T, Matthias P, Suter U. HDAC1 and HDAC2 control the transcriptional program of myelination and the survival of Schwann cells. Nature neuroscience. 2011;14:429–436. doi: 10.1038/nn.2762. [DOI] [PubMed] [Google Scholar]
- Jaegle M, Mandemakers W, Broos L, Zwart R, Karis A, Visser P, Grosveld F, Meijer D. The POU factor Oct-6 and Schwann cell differentiation. Science. 1996;273:507–510. doi: 10.1126/science.273.5274.507. [DOI] [PubMed] [Google Scholar]
- Jaros E, Jenkison M. Defective differentiation of peripheral nerves in the dystrophic mouse. Brain research. 1983;282:231–242. doi: 10.1016/0165-3806(83)90062-7. [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R. The origin and development of glial cells in peripheral nerves. Nature reviews Neuroscience. 2005;6:671–682. doi: 10.1038/nrn1746. [DOI] [PubMed] [Google Scholar]
- Jin F, Dong B, Georgiou J, Jiang Q, Zhang J, Bharioke A, Qiu F, Lommel S, Feltri ML, Wrabetz L, Roder JC, Eyer J, Chen X, Peterson AC, Siminovitch KA. N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination. Development. 2011;138:1329–1337. doi: 10.1242/dev.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuang W, Xu H, Vachon PH, Liu L, Loechel F, Wewer UM, Engvall E. Merosin-deficient congenital muscular dystrophy. Partial genetic correction in two mouse models. The Journal of clinical investigation. 1998;102:844–852. doi: 10.1172/JCI3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nature neuroscience. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Kleber M, Hari L, Brault V, Suter U, Taketo MM, Kemler R, Sommer L. Instructive role of Wnt/beta-catenin in sensory fate specification in neural crest stem cells. Science. 2004;303:1020–1023. doi: 10.1126/science.1091611. [DOI] [PubMed] [Google Scholar]
- Lee S, Chong SY, Tuck SJ, Corey JM, Chan JR. A rapid and reproducible assay for modeling myelination by oligodendrocytes using engineered nanofibers. Nature protocols. 2013;8:771–782. doi: 10.1038/nprot.2013.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Yang H, Liu Y, Huan W, Zhang S, Wu G, Lu Q, Wang Q, Wang Y. The cyclin-dependent kinase inhibitor p27(Kip1) is a positive regulator of Schwann cell differentiation in vitro. Journal of molecular neuroscience : MN. 2011;45:277–283. doi: 10.1007/s12031-011-9518-2. [DOI] [PubMed] [Google Scholar]
- Liu J, Dietz K, DeLoyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, Dupree J, Casaccia P. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nature neuroscience. 2012;15:1621–1623. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateu L, Moran O. Reversible changes in myelin structure and electrical activity during anesthesia in vivo. Biochimica et biophysica acta. 1986;862:17–26. doi: 10.1016/0005-2736(86)90464-5. [DOI] [PubMed] [Google Scholar]
- Mercuri E, Pennock J, Goodwin F, Sewry C, Cowan F, Dubowitz L, Dubowitz V, Muntoni F. Sequential study of central and peripheral nervous system involvement in an infant with merosin-deficient congenital muscular dystrophy. Neuromuscular disorders : NMD. 1996;6:425–429. doi: 10.1016/s0960-8966(96)00383-5. [DOI] [PubMed] [Google Scholar]
- Messing A, Behringer RR, Hammang JP, Palmiter RD, Brinster RL, Lemke G. P0 promoter directs expression of reporter and toxin genes to Schwann cells of transgenic mice. Neuron. 1992;8:507–520. doi: 10.1016/0896-6273(92)90279-m. [DOI] [PubMed] [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TM, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA. Axonal neuregulin-1 regulates myelin sheath thickness. Science. 2004;304:700–703. doi: 10.1126/science.1095862. [DOI] [PubMed] [Google Scholar]
- Miller DJ, Duka T, Stimpson CD, Schapiro SJ, Baze WB, McArthur MJ, Fobbs AJ, Sousa AM, Sestan N, Wildman DE, Lipovich L, Kuzawa CW, Hof PR, Sherwood CC. Prolonged myelination in human neocortical evolution. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16480–16485. doi: 10.1073/pnas.1117943109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagoe Y, Hanaoka K, Nonaka I, Hayasaka M, Nabeshima Y, Arahata K, Nabeshima Y, Takeda S. Laminin alpha2 chain-null mutant mice by targeted disruption of the Lama2 gene: a new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS letters. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Mogha A, Benesh AE, Patra C, Engel FB, Schoneberg T, Liebscher I, Monk KR. Gpr126 functions in Schwann cells to control differentiation and myelination via G-protein activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17976–17985. doi: 10.1523/JNEUROSCI.1809-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokuno K, Sobue G, Reddy UR, Wurzer J, Kreider B, Hotta H, Baron P, Ross AH, Pleasure D. Regulation of Schwann cell nerve growth factor receptor by cyclic adenosine 3′,5′-monophosphate. Journal of neuroscience research. 1988;21:465–472. doi: 10.1002/jnr.490210237. [DOI] [PubMed] [Google Scholar]
- Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, Moens CB, Talbot WS. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk KR, Oshima K, Jors S, Heller S, Talbot WS. Gpr126 is essential for peripheral nerve development and myelination in mammals. Development. 2011;138:2673–2680. doi: 10.1242/dev.062224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montani L, Buerki-Thurnherr T, de Faria JP, Pereira JA, Dias NG, Fernandes R, Goncalves AF, Braun A, Benninger Y, Bottcher RT, Costell M, Nave KA, Franklin RJ, Meijer D, Suter U, Relvas JB. Profilin 1 is required for peripheral nervous system myelination. Development. 2014;141:1553–1561. doi: 10.1242/dev.101840. [DOI] [PubMed] [Google Scholar]
- Morgan L, Jessen KR, Mirsky R. The effects of cAMP on differentiation of cultured Schwann cells: progression from an early phenotype (04+) to a myelin phenotype (P0+, GFAP-, N-CAM-, NGF-receptor-) depends on growth inhibition. The Journal of cell biology. 1991;112:457–467. doi: 10.1083/jcb.112.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Miyagoe-Suzuki Y, Ikezoe K, Miyata Y, Nonaka I, Harii K, Takeda S. Schwann cell myelination occurred without basal lamina formation in laminin alpha2 chain-null mutant (dy3K/dy3K) mice. Glia. 2001;35:101–110. doi: 10.1002/glia.1075. [DOI] [PubMed] [Google Scholar]
- Nave KA, Salzer JL. Axonal regulation of myelination by neuregulin 1. Current opinion in neurobiology. 2006;16:492–500. doi: 10.1016/j.conb.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Ness JK, Snyder KM, Tapinos N. Lck tyrosine kinase mediates beta1-integrin signalling to regulate Schwann cell migration and myelination. Nature communications. 2013;4:1912. doi: 10.1038/ncomms2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbern J, Birchmeier C. Nrg1/ErbB signaling networks in Schwann cell development and myelination. Seminars in cell & developmental biology. 2010;21:922–928. doi: 10.1016/j.semcdb.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D’Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. The Journal of cell biology. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, Peles E. N-WASP is required for membrane wrapping and myelination by Schwann cells. The Journal of cell biology. 2011;192:243–250. doi: 10.1083/jcb.201010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Occhi S, Zambroni D, Del Carro U, Amadio S, Sirkowski EE, Scherer SS, Campbell KP, Moore SA, Chen ZL, Strickland S, Di Muzio A, Uncini A, Wrabetz L, Feltri ML. Both laminin and Schwann cell dystroglycan are necessary for proper clustering of sodium channels at nodes of Ranvier. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:9418–9427. doi: 10.1523/JNEUROSCI.2068-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkaynak E, Abello G, Jaegle M, van Berge L, Hamer D, Kegel L, Driegen S, Sagane K, Bermingham JR, Jr, Meijer D. Adam22 is a major neuronal receptor for Lgi4-mediated Schwann cell signaling. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:3857–3864. doi: 10.1523/JNEUROSCI.6287-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Science signaling. 2014;7:ra76. doi: 10.1126/scisignal.2005347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton BL, Wang B, Tarumi YS, Seburn KL, Burgess RW. A single point mutation in the LN domain of LAMA2 causes muscular dystrophy and peripheral amyelination. Journal of cell science. 2008;121:1593–1604. doi: 10.1242/jcs.015354. [DOI] [PubMed] [Google Scholar]
- Pellegatta M, De Arcangelis A, D’Urso A, Nodari A, Zambroni D, Ghidinelli M, Matafora V, Williamson C, Georges-Labouesse E, Kreidberg J, Mayer U, McKee KK, Yurchenco PD, Quattrini A, Wrabetz L, Feltri ML. alpha6beta1 and alpha7beta1 integrins are required in Schwann cells to sort axons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33:17995–18007. doi: 10.1523/JNEUROSCI.3179-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Baumann R, Norrmen C, Somandin C, Miehe M, Jacob C, Luhmann T, Hall-Bozic H, Mantei N, Meijer D, Suter U. Dicer in Schwann cells is required for myelination and axonal integrity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:6763–6775. doi: 10.1523/JNEUROSCI.0801-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, Thurnherr T, Tricaud N, Meijer D, Fassler R, Suter U, Relvas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. The Journal of cell biology. 2009;185:147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen S, Luo R, Ghidineli M, Liebscher I, Giera S, Jeong S, Mogha A, Feltri M, Schöneberg T, Piao X, Monk R. The adhesion G protein-coupled receptor GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with Laminin-211. Neuron. 2015 doi: 10.1016/j.neuron.2014.12.057. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello E, Rivellini C, Dina G, Triolo D, Del Carro U, Ungaro D, Panattoni M, Feltri ML, Wrabetz L, Pardi R, Quattrini A, Previtali SC. Jab1 regulates Schwann cell proliferation and axonal sorting through p27. The Journal of experimental medicine. 2014;211:29–43. doi: 10.1084/jem.20130720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Previtali SC, Nodari A, Taveggia C, Pardini C, Dina G, Villa A, Wrabetz L, Quattrini A, Feltri ML. Expression of laminin receptors in schwann cell differentiation: evidence for distinct roles. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:5520–5530. doi: 10.1523/JNEUROSCI.23-13-05520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano-Roy S, Renault F, Romero N, Guicheney P, Fardeau M, Estournet B. EMG and nerve conduction studies in children with congenital muscular dystrophy. Muscle & nerve. 2004;29:292–299. doi: 10.1002/mus.10544. [DOI] [PubMed] [Google Scholar]
- Raphael AR, Lyons DA, Talbot WS. ErbB signaling has a role in radial sorting independent of Schwann cell number. Glia. 2011;59:1047–1055. doi: 10.1002/glia.21175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasi K, Hurskainen M, Kallio M, Staven S, Sormunen R, Heape AM, Avila RL, Kirschner D, Muona A, Tolonen U, Tanila H, Huhtala P, Soininen R, Pihlajaniemi T. Lack of collagen XV impairs peripheral nerve maturation and, when combined with laminin-411 deficiency, leads to basement membrane abnormalities and sensorimotor dysfunction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:14490–14501. doi: 10.1523/JNEUROSCI.2644-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagane K, Hayakawa K, Kai J, Hirohashi T, Takahashi E, Miyamoto N, Ino M, Oki T, Yamazaki K, Nagasu T. Ataxia and peripheral nerve hypomyelination in ADAM22-deficient mice. BMC Neurosci. 2005;6:33. doi: 10.1186/1471-2202-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito F, Masaki T, Saito Y, Nakamura A, Takeda S, Shimizu T, Toda T, Matsumura K. Defective peripheral nerve myelination and neuromuscular junction formation in fukutin-deficient chimeric mice. Journal of neurochemistry. 2007;101:1712–1722. doi: 10.1111/j.1471-4159.2007.04462.x. [DOI] [PubMed] [Google Scholar]
- Saito F, Moore SA, Barresi R, Henry MD, Messing A, Ross-Barta SE, Cohn RD, Williamson RA, Sluka KA, Sherman DL, Brophy PJ, Schmelzer JD, Low PA, Wrabetz L, Feltri ML, Campbell KP. Unique role of dystroglycan in peripheral nerve myelination, nodal structure, and sodium channel stabilization. Neuron. 2003;38:747–758. doi: 10.1016/s0896-6273(03)00301-5. [DOI] [PubMed] [Google Scholar]
- Samara C, Poirot O, Domenech-Estevez E, Chrast R. Neuronal activity in the hub of extrasynaptic Schwann cell-axon interactions. Frontiers in cellular neuroscience. 2013;7:228. doi: 10.3389/fncel.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen YA, Chen Y, Dao DQ, Mayoral SR, Wu L, Meijer D, Ullian EM, Chan JR, Lu QR. Phosphorylation of LKB1/Par-4 establishes Schwann cell polarity to initiate and control myelin extent. Nature communications. 2014;5:4991. doi: 10.1038/ncomms5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin YK, Jang SY, Park SY, Park JY, Kim JK, Kim JP, Suh DJ, Lee HJ, Park HT. Grb2-associated binder-1 is required for neuregulin-1-induced peripheral nerve myelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:7657–7662. doi: 10.1523/JNEUROSCI.4947-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorer Z, Philpot J, Muntoni F, Sewry C, Dubowitz V. Demyelinating peripheral neuropathy in merosin-deficient congenital muscular dystrophy. Journal of child neurology. 1995;10:472–475. doi: 10.1177/088307389501000610. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- Stevens B, Fields RD. Regulation of the cell cycle in normal and pathological glia. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2002;8:93–97. doi: 10.1177/107385840200800205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B, Ishibashi T, Chen JF, Fields RD. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron glia biology. 2004;1:23–34. doi: 10.1017/s1740925x04000055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling CA. Abnormalities in Schwann cell sheaths in spinal nerve roots of dystrophic mice. Journal of anatomy. 1975;119:169–180. [PMC free article] [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin alpha 2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Human molecular genetics. 1995;4:1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Taveggia C, Zanazzi G, Petrylak A, Yano H, Rosenbluth J, Einheber S, Xu X, Esper RM, Loeb JA, Shrager P, Chao MV, Falls DL, Role L, Salzer JL. Neuregulin-1 type III determines the ensheathment fate of axons. Neuron. 2005;47:681–694. doi: 10.1016/j.neuron.2005.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomassy GS, Berger DR, Chen HH, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyvodic JT. Target size regulates calibre and myelination of sympathetic axons. Nature. 1989;342:430–433. doi: 10.1038/342430a0. [DOI] [PubMed] [Google Scholar]
- Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallquist W, Plantman S, Thams S, Thyboll J, Kortesmaa J, Lannergren J, Domogatskaya A, Ogren SO, Risling M, Hammarberg H, Tryggvason K, Cullheim S. Impeded interaction between Schwann cells and axons in the absence of laminin alpha4. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:3692–3700. doi: 10.1523/JNEUROSCI.5225-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster HD, Martin R, O’Connell MF. The relationships between interphase Schwann cells and axons before myelination: a quantitative electron microscopic study. Developmental biology. 1973;32:401–416. doi: 10.1016/0012-1606(73)90250-9. [DOI] [PubMed] [Google Scholar]
- Weinstein DE, Burrola PG, Lemke G. Premature Schwann cell differentiation and hypermyelination in mice expressing a targeted antagonist of the POU transcription factor SCIP. Molecular and cellular neurosciences. 1995;6:212–229. doi: 10.1006/mcne.1995.1018. [DOI] [PubMed] [Google Scholar]
- Wolpowitz D, Mason TB, Dietrich P, Mendelsohn M, Talmage DA, Role LW. Cysteine-rich domain isoforms of the neuregulin-1 gene are required for maintenance of peripheral synapses. Neuron. 2000;25:79–91. doi: 10.1016/s0896-6273(00)80873-9. [DOI] [PubMed] [Google Scholar]
- Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin alpha 2 (Lama2) gene. Nature genetics. 1994;8:297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- Yang D, Bierman J, Tarumi YS, Zhong YP, Rangwala R, Proctor TM, Miyagoe-Suzuki Y, Takeda S, Miner JH, Sherman LS, Gold BG, Patton BL. Coordinate control of axon defasciculation and myelination by laminin-2 and -8. The Journal of cell biology. 2005;168:655–666. doi: 10.1083/jcb.200411158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun B, Anderegg A, Menichella D, Wrabetz L, Feltri ML, Awatramani R. MicroRNA-deficient Schwann cells display congenital hypomyelination. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:7722–7728. doi: 10.1523/JNEUROSCI.0876-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]