Summary
Hematopoietic stem and progenitor cell (HSPC) specification is regulated by numerous defined factors acting locally within the hemogenic niche, however, it is unclear whether production can adapt to fluctuating systemic needs. Here, we show the central nervous system controls embryonic HSPC production via the hypothalamic-pituitary-adrenal/interrenal (HPA/I) stress response axis. Exposure to serotonin or the reuptake inhibitor fluoxetine increased runx1 expression and Flk1+/cMyb+ HSPCs, independent of peripheral innervation. Inhibition of neuronal, but not peripheral, tryptophan hydroxlyase (Tph) persistently reduced HSPC number. Consistent with central HPA/I axis induction and glucocorticoid receptor (GR) activation, GR agonists enhanced, while GR loss diminished, HSPC formation. Significantly, developmental hypoxia, as indicated by HIF1α function, induced the HPA/I axis and cortisol production; furthermore, HIF1α-stimulated HSPC production was attenuated by neuronal tph or GR loss. Our data establish that embryonic HSC production responds to physiologic stress via CNS-derived serotonin synthesis and central feedback regulation to control HSC numbers.
Introduction
The formation and maintenance of hematopoietic stem and progenitor cells (HSPCs) is a complex process requiring coordination of signals from a variety of cellular inputs and a balance between self-renewal and differentiation. Definitive HSPCs first arise from hemogenic endothelium in the ventral wall of the dorsal aorta (VDA), within the aorta-gonad-mesonephros (AGM) region, via an evolutionarily conserved process termed endothelial-to-hematopoietic transition (EHT) regulated by the transcription factor Runx1. A multitude of molecular and cellular interactions required locally for hemogenic niche specification have been identified (Carroll and North, 2014). Furthermore, HSPC emergence is exquisitely timed to match embryonic growth, with local developmental cues including metabolism, hypoxia, and shear stress acting to initiate EHT (Harris et al., 2013; Imanirad et al., 2014; North et al., 2009). However, in contrast to their initial specification, the mechanisms by which the embryo coordinates the rate of HSPC production and/or expansion to developmental needs (stressors), including continued growth and hematopoietic differentiation, are relatively uncharacterized.
On an organism-wide scale, stress response initiates in the brain: the central nervous system (CNS) acts at the top of this hierarchy to sense stress signals, then communicates through neurotransmitters and neuronal circuitry to respond via neuroendocrine pathways to regulate responses in the periphery. Interestingly, several neuromodulators were identified in our prior in vivo zebrafish chemical screen for evolutionary conserved HSC regulators (North et al., 2007). Adult human CD34+ HSCs express select adrenergic, dopamine, serotonin and GABA receptors (Spiegel et al., 2007; Steidl et al., 2004; Yang et al., 2007); however, the functional requirements of each are generally unknown. Catecholamines [noradrenaline (NA), adrenaline, dopamine] can increase proliferation of CD34+ human umbilical cord blood HSCs in vitro, promote HSC motility within the murine bone marrow (BM) niche, and enhance engraftment in immunodeficient mice (Spiegel et al., 2007). GABA likewise impacts HSC migration (Zangiacomi et al., 2009), while serotonin can influence in vitro colony-formation via direct hormonal stimulation (Yang et al., 2007). Recent studies showed that the sympathetic nervous system (SNS), which innervates peripheral tissues, responds to circadian signals to mobilize adult human and murine HSCs in the BM niche via catecholaminergic signaling (Mendez-Ferrer et al., 2008); local SNS stimulation also impacts embryonic HSPC development in the murine AGM (Fitch et al., 2012). These studies highlight effects of local neuromodulation in the periphery on HSCs, particularly within the BM niche. However, it remains unclear if this influence is only exerted in a locally-restricted paracrine (hormonal) fashion, co-opted in ex vivo expansion protocols, or if HSC production and homeostasis can be impacted via distant neurotransmitter activity; in vivo utilization of central regulation during embryonic HSPC formation in response to developmental cues and/or stressors is likewise uncharacterized.
Serotonin (5-hydroxytryptamine, 5-HT), a biogenic amine that functions as neurotransmitter in the brain and hormone in the periphery, is well known for its role in stress response signaling. Serotonin is synthesized by the rate-limiting enzyme tryptophan hydroxylase (TPH); however, unique among neurotransmitters, there are two distinct isoforms of TPH that compartmentalize their functional contribution in mammals: TPH1 is responsible for peripheral synthesis, while TPH2 produces neuronal serotonin. In the CNS, serotonergic neurons cluster at the raphe nuclei and project to various brain regions to control homeostatic responses including mood, appetite, and stress. Upon stimulation, neuronal-derived serotonin is released into the synaptic cleft and binds to postsynaptic serotonin receptors (5-HTRs) on target cells to mediate downstream responses. There are 7 classes of 5-HTRs most of which are known for central functions, however some subsets exhibit additional physiological roles and expression patterns outside the CNS, including peripheral localization in the gut, cardiovascular system, and blood (Barnes and Sharp, 1999). Stress signals induce presynaptic serotonergic neurons to stimulate downstream neuroendocrine activity through the sympathetic nervous system (SNS) or the hypothalamic-pituitary-adrenal (HPA) axis leading to the release of NA or glucocorticoids (GCs), respectively, to induce peripheral responses. In the SNS, hypothalamic signals stimulate release of noradrenaline from localized sympathetic neurons directly innervating the periphery. In contrast, in the HPA axis, serotonin-stimulated neurons in the hypothalamus release corticotropin-releasing hormone (CRH), which stimulates the pituitary gland to produce and secrete adrenocorticotrophic hormone (ACTH) that then induces GC synthesis in the adrenal gland (interrenal gland in teleosts). GCs then freely circulate in the bloodstream and can bind to glucocorticoid receptors (GR). GR is a nuclear hormone receptor involved in a multitude of molecular mechanisms, including intricate feedback regulation of the HPA axis, to elicit global responses (Keller-Wood, 2015). While HSPC-specific effects of HPA axis dysregulation have not been directly examined, clinical observations imply an important role for GR in blood homeostasis. Patients with Cushing's disease who overexpress GCs develop erythrocytosis (Gursoy et al., 2006), whereas those with Addison's disease, marked by insufficient GC production, have normocytic anemia (Ellis, 2013). Acute or chronic blood loss (“erythroid stress”) also activates GR to promote erythroblast expansion (Bauer et al., 1999). These clinical studies highlight the potential role of the HPA-GR axis as a neuroendocrine regulator of hematopoiesis, allowing systemic stressors sensed in the CNS to be relayed to initiate peripheral responses, including possible impact on HSPCs.
Here, we report that exposure of zebrafish embryos to serotonin or fluoxetine, a selective serotonin reuptake inhibitor (SSRI), increased formation of runx1+ HSPCs in the VDA at 30 hours post fertilization (hpf). Conversely, treatment with a chemical inhibitor of TPH decreased HSPCs. Morpholino (MO)-knockdown of tph1 and tph2 revealed that only tph2, the neuronal isoform, was responsible for sustained effects on HSPC production, which was confirmed via an inducible selective ablation model. Modified epistasis approaches were utilized to support a role for central HPA/I axis activation, while GR-deficient embryos confirmed that neuronal-derived serotonergic signals elicit peripheral responses via the HPA/I axis and GC production. Significantly, Hif1α activation was found to increase expression of tph2 and HPA/I axis-related genes, as well as cortisol production; further, the effects of Hif1α stimulation on HSPC number was partially blocked in embryos deficient in tph2 or GR function. Together, these data indicate hypoxic stress-induced central serotonergic neurotransmitter signals affect distant embryonic HSPC production via the HPA/I axis and local GC/ GR activity.
RESULTS
Serotonin increases the formation of embryonic HSPCs
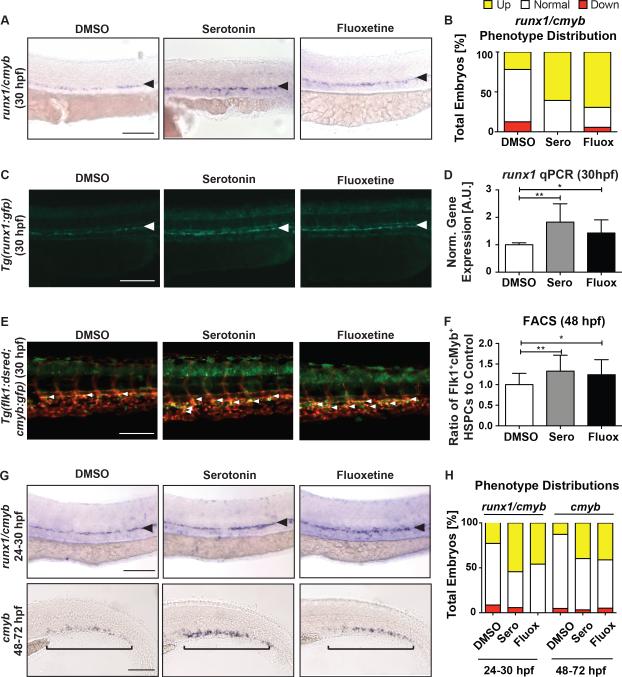
Although prior studies have described local hormonal actions of neuromodulators on adult HSCs, it remains elusive as to whether neurotransmitters acting within the CNS could remotely impact HSPC formation and expansion, particularly during embryonic hematopoiesis. Multiple neuromodulators were identified in a prior screen for regulators of HSC formation in zebrafish (North et al., 2007); serotonin (5-HT) was selected for further analysis based on its distinct spatial separation of neuronal (neurotransmitter) and peripheral (hormone) production and signaling, allowing analysis of the role of central HSPC regulation. Gene expression analysis of FACS-isolated embryonic cell fractions from Tg(flk1:dsred;cmyb:egfp) embryos indicated that the majority of 5-HTRs were not expressed in the vasculature or on HSPCs at 48hpf, however a subset exhibited some peripheral expression (Figure S1A). To confirm the preliminary screen findings, embryos were exposed to serotonin (7μM) from 12-30hpf, during the onset of definitive HSPC formation in zebrafish. Following serotonin incubation, expression of the conserved HSPC markers runx1 and cmyb was enhanced in the VDA (Figure 1A,B) relative to sibling controls as determined by whole-mount in situ hybridization (WISH) at 30hpf and confirmed by quantitative polymerase chain reaction (qPCR) for runx1 (p<0.01) (Figure 1D); exposure to representative peripheral or central 5-HTR agonists, LY344864 (5-HTR1F; 10μM) and MK212 (5-HTR2C; 7.5μM) respectively, also enhanced runx1/cmyb by WISH at 30hpf (Figure S1B,C). Treatment with the SSRI fluoxetine (30μM) elicited a similar effect on runx1 expression by WISH and qPCR (Figure 1A,B,D), implying serotonin production and function is active during HSPC formation. To clarify whether the increase in runx1 WISH expression reflected a change in HSPCs, a transgenic runx1 reporter line, Tg(runx1P1:egfp), was evaluated by fluorescence microscopy: both serotonin and fluoxetine enhanced the appearance of Runx1+ cells in the VDA (Figure 1C). To quantify HSPC number, Tg(flk1:dsred;cmyb:egfp) reporter embryos, in which HSPCs are co-labeled (yellow), were examined: serotonin exposure significantly increased Flk1:dsRed+cMyb:GFP+ HSPCs by fluorescence microscopy and FACS at 48hpf (Figure 1E,F; serotonin: p<0.01, fluoxetine: p<0.05). Notably, neither serotonin nor fluoxetine impacted vascular niche formation (flk1) or specification (ephrinb2a) by WISH (Figure S1D,E), nor were HSPCs induced in aberrant locations (Figure 1A,C,E). Further, serotonin exposure maintained the ability to enhance HSPC gene expression when initiated after hemogenic endothelium specification (>24hpf) (Figure 1G,H). Together, these data indicate elevated serotonin levels are sufficient to stimulate embryonic HSPC production, independently of alterations in hematopoietic niche formation.
Figure 1. Zebrafish Embryos Exposed to Serotonin or Fluoxetine Have Increased HSPC Formation.
(A) WISH for runx1/cmyb in the AGM following exposure to serotonin (7μM) and fluoxetine (30μM) from 12–30hpf.
(B) Qualitative phenotype distribution of embryos (n≥20 embryos/condition, ≥3 replicate experiments) from panel 1A scored with increased (up), normal, and decreased (down) runx1/cmyb in the AGM of control, serotonin-, and fluoxetine-treated embryos. Up=yellow bar; Normal=white bar; Down=red bar.
(C) Representative images of Tg(runx1P1:egfp) embryos demonstrating at 30hpf increased Runx1+ HSPCs with serotonin and fluoxetine treatment (white arrows).
(D) qPCR analysis showing serotonin and fluoxetine significantly increased runx1 gene expression at 30hpf (n≥6 replicates of 30 pooled embryos/condition).
(E) Representative images of Tg(flk1:dsred;cmyb:gfp) embryos showing dual-labelled (yellow) HSPCs (white arrows) at 48hpf.
(F) FACS analysis of (E) (n≥15 replicates of 5 pooled embryos/condition).
(G) HSPC WISH following serotonin and fluoxetine exposure from 24–30hpf (runx1/cmyb) in the AGM and 48–72hpf (cmyb) in the CHT.
(H) Qualitative phenotype distribution of (G), scored as in (B) above.
mean±SD; one-way ANOVA, Holm-Sidak post hoc: *p<0.05, **p<0.01. Scale bars=100μM.
See also Figure S1.
Inhibition of tryptophan hydroxylase 2 decreases embryonic HSPC formation
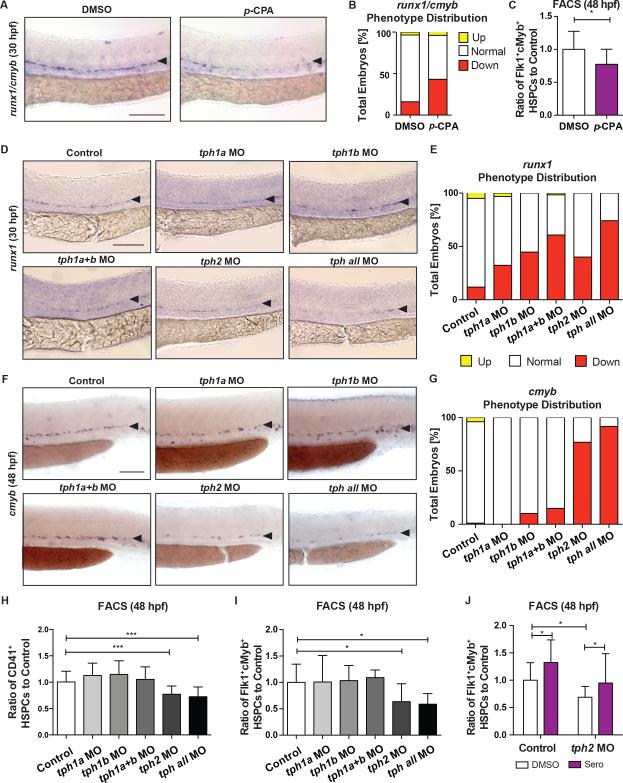
We next sought to determine whether serotonergic signaling was required for embryonic HSPC production and distinguish any differential effects of neuronal- versus peripheral-derived serotonin. TPH, the rate-limiting enzyme for serotonin biosynthesis, was inhibited globally by para-chlorophenylalanine (p-CPA; 50μM), leading to decreased runx1/cmyb expression by WISH at 30hpf (Figure 2A,B) and Flk1:dsRed+cMyb:GFP+ HSPCs by FACS at 48hpf (Figure 2C; p<0.05), with no impact on hematopoietic niche formation (flk1) or specification (ephrinb2a, Figure S2A). Serotonin production in mammals is spatially restricted by the action of distinct isoforms: TPH1 synthesizes peripheral serotonin, while TPH2 controls neuronal synthesis. Zebrafish have two copies of tph1 (tph1a, tph1b), with tph1a maternally deposited, and one copy of tph2, which is actively transcribed beginning at 20hpf (Figure S2B). Expression of tph1a is consistent with the peripheral compartment (epiphysis/pineal gland) and the spinal cord, as previously reported (Thisse, 2004), while tph1b appears ubiquitous by WISH (Figure S2C). In contrast, tph2 expression (Figure S2C) is exclusive to the raphe nuclei where neuronal serotonergic inputs originate (Yokogawa et al., 2012). Upon MO-knockdown of tph1a, tph1b, or tph2 alone or in combination [tph1a+tph1b or tph1a+tph1b+tph2 (tph all)], runx1 expression in the VDA was decreased by WISH at 30hpf (Figure 2D,E), without impact on the hemogenic niche (Figure S2D). For each functional MO (Figure S2E), both addition of the respective mRNA (Figure S2F,G) and exogenous serotonin restored runx1 expression (Figure S2H,I). Surprisingly, only tph2 knockdown (alone or in combination) caused a persistent reduction in HSPCs in the VDA and the caudal hematopoietic tissue (CHT), a secondary site of HSPC expansion, as shown by cmyb WISH at 48hpf (Figure 2F,G) and 72hpf (Figure S3A,B), respectively. The decrease in HSPCs in tph2 morphants was confirmed by FACS for CD41:GFPlo (p<0.001) using the Tg(−6.0itga2b:egfp) line and Flk1:dsRed+cMyb:GFP+ (p<0.05; Figure 2H,I) at 48hpf; further, the effect of tph2-MO was rescued by exogenous serotonin (Figure 2J). Similar findings were observed for peripheral versus central 5-HTR activity using available antagonists: exposure to the 5-HTR1A antagonist WAY100131 (2.5μM) and 5-HTR2C antagonist RS102221 (10μM) each decreased runx1/cmyb expression at 30hpf, however only the central 5-HTR2C antagonist elicited sustained reductions in cmyb at 48hpf (Figure S3C,D). Together, these data suggest neuronal Tph2-regulated serotonin production and signaling has the predominant and sustained effect on embryonic HSPC production in the VDA and CHT.
Figure 2. HSPC Production is Reduced by Chemical and Genetic Inhibition of Tryptophan Hydroxylase.
(A–C) para-Chlorophenylalanine (p-CPA)-treated embryos at 50μM had decreased HSPCs as demonstrated by (A) representative images and (B) qualitative phenotype distribution of runx1/cmyb WISH at 30hpf, and (C) FACS analysis of Flk1:dsRed+cMyb:GFP+ HSPCs at 48hpf (n≥12 replicates; mean±SD; two-tailed t-test: p<0.05).
(D) Representative images of HSPCs in embryos injected with MOs to: tph1a, tph1b, tph1a+b, tph2, and tph all as marked by runx1 WISH at 30hpf.
(E) Qualitative phenotype distribution of (D).
(F) Representative cmyb WISH images at 48hpf for embryos injected with MOs as in (D).
(G) Qualitative phenotype distribution of (F).
(H,I) FACS analysis of (I) CD41:GFP+lo and (J) Flk1:dsRed+cMyb:GFP+ HSPCs at 48hpf showing sustained HSPC deficits in tph2 morphants, but not that of tph1a/1b (n≥6 replicates; mean±SD; one-way ANOVA, Holm-Sidak post hoc: *p<0.05, ***p<0.001).
(J) Serotonin (7μM) rescued Flk1:dsRed+cMyb:GFP+ HSPCs in tph2 morphants at 48hpf by FACS analysis (n≥22 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05).
Scale bars=100μM.
See also Figure S2, S3.
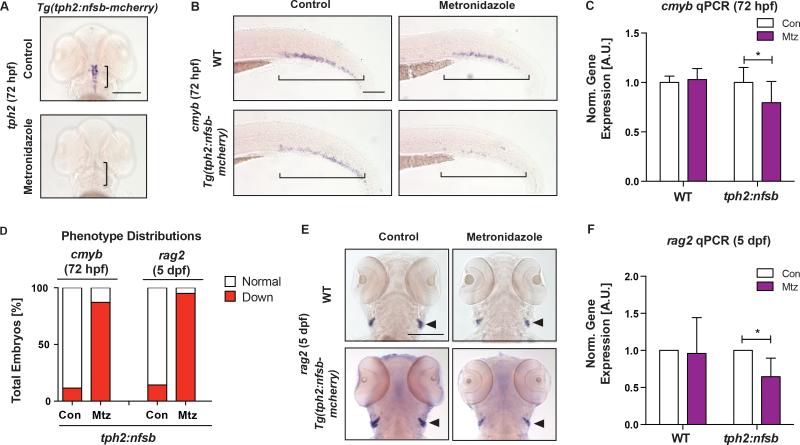
To further confirm that neuronal serotonin synthesis impacted developmental HSPC production, an inducible Tph2 ablation model, Tg(tph2:nfsb-mcherry), which drives expression of nitroreductase under the tph2 promoter specifically in serotonergic neurons was utilized (Yokogawa et al., 2012). Consistent with prior reports, metronidazole (Mtz, 10mM) exposure from 12-72hpf significantly reduced tph2 expression in the raphe nuclei by WISH (Figure 3A). Further, selective serotonergic neuron ablation decreased HSPCs as indicated by cmyb in the CHT by WISH and qPCR at 72hpf (Figure 3B–D; p<0.05). In accordance with a reduction in HSPCs, thymic rag2+ definitive lymphoid progenitors were significantly reduced as shown by WISH and qPCR analysis at 5dpf (120hpf; Figure 3D-F); myeloid cells, marked by myeloperoxidase (mpo), were also decreased, implying neither differentiation block nor bias as causal for the impact on rag2 (Figure S4A-C). Together, these findings confirm that neuronal Tph2-derived serotonin production regulates HSPC numbers during embryonic development.
Figure 3. Selective Ablation of Serotonergic Neurons Decreases HSPCs.
(A) Serotonergic neurons were effectively ablated upon metronidazole (Mtz) treatment (10mM,12–72hpf) in tph2:nfsb-mcherry embryos as marked by tph2 WISH at 72hpf. Scale bar=200μM.
(B) Representative images of cmyb+ HSPCs in the CHT of WT and tph2:nfsb-mcherry fish after treatment as in (A). Scale bar=100μM.
(C) Expression of cmyb was significantly decreased by qPCR at 72hpf in tph2:nfsb-mcherry embryos with Mtz treatment, compared to control (n≥4 replicates).
(D) Qualitative phenotype distribution of cmyb and rag2 expression in tph2:nfsb-mcherry embryos at 72hpf and 5dpf, respectively.
(E) Representative images of rag2+ lymphoid cells in WT and tph2:nfsb-mCherry fish treated from 12hpf-5dpf with Mtz. Scale bar=200μM.
(F) Expression of rag2 was significantly decreased by qPCR in tph2:nfsb-mcherry embryos by Mtz treatment at 5dpf (n≥3 replicates).
mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05.
See also Figure S4.
Serotonin selectively stimulates the HPA/I axis to impact HSPC production
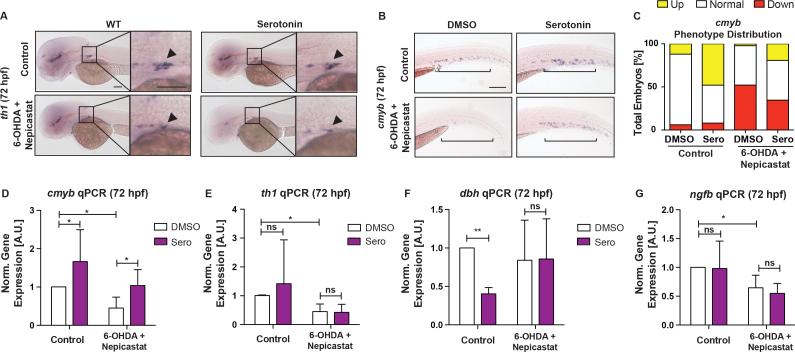
We next investigated how neuronal serotonin modulation impacts local VDA/CHT HSPC production. Serotonergic input in the CNS is relayed to the periphery via the SNS or HPA/I axis. Prior studies showed direct innervation of catecholaminergic neurons at the HSC niche regulate HSC emergence and maintenance (Fitch et al., 2012; Mendez-Ferrer et al., 2008). SNS activity is mediated by catecholamines, such as NA, and synthesized by the rate-limiting enzyme Tyrosine hydroxylase (Th1) and converted from dopamine by Dopamine β hydroxlase (Dbh). To determine if the effect of neuronal serotonin on HSPCs was mediated locally by the SNS, embryos were treated with the neurotoxic compound 6-hydroxydopamine (6-OHDA, 200μM) and DBH inhibitor (nepicastat, 30μM) to induce lesions in catecholaminergic neurons and prohibit NA synthesis, respectively (Feng et al., 2014; Stewart et al., 2004). SNS loss was confirmed by WISH for th1 at the sympathetic ganglion (Figure 4A). Antagonizing SNS function decreased cmyb expression in the CHT at 72hpf by WISH and qPCR (Figure 4B-D; S5A,B; p<0.05); however, the presence of 6-OHDA and nepicastat could not block the ability of serotonin to enhance cmyb expression (Figure 4B-D; S5A,B), nor did treatment in th1 morphant embryos (Figure S5C-E). Analyses of th1, dbh, and nerve growth factor (ngfb), a neurotrophic factor controlling sympathetic neuron maintenance (Stewart et al., 2004), indicated that, in the presence or absence of 6-OHDA/nepicastat, serotonin did not increase SNS gene expression to impact HSCs (Figure 4D-G). In sum, these findings indicate serotonergic stimulation functions independently of local SNS activity to modify HSPC production.
Figure 4. The Effect of Serotonin on HSPCs is Independent of the Sympathetic Nervous System.
(A) Representative images of tyrosine hydroxylase (th1) WISH at 72hpf demonstrating ablation of catecholaminergic neurons at the sympathetic ganglion (boxed) by 6-OHDA (200μM) and nepicastat (30μM) with or without serotonin (7μM).
(B) Representative images of cmyb+ HSPCs in the CHT at 72hpf in embryos treated with serotonin in the absence or presence of 6-OHDA and nepicastat.
(C) Qualitative phenotype distribution of (B).
(D–G) qPCR analysis at 72hpf showing serotonin significantly increased cmyb expression in the absence or presence of 6-OHDA and nepicasatat, while genes within the SNS are unaffected (n≥4 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05, **p<0.01).
Scale bars=100μM.
See also Figure S5.
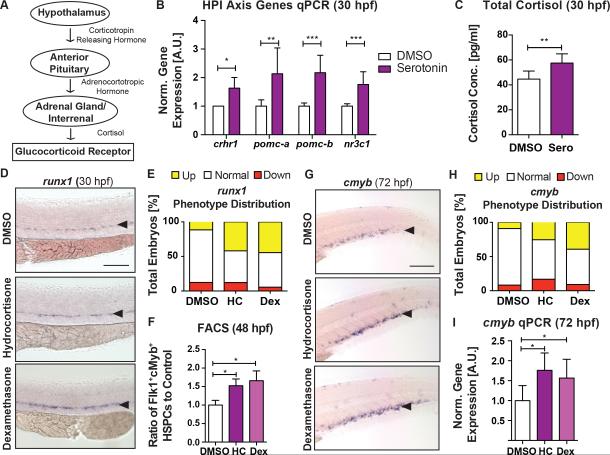
We next investigated the HPA/I axis, a classical stress response pathway that induces peripheral glucocorticoid (GC) production and signaling. Serotonergic stimulation produces a cascade of secreted hormones including CRH from the hypothalamus, ACTH from the anterior pituitary, and cortisol from the adrenal gland (interrenal organ in teleost) (Figure 5A). All components of this HPA/I axis are present in the zebrafish embryo by 20hpf (Nesan and Vijayan, 2013). Expression of nr3c1, the gene encoding glucocorticoid receptor (GR), is widespread in the embryo (Bertrand et al., 2007), including both FACS-sorted Flk1+cMyb− endothelial cells and Flk1+cMyb+ HSPCs (Figure S6A). Exposure to serotonin increased expression of corticotrophin releasing hormone receptor (crhr1), pro-opiomelanocrotin (pomc) a and b (genes encoding ACTH), and nr3c1 at 30hpf by qPCR (Figure 5B); similar observations were seen for fluoxetine, indicating endogenous serotonin is sufficient to activate the HPA/I pathway (Figure S6B). Consistent with HPA/I axis activation, total cortisol levels, as measured by ELISA at 30hpf, were increased in serotonin- and fluoxetine-treated embryos (Figure 5C, Figure S6C). Hypothalamus-associated 5-HTR2C (Schneider et al., 2012), reported to induce the HPA axis (Heisler et al., 2007) and shown here to impact HSPCs (Figure S1B,C, Figure S3C,D) despite an absence in the VDA (Figure S1A), was examined to corroborate the mechanism of serotonergic regulation. Indeed, exposure to 5-HTR2C agonist MK212 increased expression levels of HPA/I axis genes (Figure S6D). The specificity of Tph2-mediated serotonin in HPA/I axis regulation was confirmed in Mtz-treated Tg(tph2:nfsb-mcherry) embryos, where decreased expression of HPA/I axis genes was observed (Figure S6E); importantly, Tph2 ablation did not affect SNS-associated genes (Figure S6F). To investigate whether GR activation downstream of the HPA/I axis could be a functional regulator of HSPCs, zebrafish embryos were exposed directly to GR agonists hydrocortisone (25μM) and dexamethasone (1μM) resulting in significantly increased runx1 expression at 30hpf (Figure 5D,E) and Flk1+cMyb+ HSPCs by FACS at 48hpf (Figure 5F; p<0.05). Consistent with niche specification-independent effects of serotonin, GR agonist exposure from 48-72hpf similarly enhanced cmyb in the CHT at 72hpf, as evaluated by WISH and qPCR (Figure 5G-I; p<0.05). Together, these data suggest that neuronal Tph2-derived serotonin stimulates the HPA/I axis and GR production to impact HSPC development.
Figure 5. Serotonin Activates the Hypothalamic-Pituitary-Adrenal/Interrenal Axis during HSPC Formation.
(A) Schematic diagram of HPA/I axis.
(B,C) Serotonin (7μM) significantly increased HPA/I axis genes (B) by qPCR (n≥3) and total cortisol in whole embryo (C) by ELISA at 30hpf (n≥6 replicates; mean±SD; two-tailed t-test: *p<0.05)
(D) WISH for runx1 in the VDA following treatment with the GR agonists hydrocortisone (HC; 25μM) and dexamethasone (Dex; 1μM) from 12–30hpf.
(E) Qualitative phenotype distribution graph of (D).
(F) FACS analysis showing significant increases in Flk1:dsRed+cMyb:GFP+ HSPCs in hydrocortisone- and dexamethasone-treated embryos at 48hpf (n≥12 replicates; mean±SD; one-way ANOVA, Holm-Sidak post hoc:: *p<0.05).
(G–I) Hydrocortisone and dexamethasone treatment (48–72hpf) increased HSPCs as shown by (G,H) WISH for cmyb in the CHT and (I) qPCR analysis of cmyb gene expression at 72hpf (n≥3 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05).
Scale bars=100μM.
See also Figures S6.
GR activation mediates the effect of serotonin and the HPA/I axis on HSPC numbers
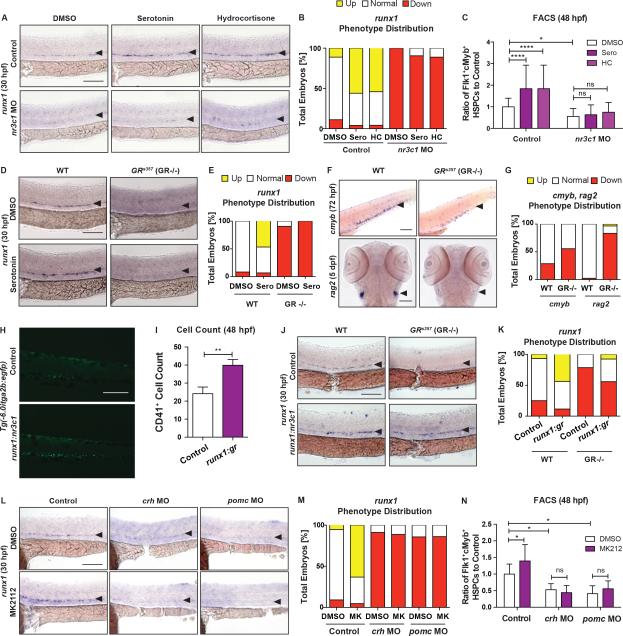
To formally establish the link between serotonin, the HPA/I axis, and cortisol production on HSPC production, modified epistasis approaches were utilized. MO-knockdown of nr3c1 significantly decreased runx1 expression at 30hpf, which could not be restored by exogenous hydrocortisone (Figure 6A-C), whereas the hemogenic niche appeared unaffected (Figure S7A). Serotonin also failed to elevate HSPCs in nr3c1 morphant embryos, by WISH at 30hpf or FACS at 48hpf (Figure 6A-C), indicating it requires downstream GR function. As confirmation, HSPCs were analyzed in nr3c1 mutant (GRs357) embryos that possess a missense mutation blocking the transcriptional activity of GR (Ziv et al., 2013). Mutants exhibited dramatically reduced expression in the presence or absence or serotonin in the VDA at 30hpf (Figure 6D,E), with no effect on the vascular niche (Figure S7B); further, cmyb expression in the CHT and HSC-derived rag2+ lymphoid cells were nearly absent at 72hpf and 5dpf, respectively (Figure 6F,G). To interrogate specificity, selective induction of nr3c1 under the runx1 promoter was examined and found to increase CD41+ HSPCs by cell counts at 48hpf (Figure 6H-I; p<0.01); runx1:nr3c1 expression also enhanced runx1 in the VDA at 30hpf and rescued the nr3c1 mutant phenotype (Figure 6J,K), indicating HSPC-associated GR activation is sufficient to modulate HSPC production. To verify that the HPA/I axis connected serotonin to GR-mediated HSPC regulation, intermediate components of the HPA/I axis were antagonized in epistatic studies: knockdown of crh and pomc (combined pomc-a and pomc-b (Figure S7C) MOs) significantly decreased HSPCs as determined by runx1 WISH at 30hpf and Flk1+cMyb+ FACS at 48hpf (Figure 6L-N; p<0.05). Importantly, exposure to dexamethasone, but not the 5-HT2RC agonist MK212, partially rescued the HPA/I morphant phenotype by WISH and FACS (Figure 6L-N, S7D-F). Collectively, these data confirm that neuronal serotonin synthesis regulates HSPC production via HPA/I axis-mediated GR activation.
Figure 6. Hypothalamic-Pituitary-Interrenal Axis and Glucocorticoid Receptor Activation Are Required for Serotonin Stimulation of HSPCs.
(A) Effect of serotonin (7μM) and hydrocortisone (25μM) treatment on runx1 WISH in control or nr3c1 MO-injected embryos at 30hpf.
(B) Qualitative phenotype distribution graph of (A).
(C) Flk1:dsRed+cMyb:GFP+ HSPCs were significantly increased in serotonin- and hydrocortisone-treated WT embryos, but not in nr3c1 morphants, at 48hpf by FACS (n≥15 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05, ****p<0.0001).
(D) GR mutant (GRs357) embryos had dramatically decreased runx1 expression in the AGM by WISH at 30hpf, which could not be rescued by serotonin (7μM).
(E) Qualitative phenotype distribution graph of (D).
(F) Altered WISH for cmyb+ HSPCs in the CHT at 72hpf and rag2+ lymphoid cells at 5dpf in the thymus in GR mutant embryos compared to controls.
(G) Qualitative phenotype distribution graph of (F).
(H) Representative images of decreased CD41+ cells in runx1:nr3c1-injected Tg(−6.0itga2b:egfp) embryos at 48hpf.
(I) Cell count of (H); n≥8; mean±SD; two-tailed t-test: **p<0.01.
(J) Injection of runx1:nr3c1 in GRs357 embryos rescued runx1+ HSPCs at 30hpf by WISH.
(K) MK212 (7.5μM) could not rescue HSPC defects seen in crh and pomc MO-injected embryos at 30hpf by runx1 WISH.
(L) Qualitative phenotype distribution graph of (J).
(M) FACS analysis of (L) with Flk1:dsRed+cMyb:GFP+ HSPCs at 48hpf by FACS (n≥3 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05).
Scale bars=100μM.
See also Figure S7.
Hypoxic stress associated Hif1α induces tph2 and HPA/I axis function to expand HSPCs
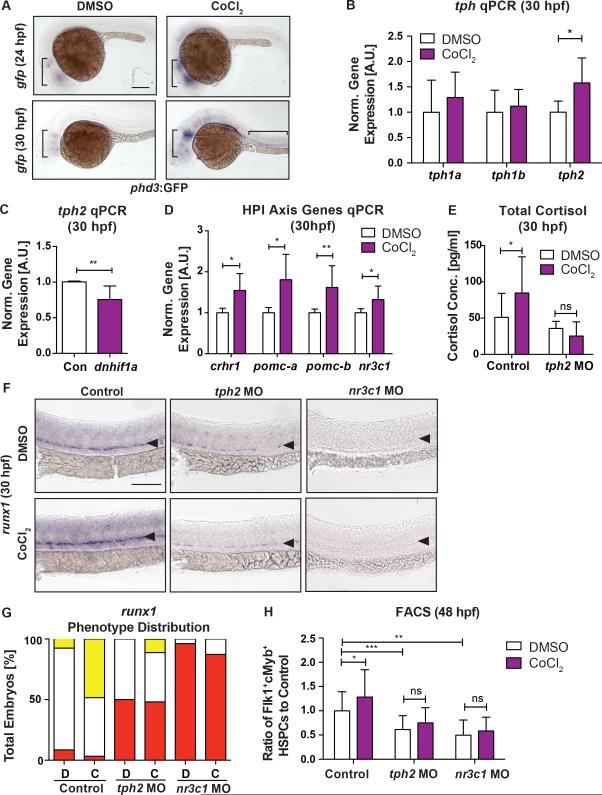
Finally, we aimed to understand the biological signals that initiate this central regulatory effect on embryonic HSPCs. The HPA/I axis is a classic stress response pathway; while behavioral stress does not induce cortisol production until 96hpf in zebrafish embryos (Alsop and Vijayan, 2009), effects of non-behavioral stressors (i.e., hypoxic, metabolic) are not well documented. As Hif1α is necessary for AGM HSPC development (Harris et al., 2013; Imanirad et al., 2014) and HPA/I axis components are expressed by 20hpf (Nesan and Vijayan, 2013), we asked if serotonergic stimulation of the HPA/I-GR axis could be induced by developmental “hypoxic” stress via Hif1α. Tg(phd3:gfp) reporter expression, indicative of Hif1α-mediated response to hypoxic stimuli (Santhakumar et al., 2012), was present predominantly in neural tissues at 24hpf and 30hpf, and further enhanced by cobalt chloride (CoCl2, 500μM), a hypoxia mimetic (Figure 7A). CoCl2 exposure increased tph2 expression, but not tph1a/1b, by qPCR (Figure 7B; p<0.05); conversely, injection of dominant-negative hif1a mRNA significantly decreased tph2 (Figure 7C; p<0.01). Consistent with Tph2 induction by Hif1α, expression of HPA/I axis intermediates and total cortisol was also elevated by CoCl2 (Figure 7D,E), while GC induction was blunted in tph2 morphants (Figure 7E). As reported (Harris et al., 2013), HSPCs were significantly increased with CoCl2 by WISH at 30hpf and FACS at 48hpf (Figure 7F-H; p<0.05); notably, this effect was partially abrogated by tph2 knockdown and impaired in nr3c1 morphants (Figure 7F-H). Collectively, our data indicate development stress cues sensed by Hif1α induce Tph2-mediated (neuronal) serotonin production to stimulate the HPA/I-axis, thereby elevating peripheral cortisol production and allowing local GR-mediated regulation of HSPC production.
Figure 7. Hypoxic Stress Activates Serotonin Production and the HPA/I Axis via Hif1α to control HSPC production.
(A) Hypoxic signals, as marked by gfp WISH in phd3:gfp fish, were present predominantly in the brain at 24hpf and 30hpf, and could be enhanced by CoCl2 (500μM) stimulation.
(B,C) CoCl2 significantly increased tph2 gene expression (B), but not that of tph1a and tph1b, at 30hpf (n≥6 replicates), while dominant negative hif1a (dnhif1α) mRNA (C) expression decreased tph2 expression in zebrafish embryos at 30hpf (n≥6 replicates; mean±SD; two-tailed t-test: *p<0.05, **p<0.01).
(D) Genes of the HPA/I axis were significantly increased at 30hpf by CoCl2 treatment (n≥6 replicates; mean±SD; two-tailed t-test: *p<0.05, **p<0.01).
(E) CoCl2 exposure significantly increased whole embryos total cortisol production at 30hpf in control but not in tph2 MO embryos (Control vs tph2 MO, **p<0.01; n≥4 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05).
(F) The effects of CoCl2 were partially abrogated in tph2 and nr3c1 morphants as marked by runx1 WISH at 30hpf.
(G) Qualitative phenotype distribution graph of (F).
(H) FACS analysis of Flk1:dsRed+cMyb:GFP+ HSPCs in (F) at 48hpf (n≥7 replicates; mean±SD; two-way ANOVA, Holm-Sidak post hoc: *p<0.05, **p<0.01, ***p<0.001).
Scale bars=100μM.
Discussion
Although direct hormonal effects on HSCs have been reported for several neuromodulators (Spiegel et al., 2007; Steidl et al., 2004; Yang et al., 2007), it is unclear if the CNS plays a role in HSC regulation, particularly during embryogenesis. Here, we demonstrate that the CNS exerts global control of HSPC production via developmental stress induced serotonergic stimulation of the HPA/I axis and GR activation. Prior evidence indicates peripheral serotonin can alter erythropoiesis and in vitro colony formation (Amireault et al., 2011; Yang et al., 2007). While we observed that local Tph1-mediated serotonin production and 5HTR activity transiently stimulates HSPC development in the VDA, our data indicate that serotonin produced exclusively in the CNS/serotonergic neurons has a sustained role in actively modulating HSPC number throughout embryogenesis. While not explored in detail here, the transient local effect of serotonin may reflect inherent differences in the inductive (VDA) versus expansive niche (CHT), including spatio-temporal function of upstream regulatory factors; indeed, our data imply that the stress sensor Hif1a only induces tph2 expression, while tph1a is maternally deposited and downregulated significantly by the onset of definitive hematopoiesis. As TPH2 contributes to <5% of the total body serotonin production in adults, revealing it as the predominant physiological mediator of HSPC regulation may be surprising; however, our data consistently indicates that serotonergic signaling modulates HSPC number rather than hemogenic endothelium specification, such that low baseline levels of neuronal production may contribute to its ability to elicit significant stress responses in vivo. In adult mice, circadian signals modulate HSC mobilization via the SNS by direct niche innervation (Mendez-Ferrer et al., 2008); here, we find that Tph2-derived serotonin affects HSPCs independently of catecholaminergic function. Instead, neuronal-derived serotonin induces the HPA/I axis and GR activity to regulate HSPC expansion. It should be noted, that we do not discount the ability of local production and serotonin receptors, such as 5-HTR1F, to mediate direct responses on HSCs; both in prior cell culture studies (Steidl et al., 2004; Yang et al., 2007), and our receptor analysis, peripheral (hormonal) activation enhanced HSPC production. However, in contrast to sustained effects of central inhibition, loss of local 5-HTR signaling had only transient impact on HSPCs, implying compensation for its role in the VDA. It is well appreciated that both the CNS and HPA/I axis function to integrate environmental stimuli to whole organism physiological responses. While contributions of additional cell types were not excluded, we show that runx1-driven GR expression is sufficient to rescue HSPC production in nr3c1 mutants, indicating that GCs can act directly on HSPCs to promote expansion. Thus, we define a mechanism by which local HSPC formation is altered by distal CNS-initiated signals during embryogenesis.
During development, embryonic growth must keep pace with oxygen supply and nutrient availability to ensure viability. HIF1α, an established stress sensor and inducible regulator of erythrocyte and vascular growth, was previously shown to control HSPC development (Harris et al., 2013; Imanirad et al., 2014). Here, we find that developmental hypoxia and HIF1α activity are correlated with tph2 expression and cortisol production. Further, the stimulatory effects of both serotonin and HIF1α activation are blunted in the absence of GR. While epistatic analysis of the conservation of serotonin-HPA-GR activity in stress-responsive HSPC regulation in mammalian embryos was beyond the scope of this study, mice defective in GR transactivation (GRdim/dim) lack increased erythroid production upon hypoxic stress, whereas WT mice had increased colony-forming unit-erythroid (CFU-E), red blood cell (RBC) counts, hemoglobin, and hematocrit (Bauer et al., 1999). Similarly, irradiated mice grafted with GRdim/dim fetal liver cells, which lack GR function exclusively in HSCs, were unresponsive to hypoxic stimuli across various blood parameters (Bauer et al., 1999). Mammalian embryos normally develop in a relatively hypoxic environment, while fish appear transiently hypoxic in localized tissues as they grow (Dunwoodie, 2009). We propose that developmental cues or stressors, such as hypoxia, sensed in the CNS and can impact distant HSPC production via the HPA/I axis and GR to meet the physiological demands of the growing organism.
While not directly investigated here, other stress signals encountered during development, like metabolic stress and inflammation, or additional neural stimuli, may contribute to or act synergistically with serotonin-induced HPA/I-GR activity to impact HSPCs, which could explain the strong GR mutant phenotype seen in our study. Further, loss of GR activity may impact additional cell types and/or physiological pathways that could collaborate with serotonin-induced HPA/I function to control HSPC number. Glucose metabolism, via downstream glycolysis/oxidative stress and HIF1α activity, is essential to HSC formation (Harris et al., 2013). Indeed, the shift in glycolysis and HIF1α activation during embryogenesis coincides with the timing of de novo HSC production (Biggers et al., 1967). GCs increase glucose levels by inhibiting insulin activity (Ferris and Kahn, 2012); therefore, activation of the HPA axis could shift metabolic sources needed for HSC expansion. Inflammatory cytokines stimulate the HPA axis; conversely, GCs inhibit expression of most inflammatory cytokines. While a role for this relationship appears counter-intuitive in regard to our findings, as recent reports show inflammatory cytokines are important for HSPC production (Espin-Palazon et al., 2014; Li et al., 2014), GCs may function to fine-tune a homeostatic balance during this process. Indeed, auto-inhibitory feedback may explain why direct exposure to dexamethasome expands HSPCs in WT embryos, but is insufficient to completely rescue HPA/I pathway morphants. We postulate that the CNS, as the master stress response regulator enables the embryo to detect and react to various signals that remain to be characterized to enable fluctuations in HSPC production; however, via intricate negative feedback mechanisms, such as those well documented for GR and the HPA/I axis (Keller-Wood, 2015), the CNS is positioned to maintain a controlled balance between expanding HSC number and differentiation requirements to maintain viability.
Our results may also provide insight into clinical implications for those with GR and psychiatric disorders if the HPA axis continuously maintains adult HSPC production and homeostasis. Analysis of GR-mediated hematopoietic regulation has focused primarily on the erythrocyte lineage (Bauer et al., 1999); however, GCs can also mobilize circulating polymorphonuclear leukocytes from the bone marrow to inflammatory sites (Nakagawa et al., 1999). Consistent with our developmental observations, humans with GC dysfunction have hematopoietic defects (Masri-Iraqi et al., 2014). Patients with Cushing's disease, who produce excess GCs, are commonly found to exhibit major depression and psychiatric disorders, often caused by neuronal serotonin imbalance (Sonino and Fava, 2001). Interestingly, SSRI use, routinely prescribed to counteract these symptoms, has been occasionally reported to cause hematological side effects (Flanagan and Dunk, 2008; Pai and Kelly, 1996). Extrapolating from our data, it will be imperative to determine if SSRIs, also frequently prescribed for depression to patients undergoing treatment for leukemia, could independently affect HSPCs, including their engraftment, function, and/or relapse potential, via inadvertent HPA-GR axis modulation. The sustained role of CNS-mediated HPA-GR axis HSPC regulation in the adult will be an important area for future prospective investigations both in the context of homeostatic regulation and during therapeutic administration of pathway modifiers.
In sum, our study uncovers an important link between the CNS and the developing hematopoietic system. We demonstrate that during embryogenesis, in response to developmental stressors such as hypoxia, central Tph2-mediated serotonin synthesis is induced to initiate HPA/I axis function and systemic GC production resulting in GR-mediated stimulation of distal HSPC production. This mechanism provides an important means by which the developing embryo can coordinate the response to environmental and/or physiological inputs to maintain health and viability, in this case utilizing the CNS to control HSPC production across developmental niches via stress-inducible signaling networks such as the HPA axis.
Experimental Procedures
Zebrafish Husbandry
Zebrafish were maintained according to Beth Israel Deaconess Medical Center IACUC protocols. Lines utilized were each previously described and are listed in Supplemental Experimental Procedures.
Chemical Treatments and Evaluation
Zebrafish embryos were exposed to select chemical regulators (dissolved in DMSO) from 5 somites to 30hpf in multiwell plates of E3 media, unless otherwise specified. The following compounds were utilized: R&D Systems (Minneapolis MN)- serotonin (7μM), fluoxetine (30μM), p-CPA (50μM), 6-OHDA (200μM), MK-212 (7.5μM), RS-102221 (10μM), dexamethasone (1μM); Sigma Aldrich (St. Louis MO)- Mtz (10mM), nepicastat (30μM), hydrocortisone (25μM), CoCl2 (500μM). WISH was performed as previously described (North et al., 2007). Qualitative phenotypes (≥20 embryos/condition × ≥3 replicate clutches, unless otherwise indicated) were evaluated as relatively high (up) /medium (normal) /low (down) in expression compared to sibling controls at 30hpf (unless otherwise specified) and graphically depicted as the % falling into each of 3 phenotypic expression bins; “normal” expression was the most representative qualitative phenotype in the bell-curve distribution of each control cohort per experiment.
Morpholino, mRNA, and Plasmid Injections
MO (2-5nl; GeneTools (Philomath OR)), mRNA, or plasmid was microinjected at the 1-cell stage as described (North et al., 2007). At the time point of interest, embryos were processed with matched controls for evaluation. MO sequences are detailed in Supplemental Experimental Procedures. dnhif1 mRNA was prepared as described (Harris et al., 2013). Cloning strategies and mRNA information are in Supplemental Experimental Procedures.
RNA Extraction and Quantitative Reverse Transcriptase Polymerase Chain Reaction
Embryos (n=30/condition × ≥3 replicates) collected at specified time points were homogenized in RNA extraction buffer by electric pestle. RNA was extracted using RNAqueous Total RNA Isolation Kit based on manufacturer protocols, samples were treated with DNaseI using Turbo DNA-free kit and 1μg of RNA was used to generate cDNA using Reverse Transcriptase Supermix (all kits: Life Technologies, Rockville MD). qPCR was performed using CFX Real-Time PCR Detection System (Biorad, Hercules CA). Ct values were determined by PCR Miner online software (Zhao and Fernald, 2005) and fold-change calculated by the R0 method with 18S as the reference. Primers sequences are listed in Supplemental Experimental Procedures.
Fluorescence Activated Cell Sorting
FACS analysis was performed using Tg(flk1:dsRed;cmyb:GFP) or Tg(−6.0itga2b:egfp) HSPC reporter embryos as previously described. Briefly, embryos (5 pooled embryo per sample × ≥3 replicates) were dissociated, resuspended in 1×PBS, and analyzed by BD FACSCanto II (BD Biosciences, San Jose CA) in the presence of SYTOX Red Dead Cell Stain (5nM; Life Technologies, Waltham MA). Data was analyzed using FlowJo X software (TreeStar, Ashland OR), and expressed as ratio of control. For Tg(−6.0itga2b:egfp) embryos, CD41lo cells were gated. For isolation of endothelial (Flk1:dsRed+cMyb:GFP−) and HSPC (Flk1:dsRed+cMyb:GFP+) fractions, pooled embryos (n>1000) were sorted at 48hpf by FACSAria (BD Biosciences, San Jose CA) with RNA isolation as above.
Cortisol Measurements
Embryos (n=30) were collected at 30hpf and homogenized in 100μL of ultrapure water by electric pestle. Ethyl acetate (500μl; Sigma Aldrich, St. Louis MO) was added to the lysate, centrifuged, and the insoluble fraction discarded. The soluble fraction was decanted, dehydrated, and re-suspended in 110μl EIA buffer; total cortisol was measured using a Cortisol EIA kit (Cayman Chemical, Ann Arbor MI) according to manufacturer's instructions.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism Software (GraphPad, La Jolla CA). Two-tailed Student's t-tests were used for pairwise comparisons and ANOVA for group analyses, with Holm-Sidak post hoc tests. Data are presented as mean±SD. P values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
We thank the BIDMC Flow Cytometry Core and Children's Hospital Genomics Core Facility for assistance. We thank K. Alexa for providing 5-HTR drugs. We thank H. Burgess (NIH), H. Baier (Max Planck Martinsried), F. van Eeden (University of Sheffield), and D. Traver (UCSD) for transgenic lines. We thank R. George (Dana Farber), B. Thisse (University of Virginia), and C. Burns (Harvard Medical School) for in situ probes. We thank T. Tsai, S.G. Megason, B. Paw, R. Karlstrom, I. Drummond, D. Langenau, and D. Robson for advice. This study was supported by a CIHR Postdoctoral Fellowship (WK) and NIH 1R01DK098241 (TEN).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
WK designed and led all experiments. MC, IF, VE, LT and SYL aided FACS, injections and WISH. MC, VE performed cloning. IF did RT-PCR and cell counts. NB assisted with zebrafish husbandry. WG provided guidance. TEN and WK analyzed data and wrote the manuscript.
References
- Alsop D, Vijayan MM. Molecular programming of the corticosteroid stress axis during zebrafish development. Comparative biochemistry and physiology Part A, Molecular & integrative physiology. 2009;153:49–54. doi: 10.1016/j.cbpa.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Amireault P, Hatia S, Bayard E, Bernex F, Collet C, Callebert J, Launay JM, Hermine O, Schneider E, Mallet J, et al. Ineffective erythropoiesis with reduced red blood cell survival in serotonin-deficient mice. Proc Natl Acad Sci U S A. 2011;108:13141–13146. doi: 10.1073/pnas.1103964108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bauer A, Tronche F, Wessely O, Kellendonk C, Reichardt HM, Steinlein P, Schutz G, Beug H. The glucocorticoid receptor is required for stress erythropoiesis. Genes & development. 1999;13:2996–3002. doi: 10.1101/gad.13.22.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand S, Thisse B, Tavares R, Sachs L, Chaumot A, Bardet PL, Escriva H, Duffraisse M, Marchand O, Safi R, et al. Unexpected novel relational links uncovered by extensive developmental profiling of nuclear receptor expression. PLoS genetics. 2007;3:e188. doi: 10.1371/journal.pgen.0030188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KJ, North TE. Oceans of opportunity: exploring vertebrate hematopoiesis in zebrafish. Experimental hematology. 2014;42:684–696. doi: 10.1016/j.exphem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwoodie SL. The role of hypoxia in development of the Mammalian embryo. Developmental cell. 2009;17:755–773. doi: 10.1016/j.devcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Ellis H. Thomas Addison: Addisonian (pernicious) anaemia, Addison's disease of the suprarenal gland. Journal of perioperative practice. 2013;23:31–32. doi: 10.1177/1750458913023001-205. [DOI] [PubMed] [Google Scholar]
- Espin-Palazon R, Stachura DL, Campbell CA, Garcia-Moreno D, Del Cid N, Kim AD, Candel S, Meseguer J, Mulero V, Traver D. Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell. 2014;159:1070–1085. doi: 10.1016/j.cell.2014.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng CW, Wen ZH, Huang SY, Hung HC, Chen CH, Yang SN, Chen NF, Wang HM, Hsiao CD, Chen WF. Effects of 6-hydroxydopamine exposure on motor activity and biochemical expression in zebrafish (Danio rerio) larvae. Zebrafish. 2014;11:227–239. doi: 10.1089/zeb.2013.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris HA, Kahn CR. New mechanisms of glucocorticoid-induced insulin resistance: make no bones about it. J Clin Invest. 2012;122:3854–3857. doi: 10.1172/JCI66180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch SR, Kimber GM, Wilson NK, Parker A, Mirshekar-Syahkal B, Gottgens B, Medvinsky A, Dzierzak E, Ottersbach K. Signaling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell. 2012;11:554–566. doi: 10.1016/j.stem.2012.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan RJ, Dunk L. Haematological toxicity of drugs used in psychiatry. Human psychopharmacology. 2008;23(Suppl 1):27–41. doi: 10.1002/hup.917. [DOI] [PubMed] [Google Scholar]
- Gursoy A, Dogruk Unal A, Ayturk S, Karakus S, Nur Izol A, Bascil Tutuncu N, Guvener Demirag N. Polycythemia as the first manifestation of Cushing's disease. Journal of endocrinological investigation. 2006;29:742–744. doi: 10.1007/BF03344186. [DOI] [PubMed] [Google Scholar]
- Harris JM, Esain V, Frechette GM, Harris LJ, Cox AG, Cortes M, Garnaas MK, Carroll KJ, Cutting CC, Khan T, et al. Glucose metabolism impacts the spatiotemporal onset and magnitude of HSC induction in vivo. Blood. 2013;121:2483–2493. doi: 10.1182/blood-2012-12-471201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O'Rahilly S, Colmers WF, Elmquist JK, et al. Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci. 2007;27:6956–6964. doi: 10.1523/JNEUROSCI.2584-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imanirad P, Solaimani Kartalaei P, Crisan M, Vink C, Yamada-Inagawa T, de Pater E, Kurek D, Kaimakis P, van der Linden R, Speck N, et al. HIF1alpha is a regulator of hematopoietic progenitor and stem cell development in hypoxic sites of the mouse embryo. Stem cell research. 2014;12:24–35. doi: 10.1016/j.scr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Wood M. Hypothalamic-Pituitary--Adrenal Axis-Feedback Control. Comprehensive Physiology. 2015;5:1161–1182. doi: 10.1002/cphy.c140065. [DOI] [PubMed] [Google Scholar]
- Li Y, Esain V, Teng L, Xu J, Kwan W, Frost IM, Yzaguirre AD, Cai X, Cortes M, Maijenburg MW, et al. Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes & development. 2014;28:2597–2612. doi: 10.1101/gad.253302.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri-Iraqi H, Robenshtok E, Tzvetov G, Manistersky Y, Shimon I. Elevated white blood cell counts in Cushing's disease: association with hypercortisolism. Pituitary. 2014;17:436–440. doi: 10.1007/s11102-013-0522-0. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Nakagawa M, Bondy GP, Waisman D, Minshall D, Hogg JC, van Eeden SF. The effect of glucocorticoids on the expression of L-selectin on polymorphonuclear leukocyte. Blood. 1999;93:2730–2737. [PubMed] [Google Scholar]
- Nesan D, Vijayan MM. Role of glucocorticoid in developmental programming: evidence from zebrafish. General and comparative endocrinology. 2013;181:35–44. doi: 10.1016/j.ygcen.2012.10.006. [DOI] [PubMed] [Google Scholar]
- North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, et al. Hematopoietic stem cell development is dependent on blood flow. Cell. 2009;137:736–748. doi: 10.1016/j.cell.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang IH, Grosser T, et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature. 2007;447:1007–1011. doi: 10.1038/nature05883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VB, Kelly MW. Bruising associated with the use of fluoxetine. The Annals of pharmacotherapy. 1996;30:786–788. doi: 10.1177/106002809603000716. [DOI] [PubMed] [Google Scholar]
- Santhakumar K, Judson EC, Elks PM, McKee S, Elworthy S, van Rooijen E, Walmsley SS, Renshaw SA, Cross SS, van Eeden FJ. A zebrafish model to study and therapeutically manipulate hypoxia signaling in tumorigenesis. Cancer research. 2012;72:4017–4027. doi: 10.1158/0008-5472.CAN-11-3148. [DOI] [PubMed] [Google Scholar]
- Schneider H, Fritzky L, Williams J, Heumann C, Yochum M, Pattar K, Noppert G, Mock V, Hawley E. Cloning and expression of a zebrafish 5-HT(2C) receptor gene. Gene. 2012;502:108–117. doi: 10.1016/j.gene.2012.03.070. [DOI] [PubMed] [Google Scholar]
- Sonino N, Fava GA. Psychiatric disorders associated with Cushing's syndrome. Epidemiology, pathophysiology and treatment. CNS drugs. 2001;15:361–373. doi: 10.2165/00023210-200115050-00003. [DOI] [PubMed] [Google Scholar]
- Spiegel A, Shivtiel S, Kalinkovich A, Ludin A, Netzer N, Goichberg P, Azaria Y, Resnick I, Hardan I, Ben-Hur H, et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol. 2007;8:1123–1131. doi: 10.1038/ni1509. [DOI] [PubMed] [Google Scholar]
- Steidl U, Bork S, Schaub S, Selbach O, Seres J, Aivado M, Schroeder T, Rohr UP, Fenk R, Kliszewski S, et al. Primary human CD34+ hematopoietic stem and progenitor cells express functionally active receptors of neuromediators. Blood. 2004;104:81–88. doi: 10.1182/blood-2004-01-0373. [DOI] [PubMed] [Google Scholar]
- Stewart RA, Look AT, Kanki JP, Henion PD. Development of the peripheral sympathetic nervous system in zebrafish. Methods in cell biology. 2004;76:237–260. doi: 10.1016/s0091-679x(04)76012-4. [DOI] [PubMed] [Google Scholar]
- Thisse B, Thisse C. Fast Release Clones: A High Throughput Expression Analysis. 2004 (Zfin Direct Data Submission ( http://zfin.org))
- Yang M, Li K, Ng PC, Chuen CK, Lau TK, Cheng YS, Liu YS, Li CK, Yuen PM, James AE, et al. Promoting effects of serotonin on hematopoiesis: ex vivo expansion of cord blood CD34+ stem/progenitor cells, proliferation of bone marrow stromal cells, and antiapoptosis. Stem Cells. 2007;25:1800–1806. doi: 10.1634/stemcells.2007-0048. [DOI] [PubMed] [Google Scholar]
- Yokogawa T, Hannan MC, Burgess HA. The dorsal raphe modulates sensory responsiveness during arousal in zebrafish. J Neurosci. 2012;32:15205–15215. doi: 10.1523/JNEUROSCI.1019-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangiacomi V, Balon N, Maddens S, Tiberghien P, Versaux-Botteri C, Deschaseaux F. Human cord blood-derived hematopoietic and neural-like stem/progenitor cells are attracted by the neurotransmitter GABA. Stem cells and development. 2009;18:1369–1378. doi: 10.1089/scd.2008.0367. [DOI] [PubMed] [Google Scholar]
- Zhao S, Fernald RD. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of computational biology : a journal of computational molecular cell biology. 2005;12:1047–1064. doi: 10.1089/cmb.2005.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv L, Muto A, Schoonheim PJ, Meijsing SH, Strasser D, Ingraham HA, Schaaf MJ, Yamamoto KR, Baier H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Molecular psychiatry. 2013;18:681–691. doi: 10.1038/mp.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.