Abstract
Background and aims
Circulating lipoprotein (a) [Lp(a)] level relates inversely to apolipoprotein (a) [apo(a)] size. Both smaller apo(a) isoforms and higher Lp(a) levels have been linked to coronary heart disease and stroke, but their independent contributions are less well defined. We examined the role of Lp(a) in younger adults with cryptogenic stroke.
Methods
Lp(a) and apo(a) isoforms were evaluated in a prospectively designed case-control study of patients with unexplained ischemic stroke and stroke-free controls, ages 18 to 64. Serum Lp(a) was measured among 255 cases and 390 controls with both apo(a)-size independent and dependent assays. Apo(a) size was determined by agarose gel electrophoresis.
Results
Cases and controls were similar in socio-demographic characteristics, but cases had more hypertension, diabetes, smoking, and migraine with aura. In race-specific analyses, Lp(a) levels showed positive associations with cryptogenic stroke in whites, but not in the smaller subgroups of blacks and Hispanics. After full adjustment, comparison of the highest versus lowest quartile in whites was significant for apo(a)-size-independent (OR=2.10 [95% CI=1.04, 4.27], p=0.04), and near-significant for apo(a)-size-dependent Lp(a) (OR=1.81 [95% CI=0.95, 3.47], p=0.073). Apo(a) size was not associated with cryptogenic stroke in any race-ethnic subgroup.
Conclusions
This study underscores the importance of Lp(a) level, but not apo(a) size, as an independent risk factor for unexplained ischemic stroke in young and middle-aged white adults. Given the emergence of effective Lp(a)-lowering therapies, these findings support routine testing for Lp(a) in this setting, along with further research to assess the extent to which such therapies improve outcomes in this population.
Keywords: Lipoprotein (a), Stroke, Case control study
Introduction
Approximately one-quarter of ischemic strokes lack a clear etiology.1 Such “cryptogenic” strokes make up an even larger proportion of ischemic strokes in younger adults, as many as 40% of which may be of indeterminate cause.2 Defining risk factors and pathogenic determinants of cryptogenic stroke has been identified as a leading research priority.2
Lipoprotein (a), formed by the attachment of low-density lipoprotein to apolipoprotein(a) [apo(a)] by a single disulfide bond, is a circulating particle of uncertain physiologic function that exhibits atherogenic and pro-thrombotic/anti-fibrinolytic properties in experimental settings.3 Plasma levels of lipoprotein (a) [Lp(a)] have been linked to coronary heart disease (CHD) and stroke in meta-analyses,4–6 with additional studies providing evidence that, in the case of CHD, the association has a causal basis.7, 8 Although the precise mechanisms by which Lp(a) heightens CVD risk remain incompletely defined, recent studies involving Lp(a)-raising genetic variants have documented associations with atherosclerotic CVD, but not with venous thrombosis.9, 10
Circulating levels of Lp(a) are highly heterogeneous, varying widely among individuals and ethnicities.11, 12 This heterogeneity is largely dependent on apo(a) isoform size, which is determined by copy number variation in the kringle IV type 2 (KIV-2) motif and bears a strong inverse relationship with plasma concentration of Lp(a).13 Because immunoassays that recognize KIV-2 epitopes preferentially detect larger apo(a) isoforms over smaller ones, assays targeting other apo(a) epitopes are recommended for more accurate quantification,14 although isoform-dependent assays remain in clinical use.15 Besides Lp(a) concentration, small apo(a) isoform size has been associated with CHD and ischemic stroke by a meta-analysis.16 It has been suggested that small apo(a) sizes may impart a greater risk of CVD independent of Lp(a) level,17 but existing evidence from studies examining circulating Lp(a) concentration and apo(a) isoform size remains sparse, particularly in the case of ischemic stroke.
Pooled analysis of studies that have examined ischemic stroke subtypes has documented a significant association of Lp(a) level with large-artery atherosclerosis, but not cardioembolic or small-artery stroke.6 Data on cryptogenic stroke are scant, however, and the association between Lp(a) level or apo(a) isoform size with this stroke subtype remains poorly defined. Nor has the relative performance of molecular weight dependent and independent Lp(a) assays been previously investigated. We sought to address these gaps in a case-control study of systematically evaluated cryptogenic stroke cases and stroke-free controls, the THrombophilia In Cryptogenic stroKe (THICK) Study.
Materials and methods
Study participants
THICK is a prospectively designed case-control study whose objective was to investigate the role of prothrombotic disorders as risk factors for cryptogenic stroke in young and middle-aged adults. Cases comprised patients 18 to 64 years old with first-ever ischemic stroke having no evident cause on evaluation at Weill Cornell Medical Center (WCMC) between 2002 and 2012. Controls included stroke-free volunteers ages 18 to 64 recruited from the medical center and surrounding area through advertisement and word of mouth during this period.
Ischemic stroke was defined by rapid onset of a focal neurological deficit of presumed vascular origin and lasting ≥24 hours or with neuroimaging evidence of cerebral infarction in the corresponding vascular territory. “Cryptogenic” stroke was defined by modification of the Trial of Org 10172 in Acute Stroke Treatment criteria,18 wherein competing stroke etiologies or incomplete diagnostic evaluation were excluded. In addition, the presence of any high or medium-risk source of cardioembolism was grounds for exclusion, with the exception of mitral valve prolapse, patent foramen ovale or atrial septal aneurysm, a hypokinetic left ventricular segment with global left ventricular ejection fraction >35%, mild mitral annular calcification, and myocardial infarction occurring >3 months earlier.
Exclusion criteria were designed to satisfy human subjects’ protections, to remove from consideration disorders known to predispose to stroke, and to achieve comparability of cases and controls. These included incapacity to provide informed consent, anticipated survival <6 months, venous thromboembolism (VTE) in the previous 6 months (excepting a new diagnosis of VTE concurrent with the index stroke), recent acute coronary syndrome (<3 months), chronic liver disease (>3-fold transaminase elevation), chronic kidney disease (serum creatinine ≥152.5 μmol/L), pregnancy or <3 months post-partum, neoplasia not in remission ≥5 years, collagen vascular disease or other chronic inflammatory disorder, HIV infection, and major trauma or surgery in the past 6 months.
Study protocol
Cases received neuroimaging and evaluation of the cervicocranial vasculature at the discretion of their primary neurologist. Vascular imaging with duplex sonography, magnetic resonance angiography, computed tomography angiography or conventional angiography to exclude a large-vessel etiology was required for study inclusion. For cases, transesophageal echocardiography was also a requirement to exclude a definite or probable cardiac source of embolism. Duplex sonography of the leg veins was encouraged following detection of an interatrial shunt. Clinical laboratory evaluation and ambulatory ECG monitoring were at the discretion of the primary physician.
All participants received a standardized interview for collection of information on demographic factors and medical history, supplemented by chart review in cases. Both cases and controls underwent transthoracic echocardiography with agitated-saline contrast based on a standardized protocol. Cases and controls also received standard laboratory testing, including a comprehensive metabolic panel, complete blood count, prothrombin and activated partial thromboplastin time, lipid profile, C-reactive protein (CRP) and a standard panel of tests for prothrombotic factors, including Lp(a). Such testing was performed ≥2 months after the index stroke in cases, or on the day of the study visit for controls. Participants were instructed to fast for ≥8 hours prior to specimen collection.
Standard clinical care calls for discontinuation of contraceptive steroids after stroke. Female cases were therefore uniformly off such therapy during their return for convalescent blood testing. This did not apply to female controls taking such medications, who were not asked to discontinue these before laboratory evaluation. Because estrogen therapy lowers Lp(a),19 we excluded female controls on contraceptive steroids (n=37) to limit potential bias.
The THICK study was approved by the institutional review board at WCMC All participants provided informed consent.
Definitions
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Hypertension was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg (at the time of the convalescent visit for cases), or use of anti-hypertensive medications or self-reported hypertension. Diabetes was defined as non-fasting blood glucose ≥11 mmol/L), fasting blood glucose ≥7 mmol/L or hemoglobin A1c ≥6.5%, or by antidiabetic treatment or self-reported diabetes. Dyslipidemia was defined as LDL≥4.1 mmol/L, or HDL<1 mmol/L in men or <1.3 mmol/L in women, or total cholesterol/HDL≥4.0, or previous use of lipid-lowering medication. Estimated glomerular filtration rate (eGFR) was calculated using a validated equation.20 Family history of CVD included premature CHD, stroke or VTE (first-degree male relative <55 or female relative <65). Migraine with aura was defined by published criteria.21
Measurement of Lp(a) and apo(a) isoforms
Serum specimens were sent to the WCMC Clinical Laboratory for determination of Lp(a) concentrations using a commercially available immunoturbidimetric assay (DiaSorin, Inc, Stillwater, MN; formerly, INCSTAR). This assay, which is sensitive to apo(a) size (molecular-weight dependent), has intra-assay and inter-assay CVs of 2.3–5.7% and 1.1–7.5%, respectively.22 Because this assay was discontinued after August 2011, only participants with measurements to this point (n=626) are included in the present analyses.
Determination of Lp(a) levels using a molecular-weight independent assay, and of apo(a) isoform size, was performed in plasma specimens stored at −70C following collection. Based on different funding sources, only specimens collected through January 2010 (n=568) underwent molecular-weight independent measurement of Lp(a), while those collected through November 2011 (n=596) had determination of apo(a) isoform size. Measurement of Lp(a) levels was done at the University of Pennsylvania with a commercially available immunoturbidimetric assay insensitive to apo(a) isoform size (Denka Seiken Co., Ltd., Tokyo, Japan)23 having an intra-assay CV of 1.3–2.2%. Apo(a) isoform size was determined by electrophoresis in 1.5% SDS agarose gels followed by immunoblotting and use of chemoluminescence for visualization, according to previously described methods.24 The results were related to standards (Immuno-France, SARL, Rungis, France) with known apo(a) isoform sizes. The size of the dominant apo(a) isoform, expressed as the number of KIV-2 repeats, was used in the analysis. These measurements were performed at the University of Vermont.
Statistical analysis
Baseline characteristics, levels of Lp(a), and apo(a) isoform size were summarized using standard descriptive statistics by case/control status. Values were compared using the Student t test or Wilcoxon rank-sum test for continuous variables and chi-square test for categorical variables, as appropriate. Pairwise correlations between molecular-weight dependent and independent Lp(a) levels, and apo(a) isoform size, were computed using Spearman coefficients. Because Lp(a) levels and apo(a) size distributions are strongly dependent on race-ethnicity, analyses relating these exposure measures to cryptogenic stroke were stratified by the three major race-ethnic groups, non-Hispanic whites, non-Hispanic blacks, and Hispanics. Small numbers of participants of Asian or other race-ethnicities precluded evaluation of these subgroups. Multivariable logistic regression was used to assess the cross-sectional relationship of molecular-weight dependent Lp(a), molecular-weight independent Lp(a), and apo(a) isoform size with cryptogenic stroke. Continuous associations were assessed after logarithmically transforming Lp(a), whose distribution was positively skewed, reporting the odds ratio (OR) per SD increment (race-ethnicity specific) of the natural logarithm of Lp(a). For apo(a) isoforms, a commonly used cutpoint of 22 KIV-2 repeats was used to dichotomize this measure. In addition, for the white subgroup, analyses were undertaken to assess the relationship by quartile of molecular-weight dependent or independent Lp(a) levels or apo(a) size with cryptogenic stroke. Subjects with KIV-2 <15 were excluded from the apo(a) isoform analyses owing to small numbers and the documented difficulty in detecting isoforms of this size.11 An initial model adjusted for age and sex, while a more fully adjusted model also included hypertension, diabetes, dyslipidemia, current smoker, migraine with aura, family history of premature CVD and CRP. The impact of mutual adjustment of Lp(a) level by either the molecular-weight dependent or independent assay and apo(a) isoform size was also examined. Assessment for interaction by age, sex, and interatrial shunt was performed by including cross-product terms in the demographics-adjusted model for each major race-ethnic group. All analyses were performed with SAS, v 9.4 (Cary, NC). A two-tailed p value <0.05 was considered statistically significant.
Results
Characteristics of cases and controls included in the analytic sample are presented in Table 1. Controls were similar to cases in demographic and several clinical characteristics, but cases more frequently had hypertension, diabetes, migraine with aura, current smoking, and interatrial shunting detected during evaluation. Overall, measurements of serum Lp(a) concentration by the molecular-weight-dependent and independent assays were highly correlated (r=0.93, p<0.001). Serum Lp(a) level, whether measured by the molecular-weight dependent or independent assay, was highest in African Americans and lowest in whites, with Hispanics falling in between (p≤0.014 for blacks or Hispanics vs. whites). Lp(a) level by either assay was or tended to be higher in white cases versus controls, but no differences in levels were observed for blacks or Hispanics. Whites had smaller apo(a) size than blacks or Hispanics (p≤0.009), but there were no differences in apo(a) size between cases and controls in any of the race-ethnic subgroups.
Table 1.
Characteristicsa of participants.
| Characteristics | Cases (n=255) | Controls (n=390) | p value |
|---|---|---|---|
| Demographic and clinical | |||
| Age, years | 46(38, 53) | 44 (35, 53) | 0.199 |
| Women, n (%) | 100(39.2) | 180 (46.1) | 0.082 |
| Race-ethnicity, n (%) | 0.797 | ||
| White | 154(60.3) | 244(62.5) | |
| Black | 33(12.9) | 45(11.5) | |
| Hispanic | 43(16.8) | 72(18.4) | |
| Asian | 17(6.6) | 19(4.8) | |
| Other | 8(3.1) | 10(2.5) | |
| Body mass index, kg/m2 | 27 ± 5.5 | 26.5 ± 5.1 | 0.483 |
| Hypertension, n (%) | 65(25.4) | 50(12.8) | <.001 |
| Diabetes, n (%) | 21(8.2) | 16(4.1) | 0.027 |
| Current smoker, n (%) | 37(14.5) | 35(8.9) | 0.029 |
| Dyslipidemia, n (%) | 89(34.9) | 138(35.3) | 0.900 |
| Menopause (in women), n (%) | 39(39) | 79(43.9) | 0.427 |
| Family history of cardiovascular disease, n (%) | 58(22.7) | 80(20.5) | 0.499 |
| Migraine with aura, n (%) | 44(17.2) | 31(7.9) | <.001 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 95.1±16.2 | 94.2±15.6 | 0.548 |
| C-reactive protein, nmol/L | 7.9(3.7, 15.1) | 8.2(4.5, 16.7) | 0.396 |
| Interatrial shunt, n (%) | 147(57.7) | 96(24.6) | <.001 |
| Lp(a), molecular-weight dependent assayb | |||
| Overall, mg/dl | 24.7(8.8, 65.4) | 16.6(6.4, 41.8) | 0.001 |
| Whites | 18.3(6.0, 54.3) | 12.3(6.0, 32.7) | 0.027 |
| Blacks | 68.8(36.2, 93.9) | 60.3(30.0, 84.8) | 0.473 |
| Hispanics | 24.6(13.6, 74.6) | 26.1(9.2, 42.2) | 0.359 |
| Lp(a), molecular-weight independent assay | |||
| Overall, nmol/L | 30.5(6.3, 79.5) | 24.0(4.3, 69.7) | 0.121 |
| Whites | 20.9(4.1, 75.3) | 11.5(3.1, 51.6) | 0.078 |
| Blacks | 70(37.7, 111.9) | 89.4(38.6, 132.9) | 0.303 |
| Hispanics | 18.4(8.3, 66.2) | 38.7(8.5, 75.9) | 0.335 |
| Apo(a), number of kringle IV type 2 repeats | |||
| Overall | 23.0(21.5, 28.0) | 23.0(22.0, 27.5) | 0.664 |
| Whites | 23.0(21.0, 27.0) | 22.0(21.0, 27.0) | 0.945 |
| Blacks | 24.0(22.0, 27.0) | 26.0(23.0, 28.0) | 0.303 |
| Hispanics | 25.0(21.0, 28.0) | 24.0(22.0, 28.0) | 0.848 |
Continuous variables presented as mean±standard deviation or median (interquartile range)
Multiply by 0.0357 to convert to μmol/L.
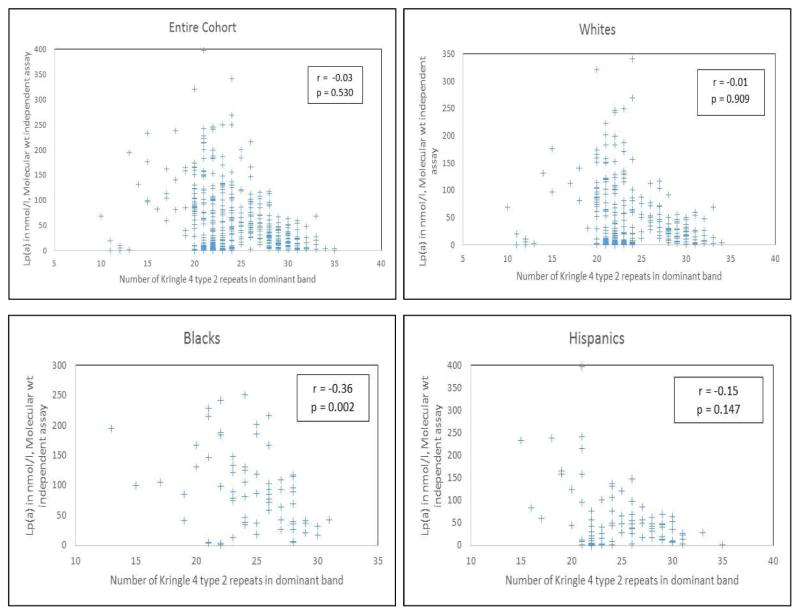
Figure 1 depicts the distribution of apo(a) size and its relationship with Lp(a) concentration (molecular-weight independent assay) among cases and controls, both overall and in the three major race-ethnic subgroups. Most apo(a) isoforms had 20 KIV-2 repeats or more, with a modest number in the 15 to 20, and a scant few in the <15 range. Apo(a) size showed a negative correlation with Lp(a) level only among African-Americans, with no significant correlations in whites and Hispanics. Findings were similar when confined to the control group.
Fig. 1.
Correlation between Lp(a) level and apo(a) size.
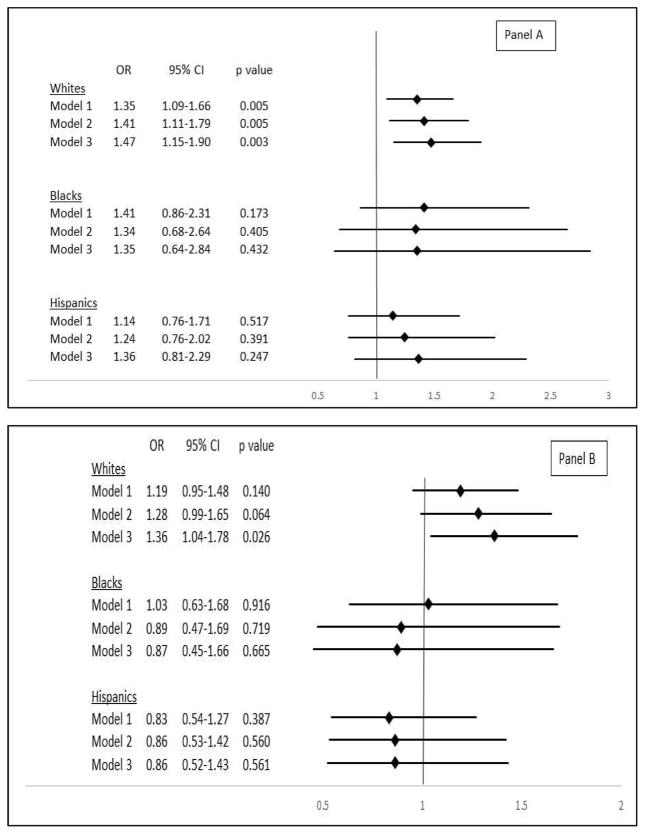
The relationship between continuous level of log-transformed Lp(a), as measured by molecular-weight-dependent assay and cryptogenic stroke in major race-ethnic subgroups, is presented in Figure 2 (Panel A). There was a significant association among whites, with a 41% (95% confidence interval [CI] 11% to 79%) higher odds of cryptogenic stroke for every SD increment in log-transformed Lp(a) after adjustment for demographic and clinical variables. This relationship persisted after additional adjustment for continuous apo(a) size. No significant association was detected for blacks or Hispanics. As shown in Table 2, assessment of the relationship for quartiles of molecular-weight-dependent Lp(a) in whites revealed a near-significant 81% (−5% to 247%) increase in odds of cryptogenic stroke for the comparison of extreme quartiles after adjustment for demographic and clinical variables. This association became significant after further adjustment for apo(a) size.
Figure 2.
Association of lipoprotein(a) and cryptogenic stroke in different ethnic groups.
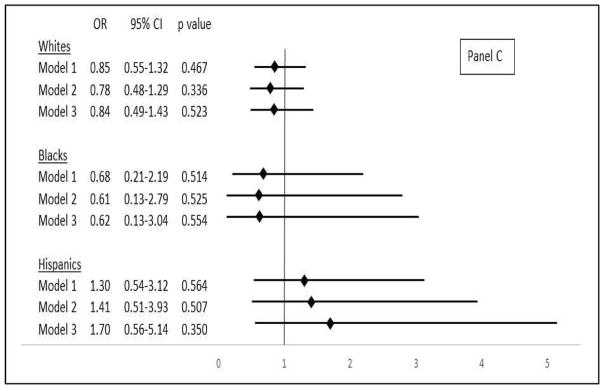
(A) Association between continuous log Lp(a), measured by molecular-weight-dependent assay, and cryptogenic stroke in major race-ethnic subgroups. Odds ratios are given per SD increment in log Lp(a) concentration. Whites, SD=0.99 (e0.99 or ~2.7-fold higher on the original scale); blacks, SD=0.88 (~2.4-fold higher); Hispanics, SD=0.99 (~2.7-fold higher). CI: confidence interval, OR: odds ratio. Model 1: adjusted for age and sex, Model 2: adjusted for age, sex, hypertension, diabetes, dyslipidemia, current smoker, migraine with aura, family history of premature CVD, and CRP. Model 3: adjusted for covariates in Model 2 plus number of kringle IV type 2 repeats. (B) Association between continuous log Lp(a), measured by molecular-weight-independent assay, and cryptogenic stroke in major race-ethnic subgroups. Odds ratios are given per SD increment in log Lp(a). Whites, SD=1.84 (~6.3-fold higher on the original scale); blacks, SD=1.16 (~3.2-fold higher); Hispanics, SD=1.74 (~5.7-fold higher). (C) Association between apo(a) size, dichotomized at KIV-2 ≤22 vs. >22, and cryptogenic stroke in major race-ethnic subgroups. Model 3 adjusts for log Lp(a) in addition to covariates in Model 2.
Table 2.
Association of quartiles of lipoprotein(a) or apolipoprotein(a) size and cryptogenic stroke in non-Hispanic whites.
| Models | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | OR (95% CI) | p value | |
| Lipoprotein(a), molecular-weight dependenta (n=391) | ||||||||
| Model 1 | Referent | NA | 0.54(0.28, 1.07) | 0.077 | 1.04(0.57, 1.88) | 0.902 | 1.63(0.93, 2.84) | 0.087 |
| Model 2 | Referent | NA | 0.57(0.26, 1.25) | 0.159 | 1.31(0.67, 2.55) | 0.437 | 1.81(0.95, 3.47) | 0.073 |
| Model 3d | Referent | NA | 0.58(0.24, 1.38) | 0.218 | 1.46(0.69, 3.07) | 0.325 | 2.02(1.01, 4.04) | 0.048 |
| Lipoprotein(a), molecular-weight independentb (n=398) | ||||||||
| Model 1 | Referent | NA | 0.61(0.30, 1.24) | 0.176 | 1.23(0.65, 2.32) | 0.533 | 1.59(0.87, 2.91) | 0.133 |
| Model 2 | Referent | NA | 0.78(0.35, 1.79) | 0.563 | 1.49(0.72, 3.06) | 0.281 | 2.10(1.04, 4.27) | 0.040 |
| Model 3d | Referent | NA | 0.70(0.28, 1.77) | 0.454 | 1.54(0.68, 3.51) | 0.299 | 2.34(1.10, 4.98) | 0.027 |
| Apolipoprotein(a), KIV-2 repeatsc (n=354) | ||||||||
| Model 1 | 0.85(0.46, 1.56) | 0.593 | 0.83(0.43, 1.58) | 0.572 | 0.97(0.53, 1.78) | 0.933 | Referent | NA |
| Model 2 | 0.85(0.43, 1.67) | 0.630 | 0.73(0.35, 1.51) | 0.394 | 1.02(0.52, 2.00) | 0.958 | Referent | NA |
| Model 3e | 0.78(0.37, 1.66) | 0.520 | 0.98(0.45, 2.11) | 0.952 | 1.06(0.51, 2.22) | 0.881 | Referent | NA |
25th, 50th and 75th percentile cutpoints are 6, 12.3 and 32.7 mg/dl, respectively. Multiply by 0.0357 to convert to μmol/L.
25th, 50th and 75th percentile cutpoints are 3.1, 11.5 and 51.6 nmol/L, respectively.
25th, 50th and 75th percentile cutpoints are 21, 22.5 and 27 KIV-2 repeats, respectively.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, hypertension, diabetes, dyslipidemia, current smoker, migraine with aura, family history of premature cardiovascular disease, C-reactive protein.
Model 3: adjusted for covariates in Model 2 plus dnumber of kringle IV type 2 repeats or elog of molecular-weight independent Lp(a).
CI: confidence interval, KIV-2: Kringle IV Type 2, NA: not applicable, OR: odds ratio.
Figure 2 (Panel B) presents the association with cryptogenic stroke for continuous concentration of log Lp(a) measured with the molecular-weight-independent assay in the major race-ethnic subgroups. After adjustment for potential demographic and clinical confounders, there was a nearly significant association with outcome among whites, with a 28% (95% CI, −1% to 65%) increased odds per SD increment in log Lp(a) concentration. This association became significant after additional adjustment for apo(a) size. There was no discernible association among blacks or Hispanics. In turn, evaluation of quartiles among whites (Table 2), showed a significant increase of 110% (4% to 327%) in odds of cryptogenic stroke for the upper versus lower quartile, which endured after further adjustment for apo(a) size.
There was no significant evidence of effect modification by age, sex or interatrial shunt of the associations of log Lp(a), as assessed with the molecular-weight dependent or independent assay, with cryptogenic stroke (p≥0.113).
Turning to apo(a) size and cryptogenic stroke, there was no evidence of association for any major race-ethnic subgroup when the number of KIV-2 repeats was dichotomized at a cutpoint of 22 (Figure 2, Panel C). Analysis of quartiles of apo(a) size among whites likewise did not show an association with cryptogenic stroke.
Discussion
In this multi-ethnic case-control study of unexplained cerebral infarction among young to middle-aged adults, we found that serum Lp(a) was associated with increased risk of cryptogenic stroke among non-Hispanic whites, with a roughly two-fold greater odds for the upper versus lower quartile after adjustment for demographic and clinical confounders. These findings were similar with use of molecular-weight-dependent and independent Lp(a) assays. We did not detect an association among non-Hispanic blacks or Hispanics, although the number of participants was smaller in these two subgroups. Evaluation of apo(a) size did not reveal an association with cerebral infarction in whites or the other race-ethnic subgroups.
The association between Lp(a) level and risk of CHD is well established,4 with Mendelian randomization analyses supporting a causal basis.7, 8 Systematic reviews have also documented a significant association of circulating Lp(a) level with stroke,5 and especially with ischemic stroke.4, 6 But in the latest meta-analysis, the association between Lp(a) and ischemic stroke was stronger for case-control studies with participants having a mean age ≤55 years compared to >55 years.6 Moreover, there was evidence of significant heterogeneity across case-control studies, which meta-regression showed was substantially related to stroke subtype.6 In particular, the strength of the association was greatest for ischemic stroke attributable to large-artery atherosclerosis or stroke of undetermined etiology.
That Lp(a) is particularly associated with large-artery atherosclerosis, but not cardioembolic or small-vessel disease subtypes of ischemic stroke, is further supported by a pooled analysis of Lp(a) genetic variants.10 A separate Mendelian randomization analysis provided evidence that the documented association between Lp(a) and carotid artery stenosis has a causal nature.9 Furthermore, the more pronounced association in younger adults noted by meta-analysis6 is consistent with findings in children and adolescents, in whom higher Lp(a) level has been related to a marked increase in risk of arterial ischemic stroke.25, 26 Previous studies of younger adults have been modest in size, however, and have included a mixture of ischemic stroke subtypes.27–29
Regarding apo(a) size, pooling of data from studies of CHD and ischemic stroke has revealed significantly increased risks of each outcome for smaller (≤22 KIV-2 repeats) versus larger (>22 KIV-2) apo(a) isoforms, although concurrent adjustment for Lp(a) level was not possible for a majority of studies.16 Of the five studies in this meta-analysis that specifically evaluated ischemic stroke, only two focused largely30 or exclusively31 on younger adults. These studies included considerable proportions of strokes with defined etiology, however, or, for the larger of the two,30 comprised mostly transient ischemic attacks.
The present findings highlight the importance of elevated Lp(a) level as a risk factor for premature cerebral infarction lacking a defined etiology among individuals of non-Hispanic white race-ethnicity. In particular, circulating Lp(a) concentration >75th percentile of the distribution in controls was associated with a doubling in the odds of cryptogenic stroke. The fact that 35.8% of non-Hispanic white patients with cryptogenic stroke in our study had Lp(a) levels above this threshold supports the high yield of Lp(a) testing in this population. Recognition of elevated Lp(a) concentration is gaining in importance in view of the growing list of emerging therapies with at least moderate Lp(a)-lowering effects. Apart from traditional options with known limitations, namely, niacin and plasma apheresis,32 anti-sense oligonucleotide therapy directed against apo-B and pro-protein convertase subtilisin kexin 9 inhibitors, drugs that have been FDA approved in certain hypercholesterolemic contexts, can achieve 30–40% reductions in Lp(a).33, 34 Even greater reductions of up to 75% have been shown by an anti-sense oligonucleotide targeting apo(a) directly,35 which is currently undergoing phase II/III clinical trials. Given the increased risk of adverse outcomes in younger patients with ischemic stroke,36, 37 use of these therapies in appropriately selected patients has the potential to meaningfully improve prognosis in this population.
The current study shows that Lp(a) level determined by a commercially available molecular-weight dependent assay in use at a tertiary medical center exhibited a similar association with cryptogenic stroke as compared with a preferred molecular-weight independent assay. There were few individuals in our study with extreme apo(a) sizes, however, and given the potential for biased measures associated with molecular-weight-dependent Lp(a) assays, use of molecular-weight-independent assays should be favored, as recommended by expert recommendations.
Unlike the findings from the previous meta-analysis of apo(a) size and ischemic stroke,16 we did not detect an association between number of KIV-2 repeats and unexplained cerebral infarction. Except in the African-American subgroup, we did not find a negative correlation between apo(a) isoform size and Lp(a) level. This could in part relate to the high variability in Lp(a) levels previously documented for small apo(a) sizes (<21 KIV-2 repeats),11 although we did not detect a significant negative correlation upon restricting to the larger isoforms. The lack of association between apo(a) isoform size and cryptogenic stroke in the present study may reflect insufficient sample size even for the non-Hispanic white subgroup, as attested by the wide 95% CI’s, particularly in light of the high variability in Lp(a) levels characteristic of small apo(a) isoforms.
Although the pathophysiologic mechanisms underlying the association between Lp(a) and CVD remain incompletely defined, experimental work points to two molecular features of Lp(a) as primarily responsible for its pathogenicity.38 The first is apo(a)’s affinity for pro-inflammatory oxidized phospholipids, which appears in large measure to account for the particle’s pro-atherogenic properties. The second is apo(a)’s homology to plasminogen, which results in competitive inhibition of fibrinolysis and leads to pro-thrombotic effects. Although earlier studies supported an association between Lp(a) and risk of venous thromboembolism,39 large collaborative studies involving genetic data have since shown that the particle is related to atherosclerosis in various vascular beds, but not to venous thrombosis.9, 10 In this regard, we did not find evidence of interaction between Lp(a) and interatrial shunting, which might have been expected if Lp(a) were a major risk factor for venous thrombosis, although our study lacked power for this type of analysis. This suggests that cryptogenic stroke in younger adults may to a sizable extent reflect occult atherothromboembolism.40
The present study is, to our knowledge, the largest multi-ethnic investigation to examine Lp(a) and apo(a) isoforms in relation to unexplained cerebral infarction in younger adults. Strengths include its prospective design and rigorous evaluation underlying the definition of cryptogenic stroke, and measurement of Lp(a) with both a commercially available apo(a)-size dependent assay, and a recommended apo(a)-size-independent assay.
Several limitations are also of note. As a cross-sectional observational study, the current case-control investigation can only show associations, but does not allow determinations of direction or causality. Mendelian randomization analyses of CHD7, 8 and carotid artery stenosis,9 however, support the premise that the relationship between Lp(a) and risk of atherosclerotic CVD is causal. Controls for the present study consisted of volunteers from the medical center and surrounding area, rather than population-based. Yet the comparable prevalences of dyslipidemia and family history of premature CVD in cases and controls suggests that this volunteer group may have self-selected on the basis of CVD risk factors, which would tend to bias differences toward the null. Last, as noted, this multi-ethnic study had diverse race-ethnic representation reflecting the race-ethnic composition of New York City and surrounding areas, yet the number of race-ethnic minority participants was not sufficient to meaningfully evaluate the associations in question. Larger studies will be required to investigate associations in these race-ethnic groups.
In this case-control study of a multi-ethnic sample, higher serum Lp(a) level, but not apo(a) isoform size, was associated with increased odds of cryptogenic stroke among non-Hispanic white participants. Larger studies are necessary to further address these questions, specifically among race-ethnic minority populations. In view of the expanding therapeutic options for Lp(a) lowering, these findings support routine measurement for Lp(a) in the context of cryptogenic stroke, at least among whites, and provide impetus for testing the impact of such therapies in improving outcomes among younger adults with ischemic, and particularly cryptogenic, stroke.
Highlights.
Lp(a) level, but not apo(a) size, was independently associated with unexplained ischemic stroke in this multi-ethnic study of young and middle-aged adults, with a ~2-fold greater odds in the highest quartile of Lp(a) concentration compared with the lowest quartile in the subset of white participants.
The findings support routine testing for Lp(a) in the setting of unexplained stroke in young adults, particularly of non-Hispanic white race-ethnicity, as more effective therapies to lower levels become available.
Acknowledgments
Financial support
This research was supported by K23 HL070854 (to Dr. Kizer) and the National Center for Advancing Translational Science under award number UL1TR000457.
Footnotes
Conflicts of interest
Dr. Kizer reports stock ownership in Pfizer, Inc, and Gilead Sciences, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, et al. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet neurology. 2014;13:429–438. doi: 10.1016/S1474-4422(13)70310-7. [DOI] [PubMed] [Google Scholar]
- 2.Putaala J, Tatlisumak T. Prime time for dissecting the entity of cryptogenic stroke. Stroke. 2014;45:950–952. doi: 10.1161/STROKEAHA.114.004676. [DOI] [PubMed] [Google Scholar]
- 3.Boffa MB, Koschinsky ML. Update on lipoprotein(a) as a cardiovascular risk factor and mediator. Curr Atheroscler Rep. 2013;15:360. doi: 10.1007/s11883-013-0360-6. [DOI] [PubMed] [Google Scholar]
- 4.Emerging Risk Factors C. Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, et al. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smolders B, Lemmens R, Thijs V. Lipoprotein (a) and stroke: A meta-analysis of observational studies. Stroke. 2007;38:1959–1966. doi: 10.1161/STROKEAHA.106.480657. [DOI] [PubMed] [Google Scholar]
- 6.Nave AH, Lange KS, Leonards CO, Siegerink B, Doehner W, Landmesser U, et al. Lipoprotein (a) as a risk factor for ischemic stroke: A meta-analysis. Atherosclerosis. 2015;242:496–503. doi: 10.1016/j.atherosclerosis.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 7.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 8.Clarke R, Peden JF, Hopewell JC, Kyriakou T, Goel A, Heath SC, et al. Genetic variants associated with lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 9.Kamstrup PR, Tybjaerg-Hansen A, Nordestgaard BG. Genetic evidence that lipoprotein(a) associates with atherosclerotic stenosis rather than venous thrombosis. Arterioscler Thromb Vasc Biol. 2012;32:1732–1741. doi: 10.1161/ATVBAHA.112.248765. [DOI] [PubMed] [Google Scholar]
- 10.Helgadottir A, Gretarsdottir S, Thorleifsson G, Holm H, Patel RS, Gudnason T, et al. Apolipoprotein(a) genetic sequence variants associated with systemic atherosclerosis and coronary atherosclerotic burden but not with venous thromboembolism. J Am Coll Cardiol. 2012;60:722–729. doi: 10.1016/j.jacc.2012.01.078. [DOI] [PubMed] [Google Scholar]
- 11.Marcovina SM, Albers JJ, Wijsman E, Zhang Z, Chapman NH, Kennedy H. Differences in lp[a] concentrations and apo[a] polymorphs between black and white americans. J Lipid Res. 1996;37:2569–2585. [PubMed] [Google Scholar]
- 12.Lanktree MB, Anand SS, Yusuf S, Hegele RA, Investigators S. Comprehensive analysis of genomic variation in the lpa locus and its relationship to plasma lipoprotein(a) in south asians, chinese, and european caucasians. Circ Cardiovasc Genet. 2010;3:39–46. doi: 10.1161/CIRCGENETICS.109.907642. [DOI] [PubMed] [Google Scholar]
- 13.Kronenberg F. Genetic determination of lipoprotein(a) and its association with cardiovascular disease: Convenient does not always mean better. J Intern Med. 2014;276:243–247. doi: 10.1111/joim.12207. [DOI] [PubMed] [Google Scholar]
- 14.Marcovina SM, Koschinsky ML, Albers JJ, Skarlatos S. Report of the national heart, lung, and blood institute workshop on lipoprotein(a) and cardiovascular disease: Recent advances and future directions. Clin Chem. 2003;49:1785–1796. doi: 10.1373/clinchem.2003.023689. [DOI] [PubMed] [Google Scholar]
- 15.Brown WV, Ballantyne CM, Jones PH, Marcovina S. Management of lp(a) J Clin Lipidol. 2010;4:240–247. doi: 10.1016/j.jacl.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Erqou S, Thompson A, Di Angelantonio E, Saleheen D, Kaptoge S, Marcovina S, et al. Apolipoprotein(a) isoforms and the risk of vascular disease: Systematic review of 40 studies involving 58,000 participants. J Am Coll Cardiol. 2010;55:2160–2167. doi: 10.1016/j.jacc.2009.10.080. [DOI] [PubMed] [Google Scholar]
- 17.Berglund L, Ramakrishnan R. Lipoprotein(a): An elusive cardiovascular risk factor. Arterioscler Thromb Vasc Biol. 2004;24:2219–2226. doi: 10.1161/01.ATV.0000144010.55563.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. Toast. Trial of org 10172 in acute stroke treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 19.Suk Danik J, Rifai N, Buring JE, Ridker PM. Lipoprotein(a), hormone replacement therapy, and risk of future cardiovascular events. J Am Coll Cardiol. 2008;52:124–131. doi: 10.1016/j.jacc.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Headache classification committee of the international headache society. Cephalalgia : an international journal of headache. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- 22.Levine DM, Sloan BJ, Donner JE, Lorenz JD, Heinzerling RH. Automated measurement of lipoprotein(a) by immunoturbidimetric analysis. Int J Clin Lab Res. 1992;22:173–178. doi: 10.1007/BF02591419. [DOI] [PubMed] [Google Scholar]
- 23.Marcovina SM, Albers JJ, Scanu AM, Kennedy H, Giaculli F, Berg K, et al. Use of a reference material proposed by the international federation of clinical chemistry and laboratory medicine to evaluate analytical methods for the determination of plasma lipoprotein(a) Clin Chem. 2000;46:1956–1967. [PubMed] [Google Scholar]
- 24.Marcovina SM, Hobbs HH, Albers JJ. Relation between number of apolipoprotein(a) kringle 4 repeats and mobility of isoforms in agarose gel: Basis for a standardized isoform nomenclature. Clin Chem. 1996;42:436–439. [PubMed] [Google Scholar]
- 25.Sultan SM, Schupf N, Dowling MM, DeVeber GA, Kirton A, Elkind MSV. Review of lipid and lipoprotein(a) abnormalities in childhood arterial ischemic stroke. International Journal of Stroke. 2014;9:79–87. doi: 10.1111/ijs.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldenberg NA, Bernard TJ, Hillhouse J, Armstrong-Wells J, Galinkin J, Knapp-Clevenger R, et al. Elevated lipoprotein (a), small apolipoprotein (a), and the risk of arterial ischemic stroke in north american children. Haematologica. 2013;98:802–807. doi: 10.3324/haematol.2012.073833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagayama M, Shinohara Y, Nagayama T. Lipoprotein(a) and ischemic cerebrovascular disease in young adults. Stroke. 1994;25:74–78. doi: 10.1161/01.str.25.1.74. [DOI] [PubMed] [Google Scholar]
- 28.Wityk RJ, Kittner SJ, Jenner JL, Hebel JR, Epstein A, Wozniak MA, et al. Lipoprotein (a) and the risk of ischemic stroke in young women. Atherosclerosis. 2000;150:389–396. doi: 10.1016/s0021-9150(99)00388-3. [DOI] [PubMed] [Google Scholar]
- 29.Rigal M, Ruidavets JB, Viguier A, Petit R, Perret B, Ferrieres J, et al. Lipoprotein (a) and risk of ischemic stroke in young adults. J Neurol Sci. 2007;252:39–44. doi: 10.1016/j.jns.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 30.Jurgens G, Taddei-Peters WC, Koltringer P, Petek W, Chen Q, Greilberger J, et al. Lipoprotein(a) serum concentration and apolipoprotein(a) phenotype correlate with severity and presence of ischemic cerebrovascular disease. Stroke. 1995;26:1841–1848. doi: 10.1161/01.str.26.10.1841. [DOI] [PubMed] [Google Scholar]
- 31.Peynet J, Beaudeux JL, Woimant F, Flourie F, Giraudeaux V, Vicaut E, et al. Apolipoprotein(a) size polymorphism in young adults with ischemic stroke. Atherosclerosis. 1999;142:233–239. doi: 10.1016/s0021-9150(98)00232-9. [DOI] [PubMed] [Google Scholar]
- 32.Nordestgaard BG, Chapman MJ, Ray K, Boren J, Andreotti F, Watts GF, et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santos RD, Raal FJ, Catapano AL, Witztum JL, Steinhagen-Thiessen E, Tsimikas S. Mipomersen, an antisense oligonucleotide to apolipoprotein b-100, reduces lipoprotein(a) in various populations with hypercholesterolemia: Results of 4 phase iii trials. Arterioscler Thromb Vasc Biol. 2015;35:689–699. doi: 10.1161/ATVBAHA.114.304549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Desai NR, Kohli P, Giugliano RP, O’Donoghue ML, Somaratne R, Zhou J, et al. Amg145, a monoclonal antibody against proprotein convertase subtilisin kexin type 9, significantly reduces lipoprotein(a) in hypercholesterolemic patients receiving statin therapy: An analysis from the ldl-c assessment with proprotein convertase subtilisin kexin type 9 monoclonal antibody inhibition combined with statin therapy (laplace)-thrombolysis in myocardial infarction (timi) 57 trial. Circulation. 2013;128:962–969. doi: 10.1161/CIRCULATIONAHA.113.001969. [DOI] [PubMed] [Google Scholar]
- 35.Tsimikas S, Viney NJ, Hughes SG, Singleton W, Graham MJ, Baker BF, et al. Antisense therapy targeting apolipoprotein(a): A randomised, double-blind, placebo-controlled phase 1 study. Lancet. 2015;386:1472–1483. doi: 10.1016/S0140-6736(15)61252-1. [DOI] [PubMed] [Google Scholar]
- 36.Rutten-Jacobs LC, Arntz RM, Maaijwee NA, Schoonderwaldt HC, Dorresteijn LD, van Dijk EJ, et al. Long-term mortality after stroke among adults aged 18 to 50 years. JAMA. 2013;309:1136–1144. doi: 10.1001/jama.2013.842. [DOI] [PubMed] [Google Scholar]
- 37.Putaala J, Haapaniemi E, Metso AJ, Metso TM, Artto V, Kaste M, et al. Recurrent ischemic events in young adults after first-ever ischemic stroke. Ann Neurol. 2010;68:661–671. doi: 10.1002/ana.22091. [DOI] [PubMed] [Google Scholar]
- 38.Koschinsky ML. Novel insights into lp(a) physiology and pathogenicity: More questions than answers? Cardiovasc Hematol Disord Drug Targets. 2006;6:267–278. doi: 10.2174/187152906779010764. [DOI] [PubMed] [Google Scholar]
- 39.Sofi F, Marcucci R, Abbate R, Gensini GF, Prisco D. Lipoprotein (a) and venous thromboembolism in adults: A meta-analysis. Am J Med. 2007;120:728–733. doi: 10.1016/j.amjmed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 40.Kizer JR. Evaluation of the patient with unexplained stroke. Coron Artery Dis. 2008;19:535–540. doi: 10.1097/MCA.0b013e32830eabb6. [DOI] [PubMed] [Google Scholar]