Abstract
Objective
Early diagnosis of epithelial ovarian cancer is critical for patient survival. The objective of this pilot study is to identify a circulating micro (mi)RNA as a potential biomarker for epithelial ovarian cancer.
Methods
A total of 135 epithelial ovarian cancer patients and 54 benign ovarian tumor patients were recruited for this study. Using customized TaqMan low density miRNA arrays, we first screened expression levels of 48 miRNAs in sera from 18 epithelial ovarian cancer patients and 16 benign ovarian tumor patients. The most significantly and differentially expressed miRNA was then further examined in all serum samples using real-time polymerase chain reaction. Its expression was further analyzed in relationship with clinicopathological factors and patient survival.
Results
Array screening data showed that expression levels of serum miRNA-20a, miRNA-125b, miRNA-126, miRNA-355, and let-7c were significantly different between malignant and benign ovarian tumor patients. Subsequent real-time polymerase chain reaction results showed that serum miRNA-125b levels were significantly higher in epithelial ovarian cancer patients compared to benign controls. Moreover, serum miRNA-125b levels were significantly higher in ovarian cancer patients in early stages I and II, and in patients having no residual tumor following surgery, but were not associated with differentiation and histological types of ovarian cancer. Notably, the higher level of miR-125b was significantly positively correlated with progression-free survival (P = 0.035) and marginally, with overall survival (P = 0.069).
Conclusions
miRNA-125b plays an important role in the pathogenesis and progression of epithelial ovarian cancer. Circulating miRNA-125b has the potential to become a novel biomarker for early diagnosis and prognosis prediction of epithelial ovarian cancer.
Key Words: Ovarian cancer, Biomarker, miRNA-125b, Diagnosis, Prognosis
Ovarian cancer is the most deadly gynecological cancer.1 The 5-year survival rate is 92% for localized epithelial ovarian cancer patients (stage I or II), and less than 30% for patients at stages III and IV.1 Serum levels of CA125 are routinely used to monitor disease progression and response to treatment in ovarian cancer patients. However, current screening tests based on serum CA125 levels are not highly predictive for early detection or monitoring of ovarian cancer progression. Of patients with ovarian cancer, 60% are diagnosed during late stages.1 Thus, it is exceptionally urgent to identify sensitive, noninvasive biomarkers to improve early detection and to guide therapy.
MicroRNAs (miRNAs) are noncoding single-stranded RNA molecules of 19 to 24 nucleotides in length. miRNAs regulate gene expression by triggering mRNA decay or by binding the 3′ untranslated region of target mRNAs to inhibit translation. A single miRNA molecule can sequentially bind to hundreds of target mRNAs to modulate various cellular processes, such as the cell cycle, differentiation, proliferation, and apoptosis.2 Expression of miRNAs is aberrant in various types of human cancers, and under these circumstances, these miRNAs can function as oncogenes or tumor suppressor genes.3–5 Serum levels of some circulating miRNAs have been shown to correspond with tumor status in some cancers and thus, have great potential for use as diagnostic tools and in predicting prognosis and response to treatments.6–10
The objective of this study was to identify 1 or more circulating miRNAs as a diagnostic and prognostic biomarker for epithelial ovarian cancer. Serum miRNA expression profiles were screened using miRNA arrays to identify miRNAs that were differentially expressed in ovarian cancer patients compared with patients with benign tumors. The most significantly and differentially expressed miRNA was then further examined in all serum samples using real-time polymerase chain reaction (PCR). Its expression was further analyzed in relationship with clinicopathological factors and patient survival. Our results showed that circulating miRNA-125b was significantly higher in early stage epithelial ovarian cancer patients, whereas significant decreased expression was seen in patients in later stages.
MATERIALS AND METHODS
Subjects and Clinical Features
From February 2009 through November 2012, a total of 135 epithelial ovarian cancer patients who underwent surgical treatment at our cancer hospital, without prior chemoradiotherapy, were recruited for this study. Their ovarian cancer was confirmed by pathological diagnosis on surgical specimens.
The median age of study participants was 54 years and ranged from 37 to 78 years. Based on International Federation of Gynecology and Obstetrics and World Health Organization standards, these patients were classified as: 33 cases in early stage (stages I and II), 102 cases in late stage (stages III and IV); 93 cases of serous adenocarcinoma (89 high grade and 4 low grade), 42 cases of nonserous ovarian cancer (24 clear cell, 12 endometrioid and 6 mucinous carcinoma); 18 cases of grades G1 and G2, 106 cases of grade G3, and 11 cases without pathological grading; 62 cases in which tumor residue was present following surgery, 71 cases with no tumor residue, and 2 cases that were uncategorized.
The control group consisted of 54 patients with benign epithelial ovarian tumors who underwent surgical treatment during the study period. Patients in this group had a median age of 55 years and ranged in age from 38 to 75 years. Based on pathological diagnosis, 19 patients had serous cystadenoma, 33 with mucinous cystadenoma, and 2 with benign cystic teratoma.
This study was approved by the ethics committee of our cancer hospital. Every patient signed an informed consent form before samples were collected.
Sample Harvesting
Whole blood (10 mL) was drawn from the ulnar vein after fasting and prior to surgery. The blood samples were left standing for 1 hour at room temperature and then centrifuged at 2500g per minute for 15 minutes at 4°C. Sample sera fractions were collected and stored at −80°C.
miRNA Microarray Assay
The miRNeasy Micro kit (Qiagen, Shanghai, China) was used to extract total RNA, including miRNA, from sera following the manufacturer’s protocol. The RNA was then reverse-transcribed into cDNA using the TaqMan MiRNA Reverse Transcription Kit (Applied Biosystems, Shanghai, China).
Based on data from previous studies, 48 miRNAs implicated in ovarian cancer were selected and TaqMan low density arrays (Applied Biosystems) were customized to detect cDNA corresponding to these 48 miRNAs. Arrays were used to screen serum samples from 18 patients with epithelial ovarian cancer and 16 negative control samples from patients with benign ovarian tumors. After mixing cDNA template with TaqMan Universal PCR Master Mix, arrays were run on the 7900HT real-time-PCR system (Applied Biosystems). Standard 7900RQ software was used for data analysis. Quantification of expression of individual miRNAs was based on 2−ΔCT where ΔCT = Ct (miRNA) − Ct (miR-16).11 The choice of miR-16 as an endogenous control against which to normalize expression of patient miRNAs was based on recommendations of the kit manufacturer.
Fluorescence-based TaqMan real-time PCR (Applied Biosystems, 7900 HT system) was used to confirm results where differential expression of a miRNA revealed in miRNA arrays.
Follow-Up
Follow-up with all ovarian cancer patients occurred by telephone once every 3 months in the first 2 years and every 6 months after surgery.
Statistics
SPSS 17.0 was used to perform statistical analysis. Data were expressed as mean ± SD. The Mann-Whitney U test was used to analyze the difference in serum miRNA expression between benign and malignant ovarian tumor patients. Mann-Whitney U, χ2 tests, and analysis of variance were used to assess correlations between serum miRNA expression levels and clinicopathological variables in epithelial ovarian cancer patients. Univariate survival analysis was assessed using the Kaplan-Meier test. For all analyses, statistical significance was characterized by P less than 0.05.
RESULTS
Differentially Expressed Serum miRNAs Between Benign Controls and Epithelial Ovarian Cancer Patients
The miRNA expression profiles were measured in sera from 18 cancer patients and 16 patients with benign tumors. Half of 18 ovarian cancer patients had cancer of stage I or stage II, and the other half had cancer classified as stage III or stage IV. Our results revealed that 5 miRNAs were significantly differentially expressed (Fig. 1). These miRNAs were miRNA-20a (8.44 ± 1.50 vs 5.28 ± 1.54; P < 0.001), miRNA-125b (0.94 ± 0.76 vs 2.33 ± 1.8; P = 0.004), miRNA-126 (1.48 ± 1.06 vs 0.66 ± 0.32; P = 0.016), miRNA-355 (0.37 ± 0.19 vs 0.17 ± 0.10; P = 0.044), and let-7c (0.24 ± 0.17 vs 0.42 ± 0.20; P = 0.037).
FIGURE 1.
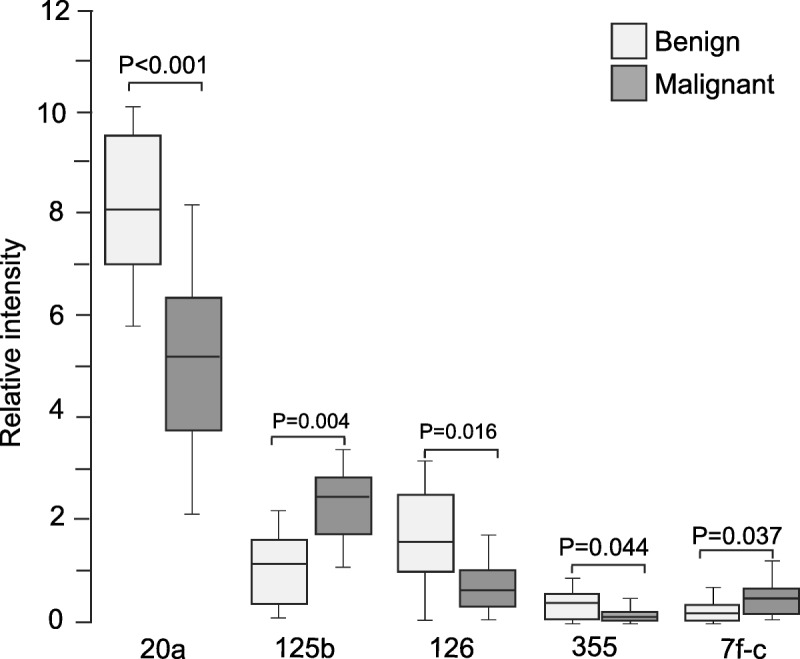
Serum miRNAs differentially expressed in benign control and epithelial ovarian cancer patients. Levels of serum miRNA were analyzed in 16 benign controls and 18 cancer patients using miRNA arrays.
Expression of the following miRNAs was extremely low or undetectable in both controls and patients: U6 snRNA, miRNA-30c-2, miRNA-32, miRNA-34b, miRNA-34c, miRNA-107, miRNA-199a, miRNA-199b, and let-7a. The list of miRNAs for which differences in expression level in ovarian cancer patients compared with benign controls was not significant is shown in Table 1.
TABLE 1.
Expression levels of nondifferentially expressed serum miRNAs
The top 3 differentially expressed miRNAs (miRNA-20a, 125b, and 126) were analyzed in the same samples using real-time PCR. Only miRNA-125b was shown significantly different between control and cancer patients (2.09 ± 1.44 vs 3.24 ± 2.12; P = 0.046), whereas miRNA-20a (8.22 ± 2.71 vs 7.52 ± 2.75; P = 0.968) and miRNA-126 (2.12 ± 1.12 vs 1.51 ± 1.21; P = 0.779) were not significant (Fig. 2). Therefore, only miRNA-125b was determined in all samples.
FIGURE 2.
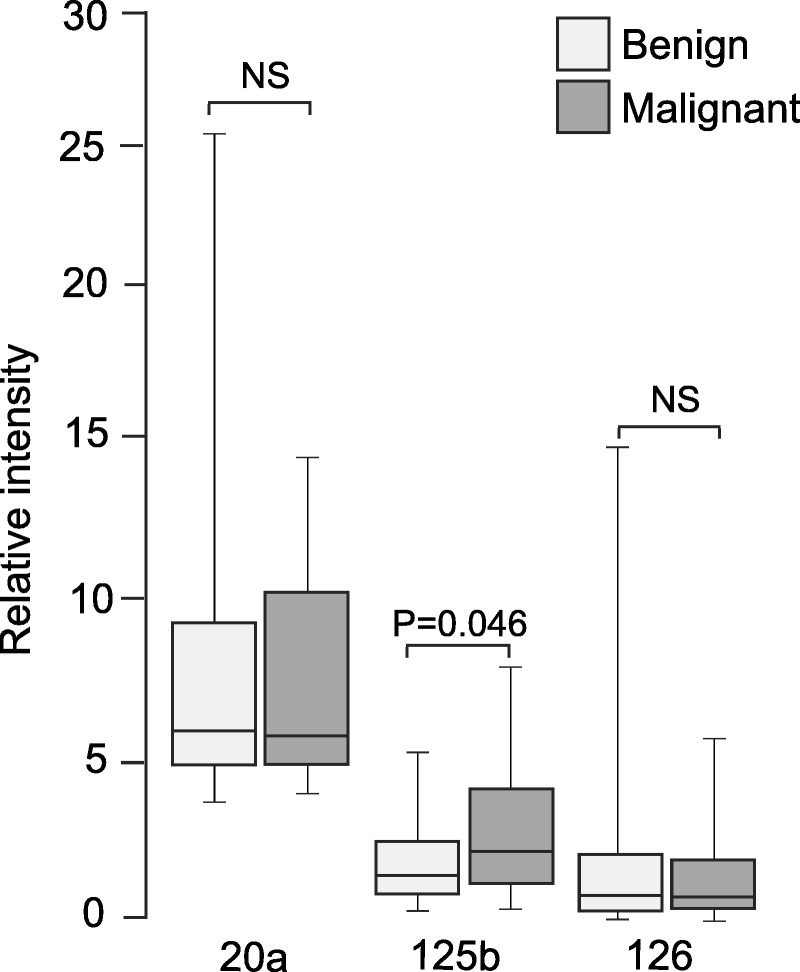
Validation of array data. Real-time PCR was applied to validate the expression of serum miRNAs in the same 16 benign controls and 18 cancer patients used in array analysis. NS, nonsignificant.
Expression of miRNA-125 Was Significantly Increased in Epithelial Ovarian Cancer Patients
Expression of miRNA-125b was then measured in serum samples of 54 controls with benign ovarian tumors and 135 patients with epithelial ovarian cancer using real-time PCR. The results showed that the expression of serum miRNA-125b in epithelial ovarian cancer patients (3.92 ± 3.28) was significantly higher than that in benign controls (2.36 ± 2.14, P = 0.002) (Fig. 3A).
FIGURE 3.
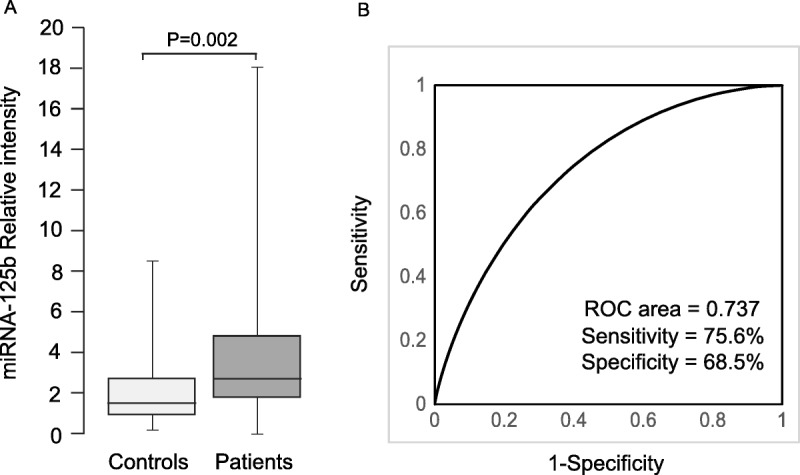
Serum miRNA-125b levels were significantly higher in epithelial ovarian cancer patients. A, Levels of serum miRNA were analyzed in 54 benign controls and 135 cancer patients by real-time PCR. B, Receiver-operating characteristic curve for serum miRNA-125b showing its potential to discriminate ovarian cancer from benign control.
The receiver-operating characteristic curve analysis demonstrated that serum miR-125b had the potential to be used as a noninvasive biomarker for ovarian cancer. As shown in Figure 3B, the sensitivity and specificity were 75.6% and 68.5%, respectively, and an area under the curve yielded a value of 0.737 for discriminating ovarian cancer patients from benign controls.
Relationship Between miRNA-125b Expression and Clinicopathological Features in Epithelial Ovarian Cancer Patients
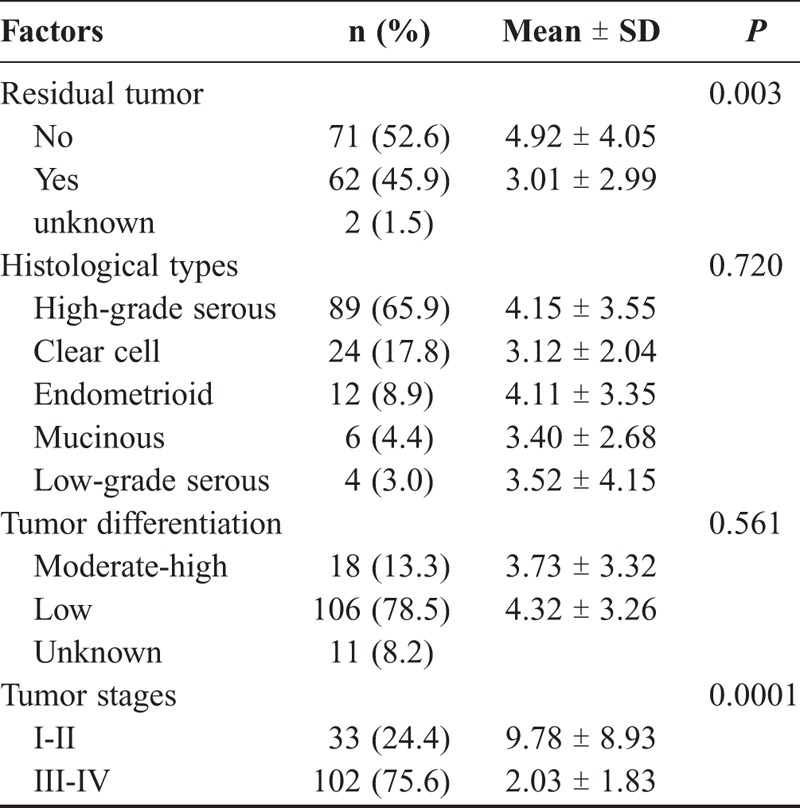
Serum miRNA-125b expression was significantly higher in patients with no residual tumors after operation than those with residual tumors (P = 0.003) (Table 2). Patients in early stages (I and II) had significantly higher levels of miRNA-125b than those in late stages (III and IV) (P = 0.0001). However, there was no significant difference in serum miRNA-125b expression among 5 histological types of epithelial ovarian cancers (P = 0.720), and between poorly differentiated (low) and moderately to highly differentiated groups (P = 0.561).
TABLE 2.
Relationship between serum mir-125b levels and clinicopathological features in epithelial ovarian cancer patients
Serum miRNA-125b Expression and Epithelial Ovarian Patient Survival
The median progression-free survival (PFS) time was 28 months and ranged from 3 to 69 months. The median overall survival (OS) time was 40 months with a range of 4 to 75 months. During the follow-up period, 58 patients relapsed and 44 patients died.
Epithelial ovarian cancer patients were divided into high and low serum miRNA-125b expression groups based on the median value of 2.57. Patients with high serum miRNA-125b levels had a significantly longer PFS time than patients with low expression levels (P = 0.035) (Fig. 4A). Patients with high serum miRNA-125b expression showed a marginally significant increase in OS time (P = 0.069) (Fig. 4B).
FIGURE 4.
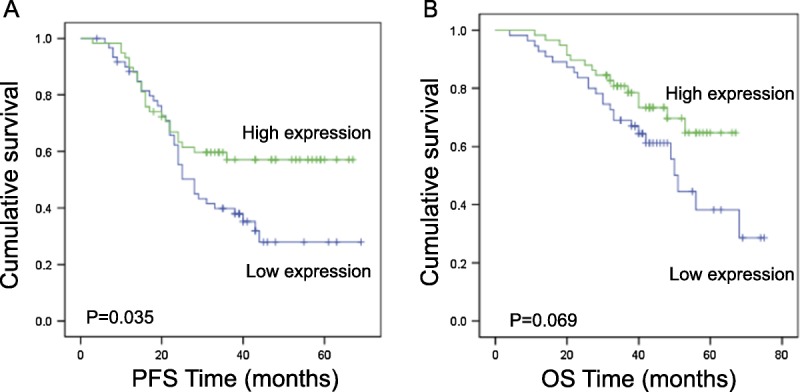
Relationship between serum miRNA-125b levels and survival of epithelial ovarian cancer patients. Univariate Kaplan-Meier survival curves of (A) PFS and (B) OS for epithelial ovarian cancer patients with high or low circulating miRNA-125b levels. A median value of 2.57 was used to group the patients.
DISCUSSION
Ovarian cancer is the most deadly gynecological cancer and one of the most common malignancies in women.1 Because of the lack of specific symptoms and accurate screening tests, ovarian cancer patients are often diagnosed during late stages and given a 5-year survival rate of less than 30%.1 Hope for early diagnosis and subsequent improvement in survival rates for ovarian cancer patients depends on identification of biomarkers specific to early-stage ovarian cancer.
Differential expression of miRNAs has shown great potential for use in identifying biomarkers for diagnosis, prognosis, and prediction of response to the treatments in cancers.12–17 Several studies have reported aberrant expression levels of miRNAs in ovarian cancer, specifically.12,18,19 Because of the invasiveness of sample collection techniques, tissue miRNAs have limitations for use as clinical biomarkers. Alternatively, collection of patient serum for miRNA expression analysis is both noninvasive and cost-effective.6–10 Serum miRNA from ovarian cancer patients can be analyzed using real-time PCR and preliminary results have shown that differential expression of miRNAs may help to facilitate early diagnosis of ovarian cancer.20 Chung et al21 found that expression levels of 88 miRNAs increased, whereas 95 miRNAs decreased in sera and ascites from ovarian cancer patients. Wei et al22 reported that ovarian cancer tissues and sera had decreased miR-212 expression based upon quantitative PCR results. Studies of ovarian cancer–specific circulating miRNAs identified in these and other studies12,23 may be used to develop diagnostic and prognostic biomarkers.
In this study, levels of 48 serum miRNAs in patients with benign ovarian tumors and patients with epithelial ovarian cancer were first screened using TaqMan low density arrays. Three miRNAs (miRNA-20a, miRNA-126, and miRNA-355) were downregulated, whereas 2 miRNAs (miRNA-125b and let-7c) were upregulated in sera from epithelial ovarian cancer patients compared to benign controls. Though both miRNA-20a and miRNA-126 were not validated in the subsequent assay using the same samples in our study, aberrant expression of serum miRNA-20a and miRNA-126 in ovarian cancer has been reported in previous studies.4,24,25 Yang et al26 reported that ovarian cancer cell lines had decreased expression of miRNA-125b compared with noncancerous immortalized ovarian epithelial cells. Changes in expression levels of serum miRNA-355, miRNA-125b, and let-7c in ovarian cancer patients were found in this study. On the other hand, many serum miRNAs reported to be differentially expressed in ovarian cancers in previous studies were not identified in this study. These inconsistencies may be the result of differences in ethnic make-up of study groups,27 as well as use of different controls and assay methods.
Because miRNA-125b was the most abundant among three novel miRNAs identified in this study, we subsequently determined its expression in serum samples from all 135 epithelial ovarian cancer patients and 54 patients with benign tumors using real-time PCR. Our results showed that serum miRNA-125b expression was significantly higher in epithelial ovarian cancer patients compared with benign controls. One intriguing finding of our study was that serum miRNA-125b levels were significantly decreased in epithelial ovarian cancer at late stages (III and IV) compared to early stages (I and II). Patients with residual tumors following surgery commonly have late stage (III and IV) epithelial ovarian cancer. Indeed, patients having residual tumors following surgery had significantly lower serum miRNA-125b levels than those without residual tumors. Notably, lower expression of serum miRNA-125b was significantly related to poorer PFS, and possibly, to poor OS. A very recent study enrolled 70 epithelial ovarian cancer patients and 70 healthy control, and determined serum miR-125b levels by real-time PCR. Their results showed that serum miRNA-125b levels were significantly upregulated in ovarian cancer compared with healthy controls. In addition, expression level of serum miR-125b was found to be significantly lower in late stages (III and IV) than early stages (I and II). Patients with lymph node and distant metastasis showed significantly lower serum miRNA levels.28 Our data were in consistence to their findings. However, possibly due to smaller sample size and shorter follow-up period, serum miRNA-125b levels were not significantly associated with OS in their study.28 These data imply that miRNA-125b may play an important role in ovarian carcinogenesis and that it might play a different role as cancer progresses. Serum miRNA-125b is a potential marker for diagnosis and prognosis prediction of epithelial ovarian cancer.
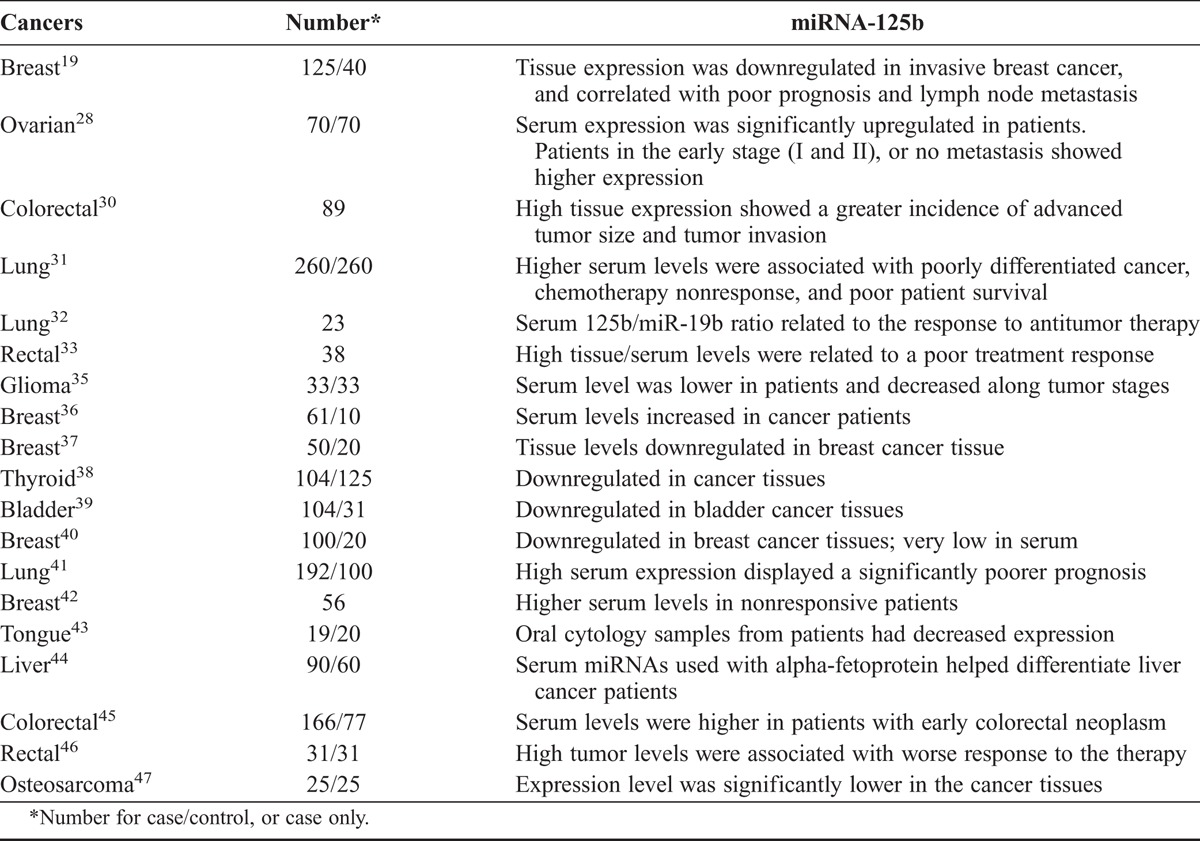
Previous studies have shown increased or decreased miRNA-125b expression related to development, progression and prognosis of other types of cancers.29 Zhang et al19 found that patients with invasive breast cancer with lymph node metastasis had higher levels of miRNA-125b expression and that this correlated with a significantly lower 5-year survival rate. Nishida et al30 found that patients with larger tumors and invasive colorectal cancer had higher expression of miR-125b, and that this was associated with poor survival. Cui et al31 analyzed the relationship between serum miR-125b expression levels and clinical features, including response to chemotherapy and prognosis, in 260 non–small-cell lung cancer patients. Their results showed that patients at stage IV, with poor tumor differentiation and nonresponsive to platinum chemotherapy had significantly higher serum levels of miRNA-125b and that this was also significantly correlated with poor survival rate, suggesting a role for miR-125b in prognosis prediction. Serum miRNA-125b and ratio of miRNA125b/miRNA19b were reported to correlate with response to chemotherapy and survival of lung cancer patients.32 High miR-125b expressions in tissue and serum were associated with a poor treatment response in rectal cancer patients.33 In contrast to these studies, Alegre et al34 reported that advanced melanoma patients had lower serum levels of miR-125b in exosomes. A more recent study showed that glioma patients had decreased serum miRNA-125b levels compared with the normal population, and lower serum miR-125b levels were related to advanced glioma stages.35 Increased or decreased miR-125b expression may contribute to carcinogenesis in different tumor entities.29 Meta-analysis suggested that the miRNA-125b could be a potential biomarker with relatively high accuracy in the diagnosis of many human cancers.35 These studies on miRNA-125 as potential biomarkers for various cancers were summarized in Table 3. Differences in the expression pattern of miRNA-125b at different stages and in different cancers are likely explained by the hypothesis that miRNA-125b, and possibly other miRNAs implicated in cancer, have the capacity to interact with several targets and effect mechanistic changes in several pathways.
TABLE 3.
Previous studies on miRNA-125b as a potential biomarker for various cancers
Multiple targets of miRNA-125b have been identified in various types of cancers in previous studies. miRNA 125b was shown to inhibit cell invasion and migration through targeting of eIF4EBP1 in ovarian cancer cells48 and to act as a transcriptional coactivator at the PSD-95, Discs-large, ZO-1-binding motif in hepatocellular carcinoma cells.49 miRNA-125b was reported to exert its tumor suppressor functions by targeting Sphk1 in bladder cancer,50 and to affect the PI3K/Akt/mTOR signaling pathway in cervical cancer.51 Nishida et al30 found that miR-125b decreased the endogenous level of p53 protein in human colorectal cancer cells. However, miR-125b was shown to suppress cervical cancer cell apoptosis through targeting of BAK1.52 Varied functions of miRNA-125b in other cancers imply a possibility to play different roles in ovarian carcinogenesis and cancer progression. More studies are needed to elucidate the specific mechanisms accounting for functions of miRNA-125b in epithelial ovarian cancer.
The limitations of this pilot study are that only patients with benign ovarian tumors were used in the control group. Our array is limited to screen 48 miRNAs. Only 1 circulating miRNA was measured in our study. The levels of serum miRNA-125b were not dynamically monitored during patient follow-up.
CONCLUSIONS
miRNA-125b may play an important role in the pathogenesis and progression of epithelial ovarian cancer. Circulating miRNA-125b has the potential to become a novel biomarker for early diagnosis and prognosis prediction of epithelial ovarian cancer.
ACKNOWLEDGMENT
The authors are immensely grateful to Dr. Rosemary Hage for her critical article review.
Footnotes
The study was sponsored by Zhejiang Natural Science Foundation (Y2111317 and LY14H160010).
The authors declare no conflicts of interest.
REFERENCES
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta: American Cancer Society; 2015. [Google Scholar]
- 2.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 3.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnick KE, Alder H, Hagan JP, et al. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol. 2009;112:55–59. [DOI] [PubMed] [Google Scholar]
- 5.Romero-Cordoba SL, Salido-Guadarrama I, Rodriguez-Dorantes M, et al. miRNA biogenesis: biological impact in the development of cancer. Cancer Biol Ther. 2014;15:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. [DOI] [PubMed] [Google Scholar]
- 8.Cheng G. Circulating miRNAs: roles in cancer diagnosis, prognosis and therapy. Adv Drug Deliv Rev. 2015;81:75–93. [DOI] [PubMed] [Google Scholar]
- 9.Gao YC, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumour Biol. 2015;36:4843–4850. [DOI] [PubMed] [Google Scholar]
- 10.Liang H, Jiang Z, Xie G, et al. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumour Biol. 2015;36:5305–5313. [DOI] [PubMed] [Google Scholar]
- 11.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. [DOI] [PubMed] [Google Scholar]
- 12.Prahm KP, Novotny GW, Hogdall C, et al. Current status on microRNAs as biomarkers for ovarian cancer. APMIS. 2016;124:337–355. [DOI] [PubMed] [Google Scholar]
- 13.Cui Z, Lin D, Song W, et al. Diagnostic value of circulating microRNAs as biomarkers for breast cancer: a meta-analysis study. Tumour Biol. 2015;36:829–839. [DOI] [PubMed] [Google Scholar]
- 14.Wu K, Li L, Li S. Circulating microRNA-21 as a biomarker for the detection of various carcinomas: an updated meta-analysis based on 36 studies. Tumour Biol. 2015;36:1973–1981. [DOI] [PubMed] [Google Scholar]
- 15.Yu X, Li Z. Serum microRNAs as potential noninvasive biomarkers for glioma. Tumour Biol. 2016;37:1407–1410. [DOI] [PubMed] [Google Scholar]
- 16.Chakraborty C, Das S. Profiling cell-free and circulating miRNA: a clinical diagnostic tool for different cancers. Tumour Biol. 2016;37:5705–5714. [DOI] [PubMed] [Google Scholar]
- 17.Zuberi M, Khan I, Gandhi G, et al. The conglomeration of diagnostic, prognostic and therapeutic potential of serum miR-199a and its association with clinicopathological features in epithelial ovarian cancer. Tumour Biol. 2016;37:11259–11266. [DOI] [PubMed] [Google Scholar]
- 18.Iorio MV, Visone R, Di Leva G, et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007;67:8699–8707. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Yan LX, Wu QN, et al. miR-125b is methylated and functions as a tumor suppressor by regulating the ETS1 proto-oncogene in human invasive breast cancer. Cancer Res. 2011;71:3552–3562. [DOI] [PubMed] [Google Scholar]
- 20.Zheng H, Zhang L, Zhao Y, et al. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One. 2013;8:e77853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung YW, Bae HS, Song JY, et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patients. Int J Gynecol Cancer. 2013;23:673–679. [DOI] [PubMed] [Google Scholar]
- 22.Wei LQ, Liang HT, Qin DC, et al. MiR-212 exerts suppressive effect on SKOV3 ovarian cancer cells through targeting HBEGF. Tumour Biol. 2014;35:12427–12434. [DOI] [PubMed] [Google Scholar]
- 23.Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110:976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xie J, Liu M, Li Y, et al. Ovarian tumor-associated microRNA-20a decreases natural killer cell cytotoxicity by downregulating MICA/B expression. Cell Mol Immunol. 2014;11:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13–21. [DOI] [PubMed] [Google Scholar]
- 26.Yang H, Kong W, He L, et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res. 2008;68:425–433. [DOI] [PubMed] [Google Scholar]
- 27.Rawlings-Goss RA, Campbell MC, Tishkoff SA. Global population-specific variation in miRNA associated with cancer risk and clinical biomarkers. BMC Med Genomics. 2014;7:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zuberi M, Khan I, Mir R, et al. Utility of serum miR-125b as a diagnostic and prognostic indicator and its alliance with a panel of tumor suppressor genes in epithelial ovarian cancer. PLoS One. 2016;11:e0153902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banzhaf-Strathmann J, Edbauer D. Good guy or bad guy: the opposing roles of microRNA 125b in cancer. Cell Commun Signal. 2014;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida N, Yokobori T, Mimori K, et al. MicroRNA miR-125b is a prognostic marker in human colorectal cancer. Int J Oncol. 2011;38:1437–1443. [DOI] [PubMed] [Google Scholar]
- 31.Cui EH, Li HJ, Hua F, et al. Serum microRNA 125b as a diagnostic or prognostic biomarker for advanced NSCLC patients receiving cisplatin-based chemotherapy. Acta Pharmacol Sin. 2013;34:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ponomaryova AA, Morozkin ES, Rykova EY, et al. Dynamic changes in circulating miRNA levels in response to antitumor therapy of lung cancer. Exp Lung Res. 2016;42:95–102. [DOI] [PubMed] [Google Scholar]
- 33.D’Angelo E, Fassan M, Maretto I, et al. Serum miR-125b is a non-invasive predictive biomarker of the pre-operative chemoradiotherapy responsiveness in patients with rectal adenocarcinoma. Oncotarget. 2016;7:28647–28657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alegre E, Sanmamed MF, Rodriguez C, et al. Study of circulating microRNA-125b levels in serum exosomes in advanced melanoma. Arch Pathol Lab Med. 2014;138:828–832. [DOI] [PubMed] [Google Scholar]
- 35.Wei X, Chen D, Lv T, et al. Serum microRNA-125b as a potential biomarker for glioma diagnosis. Mol Neurobiol. 2016;53:163–170. [DOI] [PubMed] [Google Scholar]
- 36.Mar-Aguilar F, Mendoza-Ramirez JA, Malagon-Santiago I, et al. Serum circulating microRNA profiling for identification of potential breast cancer biomarkers. Dis Markers. 2013;34:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mar-Aguilar F, Luna-Aguirre CM, Moreno-Rocha JC, et al. Differential expression of miR-21, miR-125b and miR-191 in breast cancer tissue. Asia Pac J Clin Oncol. 2013;9:53–59. [DOI] [PubMed] [Google Scholar]
- 38.Vriens MR, Weng J, Suh I, et al. MicroRNA expression profiling is a potential diagnostic tool for thyroid cancer. Cancer. 2012;118:3426–3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ichimi T, Enokida H, Okuno Y, et al. Identification of novel microRNA targets based on microRNA signatures in bladder cancer. Int J Cancer. 2009;125:345–352. [DOI] [PubMed] [Google Scholar]
- 40.Si H, Sun X, Chen Y, et al. Circulating microRNA-92a and microRNA-21 as novel minimally invasive biomarkers for primary breast cancer. J Cancer Res Clin Oncol. 2013;139:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuxia M, Zhennan T, Wei Z. Circulating miR-125b is a novel biomarker for screening non-small-cell lung cancer and predicts poor prognosis. J Cancer Res Clin Oncol. 2012;138:2045–2050. [DOI] [PubMed] [Google Scholar]
- 42.Wang H, Tan G, Dong L, et al. Circulating MiR-125b as a marker predicting chemoresistance in breast cancer. PLoS One. 2012;7:e34210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He Q, Chen Z, Cabay RJ, et al. microRNA-21 and microRNA-375 from oral cytology as biomarkers for oral tongue cancer detection. Oral Oncol. 2016;57:15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zuo D, Chen L, Liu X, et al. Combination of miR-125b and miR-27a enhances sensitivity and specificity of AFP-based diagnosis of hepatocellular carcinoma. Tumour Biol. 2016;37:6539–6549. [DOI] [PubMed] [Google Scholar]
- 45.Yamada A, Horimatsu T, Okugawa Y, et al. Serum miR-21, miR-29a, and miR-125b are promising biomarkers for the early detection of colorectal neoplasia. Clin Cancer Res. 2015;21:4234–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Svoboda M, Izakovicova Holla L, Sefr R, et al. Micro-RNAs miR125b and miR137 are frequently upregulated in response to capecitabine chemoradiotherapy of rectal cancer. Int J Oncol. 2008;33:541–547. [PubMed] [Google Scholar]
- 47.Wang F, Yu D, Liu Z, et al. MiR-125b functions as a tumor suppressor and enhances chemosensitivity to cisplatin in osteosarcoma. Technol Cancer Res Treat. 2016. [DOI] [PubMed] [Google Scholar]
- 48.Lee M, Kim EJ, Jeon MJ. MicroRNAs 125a and 125b inhibit ovarian cancer cells through post-transcriptional inactivation of EIF4EBP1. Oncotarget. 2016;7:8726–8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Fang L, Yu W, et al. MicroRNA-125b suppresses the migration and invasion of hepatocellular carcinoma cells by targeting transcriptional coactivator with PDZ-binding motif. Oncol Lett. 2015;9:1971–1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, He W, Li J, et al. MiRNA-125b inhibits proliferation and migration by targeting SphK1 in bladder cancer. Am J Transl Res. 2015;7:2346–2354. [PMC free article] [PubMed] [Google Scholar]
- 51.Cui F, Li X, Zhu X, et al. MiR-125b inhibits tumor growth and promotes apoptosis of cervical cancer cells by targeting phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol Biochem. 2012;30:1310–1318. [DOI] [PubMed] [Google Scholar]
- 52.Wang YD, Cai N, Wu XL, et al. OCT4 promotes tumorigenesis and inhibits apoptosis of cervical cancer cells by miR-125b/BAK1 pathway. Cell Death Dis. 2013;4:e760. [DOI] [PMC free article] [PubMed] [Google Scholar]


