Viral hepatitis may increase the risk of subsequent uveitis. Patients with hepatitis B virus and hepatitis C virus coinfection had the highest risk. Patients with cirrhosis had a higher risk in the multivariable model but did not attach statistic difference. Although these epidemiologic studies yielded informative results, the underlying mechanism remains to be investigated.
Key words: hepatitis B, hepatitis c, hepatitis D, liver cirrhosis, uveitis, viral hepatitis
Abstract
Purpose:
This study investigates whether patients with viral hepatitis and cirrhosis are at risk of uveitis in the years following hepatitis.
Methods:
We used data from Taiwan National Health Insurance system. The cases were patients newly diagnosed with viral hepatitis from 2000 to 2011. The end point of interest was a diagnosis of uveitis. A chi-square test was used for the difference of demographic characteristics between viral hepatitis and comparison. The risk of uveitis in hepatitis was stratified using Cox proportional hazard regression.
Results:
We selected 17,389 patients with viral hepatitis and 34,778 matched comparison. The risk of uveitis in hepatitis cohort was 1.30-fold (95% confidence interval = 1.01–1.69). Patients with hepatitis B virus and hepatitis C virus coinfection had the highest risk (hazard ratio = 2.88; 95% confidence interval = 1.07–7.78), and followed by only hepatitis C virus infection (hazard ratio = 1.75; 95% confidence interval = 1.10–2.79). Patients with cirrhosis had a higher risk in the multivariable model but did not attach statistic difference.
Conclusion:
Patients with hepatitis B virus and hepatitis C virus coinfection had the highest risk of uveitis. In patients with hepatitis C virus and/or hepatitis B virus infection, the symptoms of uveitis should be alerted. Although these epidemiologic studies yielded informative results, the underlying mechanisms and the host's genetic factors remain to be investigated.
Uveitis can be caused by various systemic diseases and the association of viral hepatitis with uveitis has been proposed.1–7 Among all the causes of viral hepatitis, hepatitis B8,9 and C10–12 are quite common and noteworthy problems in Taiwan and all over the world. Chronic viral hepatitis B, C, and D, and so on can lead to liver cirrhosis, which refers to a group of chronic liver diseases in which normal liver cells are damaged and replaced by scar tissue, decreasing the amount of normal liver tissue. Patients with hepatitis complicated with liver cirrhosis have been noted with higher aqueous flare values than noncirrhosis subjects.4
In the literature, few published reports have addressed the relationship between viral hepatitis, liver cirrhosis, and the potential risk of uveitis. Also, the estimated association between viral hepatitis, liver cirrhosis, and uveitis remains unresolved. In this study, we conducted a nationwide cohort study to measure the association by analyzing the claims data from the Taiwan National Health Insurance Research Database (NHIRD) during a follow-up period from 2000 to 2011 to with International Classification of Diseases, Ninth Revision (ICD-9) codes used to identify the correlation among these diseases in a Taiwanese population.
Patients and Methods
Data Source
Taiwan's Bureau of National Health Insurance set up the NHIRD based on the single-payer National Health Insurance program. This program was inaugurated on March 1, 1995 and provides coverage to more than 99% of all residents in Taiwan. We obtained a Longitudinal Health Insurance Database, a part of the NHIRD, which includes 1 million insurants randomly selected from the 2000 Registry for Beneficiaries. All medical claims included both inpatient and outpatient visits and medical treatment for each insurant from the start of 1996 to the end of 2011 that were contained in the Longitudinal Health Insurance Database. To comply with the Personal Information Protection Act, the identification of each insurant in the Longitudinal Health Insurance Database was recoded. This study was also approved by the Institutional Review Board of China Medical University Hospital, Taiwan.
Study Subject
We collected data of patients who were newly diagnosed with viral hepatitis (ICD-9, Clinical Modification [ICD-9-CM] 070.XX and V02.6X) from 2000 to 2011. People with at least 3 medical visits for viral hepatitis were defined as a new case and the first visit date for viral hepatitis was defined as the index date. Although the data collection period was from 2000 to 2011, we have traced back and checked all their medical records to make sure that no previous diagnosis of viral hepatitis was made even before 2000. Those with a history of uveitis (ICD-9-CM 360.00, 360.11, 360.12, 362.18, 363.00, 363.01, 363.03, 363.05–363.08, 363.1x, 363.20, 363.21, 363.4x, 364.00–364.02, 364.04 and 364.1x–364.3x), or were younger than 20 years were excluded. Patients with interferon treatment, human immunodeficiency virus infection, tuberculosis, syphilis, or autoimmune diseases were also excluded. Subjects without medical visit for eye diseases were also excluded.
Controls were randomly selected from people without viral hepatitis, interferon treatment, human immunodeficiency virus infection, tuberculosis, syphilis, or uveitis history. They were frequency matched by age group (20–24 years, 25–29 years, 30–34 years old and so on), gender, monthly income, occupation, ophthalmologic outpatient department (OPD) before the index date and index-year at a ratio 2:1. We included only patients with at least one medical visit for ophthalmology before enrolling into the study. After this restriction, we matched the ophthalmologic OPD visits between both the groups.
End point, Demographic Characteristics, and Viral Hepatitis Type
The interested end point was uveitis diagnosis. People with at least twice medical visits for uveitis, which was separated for at least 7 days, were defined as the end point to ensure the validity. All study subjects were followed from the index date until when the end point occurred. Those without endpoint development were followed until the date of withdrawal from the program or the end of 2011, whichever occurred first. In this study, the demographic characteristics included age group (20–29 years, 30–39 years, 40–49 years, 50–59 years, and 60 years and older), gender, occupation (white collar, blue collar and others), monthly income (<486, 486–810 and >810 US dollar, USD), and ophthalmologic OPD before the index date. According to the different viral hepatitis infection, viral hepatitis cohorts were grouped into five groups: only hepatitis A virus infection (HAV; ICD-9-CM 070.0x and 070.1x), only hepatitis B virus infection (HBV; ICD-9-CM 070.2x, 070.3x, and V02.61), only hepatitis C virus infection (HCV; ICD-9-CM 070.41, 070.44, 070.51, 070.54, 070.7, 070.70, 070.71 and V02.62), HBV combined HCV infection, and other (non-A, non-B, non-C hepatitis of viral cause).
Statistical Analysis
The incidence (per 10,000) and prevalence (per 10,000) of alcoholic liver disease (ALD; ICD-9-CM 571.0x-571.3x, except viral hepatitis infection), and different viral hepatitis type were calculated from 2000 to 2011. A chi-square test was used for the difference of demographic characteristics between the viral hepatitis cohort and comparison cohort. A t-test was used for the difference of the mean OPD visit for ophthalmology between the two cohorts. The incidence of uveitis was counted in 2 cohorts (per 10,000 person-years). The risk of uveitis in viral hepatitis cohort comprised with comparison cohort was stratified by age group, gender, occupation, and monthly income using Cox proportional hazard regression. A multivariable Cox model was adjusted for continuous age, gender, occupation, monthly income, and OPD visits for ophthalmology before the index date. The association between uveitis and different viral hepatitis type was estimated.
In further analysis, we also assessed the association between uveitis and cirrhosis in patients with viral hepatitis infection. Because we intended to know whether cirrhosis is an extra risk for uveitis in addition to hepatitis, we used “with or without cirrhosis” as an independent variable in the Cox model and different types of hepatitis as grouping variables. By this way, the contribution of cirrhosis to the occurrence of uveitis can be identified in different types of hepatitis. All analyses were performed using SAS statistical software, version 9.4 for Windows (SAS Institute, Cary, NC). The level of significance was set at P < 0.05 at 2-tailed test.
Results
The prevalence of all forms of hepatitis in Taiwan has generally increased from 2000 to 2011 (Figure 1A). The prevalence of viral hepatitis has also generally increased (Figure 1B). However, the incidence of viral hepatitis in Taiwan has generally decreased from 2000 to 2011 (Figure 1C). Among them, the prevalence of ALD and only HBV, only HCV, and HBV combined HCV infection increased by year, except HAV and other viral infections. The highest change of prevalence was in only HCV infection (∆2.08 = [63.73–20.66]/20.66), followed by only HBV infection (∆1.77), HBV combined HCV infection (∆1.67), and ALD (∆0.60). But the incidence of ALD and each viral hepatitis type infection decreased by year (Figure 1A). The highest change of incidence was in other viral infection (∆−0.84), followed by only HAV infection (∆−0.72), only HBV infection (∆−0.65), only HCV infection (∆−0.56), ALD (∆−0.49), and HBV combined HCV infection (∆−0.04).
Fig. 1.
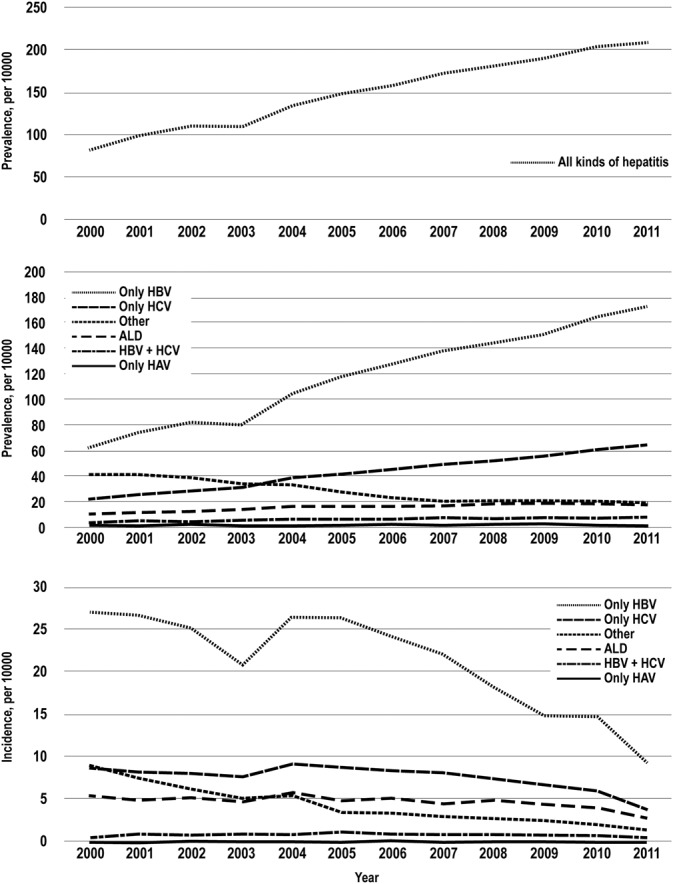
A. The prevalence of all forms of hepatitis in Taiwan has generally increased from 2000 to 2011. B. The prevalence of different viral hepatitis in Taiwan has generally increased from 2000 to 2011. C. The incidence of different viral hepatitis in Taiwan has generally decreased from 2000 to 2011.
In this retrospective cohort study, we selected 17,389 patients with viral hepatitis infection and 34,778 age, gender, monthly income, occupation, ophthalmologic OPD, and index-year–matched comparison. In patients infected with viral hepatitis, there were more men than women (56.8% vs. 43.2%) and the mean age was 46.0 years (SD = 15.0; Table 1).
Table 1.
Demographic Characteristics Between Viral Hepatitis Infection and Comparison Cohort
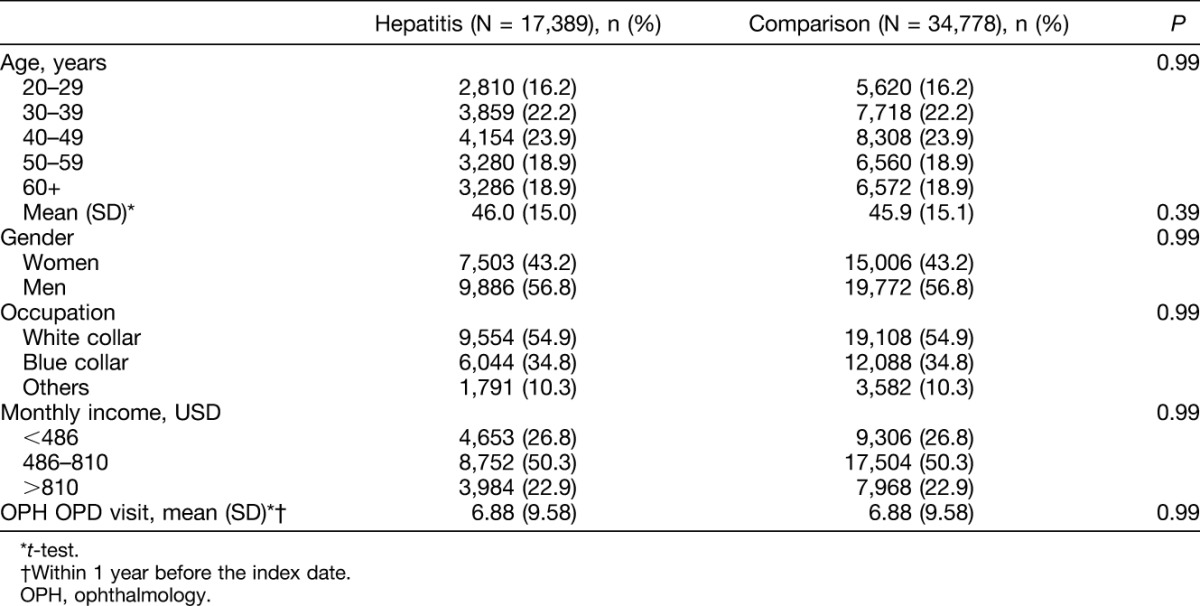
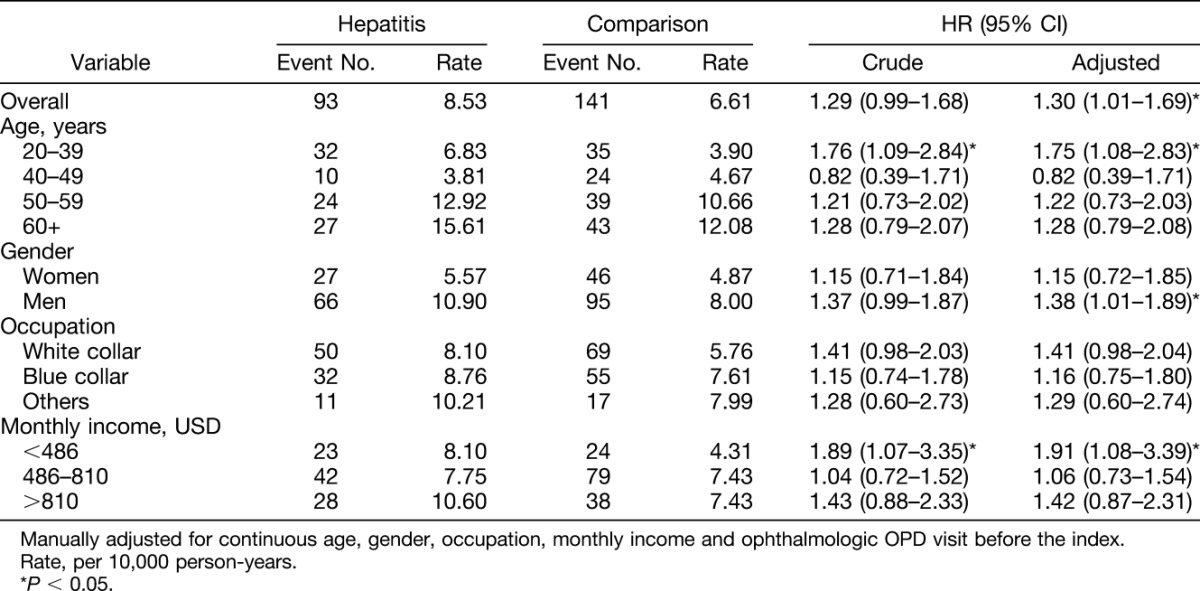
The mean follow-up years were 6.27 (SD = 3.30) and 6.13 (SD = 3.31) in hepatitis and comparison cohort, respectively. There was a significant difference of mean follow-up years between the two cohorts because of a large sample size. The mean follow-up years were 6.24, 6.22, 5.70, 6.01, and 6.80 (SD = 3.16, 3.26, 3.25, 3.27, and 3.35, respectively) in only HAV, only HBV, only HCV, HCV and HBV, respectively, and other subcohort. During the follow-up period, the incidence of uveitis in the viral hepatitis infection cohort was 1.29 times greater than that of the comparison cohort (8.53 vs. 6.61 per 10,000 person-years; Table 2). After adjusting for continuous age, gender, occupation, monthly income, and ophthalmologic OPD visits before the index date, the risk of uveitis in the viral hepatitis infection cohort was 1.30 times than that of the comparison cohort (95% confidence interval [CI] = 1.01–1.69). No matter at which age group the patients were in, gender, occupation, and monthly income, viral hepatitis infection patients had a higher risk of uveitis than comparisons, except for those at age 40 to 49 years, but did not reach statistical significance. Only men aged 20 to 39 years and those with monthly income at <486 USD presented a significantly higher risk of uveitis. The fact that uveitis events occurred more in men (N = 161) than in women (N = 73). The hepatitis infection-associated uveitis risk only in male patients presented significantly higher (HR = 1.35, 95% CI = 1.01–1.89). However, the interaction between gender and hepatitis for risk of uveitis was not significant (P = 0.53 in multivariable model).
Table 2.
Incidence and HR of Uveitis in Viral Hepatitis Infection Patients Compared With Comparisons Stratified by Gender, Age, Occupation, and Monthly Income
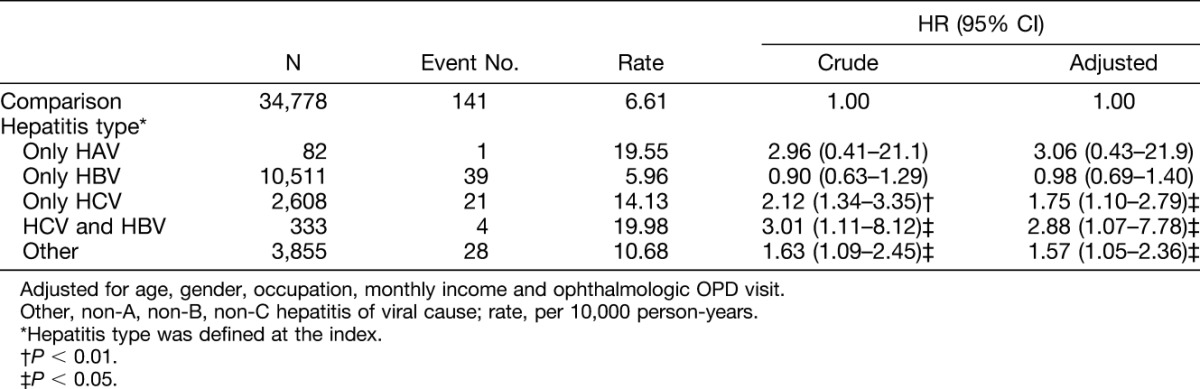
In different viral hepatitis type infections, the higher incidence of uveitis was observed in patients with HBV and HCV coinfection (19.98 per 10,000 person-years; Table 3), only HAV (19.55 per 10,000 person-years), only HCV infection (14.13 per 10,000 person-years), other causes of hepatitis (10.68 per 10,000 person-years), and HBV infection (5.96 per 10,000 person-years). In our multivariable model, a significantly higher risk of uveitis was only observed in patients with HBV and HCV coinfection (hazard ratio [HR] = 2.88, 95% CI = 1.07–7.78), patients with only HCV infection (HR = 1.75; 95% CI = 1.10–2.79), and patients with other hepatitis infection (HR = 1.57; 95% CI = 1.05–2.36) compared with the control cohort. In other hepatitis infection, there was almost unspecified viral hepatitis without mention of hepatic coma.
Table 3.
Incidence and HR of Uveitis in Different Viral Hepatitis Type Infection
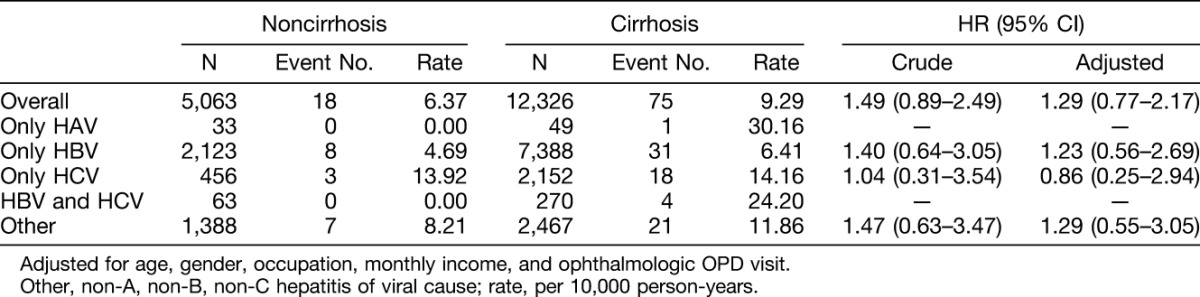
The association between uveitis and liver cirrhosis among different types of viral hepatitis infection is presented in Table 4. Compared with viral hepatitis patients without liver cirrhosis, hepatitis infection patients with liver cirrhosis had a higher risk in the multivariable model but did not attach statistical difference (HR = 1.29; 95% CI = 0.77–2.17).
Table 4.
Association Between Uveitis and Liver Cirrhosis in Cox Proportional Model Among Different Viral Hepatitis Infection
Discussion
Uveitis is one of the leading causes of visual impairment and is responsible for 5% to 20% of legal blindness in Western countries and approximately 25% of blindness in the developing world.13,14 Uveitis can be caused by a remote disease somewhere else. The uvea is rich in blood vessels, so if the immune system is activated in one organ, the inflammatory cells plus cytokines can enter the eyes hematogenously and cause inflammation. Various systemic infective agents have been postulated in the pathogenesis of uveitis, including hepatitis viruses.1–7
Viral hepatitis is the most common type of hepatitis that causes inflammation of the liver and subsequent liver damage. Viral hepatitis includes hepatitis A, hepatitis B, hepatitis C, hepatitis D, and hepatitis E. Hepatitis B and hepatitis C are the most common in Taiwan.8 In the literature, few published reports have estimated the relationship between viral hepatitis, liver cirrhosis, and the potential risk of uveitis.1–7,15,16 Grob et al1 demonstrated that 13% of patients with uveitis were sera positive for hepatitis B surface antigen in a study from Switzerland. A survey from England showed 2% of patients with various types of uveitis were sera hepatitis B surface antigen positive.2 There were 32 spontaneous reports of uveitis occurring after hepatitis B vaccine from the National Registry of Drug-Induced Ocular Side Effects, the World Health Organization, and the Food and Drug Administration between 1982 and 2009.3 A pilot study revealed significantly higher aqueous flare values and increased choroid thickness in patients with hepatitis C. The choroidal thickness also increased as the degree of anterior chamber inflammation increases.4
According to our study, the incidence of viral hepatitis in Taiwan has been generally decreased from 2000 to 2011, which may be attributed to active vaccination and public health programs.8 In addition, because of the advances in the medical care and the prolonged life expectancy, the prevalence of viral hepatitis has generally increased according to the NHIRD.
These viral infections could be associated with extrahepatic manifestations with the possibility to infect not only hepatic but also lymphatic cells and to be associated with an extrahepatic comorbidity of an autoimmune and/or lymphoproliferative nature.17,18 Ocular manifestations related to treatment and nontreatment such as dry eye syndrome, Sjögren syndrome, xerophthalmia, Mooren ulcer, cystoid macular edema, ischemic retinopathy, ischemic optic neuropathy, uveitis, scleritis, and Vogt–Koyanagi–Harada disease with hepatitis B or C have been reported.1–7,15,19,20 Nevertheless, the association between uveitis and viral hepatitis has not been extensively studied to date.
Although rare, atypical ophthalmologic complications associated with interferon treatment, including uveitis, have been reported.15,16,21,22 There were also few case reports of Vogt–Koyanagi–Harada disease occurring during interferon-α and ribavirin therapy for HCV.19,23–25
Our study concludes that viral hepatitis may increase the risk of subsequent uveitis. The risk of uveitis in the viral hepatitis infection cohort was 1.30-fold in comparison with that of the cohort (95% CI = 1.01–1.69). In the multivariable model, patients with HBV and HCV coinfection had the highest risk (HR = 2.88; 95% CI = 1.07–7.78), followed by only HCV infection (HR = 1.75; 95% CI = 1.10–2.79). Furthermore, viral hepatitis infection complicated with liver cirrhosis had a higher risk in the multivariable model but did not attach statistical difference (HR = 1.29; 95% CI = 0.77–2.17).
The uveitis events were observed more in men (N = 161) than in women (N = 73), which is the reason to demonstrate the relatively higher risk of uveitis in men and not in women. However, the interaction between gender and hepatitis for risk of uveitis was not significant in the multivariable model (P = 0.53). It means that the association between uveitis and hepatitis was similar in men and women.
HBV is a 42-nanometer, partially double-stranded DNA virus classified in the Hepadnaviridae family. The HCV is a 55-nanometer, positive-strand RNA virus classified in the Flaviviridae family. HBV can be detected in the eye as a number of studies have revealed hepatitis B surface antigen in tears and aqueous humor of hepatitis B surface antigen seropositive patients.26 Researchers have also detected the presence of HCV RNA in tear fluid.27 Uveitis might be a physiologic immune response to fight an infection within the eye.
The other possible mechanism of comorbidity with uveitis includes the complement-mediated immune pathway leading to granulocyte aggregation within the retinal vasculature, which, in turn, causes the release of inflammatory mediators and uveoretinal inflammation.28,29 Significantly higher levels of immunoglobulin G antiuveal autoantigen with coiled coil domains and ankyrin-repeat autoantibodies were found in patients with panuveitis, compared with healthy controls, indicating that autoimmunity is a common phenomenon in these diseases.30 Hepatitis C virus itself has also been reported to have the capability to modulate genes involved in ocular pathologies.31 The statistical significance of both HBV and HCV increasing the risk of uveitis also suggests that this association exists.
Our study has a few limitations. First, diagnoses of viral hepatitis, liver cirrhosis, and uveitis are identified completely dependent on ICD codes. However, Taiwan's Bureau of National Health Insurance has established a mechanism to interview patients and it reviews medical charts to verify diagnosis validity and quality of care. Hospitals receive heavy penalties from the National Health Insurance Bureau when discrepancies, overcharging, and malpractice are discovered. Comorbidity between nonuveitic ocular manifestations and uveitis cannot be definitely excluded. However, the diagnostic bias could be lessened because of codes specific for uveitis, Sjögren syndrome, dry eye syndrome, and ischemic retinopathy in NHIRD. Second, these findings may only be related to the Taiwanese population and that other similar studies should be performed in different countries to see if the association holds.
Because the incidence of HBV and HCV is relatively low in western countries, the literature with large case number and similar findings is difficult to obtain. Nevertheless, this also demonstrates the originality of this research. However, more robust evidence from further investigations about different viral infections or hepatic diseases is needed to explain the findings in this study.
In conclusion, this study suggests that viral hepatitis may increase the risk of subsequent uveitis. No matter at which age group, gender, occupation, and monthly income, patients with viral hepatitis infection had a higher risk of uveitis than comparisons, except those in the age group of 40 years to 49 years, but it did not reach statistical significance. The association between gender and hepatitis for risk of uveitis was not significant in multivariable model. Patients with HBV and HCV coinfection had the highest risk of uveitis. Viral hepatitis infection complicated with liver cirrhosis had a higher risk but did not attach statistical difference. In patients with HCV and/or HBV infection, the symptomatic condition of uveitis should be alerted. Although these epidemiologic studies yielded informative results, the underlying mechanisms and the host's genetic factors remain to be investigated.
Footnotes
Supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212–133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 104–2325-B-039–005), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Paper presented at the 13th Congress of the International Ocular Inflammation Society, San Francisco, CA, September 25–27, 2015.
The authors were involved in design and conduct of study; data collection; analysis, management, and interpretation of data; and preparation, review, and approval of manuscript. None of the authors have any financial/conflicting interests to disclose.
References
- 1.Grob PJ, Martenet AC, Witmer R. Nonspecific immune parameters and hepatitis B antigens in patients with uveitis. Mod Probl Ophthalmol 1976;16:254–258. [PubMed] [Google Scholar]
- 2.Murray PI, Prasad J, Rahi AHS. Status of hepatitis B virus in the aetiology of uveitis in Great Britain. Br J Ophthalmol 1983;67:685–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraunfelder FW, Suhler EB, Fraunfelder FT. Hepatitis B vaccine and uveitis: an emerging hypothesis suggested by review of 32 case reports. Cutan Ocul Toxicol 2010;29:26–29. [DOI] [PubMed] [Google Scholar]
- 4.Strobbe E, Cellini M, Campos EC. Aqueous flare and choroidal thickness in patients with chronic hepatitis C virus infection. Ophthalmology 2013;120:2258–2263. [DOI] [PubMed] [Google Scholar]
- 5.Maya R, Gershwin ME, Shoenfeld Y. Hepatitis B virus (HBV) and autoimmune disease. Clin Rev Allergy Immunol 2008;34:85–102. [DOI] [PubMed] [Google Scholar]
- 6.Köse Ş, Senger SS, Çavdar G, et al. A case with chronic hepatitis B and anterior uveitis—is there any connection? J Microbiol Infect Dis 2011;1:78–79. [Google Scholar]
- 7.Tsoumani A, Theopistos V, Katsanos K, et al. Treatment and non-treatment related ocular manifestations in patients with chronic hepatitis B or C. Eur Rev Med Pharmacol Sci 2013;17:1123–1131. [PubMed] [Google Scholar]
- 8.Chen DS. Fighting against viral hepatitis: lessons from Taiwan. Hepatology 2011;54:381–392. [DOI] [PubMed] [Google Scholar]
- 9.Locarnini S, Hatzakis A, Chen DS, et al. Strategies to control hepatitis B: public policy, epidemiology, vaccine and drugs. J Hepatol 2015;62(1 suppl):S76–S86. [DOI] [PubMed] [Google Scholar]
- 10.Gower E, Estes C, Blach S, et al. Global epidemiology and genotype distribution of the hepatitis C virus infection. J Hepatol 2014;61(1 suppl):S45–S57. [DOI] [PubMed] [Google Scholar]
- 11.Suthar AB, Harries AD. A public health approach to hepatitis C control in low- and middle-income countries. Plos Med 2015;12:e1001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham CS, Swan T. A path to eradication of hepatitis C in low- and middle-income countries. Antivir Res 2015;119:89–96. [DOI] [PubMed] [Google Scholar]
- 13.Bodaghi B, Cassoux N, Wechsler B, et al. Chronic severe uveitis: etiology and visual outcome in 927 patients from a single center. Medicine (Baltimore) 2001;80:263–270. [DOI] [PubMed] [Google Scholar]
- 14.Rothova A, Suttorp-van Schulten MS, Frits Treffers W, et al. Causes and frequency of blindness in patients with intraocular inflammatory disease. Br J Ophthalmol 1996;80:332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Day R, Gillies MC, Ahlenstiel G. Ophthalmologic complications of antiviral therapy in hepatitis C treatment. World J Gastroenterol 2013;19:8227–8237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.London NJ, Garg SJ, Moorthy RS, et al. Drug-induced uveitis. J Ophthalmic Inflamm Infect 2013;3:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zignego AL, Piluso A, Giannini C. HBV and HCV chronic infection: autoimmune manifestations and lymphoproliferation. Autoimmun Rev 2008;8:107–111. [DOI] [PubMed] [Google Scholar]
- 18.Pyrsopoulos NT, Reddy KR. Extrahepatic manifestations of chronic viral hepatitis. Curr Gastroenterol Rep 2001;3:71–78. [DOI] [PubMed] [Google Scholar]
- 19.Sène D, Touitou V, Bodaghi B, et al. Intraocular complications of interferon-α and ribavirin therapy in patients with chronic viral hepatitis C. World J Gastroenterol 2007;13:3137–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zegans ME, Anninger W, Chapman C, et al. Ocular manifestations of hepatitis C virus infection. Curr Opin Ophthalmol 2002;13:423–427. [DOI] [PubMed] [Google Scholar]
- 21.Narkewicz MR, Rosenthal P, Schwarz KB, et al. PEDS-C Study Group. Ophthalmologic complications in children with chronic hepatitis C treated with pegylated interferon. J Pediatr Gastroenterol Nutr 2010;51:183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nigam N, Hedaya J, Freeman WR. Interferon induced sarcoid uveitis with papillitis and macular edema. Retin Cases Brief Rep 2009;3:102–104. [DOI] [PubMed] [Google Scholar]
- 23.Touitou V, Bodaghi B, Cassoux N, et al. Vogt-Koyanagi-Harada disease in patients with chronic hepatitis C. Am J Ophthalmol 2005;140:949–952. [DOI] [PubMed] [Google Scholar]
- 24.Papastathopoulos K, Bouzas E, Naoum G, et al. Vogt-Koyanagi-Harada disease associated with interferon-A and ribavirin therapy for chronic hepatitis C infection. J Infect 2006;52:e59–e61. [DOI] [PubMed] [Google Scholar]
- 25.Sylvestre DL, Disston AR, Bui DP. Vogt-Koyanagi-Harada disease associated with interferon alpha-2b/ribavirin combination therapy. J Viral Hepat 2003;10:467–470. [DOI] [PubMed] [Google Scholar]
- 26.Koksal I, Cetinkaya K, Aker F. Hepatitis B surface antigen in tears and aqueous humor: a comparative study of serum hepatitis B surface antigen levels. Ophthalmologica 1992;204:19–22. [DOI] [PubMed] [Google Scholar]
- 27.Mendel I, Muraine M, Riachi G, et al. Detection and genotyping of the hepatitis C RNA in tear fluid from patients with chronic hepatitis C. J Med Virol 1997;51:231–233. [DOI] [PubMed] [Google Scholar]
- 28.Myers JP, Di Bisceglie AM, Mann ES. Cryoglobulinemia associated with Purtscher-like retinopathy. Am J Ophthalmol 2001;131:802–804. [DOI] [PubMed] [Google Scholar]
- 29.Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryglobulinemia. N Engl J Med 1992;327:1490–1495. [DOI] [PubMed] [Google Scholar]
- 30.Yamada K, Senju S, Nakatsura T. Identification of a Novel autoantigen UACA in patients with panuveitis. Biochem Biophys Res Commun 2001;280:1169–1176. [DOI] [PubMed] [Google Scholar]
- 31.Pazienza V. Ophthalmological complications in hepatitis C virus infection: side effect of interferon therapy or a direct role of HCV? Biomed Pharmacother 2011;65:317–318. [DOI] [PubMed] [Google Scholar]


