Abstract
Background
Shigella is a leading cause of childhood diarrhea mortality in sub-Saharan Africa. Current World Health Organization guidelines recommend antibiotics for children in non cholera-endemic areas only in the presence of dysentery, a proxy for suspected Shigella infection.
Methods
To assess the sensitivity and specificity of the syndromic diagnosis of Shigella-associated diarrhea, we enrolled children aged 6 months to 5 years presenting to 1 of 3 Western Kenya hospitals between November 2011 and July 2014 with acute diarrhea. Stool samples were tested using standard methods for bacterial culture and multiplex polymerase chain reaction for pathogenic Escherichia coli. Stepwise multivariable logit models identified factors to increase the sensitivity of syndromic diagnosis.
Results
Among 1360 enrolled children, median age was 21 months (interquartile range, 11–37), 3.4% were infected with human immunodeficiency virus, and 16.5% were stunted (height-for-age z-score less than −2). Shigella was identified in 63 children (4.6%), with the most common species being Shigella sonnei (53.8%) and Shigella flexneri (40.4%). Dysentery correctly classified 7 of 63 Shigella cases (sensitivity, 11.1%). Seventy-eight of 1297 children without Shigella had dysentery (specificity, 94.0%). The combination of fecal mucous, age over 23 months, and absence of excessive vomiting identified more children with Shigella-infection (sensitivity, 39.7%) but also indicated antibiotics in more children without microbiologically confirmed Shigella (specificity, 82.7%).
Conclusions
Reliance on dysentery as a proxy for Shigella results in the majority of Shigella-infected children not being identified for antibiotics. Field-ready rapid diagnostics or updated evidence-based algorithms are urgently needed to identify children with diarrhea most likely to benefit from antibiotic therapy.
Keywords: algorithms for diarrhea, antibiotic indication, dysentery, pediatric diarrhea, Shigella, syndromic
Shigella is a leading cause of diarrheal disease in children worldwide [1]. Children with Shigella-associated diarrhea are at risk of linear growth faltering and the consequences of this Gram-negative bacteria can be fatal [2–5]. Risk of death from Shigella is associated with young age, malnutrition, human immunodeficiency virus (HIV)-infection, and complications including encephalopathy, hyponatremia, and seizures [6–9]. Historically, Shigella dysenteriae type 1 infections were thought to be responsible for most Shigella-attributed deaths [10]. However, S dysenteriae type 1 prevalence appears to be decreasing globally, and evidence suggests that risk of death and other potentially lethal sequelae may not be Shigella species-specific [7, 8, 11–14]. Although a key clinical feature of S dysenteriae type 1 is bloody stool (dysentery), other species of Shigella are less likely to be dysenteric [8, 15]. For reasons possibly related to the virtual disappearance of S dysenteriae type 1 or more careful management of children presenting with dysenteric diarrhea, dysentery no longer appears to be associated with poor Shigella outcomes [2, 7, 9]. Reducing Shigella-associated morbidity and mortality may require increased attention to Shigella-infected children without dysentery.
Antibiotics reduce time to diarrhea resolution and bacterial clearing in children with dysentery due to Shigella [16, 17]. Antibiotic treatment of nondysenteric Shigella in patients with travelers’ diarrhea and in Shigella-challenged volunteers also confers similar benefits [18, 19]. Given the risk of poor outcome associated with nondysenteric species of Shigella, antibiotic treatment of all childhood Shigella infections may reduce morbidity and mortality attributed to this infection.
In resource-limited settings, where the highest morbidity and mortality burden from Shigella occurs, most clinical facilities lack access to pathogen detection diagnostics. In the absence of such laboratory capacity, health workers rely on clinical judgment and syndrome-based guidelines for management of diarrhea [20]. In World Health Organization (WHO) guidelines, including the updated 2014 Integrated Management of Childhood Illness (IMCI) algorithm for diarrhea, antibiotics (a 3-day course of ciprofloxacin) are only recommended for children with suspected Shigella infection, as determined by presence or history of dysentery, as well as for children with suspected cholera [20, 21].
Within a large cohort of children presenting with acute diarrhea at 3 hospitals in Western Kenya, we sought to determine the sensitivity and specificity of the syndromic definition of dysentery for diagnosing Shigella-associated diarrhea against the gold standard of stool culture. We compared dysentery-alone with alternative classifications of Shigella infection based on sociodemographic factors, clinical history, clinical presentation, and stool examination with the goal of improving the sensitivity of syndromic algorithms for identifying Shigella.
METHODS
Population
We enrolled children aged 6 months to 5 years presenting with acute diarrhea to outpatient departments at 3 hospitals in the Nyanza province of Western Kenya (Kisii Provincial, Homa Bay District, and Migori District Hospital) as part of an ongoing diarrhea and fever surveillance study from November 28, 2011 to July 31, 2014. Acute diarrhea was defined as 3 or more loose or watery stools in the last 24 hours lasting less than 14 continuous days. Written informed consent was obtained from primary caregivers of enrolled children and all study procedures were approved by the University of Washington and Kenya Medical Research Institute (KEMRI) Ethics Committees.
Data Collection
Clinical history and sociodemographic information were collected from the accompanying caregivers. Integrated Management of Childhood Illness-specified clinical signs by physical examination were sought, including general danger signs (not able to drink or breastfeed, excessive vomiting, convulsions, lethargy, stiff neck), dehydration signs (sunken eyes, slow recoil skin pinch, restlessness or irritability, drinking eagerly or thirsty), as well as dysentery (reported history of blood in the stool since the diarrhea episode began). Height, weight, and mid-upper arm circumference were measured by the nursing staff and height-for-age z-scores (HAZ), weight-for-age z-scores (WAZ), and weight-for-height z-scores (WHZ) were calculated using WHO ANTHRO software [22]. Stunting and wasting were defined as HAZ less than −2 and WHZ less than −2, respectively.
All caregivers were provided with stool collection kits and instructions for collection. If a child could not produce stool within 1 hour, 2 rectal swabs were obtained. Blood was collected from children for malaria and HIV testing (per Kenyan National Guidelines). Human immunodeficiency virus status was determined using antibody testing (Abbott Determine rapid test kit and confirmed using Uni-Gold) or HIV DNA polymerase chain reaction (PCR) assays if the child was <18 months old. Malaria parasitemia was assessed by both rapid testing ([RDT] Paracheck Pf Orchid Biomedical Services, India) and microscopy. The HIV status of consenting accompanying biological mothers was ascertained by self-report and confirmed with antibody testing if unknown or HIV negative.
Stool Specimen Processing
Rectal swabs or a portion of stool samples were transferred into Cary-Blair transport medium for bacterial culture; remaining stool samples were placed in 10% formalin for parasite determination. Samples were stored and shipped on cold packs daily to the KEMRI/United States Army Medical Research Unit Microbiology Hub laboratory in Kericho, Kenya, within 24 hours of collection. Upon arrival at the laboratory, stool was examined for gross blood, mucous, and appearance (watery). Bacteria culture and microscopy for parasite detection were performed as described elsewhere [23]. Four lactose fermenting and 2 sorbitol nonfermenting Escherichia coli isolates from children enrolled before October 2013 were used for identification of pathogenic E coli with multiplex PCR [24]. Antibiotic susceptibility testing was performed using the MicroScan Walkaway40 Plus automated bacterial identification platform.
Statistical Analysis
Among enrolled children, we described sociodemographic, clinical history, clinical presentation, macroscopic stool investigation, and enteric pathogen detection with frequencies and percentages or medians and interquartile ranges (IQR). Because enteroinvasive E coli (EIEC) and Shigella share the same virulence gene, ipaH, we also calculated the frequency of overlap between the 2 types of infections. We determined the diagnostic accuracy (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) of dysentery for identifying children infected with culture-confirmed Shigella. Dysentery was defined as either history or presence of bloody stool. For each measure of diagnostic performance, 95% confidence intervals (CIs) were calculated assuming a binomial distribution. We described the frequencies and percentages of various factors in children with and without Shigella infections and calculated the discriminatory ability of each factor using area under the curve (AUC) and associated bootstrapped 95% CIs.
Multivariable logit regression models were fit to determine the minimal set of variables that improved upon the discriminatory ability of dysentery for syndrome-based identification of Shigella infections. The following variables were considered as plausible discriminatory indicators of Shigella infection and ordered by increasing the level of resource and training requirements: Model 1, dysentery; Model 2–Model 1 with laboratory-observed mucous or watery stool; Model 3–Model 2 with easily collected history and presentation information (IMCI general danger signs, IMCI-dehydration signs, age, sex, history of fever, recent antibiotic use, current breastfeeding, and number months of exclusive breastfeeding); Model 4–Model 3 plus measures that require slightly more time, training, and equipment (axillary temperature, HAZ, and WHZ); and Model 5–Model 4 factors in addition to HIV and malaria RDT results, which, although becoming more frequently available in resource-limited settings, may not be feasible for the low-level health facilities. For each model, factors were added stepwise (starting with smallest P value in univariate analyses) into a logistic regression model, and variables were maintained in the model as long as the variable contributed to the discrimination of Shigella infections beyond the previously added variables (as measured by a decreasing Akaike Information Criteria [AIC] value estimated at each model iteration) [25]. The AUCs of the 5 models were compared, pairwise, using nonparametric methods based on a generalized U-statistic. Children were reclassified using the model that was a meaningful improvement on the previous model, but it was not statistically different from the subsequent model. The diagnostic accuracy of the newly created model was assessed.
RESULTS
Study Population
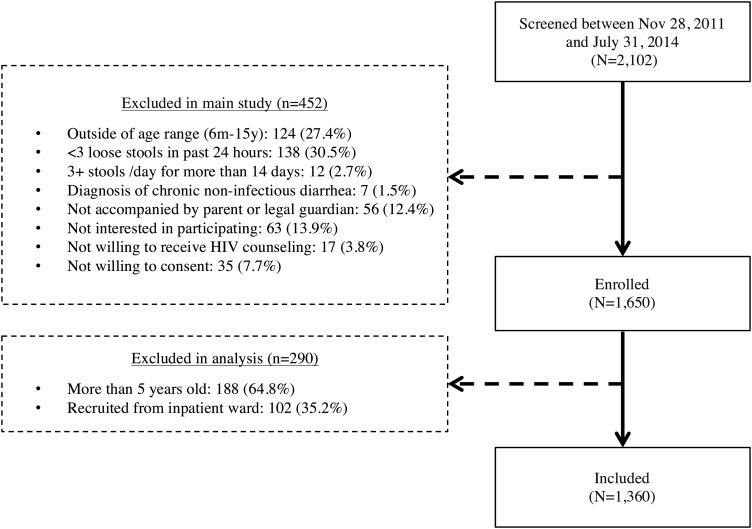
Among 2102 screened children, 742 did not meet prespecified inclusion criteria or declined participation (Figure 1). As a result, 1360 children were included in this analysis. Included children were a median of 21 months old (IQR, 11–37 months), approximately half (47.1%) were female, and the majority (94.4%) enrolled from Kisii or Homa Bay hospitals. Less than half (40.2%) reported a monthly household income of less than 5000 Kenyan shillings (∼$60.00), and 40.0% lived in overcrowded households (≥2 people per room). The median WAZ, WHZ, and HAZ was −0.6 (IQR, −1.4 to 0.3), −0.4 (IQR, −1.6 to 0.6), and −0.5 (IQR, −1.6 to 0.6), respectively; 10.4% had an HIV-infected mother, and 3.4% were infected with HIV (Table 1).
Figure 1.
Number of screened, enrolled, and included participants.
Table 1.
Characteristics of Enrolled Children (N = 1360)
| Characteristic | n (%) |
|---|---|
| Median (IQR) | |
| Sociodemographic | |
| Median age (months) | 21 (11–37) |
| Male | 720 (52.9%) |
| Site | |
| Kisii | 613 (45.1%) |
| Homa Bay | 671 (49.3%) |
| Migori | 76 (5.6%) |
| Monthly household income <5000 Kenyan Shillings | 545 (40.2%) |
| Crowdinga | 507 (40.0%) |
| Livestockb ownership | 1004 (73.9%) |
| Unprotected water sourcec | 186 (13.7%) |
| Reports treating drinking waterd | 1069 (78.9%) |
| Clinical Historye | |
| Bloody stoolf | 79 (6.5%) |
| History of fever within last 48 hours | 597 (43.9%) |
| Antibiotic used in last 7 days | 167 (12.3%) |
| Median no. of months exclusively breastfed | 6 (4–6) |
| Currently breastfeeding (among ≤24 monthsg) | 545 (74.3%) |
| Clinical Presentation | |
| Presenting with any IMCI danger signs | 396 (29.4%) |
| Unable to drink or breastfeed | 55 (4.1%) |
| Excessive vomiting | 344 (25.5%) |
| Convulsions | 6 (0.5%) |
| Lethargy/Unconscious | 24 (1.8%) |
| Presenting with any dehydration signs | 419 (30.8%) |
| Restless/Irritable | 222 (16.3%) |
| Sunken eyes | 255 (18.8%) |
| Drinks eagerly, thirsty | 155 (11.4%) |
| Skin pinch goes back slowly | 83 (6.1%) |
| Sunken fontanelle | 91 (6.7%) |
| IMCI Dehydration Classifications | |
| Severeh | 81 (6.0%) |
| Somei | 188 (13.8%) |
| None | 1091 (80.2%) |
| Stuntedj (HAZ≤) | 220 (16.5%) |
| Wastedk (WHZ≤2) | 243 (18.0%) |
| Axillary temperature ≥37.5°C at presentation | 451 (33.2%) |
| HIV-exposed uninfectedl | 128 (10.4%) |
| HIV-infected | 46 (3.4%) |
| HIV-associated immunosuppressionm | 16 (37.2%) |
| Stool Examinationn | |
| Blood observed in stool | 15 (1.2%) |
| Mucous observed in stool | 696 (55.8%) |
| Watery | 863 (69.2%) |
Abbreviations: CRF, case report form; HAZ, height-for-age z-scores; HIV, human immunodeficiency virus; IMCI, Integrated Management of Childhood Illness; IQR, interquartile range; WHZ, weight-for-height z-scores.
a≥2 people per room living in house.
bOwnership of cows, goats, or chickens.
cUnprotected well/spring/surface water.
dWater treatment method used most often: boil (30.3%), bleach/chlorine (47.8%), strain through cloth (0.9%,) use water filter (0.6%), let it stand and settle (7.8%), other (0.3%), missing (12.4%).
eReported by accompanying caregiver.
fQuestion added to CRF in March 2012, therefore missing for first 147 patients.
gAmong 736 children who were ≤24 months of age.
hTwo or more of the following signs: lethargic or unconscious, sunken eyes, not able to drink or drinking poorly, skin pinch goes back very slowly.
iTwo or more of the following signs: restless/irritable, sunken eyes, drinks eagerly/thirsty, skin pinch goes back slowly.
jHAZ less than −6 and greater than 6 were considered to be implausible values and considered missing.
kWHZ less than −6 and greater than 6 were considered to be implausible values and considered missing.
lAmong children known to be HIV-uninfected, who were accompanied by their biological mother whose HIV status was known (by antibody test or self-report if positive) (N = 1231).
mDefined in terms of CD4% (age ≤11 months: <25%, 12 months–35 months: <20%, 36+ months: <15%) or, in absence of CD4 % data, in terms of CD4 count (age ≤11 months: <1500 cells/mm3, 12 months–35 months: <750 cells/mm3, 36+ months <350 cells/mm3).
nPerformed by laboratory technician upon receipt of whole stools samples (could not be done on rectal swabs) N = 1159.
The majority (90%) of primary caregivers reported that the child had less than 4 continuous days of 3 or more loose stools in a 24-hour period (median number of days of loose stool, 2; IQR, 2–3 days), and the median number of loose stools in the last 24 hours was 5 (IQR, 4–6). Blood in stool was reported by 6.5% of caregivers, and bloody stool lasted a median of 1 day (IQR, 1–2 days). At presentation, 29.4% of children had at least 1 IMCI general danger sign, with 13.8% classified as having some dehydration and 6.0% having severe dehydration (children with severe dehydration were subsequently hospitalized).
Stool Evaluation
Almost all children (91.7%) were able to provide a whole stool sample. Of the 1247 whole stool samples, 1.2%, 55.8%, and 69.2% had evidence of blood, mucous, and watery appearance upon inspection, respectively. Among the 1360 stool samples, 63 (4.6%) were positive for Shigella by microbiologic isolation. We were able to further speciate 52: S sonnei in 28 (53.8%), S flexneri in 21 (40.4%), S dysenteriae in 2 (3.9%), and Shigella boydii in 1 (1.9%) child (Table 2). Of the 43 Shigella-infected children with specimens tested for pathogenic E coli, 5 (11.6%) had EIEC, and these 5 represented 20.8% of the 24 children in whom EIEC was detected. None of the 63 Shigella isolates were resistant to ciprofloxacin, but 57 (90.5%) were resistant to cotrimoxazole, 30 (47.6%) to ampicillin, 47 (74.6%) to tetracycline, and 22 (34.9%) were multidrug (cotrimoxazole, ampicillin, and tetracycline) resistant.
Table 2.
Prevalence of Enteric Infections Among Enrolled Children Overall and by Dysenteric History/Presentation
| Organism Identified | Overall N = 1360 |
History or Current Visible Blood in Stool N = 85 |
No History or Current Visible Blood in Stool N = 1275 |
|---|---|---|---|
| Bacteria (N = 1360) | N % | n % | n % |
| No bacteriaa | 589 (64.7%) | 37 (67.3%) | 552 (64.6%) |
| Any pathogenic Escherichia colib | 222 (22.5%) | 14 (25.5%) | 208 (24.3%) |
| EAEC | 124 (12.6%) | 4 (7.3%) | 120 (14.0%) |
| EHEC | 2 (0.2%) | 0 | 2 (0.2%) |
| EIEC | 24 (2.5%) | 3 (5.5%) | 21 (2.5%) |
| EPEC (atypical)c | 18 (2.3%) | 2 (4.1%) | 16 (2.2%) |
| EPEC (typical) | 38 (3.9%) | 2 (3.6%) | 36 (4.2%) |
| ETEC | 41 (4.2%) | 3 (5.5%) | 38 (4.4%) |
| Campylobacter sppd | 101 (7.4%) | 3 (3.5%) | 98 (7.7%) |
| Shigella spp | 63 (4.6%) | 7 (8.2%) | 56 (4.4%) |
| Shigella boydii | 1 (1.6%) | 0 | 1 (0.08%) |
| Shigella dysentariae | 2 (0.17%) | 1 (1.2%) | 1 (0.08%) |
| Shigella flexneri | 21 (1.8%) | 5 (5.9%) | 16 (2.2%) |
| Shigella sonnei | 28 (2.1%) | 1 (1.2%) | 27 (2.1%) |
| Undeterminede | 11 (0.8%) | 0 | 11 (0.9%) |
| Salmonella sppf | 13 (1.0%) | 1 (1.2%) | 12 (0.9%) |
| Aeromonas hyrophila | 3 (0.2%) | 0 | 3 (0.2%) |
| Plesiomonas shigelloides | 1 (0.07%) | 0 | 1 (0.1%) |
| Parasites (N = 1247g) | |||
| Giardia spp | 127 (10.2%) | 4 (4.8%) | 123 (10.6%) |
| Cryptosporidium spp | 56 (4.5%) | 1 (1.2%) | 55 (4.7%) |
| Helminthsh | 39 (3.1%) | 5 (6.0%) | 34 (2.9%) |
| Entamoeba histolytica/dispar | 5 (0.4%) | 0 | 5 (0.4%) |
| Isospora spp | 1 (0.08%) | 0 | 1 (0.1%) |
| No bacteria or parasite (N = 813i) | 423 (52.0%) | 33 (61.1%) | 390 (51.4%) |
Abbreviations: EAEC, enteroaggregative E coli; EHEC, enterohemorrhagic E coli; EIEC, enteroinvasive E coli; EPEC, enteropathogenic E coli; ETEC, enterotoxigenic E coli.
aAmong the 910 children whose stool was cultured and PCR tested for pathogenic E. coli; among those with dysentery (n=55).
bTested only in children enrolled before October 2013 (n=910 samples, among those with dysentery [n=55]).
cAmong those in which eae gene was tested (n=770, among those with dysentery [n=49]).
dCampylobacter jejuni (n=76), Campylobacter spp. other than jejuni (n=25).
eNot speciated due to antisera stock out.
fNon-typhoidal species (n=12), Salmonella typhi (n=1).
gTestable only among those who provided a whole stool sample (as opposed to rectal swab); among those with dysentery (n=83).
hOnly able to detect Ascaris lumbricoides infections.
iAmong those who were able to provide a stool sample (rather than rectal swab) and who were tested for all bacteria (including pathogenic E.coli); among those with dysentery (n=54).
Sensitivity and Specificity of Dysentery for Shigella Identification
Eight-five (6.3%) children had dysentery by history or stool observation. Among the 63 children with Shigella-positive stools, 7 had dysentery (sensitivity, 11.1% [95% CI, 4.6%–21.6%] and PPV, 8.2% [95% CI, 3.4%–16.2%]). Among the 1297 children without microbiologic isolation of Shigella, 1219 did not have a history of bloody stools (specificity, 94.0% [95% CI, 92.6%–95.2%] and NPV, 95.6% [95% CI, 94.3%–96.7%]). When considering the 23 children with either S dysenteriae or S flexneri infections only, the diagnostic performance of dysentery was slightly higher, because 6 children with 1 of these 2 Shigella species had dysentery (sensitivity, 26.1% [95% CI, 10.2%–48.4%]; specificity, 94.1% [95% CI, 92.7%–95.3%]; PPV, 7.1% [95% CI, 2.6%–14.7%], and NPV, 98.7% [95% CI, 97.9%–99.2%]). The most common bacteria and parasites other than Shigella identified in the stools of the 85 children with dysentery included the following: enteroaggregative E coli (7.3%), Ascaris lumbricoides (6.0%), enterotoxigenic E coli (ETEC) (5.5%), and Giardia lamblia (4.8%) (Table 2).
Predictors of Shigella Infections
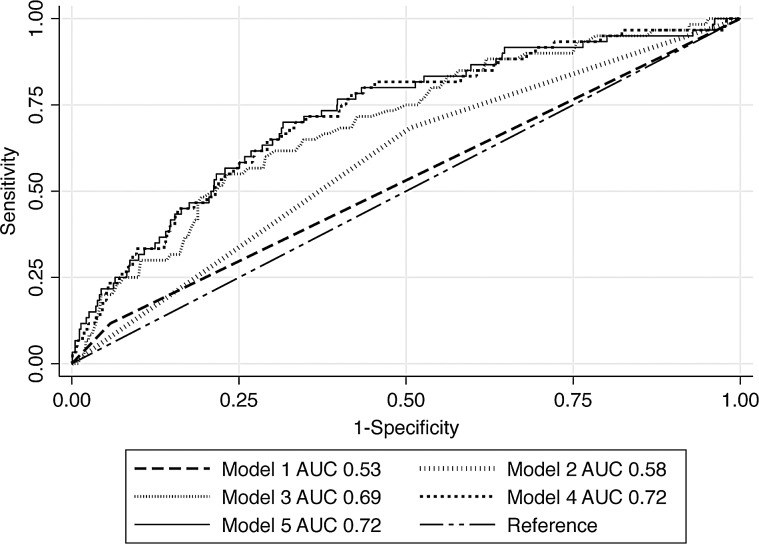
No single characteristic discriminated well between children with and without Shigella infections, with AUC values ranging from 0.50 to 0.54 (Table 3). The AUC for dysentery (Model 1) was 0.53 (95% CI, 0.50–0.59) (Figure 2; Supplementary Table 1). In Model 2, containing additional observable stool factors, fecal mucous was the only predictor maintained in the model (Model 2 AUC, 0.57 [95% CI, 0.50–0.64]) with dysentery not being included based on prespecified model building criteria. Model 2 discriminated somewhat better than Model 1 (P value comparing AUC of Model 1 to Model 2 = .086). Of factors considered for Model 3, fecal mucous, age, and absence of excessive vomiting (as defined by IMCI) were retained in the model, resulting in an AUC of 0.69 (95%CI, 0.61–0.75), a substantial improvement from Model 2 (P = .0015). More intense examination of the child (Model 4) and malaria and HIV status (Model 5) resulted in slight improvements in the AUC values but did not reach statistical significance (Model 4 vs Model 3, P = .10 and Model 5 vs Model 3, P = .15). Presence of mucous in stool, older age, and absence of excessive vomiting constituted the minimal set of discriminatory variables for identifying children with Shigella infections. If children presenting with mucous in stool, without excessive vomiting, and between the ages of 24 and 59 months were considered the group of children with suspected Shigella infection instead of dysentery, then 249 of the children enrolled in this study would have met criteria for ciprofloxacin indication, 25 of whom were infected with Shigella (PPV, 10.0%; sensitivity, 39.7%). Using this criteria, 1111 children would not have met criteria for ciprofloxacin treatment, despite 38 (3.4%) being infected with Shigella (NPV, 96.6%; specificity, 82.7%).
Table 3.
Ability of Sociodemographic and Clinical Factors to Discriminate Between Children With and Without Shigella Infections
| Characteristic |
Shigella spp (N = 63) |
No Shigella (N = 1297) |
Discriminatory Ability |
|---|---|---|---|
| N (%) | N (%) | AUCa 95% CI | |
| Sociodemographic | |||
| Age | |||
| 6–11 months | 7 (11.1%) | 367 (28.3%) | |
| 12–23 months | 12 (19.1%) | 364 (28.1%) | 0.52 (0.51–0.54) |
| 24–59 months | 44 (69.8%) | 566 (43.6%) | |
| Monthly household income <5000 Kenyan Shillings | 15 (23.8%) | 499 (38.5%) | 0.51 (0.50–0.52) |
| Crowding | 17 (27.0%) | 528 (40.8%) | 0.51 (0.50–0.52) |
| Livestock ownership | 49 (77.8%) | 955 (73.8%) | 0.50 (0.50–0.52) |
| Household treats drinking water | 53 (84.1%) | 1016 (78.4%) | 0.51 (0.49–0.52) |
| Unprotected water source | 7 (11.1%) | 179 (13.8%) | 0.51 (0.50–0.52) |
| Clinical Presentation | |||
| Unable to drink or breastfeed | 4 (6.5%) | 51 (4.0%) | 0.51 (0.50–0.55) |
| Excessive vomiting | 7 (11.3%) | 337 (26.2%) | 0.52 (0.51–0.53) |
| Lethargic/unconscious | 0 (0%) | 24 (1.9%) | 0.52 (0.52–0.53) |
| Restless | 9 (14.3%) | 213 (16.4%) | 0.51 (0.50–0.52) |
| Sunken eyes | 11 (17.5%) | 244 (18.8%) | 0.50 (0.50–0.52) |
| Thirsty | 6 (9.5%) | 149 (11.5%) | 0.50 (0.50–0.52) |
| Slow skin pinch | 1 (1.6%) | 82 (6.3%) | 0.52 (0.51–0.53) |
| Sunken fontanelle | 2 (3.2%) | 89 (6.9%) | 0.51 (0.50–0.53) |
| No dehydration | 52 (82.5%) | 1039 (80.1%) | |
| Some dehydration | 8 (12.7%) | 1807 (13.9%) | |
| Severe dehydration | 2 (4.8%) | 8 (6.0%) | 0.51 (0.50–0.53) |
| Blood in stool | 2 (3.2%) | 13 (1.0%) | 0.54 (0.50–0.63) |
| Mucous in stool | 42 (66.7%) | 654 (50.4%) | 0.51 (0.50–0.53) |
| Stunted (HAZ less than −2) | 7 (11.3%) | 213 (16.8%) | 0.51 (0.50–0.52) |
| Wasted (WHZ less than −2) | 8 (12.9%) | 235 (18.3%) | 0.51 (0.50–0.52) |
| MUAC <12.5 cm | 1 (1.6%) | 79 (6.1%) | 0.52 (0.50–0.53) |
| Malaria | 4 (6.4%) | 139 (10.7%) | 0.51 (0.50–0.53) |
| Current fever (≥37.5°C) | 27 (42.9%) | 424 (32.7%) | 0.51 (0.50–0.52) |
| Clinical History | |||
| Fever in last 48 hours | 32 (50.8%) | 565 (43.6%) | 0.51 (0.50–0.52) |
| History of bloody stool | 7 (12.7%) | 72 (6.2%) | 0.52 (0.50–0.56) |
| Currently breastfeeding among ≤24 month olds | 13 (59.1%) | 557 (74.0%) | 0.51 (0.50–0.53) |
| Exclusively breastfed for less than 6 months | 28 (45.9%) | 461 (36.6%) | 0.51 (0.50–0.52) |
| HIV-infected | 3 (4.8%) | 43 (3.4%) | 0.51 (0.50–0.55) |
| HIV-exposed uninfected | 1 (0.8%) | 51 (4.6%) | 0.52 (0.51–0.53) |
| Antibiotic use within the last 7-days | 5 (7.9%) | 162 (12.5%) | 0.51 (0.50–0.52) |
| Hospitalization within the last year | 4 (6.4%) | 96 (7.4%) | 0.50 (0.50–0.52) |
Abbreviations: AUC, area under the curve; CI, confidence interval.
aIn cases when presence of factor is more common in non-Shigella cases (inversely associated with Shigella), the AUC is calculated based on absence (rather than presence) of that factor.
Figure 2.
Comparison of Integrated Management of Childhood Illness (IMCI) classification of dysentery (suspected Shigella infection) (Model 1) to classifications that consider additional factors ordered from least resource-demanding to most Models 2–5). Model 2–Model 1 with additionally observable stool characteristics (mucous and watery). Model 3–Model 2 with easily collected clinical history (IMCI danger and dehydration signs, breastfeeding history, age, sex). Model 4–Model 3 factors plus additional information collected from physical exam (axillary temperature, weight-for-height-z, height-for-age-z. Model 5–Model 4 in addition to rapid human immunodeficiency virus and malaria blood test results. AUC, area under the curve.
DISCUSSION
In this study of children presenting to Kenyan hospitals for acute diarrhea, IMCI dysentery-based treatment guidelines identified few children infected with Shigella spp. In the absence of laboratory diagnosis, very few of the children infected with Shigella (11%) would have an indication for antibiotic treatment. Given recent evidence that S flexneri and S sonnei outnumber S dysenteriae type 1 infections in Asia and Africa, that these species are less likely than S dysenteriae type 1 to present with visible bloody stool, and that host factors such as malnutrition may play a more important role than species in determining risk of death associated with Shigella infection, the rationale for limiting syndrome-based antibiotic indication to dysentery alone may need to be revisited [5, 8, 12, 15]. We identified additional factors, namely presence of mucous, older age, and absence of excessive vomiting, that did improve discrimination between children with and without Shigella infections. However, the sensitivity and PPV remained fairly poor with this new clinical algorithm and would miss children at the highest risk of diarrhea-associated mortality (such as children under 2 years) [1]. Evidence-based strategies for Shigella identification and risk stratification are urgently needed to identify groups of children most likely to benefit from antibiotic therapy during a diarrheal episode.
International diarrhea management guidelines, including the IMCI guidelines, limit the recommended use of antibiotics to relatively few indications. This may be due to potential prolonged Salmonella carriage and increased morbidity in enterohemorrhagic E coli infections, the high prevalence of viral diarrheal pathogens, and the need for antibiotic stewardship in an era of increasing antimicrobial resistance [26]. After rotavirus, the most common causes of moderate-to-severe diarrhea (MSD) in Africa and Asia are bacterial (ETEC, Shigella spp, enteropathogenic E coli [EPEC], Campylobacter spp) and parasitic (Cryptosporidium), and many of these pathogens are also associated with linear growth faltering and death [1, 3, 27–29]. Antimicrobials have demonstrated efficacy in treating gastroenteritis caused by specific pathogens, namely Shigella spp, Vibrio cholerae, Cryptosporidium spp, Campylobacter spp, and in the context of traveler's diarrhea, commonly caused by ETEC, EPEC, and Campylobacter spp [17, 30–32]. In addition, in resource-limited settings, empiric antibiotic use among children with severe acute malnutrition and/or HIV is associated with improvements in growth and development, reductions in diarrhea episodes, and reduced risk of death [33–35].
Although there is rational for possibly expanding antibiotic use in the management of diarrhea beyond dysentery and suspected cholera, there are substantial risks. A rapid increase in fluoroquinolone-resistant and multidrug-resistant enteric Shigella infections has been observed in parallel with the increase in fluoroquinolone availability and use in Asia [36]. In the United States, antibiotic-associated enteric infections such as Clostridium difficile cause severe and sometimes fatal forms of enteritis [37]. Finally, in many resource-limited settings such as Kenya, antibiotic use for diarrhea is common despite existing guidelines, and additional indications might not change prescribing practices [38, 39]. Therefore, careful consideration of the costs and benefits associated with changes to guidelines recommending antibiotics for pediatric diarrhea need to be considered.
The poor sensitivity (11%) of visible blood in stool for identifying Shigella infection in this population of Kenyan children is consistent with studies from Asia. Among 56 958 adults and children with diarrhea, the sensitivity of visible blood in stool for identifying culture-confirmed Shigella infections was 27% [15]. In Bangladesh, the sensitivity of visible blood in stool was 42% and 17% in hospitalized children and infants, respectively [8, 40]. In 2 studies that evaluated fecal blood as a potential risk predictor of case fatality, fecal blood was not associated with death [9, 40]. However, other risk factors for death among patients with Shigella infections include easily determined factors such as young age, malnutrition, severe dehydration, and signs of severe illness, such as convulsions or unconsciousness [8, 9, 13, 41, 42]. Determining the ideal set of characteristics observed at diarrhea presentation most predictive of a child's likelihood of benefiting from antibiotics will require additional validation studies conducted in sub-Saharan Africa and Asia, where these guidelines are most commonly used.
There were important limitations to this study. First, the generalizability of the population may be limited by the fact that 8.1% of the 2102 children screened were not enrolled because they were not accompanied by a legal guardian or were with a guardian who refused participation. Diarrhea duration of more than 14 days was an exclusion criteria that may have resulted in reduced detection of Shigella infections; however, persistent diarrhea was an uncommon presentation in our facilities (2.7% of 2102 screened). In addition, due to the age restrictions of the parent study inclusion criteria, we excluded children under 6 months of age, a group of children at high risk of diarrhea-associated mortality and in whom dysentery may be a particularly poor indicator of Shigella infection [40].
Moreover, because the study was cross-sectional, we were not able to evaluate predictors of poor outcomes among children infected with Shigella. It could be that guideline-indicated treatment decisions based on known risk factors for poor outcome rather than identification of specific pathogens could result in fewer diarrhea-associated deaths. We were also unable to determine whether identified S dysenteriae infections were type 1, the type most commonly associated with dysentery; however, only 2 children in this study were infected with S dysenteriae by culture. Furthermore, S dysenteriae type 1 was not identified among the 1130 Shigella isolates from MSD cases in the recent Global Enteric Multicenter Study, which included 105 Shigella isolates from Western Kenya [12]. The total number of Shigella infections in the present study was also small, which limited our ability to conduct internal validation of the clinical models. More sensitive diagnostic methods, such as molecular tools, would have increased the number of Shigella infections identified [43]. However, the clinical relevance of lower concentration infections for sequelae, such as death, stunting, and diarrhea persistence, are largely unknown. Until such thresholds are determined, clinical decisions based on molecular diagnostics may not be useful.
CONCLUSIONS
Despite these limitations, our study is strengthened by the systematic and detailed clinical characterization of enrolled children, enabling comparison of various sets of factors suggestive of Shigella infections among children with acute diarrhea. Our results suggest that diagnostic discrimination between children with and without Shigella infections could be improved by considering factors other than dysentery in the syndromic diagnosis of a child presenting with acute diarrhea. Although the combination of age over 23 months, mucous in stool, and absence of excessive vomiting increased the number of Shigella-infected children who would receive syndromic antibiotic treatment, it came at the cost of indicating antibiotics for a substantial proportion of children without Shigella infections. These findings suggest that the use of dysentery as the syndromic-indicator for suspected Shigella infection may need to be reconsidered as WHO guidelines for the treatment of diarrhea are re-evaluated. Emerging evidence highlighting the burden of bacterial enteric infections and the consequences that these infections have on growth and survival suggest that further research is needed to elucidate potential harms and benefits of treating diarrhea and in identifying optimal ways to distinguish children most likely to benefit from antibiotic therapy.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that were published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Acknowledgments
We thank all of the participants and clinics who participated in this study. We also acknowledge the staff of the University of Washington/Kenya Medical Research Institute collaboration and Drs. Brett Swierczewski and Brook A. Danboise from the Walter Reed Project, United States Army Medical Research Unit-Kenya. In addition, we thank the UW Global Center for Integrated Health of Women, Adolescents and Children (Global WACh) and the Kenya Research Program for support during the preparation of this article. We also thank Dr. Solomon Mpoke, the director of the Kenya Medical Research Institute, for support.
Author contributions. The study was designed and implemented by the study investigators, and the investigators conducted the analysis and prepared the manuscript.
Disclaimer. The findings and conclusions in this paper are those of the authors and are not to be construed as official, or as reflecting true views of the Department or the Army of the Department of Defense, the Kenya Medical Research Institute, the University of Washington, or other affiliated institution. The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Financial support. This research and publication were made possible with support from the Walter Reed Army Institute of Research, University of Washington Center for AIDS Research, a National Institutes of Health (NIH)-funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (National Institute of Allergy and Infectious Diseases, National Cancer Institute, National Institute of Mental Health, National Institute on Drug Abuse, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Heart, Lung, and Blood Institute, National Institute on Aging). Funding was provided by the NIH (grant number U19-A2090882). P. B. P. is supported by the University of Washington STD/AIDS Research Training Program (grant number T32-AI007140) and through funding from the Bill and Melinda Gates Foundation. G. C. J.-S. is supported by an NIH mentoring award (grant number K24-HD054314).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Kotloff KL, Nataro JP, Blackwelder WC et al. . Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 2.O'Reilly CE, Jaron P, Ochieng B et al. . Risk factors for death among children less than 5 years old hospitalized with diarrhea in rural Western Kenya, 2005–2007: a cohort study. PLoS Med 2012; 9:e1001256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee G, Paredes Olortegui M, Penataro Yori P et al. . Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J 2014; 33:1004–9. [DOI] [PubMed] [Google Scholar]
- 4.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 1984; 73:799–805. [PubMed] [Google Scholar]
- 5.Kotloff KL, Winickoff JP, Ivanoff B et al. . Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ 1999; 77:651–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Keddy KH, Sooka A, Crowther-Gibson P et al. . Systemic shigellosis in South Africa. Clin Infect Dis 2012; 54:1448–54. [DOI] [PubMed] [Google Scholar]
- 7.Chisti MJ, Faruque AS, Khan WA et al. . Characteristics of children with Shigella encephalopathy: experience from a large urban diarrhea treatment center in Bangladesh. Pediatr Infect Dis J 2010; 29:444–7. [DOI] [PubMed] [Google Scholar]
- 8.Khan WA, Griffiths JK, Bennish ML. Gastrointestinal and extra-intestinal manifestations of childhood shigellosis in a region where all four species of Shigella are endemic. PLoS One 2013; 8:e64097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Broek JM, Roy SK, Khan WA et al. . Risk factors for mortality due to shigellosis: a case-control study among severely-malnourished children in Bangladesh. J Health Popul Nutr 2005; 23:259–65. [PubMed] [Google Scholar]
- 10.World Health Organization. Guidelines for the Control of Shigellosis, Including Epidemics due to Shigella dysenteriae type 1. Geneva: World Health Organization; 2005. [Google Scholar]
- 11.Khatun F, Faruque AS, Koeck JL et al. . Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008). Epidemiol Infect 2011; 139:446–52. [DOI] [PubMed] [Google Scholar]
- 12.Livio S, Strockbine NA, Panchalingam S et al. . Shigella isolates from the global enteric multicenter study inform vaccine development. Clin Infect Dis 2014; 59:933–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bennish ML, Harris JR, Wojtyniak BJ, Struelens M. Death in shigellosis: incidence and risk factors in hospitalized patients. J Infect Dis 1990; 161:500–6. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed F, Ansaruzzaman M, Haque E et al. . Epidemiology of postshigellosis persistent diarrhea in young children. Pediatr Infect Dis J 2001; 20:525–30. [DOI] [PubMed] [Google Scholar]
- 15.von Seidlein L, Kim DR, Ali M et al. . A multicentre study of Shigella diarrhoea in six Asian countries: disease burden, clinical manifestations, and microbiology. PLoS Med 2006; 3:e353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst Rev 2010; 8:CD006784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Das JK, Ali A, Salam RA, Bhutta ZA. Antibiotics for the treatment of Cholera, Shigella and Cryptosporidium in children. BMC Public Health 2013; 13 (Suppl 3):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuPont HL, Reves RR, Galindo E et al. . Treatment of travelers’ diarrhea with trimethoprim/sulfamethoxazole and with trimethoprim alone. N Engl J Med 1982; 307:841–4. [DOI] [PubMed] [Google Scholar]
- 19.Taylor DN, McKenzie R, Durbin A et al. . Rifaximin, a nonabsorbed oral antibiotic, prevents shigellosis after experimental challenge. Clin Infect Dis 2006; 42:1283–8. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Chart Booklet: Integrated Management of Childhood Illness. Geneva: World Health Organization; 2014. [Google Scholar]
- 21.World Health Organization. Pocket Book of Hospital Care for Children: Guidelines for the Management of Common Illnesses Second Edition. Geneva: World Health Organization; 2013. [PubMed] [Google Scholar]
- 22.World Health Organization. WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards: Length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: Methods and development. 2006. pp 312 Available at: http://www.who.int/childgrowth/publications/en/). Accesed October 17, 2013. [Google Scholar]
- 23.Pavlinac PB, John-Stewart GC, Naulikha JM et al. . High-risk enteric pathogens associated with HIV infection and HIV exposure in Kenyan children with acute diarrhoea. AIDS 2014; 28:2287–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aranda KR, Fabbricotti SH, Fagundes-Neto U, Scaletsky IC. Single multiplex assay to identify simultaneously enteropathogenic, enteroaggregative, enterotoxigenic, enteroinvasive and Shiga toxin-producing Escherichia coli strains in Brazilian children. FEMS Microbiol Lett 2007; 267:145–50. [DOI] [PubMed] [Google Scholar]
- 25.Picard RR, Cook RD. Cross-validation of regression-models. J Am Stat Assoc 1984; 79:575–83. [Google Scholar]
- 26.Isaacs D. Gastrointestinal infections. In: Elliot E, Gilbert R, Moyer V, Pichichero M (eds). Evidence-Based Pediatric Infectious Diseases. Chapter 7 Malden, Massachusetts: Blackwell Publishing; 2007; pp 74–101. [Google Scholar]
- 27.Lanata CF, Fischer-Walker CL, Olascoaga AC et al. . Global causes of diarrheal disease mortality in children <5 years of age: a systematic review. PLoS One 2013; 8:e72788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee G, Pan W, Penataro Yori P et al. . Symptomatic and asymptomatic Campylobacter infections associated with reduced growth in Peruvian children. PLoS Negl Trop Dis 2013; 7:e2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platts-Mills JA, Gratz J, Mduma E et al. . Association between stool enteropathogen quantity and disease in Tanzanian children using TaqMan array cards: a nested case-control study. Am J Trop Med Hyg 2014; 90:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ternhag A, Asikainen T, Giesecke J, Ekdahl K. A meta-analysis on the effects of antibiotic treatment on duration of symptoms caused by infection with Campylobacter species. Clin Infect Dis 2007; 44:696–700. [DOI] [PubMed] [Google Scholar]
- 31.De Bruyn G, Hahn S, Borwick A. Antibiotic treatment for travellers’ diarrhoea. Cochrane Database Syst Rev 2000; 3:Cd002242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah N, DuPont HL, Ramsey DJ. Global etiology of travelers’ diarrhea: systematic review from 1973 to the present. Am J Trop Med Hyg 2009; 80:609–14. [PubMed] [Google Scholar]
- 33.Gough EK, Moodie EE, Prendergast AJ et al. . The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ 2014; 348:g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trehan I, Goldbach HS, LaGrone LN et al. . Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013; 368:425–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S et al. . A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med 2014; 370:41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Azmi IJ, Khajanchi BK, Akter F et al. . Fluoroquinolone resistance mechanisms of Shigella flexneri isolated in Bangladesh. PLoS One 2014; 9:e102533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dubberke ER, Olsen MA. Burden of Clostridium difficile on the healthcare system. Clin Infect Dis 2012; 55 (Suppl 2):S88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwisler G, Simpson E, Moodley M. Treatment of diarrhea in young children: results from surveys on the perception and use of oral rehydration solutions, antibiotics, and other therapies in India and Kenya. J Glob Health 2013; 3:010403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karambu S, Matiru V, Kiptoo M, Oundo J. Characterization and factors associated with diarrhoeal diseases caused by enteric bacterial pathogens among children aged five years and below attending Igembe District Hospital, Kenya. Pan Afr Med J 2013; 16:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huskins WC, Griffiths JK, Faruque AS, Bennish ML. Shigellosis in neonates and young infants. J Pediatr 1994; 125:14–22. [DOI] [PubMed] [Google Scholar]
- 41.Mitra AK, Engleberg NC, Glass RI, Chowdhury MK. Fatal dysentery in rural Bangladesh. J Diarrhoeal Dis Res 1990; 8:12–7. [PubMed] [Google Scholar]
- 42.Nathoo KJ, Porteous JE, Siziya S et al. . Predictors of mortality in children hospitalized with dysentery in Harare, Zimbabwe. Cent Afr J Med 1998; 44:272–6. [PubMed] [Google Scholar]
- 43.Lindsay B, Ochieng JB, Ikumapayi UN et al. . Quantitative PCR for detection of Shigella improves ascertainment of Shigella burden in children with moderate-to-severe diarrhea in low-income countries. J Clin Microbiol 2013; 51:1740–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.