Abstract
Background
The goal of this study was to obtain representative Kenyan data on the point prevalence of acute otitis media (AOM) and its sequelae (otitis media with effusion [OME] and chronic suppurative otitis media [CSOM]), a major cause of preventable hearing loss in children in developing countries. In Africa, there are limited studies on the prevalence of AOM and its sequelae in children.
Methods
Study subjects were children aged 2 to 15 years and were enrolled from randomly selected preprimary and primary schools. After parental or guardian consent, subjects had a questionnaire administered, otoscopy and tympanometry were done, and audiometry was performed on those with ear problems detected on these examinations.
Results
A total of 9825 (75%) children was from rural schools. The prevalence of CSOM was 15 of 1000, OME was 15 of 1000, and AOM was 7 of 1000 children. Rural Rift Valley schoolchildren had the highest prevalence of CSOM (24 of 1000) compared with other regions (12 of 1000; P < .0001). Ear discharge occurred before 3.5 years in 50% of 901 children with ear discharge. A history of ear discharge was associated with abnormal tympanograms (odds ratio [OR], 11.9–19.2) and mild-to-severe hearing loss (OR, 21.6–38.6), even in children without ear disease (OR, 10.7–24.4).
Conclusions
The burden of AOM sequelae in Kenyan preschool and schoolchildren is significant, and it occurs mostly in the first 4 years of life. By preventing early recurrent AOM, pneumococcal vaccination might partly avert nonreversible sequelae.
Keywords: acute otitis media, chronic suppurative otitis media, otitis media with effusion, otoscopy, perforation, tympanic membrane
Chronic suppurative otitis media (CSOM) is the most common cause of hearing disability in developing countries [1–4]. In Africa, the prevalence of CSOM among schoolchildren is high with rates ranging from 16 of 1000 in Tanzania [1] to 23 of 1000 in Nigeria [2] and 24 of 1000 in Kenya [3]. Thus, an estimated 2.4 million African schoolchildren suffer from CSOM and its sequelae [4].
Despite this burden, there are few clinic-based studies of otitis media (OM) in Africa, and there are no recent population-based studies. Thus, in a study conducted in Ilorin, Nigeria, 64 of 200 febrile children had OM, 40 (2 of 3) of whom had purulent discharge associated with a tympanic membrane perforation [5]. Likewise, in Addis Ababa, 1232 of 1360 children with OM had purulent discharge [6]. These high rates reflect the fact that early OM is not commonly recognized in Africa, and most children present late in the course of the illness when a perforation has occurred. Repeated perforations lead to subsequent scarred and sclerotic tympanic membranes. A study of 854 schoolchildren in Tanzania found such sequelae in 11% of urban and 15% of rural school children [1].
Because CSOM most likely follows 1 or repeated attacks of acute OM (AOM) [5], the problem of underrecognized and inadequately treated AOM would have a direct impact on the burden of chronic OM and its sequelae. The prototype of a 10-valent pneumococcal vaccine conjugated to protein D of Haemophilus influenzae (PHiD-CV) prevents AOM caused by both Streptococcus pneumoniae and nontypeable H. influenzae [6–8] and is more effective than pneumococcal conjugated vaccine (PCV7) in Indigenous Australian children in preventing CSOM [9]. Because this vaccine has recently been introduced into the routine immunization schedule in infants in Kenya, there is potential for preventing the suppurative sequelae of AOM and subsequent hearing impairment. Hence, we conducted this study in Kenyan children, with the ultimate goal of deriving a population-based estimate of childhood CSOM and hearing impairment before introduction of the PHiD-CV vaccine. We intend to follow up this baseline study in the same areas 5–6 years after vaccine introduction, to determine the potential impact of the vaccine on OME, CSOM, and on hearing impairment.
METHODS
Study Description
We conducted a prospective study between June 2012 and December 2012 to obtain the prevalence of OM and its sequelae in preschool, elementary, and secondary school students aged 2–15 years. June to October are wetter months. Ethical approval was obtained from Institutional Review Boards at the University of Nairobi and the University of Colorado Denver.
Selecting Schools for the Study
We conducted the study in 9 districts in Kenya, which were chosen using random stratified sampling. Five districts had mixed rural and urban schools, 3 were purely rural, and 1 was purely urban. In each district, a list of all public preprimary and primary schools was obtained from the office of the District Education Officer. The study excluded schools (1) with enrollment of <300 children, (2) with unique characteristics such as those for the deaf or for the blind, and (3) the top and bottom 10% of schools in size. The remaining schools were stratified according to their rural/urban status and the geographical/educational zone in which they were located within the district. A total of 6–7 schools were then proportionately and randomly selected from these district strata. In addition, eligible children attended to in the outpatient departments of the nearest public health facilities on the day of screening were referred to the schools for screening.
Selection and Description of Participants
Parents or legal guardians of children who had signed informed consent were administered a standard questionnaire by trained nurses. Their children then underwent a detailed otoscopic examination by a study clinical officer in the school classrooms. Those with a past history of ear disease and/or abnormal otoscopic findings were sent for a hearing evaluation, which was done in a quiet schoolroom within each school. If the parent indicated that the child currently had or had in the past hearing loss, ear discharge, and/or any of the problems relating to the ear or hearing, he/she was considered to have a medical history of ear problems.
Physical Examination Including Otoscopy and Hearing Screening
Six practicing clinical officers who were trained as ear, nose and throat specialists (ENTs) completed otoscopic and tympanometric examination of all study subjects. Clinical officers were retrained in standardized definitions of findings on otoscopy. Otoscopic examination was done with a headlight and Heine otoscopes. Interacoustics handheld tympanometers (Assens, Denmark) were used for tympanometery, and the type was determined as A, As, Ad, C, B, or High Volume Flat, according to the Jerger classification [10]. Those children found to have obstructive earwax or foreign bodies had their ears cleaned or foreign bodies removed. A formal audiologic evaluation was performed on select children, as described below.
Audiological Testing and Referral
The audiologist performed pure-tone audiometry, using a MAICO (Berlin Germany) Air/Bone/SP audiometer, on all children with any of the following: (1) a report of impaired hearing, (2) any abnormality seen on otoscopic examination, or (3) an abnormal tympanogram.
The hearing-screening threshold was set at 30 decibel (dB) hearing level, and air conduction testing was done through headphones at 4 frequencies: 500, 1000, 2000, and 4000 kHz. A semi sound proof room was set up in the most quiet area of the school where the ambient noise level did not exceed 30 dB.
After audiologic evaluation, parents of children found to have hearing impairment were informed and counseled appropriately. Those children in need of surgical repair of perforated ear drums, hearing aids, or any other follow up were referred to the nearest hospital with a functional ENT unit. Those children with ear infections were treated appropriately.
Definitions
Acute otitis media is defined as abnormal tympanic membrane on otoscopy and/or tympanometry (erythema of the tympanic membrane, bulging and reduced or no movement on tympanogram) with at least 1 of the following signs: ear pain or ear discharge for less than 2 weeks in absence of an otitis externa [11, 12].
Otitis media with effusion is defined as a visually abnormal tympanic membrane on otoscopy and/or tympanometry (no perforation, but with dullness, not red, not bulging) and/or flat tympanogram [13].
Chronic suppurative otitis media is defined as chronic inflammation of the middle ear and mastoid cavity, with otorrhea for at least 2 weeks duration through a perforated tympanic membrane. Chronic suppurative otitis media was considered to be active if there was otorrhea and inactive if there was a perforation without otorrhea and/or the presence of tympanosclerosis [14]. This World Health Organization (WHO) definition was chosen to be comparable with other studies done in developing countries [1–6].
Data Management and Statistical Analysis
Study data were collected and managed using REDCap electronic data capture tools hosted at the University of Colorado at Denver (Aurora, CO) [15]. SAS version 9.4 (SAS Institute, Cary, NC) and SPSS version 22 (IBM, New York, NY) were used for statistical analyses.
Rate comparisons were used in statistical evaluations of otoscopy and ear disease [16]. Confidence intervals for rates were calculated with a normal approximation to the Poisson distribution (N > 10) or an exact Poisson test (N < 10) [16]. Rates were statistically compared using a normal approximation to the binomial distribution when sufficient N, and an exact binomial test was used in all other instances [16].
A Mantel-Haenszel χ2 test was used to assess any statistical associations between conductive and mixed hearing loss and/or abnormal tympanogram with parent-reported ear discharge history. Children who had earwax but no ear disease and those with ear diseases were excluded from this analysis. The categories mild, moderate, severe, and profound were per WHO definitions for children and corresponded to hearing loss in the following dB ranges, respectively: 16–30, 31–60, 61–80, and >80 [17]. Hearing loss categories severe and profound were combined because the numbers were sparse.
RESULTS
We screened 13 244 children in 58 schools; 135 were lost to follow up after day 1 and 13 109 completed the study. Most (9825; 75%) of the children were from rural schools, and approximately 40% were in the 6–9 year age group with an approximately equal numbers of boys and girls in all age groups (Table 1).
Table 1.
Demographic Characteristics of Study Subjects By Region
| Region | Nakuru North | Nyandarua Central | Kitui Central | Embu West | Kisii South | Nyando | Kakamega East | Eldoret East | Starehe | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age Group | N (%)a | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Urban Female | <6 years | 20 (4.6) | 20 (16.4) | 10 (7.9) | 14 (6.7) | 15 (10.9) | – | – | – | 39 (5.6) | 118 (6.8) |
| 6–9 years | 179 (41.1) | 54 (44.3) | 57 (45.2) | 113 (54.1) | 48 (35) | – | – | 4 (80) | 250 (35.7) | 705 (40.6) | |
| 10–12 years | 161 (37) | 31 (25.4) | 39 (31) | 68 (32.5) | 50 (36.5) | – | – | – | 268 (38.2) | 617 (35.6) | |
| 13–15 years | 75 (17.2) | 17 (13.9) | 20 (15.9) | 14 (6.7) | 24 (17.5) | – | – | 1 (20) | 144 (20.5) | 295 (17) | |
| Total | 435 (100) | 122 (100) | 126 (100) | 209 (100) | 137 (100) | – | – | 5 (100) | 701 (100) | 1735 (100) | |
| Urban Male | <6 years | 28 (6.8) | 19 (18.1) | 13 (9.8) | 13 (7.3) | 15 (13.8) | – | – | – | 53 (8.8) | 141 (9.1) |
| 6–9 years | 146 (35.5) | 39 (37.1) | 54 (40.9) | 83 (46.4) | 38 (34.9) | – | – | 3 (37.5) | 215 (35.5) | 578 (37.3) | |
| 10–12 years | 160 (38.9) | 35 (33.3) | 45 (34.1) | 67 (37.4) | 45 (41.3) | – | – | 4 (50) | 231 (38.2) | 587 (37.9) | |
| 13–15 years | 77 (18.7) | 12 (11.4) | 20 (15.2) | 16 (8.9) | 11 (10.1) | – | – | 1 (12.5) | 106 (17.5) | 243 (15.7) | |
| Total | 411 (100) | 105 (100) | 132 (100) | 179 (100) | 109 (100) | – | – | 8 (100) | 605 (100) | 1549 (100) | |
| Rural Female | <6 years | 30 (8.1) | 112 (15.2) | 102 (14.2) | 51 (11.2) | 99 (18.5) | 126 (19.6) | 94 (13.3) | 140 (16.2) | – | 754 (15) |
| 6–9 years | 147 (39.8) | 288 (39.1) | 271 (37.7) | 208 (45.6) | 211 (39.4) | 233 (36.2) | 281 (39.7) | 321 (37.2) | – | 1960 (39) | |
| 10–12 years | 121 (32.8) | 210 (28.5) | 217 (30.2) | 138 (30.3) | 153 (28.5) | 202 (31.4) | 239 (33.8) | 261 (30.3) | – | 1541 (30.6) | |
| 13–15 years | 71 (19.2) | 126 (17.1) | 128 (17.8) | 59 (12.9) | 73 (13.6) | 82 (12.8) | 94 (13.3) | 140 (16.2) | – | 773 (15.4) | |
| Total | 369 (100) | 736 (100) | 718 (100) | 456 (100) | 536 (100) | 643 (100) | 708 (100) | 862 (100) | – | 5028 (100) | |
| Rural Male | <6 years | 21 (6.1) | 93 (13.4) | 88 (12.8) | 58 (12.1) | 92 (19) | 119 (18.8) | 90 (13.3) | 128 (16) | – | 689 (14.4) |
| 6–9 years | 138 (40.1) | 270 (39) | 240 (34.8) | 224 (46.6) | 187 (38.6) | 224 (35.4) | 286 (42.4) | 287 (36) | – | 1856 (38.7) | |
| 10–12 years | 131 (38.1) | 232 (33.5) | 223 (32.4) | 152 (31.6) | 145 (30) | 206 (32.5) | 201 (29.8) | 259 (32.5) | – | 1549 (32.3) | |
| 13–15 years | 54 (15.7) | 98 (14.1) | 138 (20) | 47 (9.8) | 60 (12.4) | 84 (13.3) | 98 (14.5) | 124 (15.5) | – | 703 (14.7) | |
| Total | 344 (100) | 693 (100) | 689 (100) | 481 (100) | 484 (100) | 633 (100) | 675 (100) | 798 (100) | – | 4797 (100) | |
| Grand Total | 1559 | 1656 | 1665 | 1325 | 1266 | 1276 | 1383 | 1673 | 1306 | 13109 |
aPercentage represents the proportion of all children from each site in the age/sex categories indicated.
Wax was found in 4.2% of children and 1.1% had a tympanic membrane perforation. Except for retracted or bulging tympanic membranes, or healed scarred tympanic membranes, which were more common in urban children (P < .05; Table 2), the rates of other otoscopic finding were similar in both urban and rural schoolchildren.
Table 2.
Otoscopic Findings in 13 109 Kenyan Children by Urban and Rural Schools
| Urban (N = 3284) |
Rural (N = 9825) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Ear Problem | N | Rate | (95% CI) | N | Rate | (95% CI) | P Valuea | |
| Wax or not seen both ears | 190 | 57.9 | (49.6–66.1) | 354 | 36.0 | (32.3–39.8) | <.001 | |
| Intact TM | Dull | 46 | 14.0 | (10–18.1) | 139 | 14.1 | (11.8–16.5) | .49 |
| Retracted | 11 | 3.4 | (1.4–5.3) | 6 | 0.6 | (0.2–1.3) | <.001 | |
| Red | 23 | 7.0 | (4.1–9.9) | 78 | 7.9 | (6.2–9.7) | .66 | |
| Bulging | 20 | 6.1 | (3.4–8.8) | 31 | 3.2 | (2–4.3) | .02 | |
| Healed TM | 6 | 1.8 | (0.7–4) | 4 | 0.4 | (0.1–1) | .02 | |
| Perforation | Small | 16 | 4.9 | (2.5–7.3) | 36 | 3.7 | (2.5–4.9) | .21 |
| Large | 18 | 5.5 | (2.9–8) | 41 | 4.2 | (2.9–5.4) | .21 | |
| Subtotal | 9 | 2.7 | (1.3–5.2) | 22 | 2.2 | (1.3–3.2) | .38 | |
| Total | 1 | 0.3 | (0–1.7) | 7 | 0.7 | (0.3–1.5) | .37 | |
| Attic | 0 | 0.0 | (0–1.1) | 5 | 0.5 | (0.2–1.2) | .24 | |
| Dry | 6 | 1.8 | (0.7–4) | 20 | 2.0 | (1.1–2.9) | .51 | |
| Other | Tympanosclerosis | 3 | 0.9 | (0.2–2.7) | 6 | 0.6 | (0.2–1.3) | .83 |
| Cholesteatoma | 0 | 0.0 | (0–1.1) | 6 | 0.6 | (0.2–1.3) | .18 | |
Abbreviations: CI, confidence interval; TM, tympanic membrane.
aComparison of incidence rates [16].
The overall prevalence among schoolchildren with AOM was 7 of 1000, 15 of 1000 for OME, and 15 of 1000 for CSOM. There was no difference in the rates between rural and urban schoolchildren for AOM (7.6 and 5.2 of 1000, respectively), CSOM (14.8 of 1000 vs 17.7 of 1000), or OME (15 of 1000) in both rural and urban areas (Table 3). When these rates were examined by region, however, children in the Rift Valley had a significantly higher prevalence of OME and CSOM than children in the other 4 regions combined (Table 4). This difference was even more noticeable in rural areas (24 of 1000 vs 12 of 1000, respectively; P < .0001).
Table 3.
Age-Specific Rates/1000 Children (With 95% CIs) of Otitis Media Diagnoses by Age Group and Urban/Rural Residence in Kenyan Children
| <6 yrs | 6–9 yrs | 10–12 yrs | 13–15 yrs | Total | |||
|---|---|---|---|---|---|---|---|
| Age-Specific Population (N) |
Urban | 259 | 1283 | 1204 | 538 | 3284 | |
| Rural | 1443 | 3816 | 3090 | 1476 | 9825 | ||
| Acute Otitis Media | Urban | 19.3 (6.3–45.1) | 6.2 (2.7–12.3) | 2.5 (0.5–7.3) | 1.9 (0–10.4) | 5.2 (2.7–7.6) | |
| Rural | 27.7 (19.1–36.3) | 5.8 (3.4–8.2) | 4.2 (1.9–6.5) | 0 (0–2.5) | 7.6 (5.9–9.4) | ||
| Otitis Media With Effusion | Urban | 54.1 (25.7–82.4) | 14.8 (8.2–21.5) | 8.3 (3.2–13.5) | 11.2 (4.1–24.3) | 14.9 (10.7–19.1) | |
| Rural | 35.3 (25.6–45) | 14.4 (10.6–18.2) | 9.1 (5.7–12.4) | 6.8 (2.6–11) | 14.7 (12.3–17) | ||
| Chronic Suppurative Otitis Media | Active | Urban | 11.6 (2.4–33.9) | 3.9 (1.3–9.1) | 2.5 (0.5–7.3) | 11.2 (4.1–24.3) | 5.2 (2.7–7.6) |
| Rural | 5.5 (2.4–10.9) | 5.2 (2.9–7.5) | 6.5 (3.6–9.3) | 6.8 (2.6–11) | 5.9 (4.4–7.4) | ||
| Inactive | Urban | 19.3 (6.3–45.1) | 6.2 (2.7–12.3) | 13.3 (6.8–19.8) | 14.9 (6.4–29.3) | 11.3 (7.6–14.9) | |
| Rural | 6.9 (2.6–11.2) | 6.8 (4.2–9.4) | 8.1 (4.9–11.3) | 16.2 (9.7–22.8) | 8.6 (6.8–10.5) | ||
| Healed | Urban | 0 (0–14.2) | 0 (0–2.9) | 0 (0–3.1) | 1.9 (0–10.4) | 0.3 (0–1.7) | |
| Rural | 0.7 (0–3.9) | 0 (0–1) | 0.3 (0–1.8) | 0.7 (0–3.8) | 0.3 (0.1–0.9) | ||
| Tympanosclerosis | Urban | 0 (0–14.2) | 0.8 (0–4.3) | 1.7 (0.2–6) | 5.6 (1.2–16.3) | 1.8 (0.7–4) | |
| Rural | 0 (0–2.6) | 0.3 (0–1.5) | 1.6 (0.5–3.8) | 6.8 (2.6–11) | 1.6 (0.8–2.4) | ||
| All CSOMa | Urban | 30.9 (13.3–60.8) | 10.1 (4.6–15.6) | 17.4 (10–24.9) | 29.7 (15.1–44.3) | 17.7 (13.1–22.2) | |
| Rural | 12.5 (6.7–18.2) | 11.8 (8.3–15.2) | 15.2 (10.9–19.6) | 23.7 (15.8–31.5) | 14.8 (12.4–17.2) | ||
Abbreviations: CI, confidence interval; CSOM, chronic suppurative otitis media.
aCholesteatoma is included in with the All CSOM group.
Table 4.
Final Classification of Otologic Diagnosis in 13 109 Kenyan Children by District With Rate/1000 Children
| AOM | OME | CSOM | Tympanosclerosis | |
|---|---|---|---|---|
| Region | N [Rate; 95% CIa] | N [Rate; 95% CI ] | N [Rate; 95% CI] | N [Rate; 95% CI ] |
| Rift Valley N = 3232 | 23 [7.1; 4.2–10] | 66 [20.4; 15.5–25.3] | 72 [22.3; 17.1–27.4] | 3 [0.9; 0.2–2.7] |
| Other Districts N = 9877 | 69 [7; 5.3–8.6] | 127 [12.9; 10.6–15.1] | 131 [13.3; 11–15.5] | 6 [0.6; 0.2–1.3] |
| P valuea | .52 | .001 | <.001 | .39 |
Abbreviations: AOM, acute otitis media; CI, confidence interval; CSOM, chronic suppurative otitis media; OME, otitis media with effusion.
aComparison of incidence rates [16].
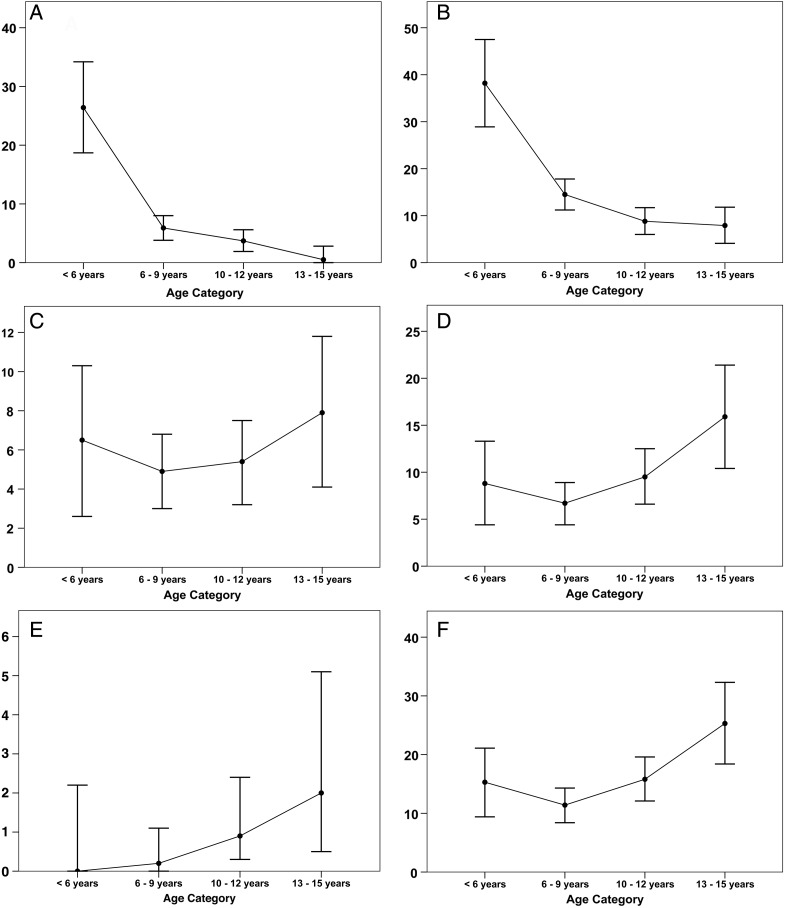
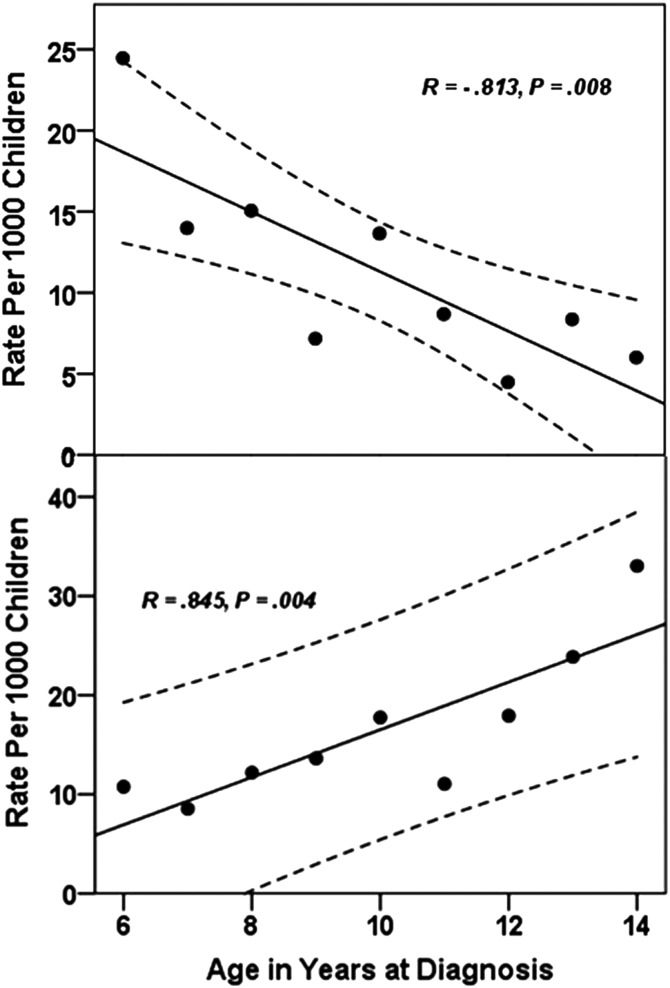
The prevalence of AOM and OME was highest in children under the age of 6 years, and it steadily declined as children got older (Figure 1A and B). There were higher rates of CSOM (active and inactive) in the early age groups and the lowest rates in children 6–9 years of age (Figure 1C and D). However, subsequent to that age, the rates of active and inactive CSOM increased steadily (Figure 1C and D). The sequelae of CSOM (tympanosclerosis) increased steadily with age (Figure 1E). The rate of decline in OME, between the ages of 6 and 15, almost mirrored the increase in the rate of CSOM in those age groups (Figure 2).
Figure 1.
Ear disease rate (95% confidence limits) per 1000 children by age at diagnosis for (A) acute otitis media, (B) otitis media with effusion, (C) active chronic suppurative otitis media (CSOM), (D) inactive CSOM, (E) tympanosclerosis, and (F) total CSOM.
Figure 2.
Ear disease rate per 1000 children by age at diagnosis for (A) acute otitis media (R2 = 0.661; model: y = 29.71–1.84x) and (B) total chronic suppurative otitis media (R2 = 0.714; y = − 7.46 + 2.4x).
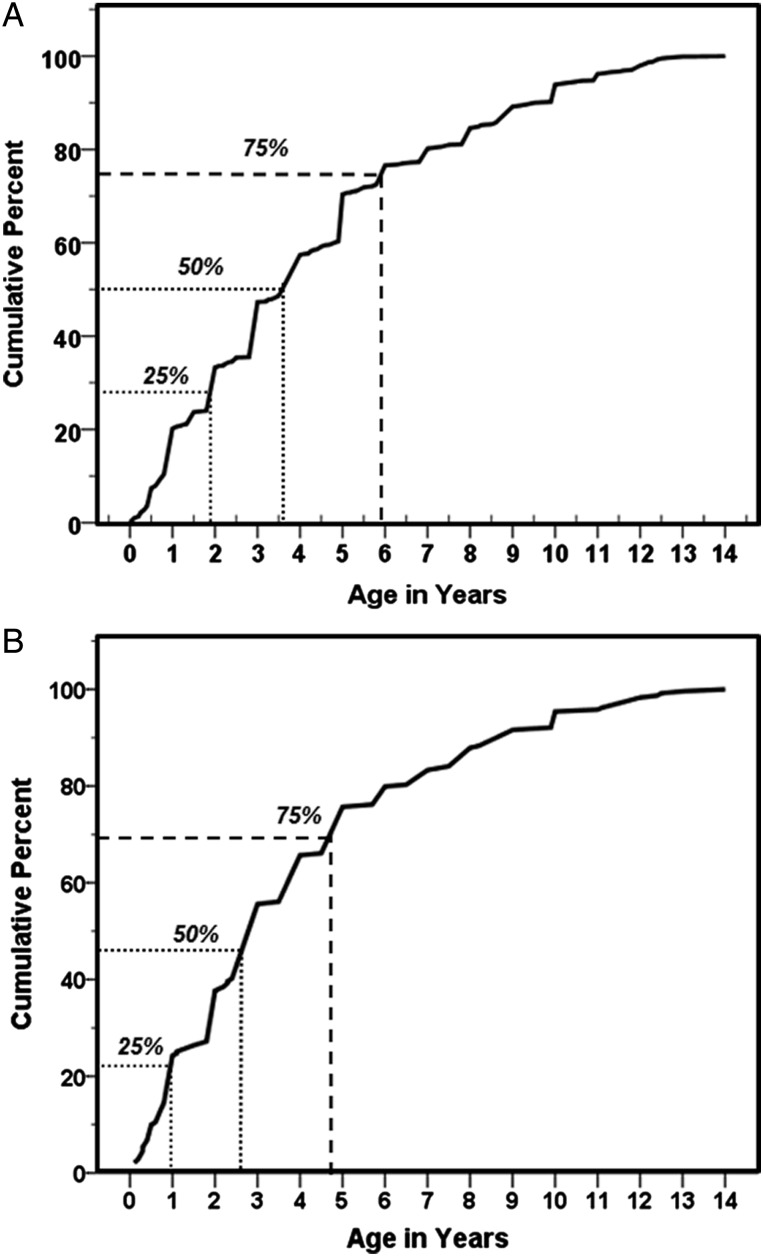
In those children with a history of ear discharge by parental recall (N = 901), the age of onset was at about 3.5 years in 50% and before 6 years in 75% (Figure 3A). When we restricted the analysis to the children with an abnormal ear diagnosis (N = 239; Figure 3B), the age of onset was before 3 years of age in 50% of children and before 5 years of age in 75%. Younger children with CSOM (<10 years) had more ear discharge than expected starting before the age of 3 years (37 of 65; 56.9%), whereas older children with CSOM (>10 years) had less ear disease than expected starting before 3 years of age (33 of 88; 37.5%) (P = .027; Mantel-Haenszel χ2 test).
Figure 3.
Age of ear discharge in the past by parental recall for (A) all children with history of ear discharge, and (B) excluding those children with no abnormal ear diagnosis.
A history of ear discharge was significantly associated with abnormal tympanograms (odds ratio [OR], 11.9–19.2; Table 5). This also occurred in those without obvious clinical ear disease (OR, 10.4–11.5; Table 5). This association was even more striking with hearing loss (Table 5). In all children with a history of ear discharge, the odds of having mild-to-severe hearing loss varied between 21.6 and the 38.6, and these results were highly significant even in children without ear disease with odds ratios ranging from 10.7 to 24.4 (Table 5).
Table 5.
Tympanogram and Hearing Loss Results by History of Ear Discharge
| Tympanogram Type Result | No History of Ear Discharge N (%) | History of Ear Discharge N (%) | Odds Ratio (95% CI) | P Valuea |
|---|---|---|---|---|
| Includedb: Those With Ear Disease | ||||
| Normal | 11025 (97.3) | 553 (68.3) | ||
| B or High Volume Flat | 203 (1.8) | 196 (24.2) | 19.2 (15.5–23.8) | <.001 |
| As, Ad, or C | 102 (0.9) | 61 (7.5) | 11.9 (8.6–16.6) | <.001 |
| Excludedc: Those With Ear Disease | ||||
| Normal | 10954 (98.7) | 518 (87.5) | ||
| B or High Volume Flat | 71 (0.6) | 35 (5.9) | 10.4 (6.9–15.8) | <.001 |
| As, Ad or C | 72 (0.6) | 39 (6.6) | 11.5 (7.7–17.1) | <.001 |
| Hearing Loss Categoryd | No History of Ear Discharge | History of Ear Discharge | Odds Ratio (95% CI) | P Valuea |
| Includede: Those With Ear Disease | ||||
| None | 11397 (94.2) | 345 (38.8) | ||
| Mild | 558 (4.6) | 394 (44.3) | 23.3 (19.7–27.6) | <.001 |
| Moderate | 112 (0.9) | 131 (14.7) | 38.6 (29.4–50.8) | <.001 |
| Severe/Profound | 29 (0.24) | 19 (2.1) | 21.6 (12–39) | <.001 |
| Excludedf: Those With Ear Disease | ||||
| None | 11321 (95.6) | 310 (47.7) | ||
| Mild | 447 (3.8) | 299 (46) | 24.4 (20–29) | <0.001 |
| Moderate | 56 (0.5) | 34 (5.2) | 22.2 (14–34) | <0.001 |
| Severe/Profound | 24 (0.2) | 7 (1.1) | 10.7 (4.5–25) | <0.001 |
Abbreviations: CI, confidence interval; WHO, World Health Organization.
aPer Mantel-Haenszel χ2.
bExcluded were those with ear wax and/or other obstructions with no other signs or symptoms to cause abnormal tympanograms or hearing loss. Eight hundred forty-five of the remaining 12 985 did not have a tympanogram.
cExcluded were those with ear wax and/or other obstructions with no other signs or symptoms to cause abnormal tympanograms or hearing loss; excluded also were those with ear disease. Eight hundred nine of the remaining 12 498 did not have a tympanogram.
dCategory for the Worse Ear per WHO pediatric thresholds.
eExcluded were those with ear wax and/or other obstructions with no other signs or symptoms to cause abnormal tympanograms or hearing loss.
fExcluded were those with ear wax and/or other obstructions with no other signs or symptoms to cause abnormal tympanograms or hearing loss; excluded also were those with ear disease.
DISCUSSION
This study is one of the largest population-based, representative national samples of OM and its sequelae, in any developing country. Our major findings were that OM was found in 37 of 1000 schoolchildren, the majority being due to OME and CSOM (15 of 1000), with higher OME rates in younger children and higher CSOM rates in older children. A history of ear discharge, even in children with no ear pathology, predicted abnormal tympanograms and hearing loss in later childhood.
Previous smaller studies done in Kenya in 1992 and 2002 showed a prevalence of CSOM of 11 of 1000 among schoolchildren in the Kiambu district (Southern Kenya) [3] and 12 of 1000 in the Kisumu district (Western Kenya) [18]. Our study, which covered a broader geographic range and larger diversity of urban and rural populations in Kenya, has confirmed a prevalence of CSOM of 13 in 8 districts but 22 in the Rift Valley. The previous Kenyan studies [3, 18] and others from Africa [19, 20] did not include dry perforations in calculating the prevalence of CSOM, and hence our rates might be higher in older children. It is possible that dry perforations may also be sequelae of AOM in an otherwise currently healthy ear. However, overall, our rates of CSOM (15 of 1000) are consistent with rates per 1000 schoolchildren in Africa and the Middle East: Saudi Arabia 13 [21], Gambia 15 [22], Tanzania 16 [1], but lower than in the Asia Pacific region: Indonesia 27 [23], India 36–48 [24, 25], Nepal 50–76 [26, 27], and Solomon Islands 60 [28].
There are African studies with higher rates of CSOM than reported here [29, 30], which included children at the 2 ends of the age spectra that we studied. In a Nigerian study of 101 preschool children aged 3.5–6 years, the rate of perforated tympanic membranes was 119 of 1000 children [29]. In a separate Nigerian study, in children aged 9–15, the rate was 23 of 1000 children [30]. In that study, there was a clear difference in the rates in schools catering to lower socioeconomic strata (40 of 1000 vs 15 of 1000) versus schools catering to a higher socioeconomic strata, similar to a study from India [24]. We are unsure of the significance of the very high rate of perforated tympanic membrane in the former study [29], but no other studies from Nigeria [2, 30] or other countries in Africa or Asia [3, 18–28, 30] have shown similar rates. In our study, the rates of CSOM in children under the age of 6 or in the 13–15 year age group (29.7–30.9) are the highest rates of all age groups (Table 3; Figure 1).
The effect of urbanization on CSOM was not obvious in our study as has been found in studies in Nigeria and Tanzania, where rates of CSOM were higher in rural schoolchildren than urban ones [1, 31]. A recent study by our group, conducted in 2011–2012 using the same protocol as in this study in 6 sites in Indonesia, showed an overall rate of CSOM of 17 of 1000 children, similar to the rate we found of 15 of 1000 children [23]. However, the rates of CSOM in rural Indonesia were 27 of 1000 children compared with 7 of 1000 in urban areas (P < .001). We were surprised to find that there was a strikingly high incidence of CSOM in rural areas of Bali (75 of 1000 children), with rates as high as 84 of 1000 in children aged 6–9 years. These studies were done in settings where there were obvious higher socioeconomic levels in the urban schools than in the rural schools. In this study, many of the urban areas are economically comparable with rural areas because these urban areas are in predominantly rural districts and not from large metropolitan areas, except for 1 district (Starehe), in the poorer parts of the capital, Nairobi.
However, we did find that the rates of CSOM in rural areas of the Rift Valley were twice the rate in the rest of Kenya. The rate of 24 of 1000 was similar to contemporary rates in rural Indonesia. The population in the Rift Valley comprises a high proportion of communities that lead a mostly pastoral existence. Perhaps delayed care seeking behavior underlies this observation; however, it is also possible that environmental (smoke exposure in poorly ventilated traditional huts) and other factors contribute to this high rate.
The prevalence of OME has varied in several epidemiological studies mainly due to differences in population characteristics, methodology, seasonal variations, and diagnostic criteria [13, 32]. The prevalence of 15 of 1000 found in our study is similar to the rate of 12 of 1000 found by Ogisi among 5- and 6-year-old Nigerian children [33] and 20 of 1000 in 6- and 7-year-old Chinese children in Hong Kong [34]. A recent study from China in 2902 children found that the highest prevalence of OME was in children 2 years of age at 140 of 1000 but steadily decreased to 17 of 1000 in children 7 years of age [35]. According to De Ru and Grote [36], the greatest risk of OME is in the first 2 years of life. This age group would have been missed in our study, although we did show that children 3–6 years of age had the highest rates of OME that steadily decreased in older age groups (Figure 1). In our study from Indonesia [23], the prevalence of OME (3.8 of 1000 children) was much lower than in the present study. Perhaps this reflects on a different pathogenesis leading to CSOM in Kenya and Indonesia. Chronic suppurative otitis media can arise from nonhealing of an acute perforation of the tympanic membrane, recurrent perforations of the tympanic membrane, or superinfection of persistent OME [11]. The observation of higher rates of OME early in life, decreasing as children get older in Kenya, that correlate with increasing rates of CSOM suggest this pathway in Kenya. In addition, the observation of a later age of onset of perforation in older children with CSOM in Kenya suggests that recurrent perforations of the tympanic membrane starting in early life is uncommon in Kenyan children with CSOM. On the other hand, in Indonesia, the much lower rates of OME, but significantly higher rates of CSOM, suggests that acute perforation or recurrent perforations of the tympanic membrane might be the pathway. This might also explain the much higher rates of CSOM in rural areas in Indonesia but relatively lower rates of CSOM in both rural and urban areas in Kenya.
Atticoantral disease has been associated with a high incidence of ear cholesteatoma, but acquired cholesteatoma has been reported to be uncommon in populations that have high rates of CSOM [10]. In India, atticoantral disease was seen in 7 of 1000 preschool children and 15 of 1000 primary schoolchildren [37] with similar rates being reported from Malaysia with 5.3 of 1000 [38]. In Africa, there are no prevalence data, but little atticoantral disease is seen in studies from Nigeria [39]. In a study from Sierra Leone, the rate of CSOM in 2015 children was found to be 6.4%, but only 1 child had a cholesteatoma [40]. We found 6 children with otoscopically obvious cholesteatoma in the 13 109 studied, a rate of 0.45 of 1000, compared with none of 7002 children in Indonesia [23]. Perhaps this difference reflects the different putative pathways for the development of CSOM in the 2 countries, and perhaps it explains the differences in disease presentations in Asia and Africa.
CONCLUSIONS
That CSOM has its origin in early childhood is borne out by the fact that according to parental recall, the age at first discharge was less than 3 years in 50% of children and 6 years in more than 75% in children with history of ear discharge. It is possible that some of this ear discharge might have been due to nonotopathologic causes such as cerumen, seborrheic dermatitis, etc. Nevertheless, there were significant differences in tympanometry and hearing loss in children with a history of ear discharge in the past, regardless of whether they had current ear disease. Children with a history of ear discharge have a 10-fold higher rate of abnormal tympanograms and a 20-fold higher rate of hearing loss. This would suggest that vaccination of infants with a conjugate pneumococcal vaccine, if it prevents AOM, OME, and tympanic perforations in Kenyan infants, might be a significant added benefit of pneumococcal vaccination, in preventing hearing impairment in Kenyan schoolchildren. Therefore, the implications of this study have significant global import, for policymakers in developing countries.
Acknowledgments
We thank the Kenyan schools, parents, and children for their participation in this study, and the clinical officers in the Ear, Nose and Throat Department of Surgery at the College of Health Sciences, University of Nairobi, Kenyatta National Hospital for contributions to this study.
Disclaimer. The contents are the authors' sole responsibility and do not necessarily represent the views of the National Institutes of Health (NIH). GlaxoSmithKline (GSK) Biologicals SA was provided the opportunity to review a preliminary version of this manuscript for factual accuracy, but the authors were solely responsible for final content and interpretation.
Financial support. This work was supported in part by the NIH/National Center for Advancing Translational Sciences, Colorado Clinical and Translational Sciences Institute (Grant Number UL1 TR000154). This work was also supported by research grants from GSK Biologicals SA to the following authors' institution: University of Colorado Denver, Aurora, CO (to E. A. F. S.); University of Nairobi, Kenyatta National Hospital, Nairobi (to F. K., S. N. N., J. A., and I. M. M.); and Children's Hospital Colorado, Aurora, CO (to P. C.-L.).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Bastos I, Mallya J, Ingvarsson L et al. Middle ear disease and hearing impairment in northern Tanzania. A prevalence study of schoolchildren in the Moshi and Monduli districts. Int J Pediatr Otorhinolaryngol 1995; 32:1–12. [DOI] [PubMed] [Google Scholar]
- 2.Olatoke F, Ologe FE, Nwawolo CC, Saka MJ. The prevalence of hearing loss among schoolchildren with chronic suppurative otitis media in Nigeria and its effect on academic performance. Ear Nose Throat J 2008; 87:E19. [PubMed] [Google Scholar]
- 3.Hatcher J, Smith A, Mackenzie I et al. A prevalence study of ear problems in school children in Kiambu district, Kenya, May 1992. Int J Pediatr Otorhinolaryngol 1995; 33:197–205. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Report by the Director General. Prevention of Deafness and Hearing Impairment. Document A39/14. Geneva: World Health Organization; 1986. [Google Scholar]
- 5.Roland PS. Chronic suppurative otitis media: a clinical overview. Ear Nose Throat J 2002; 81(8 Suppl 1):8–10. [PubMed] [Google Scholar]
- 6.Prymula R, Peeters P, Chrobok V et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740–8. [DOI] [PubMed] [Google Scholar]
- 7.Schuerman L, Borys D, Hoet B et al. Prevention of otitis media: now a reality? Vaccine 2009; 27:5748–54. [DOI] [PubMed] [Google Scholar]
- 8.Diel M, Laurenz M, Krause K et al. Impact of pneumococcal conjugate vaccines on acute otitis media among children in Germany. In: 31st Annual Meeting of the European Society for Paediatric Infectious Diseases; May 28–June 1, 2013. (Abstract 997); Milan, Italy. [Google Scholar]
- 9.Leach AJ, Wigger C, Andrews R et al. Otitis media in children vaccinated during consecutive 7-valent or 10-valent pneumococcal conjugate vaccination schedules. BMC Pediatr 2014; 14:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jerger J. Clinical experience with impedance audiometry. Arch Otolaryngol 1970; 92:311–4. [DOI] [PubMed] [Google Scholar]
- 11.Bluestone CD. Epidemiology and pathogenesis of chronic suppurative otitis media: implications for prevention and treatment. Int J Pediatr Otorhinolaryngol 1998; 42:207–23. [DOI] [PubMed] [Google Scholar]
- 12.Klein JO, Bluestone CD. Acute otitis media. Pediatr Infect Dis 1982; 1:66–73. [PubMed] [Google Scholar]
- 13.Bluestone CD, Klein JO, McCracken GH Jr et al. Panel discussion: management of children with recurrent or chronic otitis media with effusion. Pediatr Infect Dis 1984; 4:397–400. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Chronic suppurative otitis media: Burden of Disease and Management Options; 2004. Available at: http://www.who.int/pbd/deafness/activities/hearing_care/otitis_media.pdf Accessed 1 November 2014. [Google Scholar]
- 15.Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosner BA. Chapter 14. Hypothesis testing: person time data In: Crockett C, ed. Fundamentals of Biostatistics. 5th ed Pacific Grove, CA: Duxbury Thompson Learning; 2000: pp 681–688, 761. [Google Scholar]
- 17.World Health Organization. Deafness and Hearing Loss. Available at: http://www.who.int/mediacentre/factsheets/fs300/en/ Accessed 1 November 2014. [Google Scholar]
- 18.Macfadyen C, Gamble C, Garner P et al. Topical quinolone vs. antiseptic for treating chronic suppurative otitis media: a randomized controlled trial. Trop Med Int Health 2005; 10:190–7. [DOI] [PubMed] [Google Scholar]
- 19.Alabi BS, Abdulkarim AA, Fatai O, Abdulmajeed SO. Prevalence of acute otitis media among children with pyrexia in a Nigerian hospital. Auris Nasus Larynx 2009; 36:532–5. [DOI] [PubMed] [Google Scholar]
- 20.Tessema G. Otitis media seen in Yekati 12 Hospital. Ethiop Med J 2001; 39:113–22. [PubMed] [Google Scholar]
- 21.Zakzouk SM, Hajjaj MF. Epidemiology of chronic suppurative otitis media among Saudi children–a comparative study of two decades. Int J Pediatr Otorhinolaryngol 2002; 62:215–8. [DOI] [PubMed] [Google Scholar]
- 22.McPherson B, Holborow A. A study of deafness in West Africa: the Gambian Hearing Health Project. Int J Pediatr Otorhinolaryngol 1985; 10:115–35. [DOI] [PubMed] [Google Scholar]
- 23.Anggraeni R, Hartanto WW, Djelantik B et al. Otitis media in Indonesian urban and rural school children. Pediatr Infect Dis J 2014; 33:1010–5. [DOI] [PubMed] [Google Scholar]
- 24.Chadha SK, Sayal A, VMalhotra V, Agarwal AK. Prevalence of preventable ear disorders in over 15000 school children in northern India. J Laryngol Otol 2013; 127:28–32. [DOI] [PubMed] [Google Scholar]
- 25.Rupa V, Jacob A, Joseph A. Chronic suppurative otitis media: prevalence and practices among rural South Indian children. Int J Pediatr Otorhinolaryngol 1999; 48:217–22. [DOI] [PubMed] [Google Scholar]
- 26.Adhikari P, Joshi S, Baral D, Kharel B. Chronic suppurative otitis media in urban private school children of Nepal. Braz J Otorhinolaryngol 2009; 75:669–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adhikari P. Pattern of ear diseases in rural school children: experiences of free health camps in Nepal. Int J Pediatr Otorhinolaryngol 2009; 73:1278–80. [DOI] [PubMed] [Google Scholar]
- 28.Eason RJ, Harding E, Nicholson R et al. Chronic suppurative otitis media in the Solomon Islands: a prospective, microbiological, audiometric and therapeutic survey. N Z Med J 1986; 99:812–5. [PubMed] [Google Scholar]
- 29.Adebola SO, Ayodele SO, Oyelakin OA et al. Pre-school hearing screening: profile of children from Ogbomoso, Nigeria. Int J Pediatr Otorhinolaryngol 2013; 77:1987–91. [DOI] [PubMed] [Google Scholar]
- 30.Ologe FE, Nwawolo CC. Chronic suppurative otitis media in school pupils in Nigeria. East Afr Med J 2003; 80:130–4. [DOI] [PubMed] [Google Scholar]
- 31.Minja BM, Machemba A. Prevalence of otitis media, hearing impairment and cerumen impaction among school children in rural and urban Dar es Salaam, Tanzania. Int J Pediatr Otorhinolaryngol 1996; 37:29–34. [DOI] [PubMed] [Google Scholar]
- 32.Castaquo LA, Lavinsky L. Otitis media in children: seasonal changes and socio-economic level. Int j Pediatr Otorhinolaryngol 2002; 62:129–34. [DOI] [PubMed] [Google Scholar]
- 33.Ogisi FO. Impedance screening for otitis media with effusion in Nigerian children. J Laryngol Otol 1988; 102:986–8. [DOI] [PubMed] [Google Scholar]
- 34.Tong MC, Yue V, Ku PK et al. Screening for otitis media with effusion to measure its prevalence in Chinese children in Hong Kong. Ear Nose Throat J 2000; 79:626–30. [PubMed] [Google Scholar]
- 35.Zhang Q, Wei J, Xu M et al. Prevalence of otitis media with effusion among children in Xi'an, China: a randomized survey in China's mainland. Ann Otol Rhinol Laryngol 2011; 120:617–21. [DOI] [PubMed] [Google Scholar]
- 36.De Ru JA, Grote JJ. Otitis media with effusion: disease or defense? Int J Pediatr Otorhinolaryngol 2004; 68:331–9. [PubMed] [Google Scholar]
- 37.Sophia A, Isaac Rita, Rebekah Grace et al. Risk factors for otitis media among preschool, rural Indian children. Int J Pediatr Otorhinolaryngol 2010; 74:677–83. [DOI] [PubMed] [Google Scholar]
- 38.Elango S, Purohit GN, Hashim M, Hilmi R. Hearing loss and ear disorders in Malaysian school children. Int J Pediatr Otorhinolaryngol 1991; 22:75–80. [DOI] [PubMed] [Google Scholar]
- 39.Amusa YB, Jaduwola IK, Owayade OO. Epidemiology of otitis media in a local tropical African population. West Afr J Med 2005; 24:227–30. [DOI] [PubMed] [Google Scholar]
- 40.Seely DR, Gloyd SS, Omope AD, Norton SJ. Hearing loss prevalence and risk factors among Sierra Leonean children. Arch Otolaryngol Head Neck Surg 1995; 121:853–8. [DOI] [PubMed] [Google Scholar]