Abstract
G protein-coupled receptors (GPCRs) modulate cytoplasmic signalling in response to extracellular stimuli, and are important therapeutic targets in a wide range of diseases. Structure determination of GPCRs in all activation states is important to elucidate the precise mechanism of signal transduction and to facilitate optimal drug design. However, due to their inherent instability, crystallisation of GPCRs in complex with cytoplasmic signalling proteins, such as heterotrimeric G proteins and β-arrestins, has proved challenging. Here, we describe the design of a minimal G protein, mini-Gs, which is composed solely of the GTPase domain from the adenylate cyclase stimulating G protein Gs. Mini-Gs is a small, soluble protein, which efficiently couples GPCRs in the absence of Gβγ subunits. We engineered mini-Gs, using rational design mutagenesis, to form a stable complex with detergent-solubilised β1-adrenergic receptor (β1AR). Mini G proteins induce similar pharmacological and structural changes in GPCRs as heterotrimeric G proteins, but eliminate many of the problems associated with crystallisation of these complexes, specifically their large size, conformational dynamics and instability in detergent. They are therefore novel tools, which will facilitate the biochemical and structural characterisation of GPCRs in their active conformation.
Keywords: complex, G protein, G protein-coupled receptor, GPCR, Gs, mini G protein, mini-Gs
Introduction
G protein-coupled receptors (GPCRs) modulate cytoplasmic signalling, through heterotrimeric G proteins and β-arrestins, in response to extracellular stimuli, such as hormones and neurotransmitters (Rosenbaum et al., 2009). The central role of GPCRs in regulating cellular responses makes them an important therapeutic target (Lagerstrom and Schioth, 2008). GPCRs adopt different conformational states in response to binding different classes of ligand and coupling to cytoplasmic signalling proteins. Therefore, structure determination of GPCRs in all activation states is important to decipher the molecular mechanisms of signal transduction, and to facilitate optimal drug design.
Heterotrimeric G proteins are composed of α, β and γ subunits. Gα consists of a GTPase domain (GαGTPase), which is analogous to members of the small GTPase superfamily of proteins, and an α-helical domain (GαAH), which is unique to heterotrimeric G proteins (Sprang, 1997). In the inactive, GDP-bound state, Gα binds Gβγ, forming a heterotrimer with low basal nucleotide exchange activity (Higashijima et al., 1987). The trimer is anchored to the cell membrane, through lipid modifications of both Gα and Gγ (Spiegel et al., 1991). GPCRs catalyse rapid nucleotide exchange on heterotrimeric G proteins, but only weakly activate the isolated α subunit (Herrmann et al., 2006; Phillips et al., 1992).
Agonist binding to a GPCR promotes its transition to a structural state that can efficiently interact with heterotrimeric G proteins (Lebon et al., 2011b; Rasmussen et al., 2011b; Rosenbaum et al., 2011; Xu et al., 2011). The agonist-bound receptor engages the C-terminal region of Gα (Hamm et al., 1988), initiating a rotation and displacement of the α5 helix (Oldham et al., 2006). This ultimately destabilises the nucleotide-binding pocket and the GαGTPase–GαAH domain interface, allowing GDP to dissociate (Alexander et al., 2014; Dror et al., 2015; Flock et al., 2015; Kaya et al., 2014; Sun et al., 2015; Van Eps et al., 2011). The resulting nucleotide-free ternary complex displays large, mutually induced structural changes in both the receptor and G protein (Rasmussen, et al., 2011b), and is often characterised by increased agonist binding affinity of the receptor (Delean et al., 1980). This complex can be trapped in the absence of guanine nucleotides (Bornancin et al., 1989), but is extremely short-lived in vivo due to rapid binding of GTP to Gα (Vuong et al., 1984). GTP binding triggers dissociation of the G protein from the receptor (Kuhn, 1981) and separation of Gα from Gβγ (Fung et al., 1981).
GPCR–G protein complexes are difficult targets for structural studies due to their large size, conformational dynamics, and instability in detergent. To date, only a single structure of a GPCR–G protein complex has been reported, namely the β2-adrenergic receptor (β2AR) bound to the adenylate cyclase stimulating G protein Gs (Rasmussen et al., 2011b). This structure provided the first atomic resolution insight into the organisation of the ternary complex, but further structures are required to fully decipher the molecular mechanisms of signal transduction and the specificity for G protein coupling. Given the difficulties in crystallising GPCR–G protein complexes, novel tools are needed to facilitate their high-throughput crystallisation.
Here, we report the design of a minimal G protein, termed mini-Gs, which is composed solely of the GαGTPase domain from Gs. Mini-Gs closely mimics the pharmacological and structural changes induced in GPCRs by heterotrimeric Gs. It is therefore a novel tool, which will facilitate the characterisation of GPCRs in their active conformation, and has allowed the structure determination of the adenosine A2A receptor in the fully active state (Carpenter et al., 2016).
Materials and Methods
Cloning
Details of G protein, β1AR and mini-Gs constructs used in this work are provided in Supplementary Tables SI–SIII, respectively. All G proteins used in this study were mutated to remove sites of lipid modification. G protein cDNAs were cloned into the transfer vector pBacPAK8 (Clontech), and baculoviruses were prepared using the flashBAC ULTRA system (Oxford Expression Technologies). Synthetic genes (Integrated DNA Technologies) for Nb80 (Rasmussen et al., 2011a) and Nb35 (Westfield et al., 2011) were cloned into pET26b (Novagen) for periplasmic expression in Escherichia coli. Mini-Gs constructs, which were derived from the long isoform of the human Gαs gene, were cloned into the pET15b vector (Novagen) for expression in E. coli.
Expression and purification of β1AR
β1AR constructs were expressed in insect cells using the baculovirus expression system, and purified as described previously (Warne et al., 2003; Warne et al., 2011; Warne et al., 2009).
Baculovirus expression of G proteins
Trichoplusia ni cells (Expression Systems) were grown in ESF921 serum-free media (Expression Systems) in 5 L optimum growth flasks (Thompson Instrument Company). Immediately before infection, heat-inactivated foetal bovine serum (Sigma) was added to a final concentration of 5%. Cells were infected with third passage virus at a final concentration of 3%. In the case of co-infection with multiple viruses (for heterotrimeric Gs or Gβγ) each virus was added to a final concentration of 3%. The final volume of culture was 3 L per flask and the final cell density was 3 × 106 cells/ml. Cells were harvested 48 h post-infection by centrifugation at 5000 g for 5 mins, flash-frozen in liquid nitrogen and stored at −80°C.
Protein purification
Details of G protein, nanobody and mini-Gs purifications are provided in the Supplementary material.
Saturation binding assay
Insect cell membranes containing β1AR were resuspended in assay buffer (20 mM HEPES pH 7.5, 100 mM NaCl). The sample was aliquoted and [3H]-dihydroalprenolol was added (to give final concentrations in the range of 0.25 nM to 256 nM), alprenolol was added to the negative control (1 mM final concentration). Samples were incubated at 20°C for 2 h, before filtering through 96-well glass fibre filter plates (Merck Millipore) and washing with ice-cold assay buffer. Radioactivity was quantified by scintillation counting and apparent Kd values were determined using GraphPad Prism version 5.0 (GraphPad Software, San Diego, CA).
Competition binding assay
Insect cell membranes containing β1AR were resuspended in assay buffer (25 mM HEPES pH 7.5, 100 mM NaCl, 1 mM MgCl2, 1 mM ascorbate). The sample was aliquoted and binding partner (25 μM final concentration), isoprenaline (final concentrations in the range of 1 pM–100 mM) and apyrase (0.1 U/ml final concentration) were added. Alprenolol was added to the negative control (100 μM final concentration). Samples were incubated at 20°C for 1.5 h, before adding [3H]-dihydroalprenolol (5 or 20 nM final concentrations for β1ARΔNC or β1AR-84, respectively). Samples were incubated at 20°C for 1.5 h, before filtering through 96-well glass fibre filter plates and washing with ice-cold assay buffer. Radioactivity was quantified by scintillation counting and Ki values were determined using GraphPad Prism version 5.0.
Competition binding assays using detergent-solubilised β1AR-84 were performed using a similar protocol, except: all steps were performed at 4°C; membranes were solubilised with dodecyl maltoside (DDM; 0.1% final concentration) for 30 min, prior to addition of binding partner and ligands; separation of bound from free ligand (by gel filtration) was performed exactly as described in the thermostability assay protocol (below).
Thermostability measurement of β1AR∆NC–mini-Gs complexes
Thermostability assays were performed using a modified version of previously described methods (Lebon et al., 2011a; Serrano-Vega et al., 2008). Insect cell membranes containing β1AR∆NC were resuspended in assay buffer (25 mM HEPES pH 7.5, 400 mM NaCl, 1 mM MgCl2, 1 mM ascorbate, 0.1% BSA, 0.004% bacitracin). The sample was aliquoted and binding partner (25 μM final concentration), 3H-norepinephrine (200 nM final concentration) and apyrase (0.1 U/ml final concentration) were added. Norepinephrine was added to the negative control (200 μM final concentration). Samples were incubated at 4°C for 1 h, before solubilisation with detergent for 1 h on ice. The detergents dodecyl maltoside (DDM), decyl maltoside (DM), nonyl glucoside (NG) or octyl glucoside (OG) were used at final concentrations of 0.1, 0.13, 0.3 or 0.8%, respectively. Samples were heated to different temperatures (between 4 and 50°C) for exactly 30 min, followed by quenching on ice for 30 min. Samples were separated by gel filtration through Toyopearl HW-40F resin packed in a 96-well filter plate (Merck Millipore). Radioactivity was quantified by scintillation counting and apparent melting temperature (Tm) values were determined using GraphPad Prism version 5.0.
Thermostability measurement of GDP-bound mini-Gs mutants by differential scanning fluorimetry
Differential scanning fluorimetry (DSF) was performed essentially as described previously (Niesen et al., 2007). Mini-Gs mutants (30 μg) were diluted with assay buffer (10 mM HEPES pH 7.5, 100 mM NaCl, 1 mM MgCl2, 1 mM GDP, 2 mM DTT). SYPRO-orange was added to give a final concentration of ×2. Thermostability measurements were performed using a Rotor-Gene Q (Qiagen). Samples were equilibrated for 90 s at 25°C before ramping from 25 to 99°C at 4 s/°C. The apparent melting temperature (Tm), corresponding to the inflection point of the curve, was derived from analysis using the Rotor-Gene Q software.
Gel filtration analysis of mini G protein complexes
The mini-Gs–βγ complex was prepared using mini-Gs399, a construct in which the N-terminal residues 6–25 were replaced and the L272D mutation was reversed (Supplementary Table SIII). Purified mini-Gs399 was mixed with non-lipidated Gβ1γ2 dimer in an equimolar ratio and incubated on ice for 4 h. The sample was loaded onto a Superdex-200 10/300 gel filtration column (GE healthcare), equilibrated with gel filtration buffer (10 mM HEPES pH 7.5, 100 mM NaCl, 1 mM MgCl2, 1 μM GDP, 0.1 mM TCEP).
The β1AR–mini-Gs complex was prepared using β1AR∆NC purified in lauryl maltose neopentyl glycol (LMNG) detergent. Purified β1AR∆NC was mixed with a 1.2-fold molar excess of mini-Gs393 and incubated on ice for 4 h. The sample was loaded onto a Superdex-200 10/300 gel filtration column, equilibrated with gel filtration buffer (10 mM HEPES pH 7.5, 100 mM NaCl, 1 mM MgCl2, 1 μM ascorbic acid, 1 μM isoprenaline, 0.002% LMNG). Peak fractions were analysed by SDS-PAGE on a 4–20% Tris-glycine gel (Thermo Fisher).
The gel filtration column was calibrated using molecular weight standards (Sigma), and the apparent molecular weight of samples was calculated using the calibration curve shown in Supplementary Fig. S9.
Statistical analysis
Unpaired, two-tailed t-tests were used to compare two data sets, and P values are quoted in the text.
Results
Strategy to develop a minimal G protein
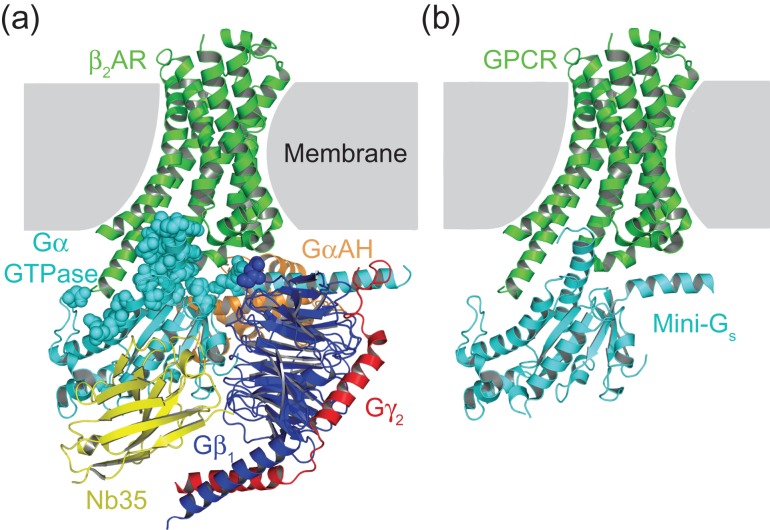
The aim of this work was to isolate the minimum component of Gs that could be used to crystallise native-like GPCR–G protein complexes. The molecular weight of Gs is 90 kDa, however, the β2AR–Gs complex (Rasmussen et al., 2011b) revealed that more than 97% of direct contacts between the G protein and receptor are mediated by the 27 kDa GαGTPase domain (Fig. 1a). We hypothesised that this domain would be sufficient to stabilise GPCRs in their fully active state, i.e. the conformation adopted by β2AR in the β2AR–Gs complex (Rasmussen et al., 2011b), and it was therefore used as the starting point to engineer a minimal G protein (mini-Gs; Fig. 1b).
Fig. 1.
Design of a minimal G protein. (a) Crystal structure of the β2AR–Gs complex (PDB code 3SN6; Rasmussen et al., 2011b). The intracellular component of this complex, which is composed of Gαs, Gβ1, Gγ2 and Nb35, totals over 100 kDa in molecular weight. However, over 97% of direct contacts (3.9 Å cut-off) between β2AR and Gs are formed by the GαGTPase domain (cyan). Residues from Gs that form contacts with β2AR are shown as spheres. (b) Model of the proposed complex between a GPCR and mini-Gs (isolated GαGTPase domain). The intracellular component of this complex is a single protein with a molecular weight of approximately 27 kDa. Figures were prepared using PyMOL (The PyMOL Molecular Graphics System, Version 1.7.4 Schrödinger, LLC)
Three binding partners were used as controls during this work: Nb80, a nanobody that binds to β2AR and induces a comparable shift in agonist binding affinity to heterotrimeric Gs (Rasmussen et al., 2011a); soluble heterotrimeric Gs that was mutated to remove all potential lipidation sites, referred to herein as Gs (Supplementary Table SI); and Nb35, a nanobody that stabilises Gs in its GPCR-bound conformation (Westfield et al., 2011). Gs was either used alone (referred to as Gs) or in the presence of Nb35 (referred to as Gs–Nb35). As active state structures of β2AR bound to either Nb80 or Gs–Nb35 have been determined (Rasmussen et al., 2011a, 2011b), the stability and pharmacological activity of an engineered mini-Gs should, at a minimum, reflect the analogous properties of these binding partners.
The β1-adrenergic receptor (β1AR) was used as a model GPCR in the development of mini-Gs. Both β1AR and β2AR are able to bind Gs and Nb80 and exhibit a significant increase in agonist binding affinity. Either receptor could have been used for the development of mini-Gs, but because our laboratory has developed a large number of thermostabilised β1AR variants (Miller and Tate, 2011; Serrano-Vega et al., 2008), we chose to work with β1AR. We did not use the adenosine A2A receptor for the development of mini-Gs, because there is only a small increase in agonist affinity upon binding a G protein (Carpenter et al., 2016).
To be most useful for the characterisation and structure determination of GPCRs in their active state, mini-Gs needed to fulfil a number of criteria. Mini-Gs should be stable enough in its basal conformation to allow high-yield expression and purification, promote the transition of β1AR to the high-affinity agonist-bound state, and form a stable complex with detergent-solubilised β1AR. The development of mini-Gs involved a number of key steps, some of which only became apparent as the work progressed: (i) development of a sensitive assay to detect G protein coupling to β1AR; (ii) isolation of the GαGTPase domain (mini-Gs) and demonstration of binding to β1AR; (iii) thermostabilisation of the β1AR–mini-Gs complex in membranes; (iv) thermostabilisation of the β1AR–mini-Gs complex in detergent; and (v) validation of the final mini-Gs construct. Details of each of these steps are given under the corresponding subheadings later.
Development of a sensitive assay to detect G protein coupling to β1AR
A sensitive competition binding assay was developed that could detect the interaction of different binding partners with β1AR, by measuring the affinity of agonist binding to the receptor. A heterologous competition format was used to determine the agonist binding affinity (Ki) of β1AR by measuring binding of the antagonist 3H-dihydroalprenolol (3H-DHA; Supplementary Fig. S1) in the presence of increasing concentrations of the agonist isoprenaline. The concentration of binding proteins used in the assays was standardised to 25 μM, which was approximately 30-fold above the equilibrium dissociation constant (KD) for Nb80 binding to β1AR (Miller-Gallacher et al., 2014). Initially, we used a truncated form of turkey β1AR (Warne et al., 2003), which was designated β1AR∆NC (Supplementary Table SII). This construct did not contain any thermostabilising mutations, and behaved identically to full-length receptor in cell-signalling assays (Baker et al., 2011), despite containing truncations of disordered regions in the N-terminus and C-terminus. The Ki for isoprenaline binding to β1AR∆NC was 40 ± 0 nM in the absence of a binding partner, which shifted to 5.8 ± 0.8 nM, 17 ± 2 nM or 6.8 ± 0.6 nM in response to Nb80, Gs or Gs–Nb35, respectively (Supplementary Fig. S2). The shift in isoprenaline Ki upon G protein binding to β1AR∆NC was relatively small, which was unsuitable for detecting potentially small changes elicited upon binding of unstable G protein derivatives during the development of mini-Gs. We therefore used a minimally thermostabilised β1AR construct (β1AR-84; Supplementary Table SII), which contained four mutations that increased the stability preferentially of the inactive state of the receptor (Miller-Gallacher et al., 2014; Warne et al., 2011), in addition to the truncations at the N-terminus and C-terminus. β1AR-84 had a lower affinity for isoprenaline (Ki of 2.6 ± 0.3 μM) in its uncoupled state than β1AR∆NC, but showed a larger shift in agonist binding affinity when coupled to either Nb80, Gs or Gs–Nb35 (Ki of 28 ± 1 nM, 271 ± 54 nM or 16 ± 4 nM, respectively; Table I and Supplementary Fig. S2). The competition binding data fitted best to single-site binding parameters. Therefore, the partial shift in isoprenaline Ki observed for some binding partners most likely reflected incomplete stabilisation of the high-affinity agonist-bound state, rather than indicating partial coupling or mixed receptor populations. Although Gs was able to couple β1AR, Nb35 was required to stabilise the Gs complex, resulting in β1AR–Gs–Nb35 complexes with similar affinity for isoprenaline compared to the β1AR–Nb80 complexes (Table I and Supplementary Fig. S2). The competition binding assay using β1AR-84 showed a 162-fold increase in isoprenaline affinity upon Gs–Nb35 coupling to the receptor, which was far larger than the 6-fold shift in affinity induced by Gs–Nb35 binding to β1AR∆NC. We therefore used β1AR-84 in all subsequent competition binding assays during the development of mini-Gs.
Table I.
β1AR-84 competition binding data
| Binding partner | Mutation | CGN codea | β1AR-84 isoprenaline Ki (nM) | Effect on expression | |
|---|---|---|---|---|---|
| 4°C | 20°C | ||||
| None | n.a.b | n.a. | 2100 ± 180 (n = 12) | 2600 ± 270 (n = 15) | n.a. |
| Nb80 | n.a. | n.a. | n.d.c | 28 ± 1 (n = 2) | n.a. |
| Gs | n.a. | n.a. | 420 ± 80 (n = 2) | 271 ± 54 (n = 2) | n.a. |
| Gs–Nb35 | n.a. | n.a. | n.d. | 16 ± 4 (n = 3) | n.a. |
| Mini-Gs77 | Parental | n.a. | 99 ± 12 (n = 4) | 1900 ± 230 (n = 3) | n.a. |
| H41I | 41G.S1.2 | 32 | 390 | = | |
| H41V | 41G.S1.2 | 51 | 490 | = | |
| A48L | 48G.s1h1.2 | 43 | 170 | = | |
| G49D | 49G.s1h1.3 | 25 | 280 | = | |
| E50N | 50G.s1h1.4 | 37 | 720 | = | |
| R201A | 201G.hfs2.2 | 31 | 1480 | − | |
| G226A | 227G.s3h2.2 | 86 | 820 | = | |
| 227–230 subd | 227G.s3h2.3 | 23 | 530 | = | |
| E230A | 230G.H2.3 | 51 | 540 | − | |
| A249D | 249G.S4.7 | 10 | 35 | + | |
| A249E | 249G.S4.7 | 70 | 390 | = | |
| S252D | 252G.s4h3.3 | 14 | 94 | + | |
| S252E | 252G.s4h3.3 | 38 | 380 | + | |
| 255–264 dele | 254G.s4h3.5 | 21 | 20 | + | |
| L272D | 272G.H3.8 | 7 | 310 | = | |
| L272E | 272G.H3.8 | 28 | 750 | = | |
Competition binding data showing the isoprenaline Ki of β1AR-84 in the presence of different binding partners. Data are from a single experiment performed in duplicate unless otherwise stated; where two or more independent experiments were performed, data represent mean ± SEM, from the number (n) of independent experiments performed in duplicate. The effect of mutations on the expression level of mini-Gs was estimated from SDS-PAGE gels. Expression levels are described as being equal to (=), more than 2-fold lower than (−), or more than 2-fold higher than (+) the parental construct.
aCommon Gα numbering (CGN) system (Flock et al., 2015).
bNot applicable.
cNot determined.
dSubstitution of switch II residues 227–230 with two glycine residues.
eDeletion of switch III residues 255–264.
Isolation of the Gαs GTPase domain and measuring binding to β1AR-84
The GαGTPase domain from Gαs had previously been expressed as an isolated protein, in order to determine its role in guanine nucleotide binding and hydrolysis (Markby et al., 1993), but its ability to couple to GPCRs had never been investigated. We isolated the GTPase domain by replacing the sequence corresponding to GαAH with a short glycine linker (Supplementary Table SIII). This construct, mini-Gs77, expressed poorly in E. coli and could not be purified to homogeneity, indicating that it was very unstable. Nevertheless, a small amount of partially pure protein could be prepared (Supplementary Fig. S3), and was tested for its ability to couple β1AR-84 (at 20°C) in either the presence or absence of Gβγ–Nb35. No significant shift in the isoprenaline Ki of β1AR-84 (2.6 ± 0.3 μM) was observed in the presence of mini-Gs77 (1.9 ± 0.2 μM; P = 0.254), but mini-Gs77–Gβγ–Nb35 induced a large shift in isoprenaline affinity to 3.6 ± 0.8 nM (P = 0.004; Table I and Supplementary Fig. S2). Thus partially purified mini-Gs77 was functional, but the data also suggested that it was unable to couple β1AR-84 in the absence of Gβγ when assayed at 20°C. In contrast, when the assay was performed at 4°C mini-Gs77 induced a significant shift in isoprenaline Ki from 2.1 ± 0.2 μM for uncoupled β1AR-84 (at 4°C) to 99 ± 12 nM (P < 0.001; Table I and Supplementary Fig. S2). This demonstrated that the isolated GαGTPase domain (mini-Gs77) could couple to β1AR-84 in the absence of Gβγ, but suggested that the thermostability of the GαGTPase domain was a limiting factor in its ability to stabilise the high-affinity agonist-bound state of the receptor.
Thermostabilisation of the β1AR–mini-Gs complex in membranes
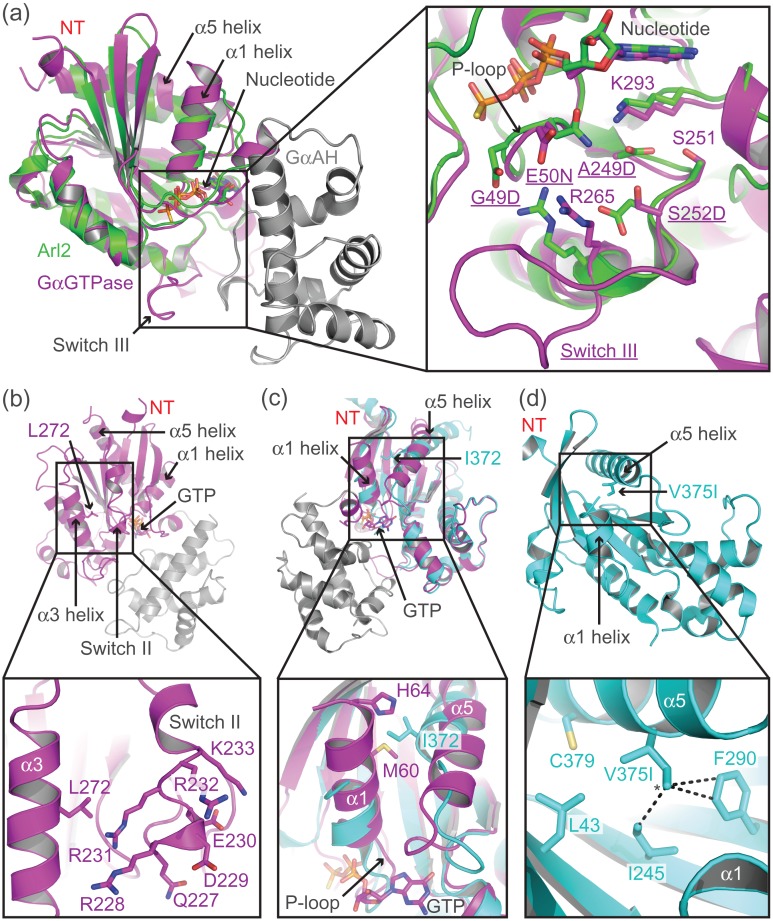
Rational design mutagenesis was employed to thermostabilise mini-Gs in complex with membrane-embedded β1AR. Mutations were designed based on structural alignments (Fig. 2a and Supplementary Fig. S4) between Gαs (Sunahara et al., 1997) and Arl2 (Hanzal-Bayer et al., 2002); Arl2 is the small GTPase with the greatest structural similarity to Gαs. This initial mutagenic screen primarily targeted regions of Gαs that were close to the GαAH domain interface or that were known to be conformationally dynamic. Mutants were screened using the competition binding assay at both 4 and 20°C. Due to the low, and variable expression level of the mutants, it was not easy to standardise the concentration of mini-Gs mutants used in the assays. Therefore, the total mini-Gs purified from 1 L of E. coli culture was used per competition curve (Table I). Approximately 100 mutants were tested during this initial screen. Mutations that shifted the isoprenaline Ki of β1AR-84 more than 2-fold compared to the parental mini-Gs construct (mini-Gs77) at either temperature were classed as positive. A total of 16 positive mutations, covering 12 unique positions were identified (Table I).
Fig. 2.
Rational design of mutations to stabilise mini-Gs. (a) Structural alignment of Gαs (PDB code 1AZT; Sunahara et al., 1997), coloured magenta and grey, and Arl2 (PDB code 1KSH; Hanzal-Bayer et al., 2002), coloured green. The Gαs GTPase domain aligns to Arl2 with an RMSD of 1.9 Å, despite sharing sequence identity of only 25%, determined using the Dali server (Holm and Rosenstrom, 2010). See Supplementary Fig. S4 for a sequence alignment between Gαs and Arl-2. The inset shows an expanded view of mini-Gs residues (shown as sticks and underlined name) that were mutated (G49D, E50N, A249D, and S252D) to match the corresponding residue in Arl2. Residues with which the mutations potentially interact are shown as sticks. (b) Mutation of Leu272, which is located within the α3 helix of Gαs (PDB code 1AZT; Sunahara et al., 1997), to aspartic acid allows potential interactions with a cluster of charged and polar residues (227–233) in the N-terminal region of switch II. (c) Alignment of Gαs in its GTP-bound conformation (PDB code 1AZT; Sunahara et al., 1997), coloured magenta, and GPCR-bound conformation (PDB code 3SN6; Rasmussen et al., 2011b), coloured cyan. In the GPCR-bound conformation Ile372 (α5 helix) sterically clashes with Met60 and His64 (α1 helix), preventing close packing of the α1 helix against the core of the GαGTPase domain. (d) The V375I mutation (modelled using PyMOL) was designed to increase hydrophobic contacts between the core of the GαGTPase domain and the α5 helix in its GPCR-bound conformation (PDB code 3SN6; Rasmussen et al., 2011b). Residues that interact with Val375 are shown as sticks, additional contacts (less than 4.2 Å), which are predicted to be formed by the δ-carbon (*) of the isoleucine mutation are displayed as dashed lines.
Some of the single mini-Gs mutants produced a near maximal shift in the agonist binding affinity of β1AR-84 in the competition binding assay, therefore, mutation combinations could not be reliably tested using this assay, as further small increases in agonist affinity would be difficult to accurately measure. Instead, the mutation combinations were tested in complex with detergent-solubilised β1AR∆NC using a thermostability (Tm) assay (Table II). The agonist 3H-norepinephrine (3H-NE) was used in the Tm assay, however due to the high background signal associated with this ligand, a maximum concentration of 200 nM could be used. This was approximately equal to the Ki of uncoupled β1AR∆NC, but approximately 250-fold above the Ki of β1AR∆NC complexed with Nb80 or Gs–Nb35 (Supplementary Fig. S5). Therefore, apparent Tm values quoted for uncoupled β1AR∆NC are under non-saturated agonist conditions, but β1ARΔNC complexes, which have higher agonist binding affinity, are under agonist-saturated conditions.
Table II.
β1AR∆NC thermostability data
| Binding partner | Mutation | CGN codea | Apparent Tm of β1AR∆NC in DDM, measured by 3H-NE binding (°C) | Stability of GDP-bound mini-Gs measured by DSF (°C) |
|---|---|---|---|---|
| None | n.a.b | n.a. | 25.9 ± 0.0 (n = 3) | n.a. |
| Nb80 | n.a. | n.a. | 32.0 ± 0.0 (n = 3) | n.a. |
| Gs–Nb35 | n.a. | n.a. | 35.8 ± 0.1 (n = 3) | n.a. |
| Gαs | n.a. | n.a. | n.d.c | 50.1 ± 0.1 (n = 3) |
| Mini-Gs162 | A249D | 25.1 (n = 1) | 60.6 ± 0.1 (n = 3) | |
| Mini-Gs164 | A249D, SIIId | 28.6 (n = 1) | 66.5 ± 0.0 (n = 3) | |
| Mini-Gs165 | A249D, S252D, SIII | 28.5 ± 0.2 (n = 2) | 68.7 ± 0.0 (n = 3) | |
| Mini-Gs169 | A249D, S252D, SIII, L272D | 28.8 (n = 1) | 67.1 ± 0.0 (n = 3) | |
| Mini-Gs183 | G49D, E50N, A249D, S252D, SIII, L272D | 28.7 ± 0.2 (n = 4) | 72.5 ± 0.0 (n = 3) | |
| Mini-Gs199e | G49D, E50N, A249D, S252D, SIII, L272D | 29.2 ± 0.2 (n = 17) | 72.5 ± 0.0 (n = 3) | |
| Mini-Gs254 | Mini-Gs199 + M60A | 60G.H1.8 | 31.5 ± 0.3 (n = 5) | 70.3 ± 0.0 (n = 3) |
| Mini-Gs350 | Mini-Gs199 + L63Y | 63G.H1.11 | 30.9 ± 0.4 (n = 2) | 70.7 ± 0.0 (n = 3) |
| Mini-Gs340 | Mini-Gs199 + I372A | 372G.H5.4 | 34.0 (n = 1) | 66.6 ± 0.1 (n = 3) |
| Mini-Gs303 | Mini-Gs199 + V375I | 375G.H5.7 | 31.5 ± 0.6 (n = 3) | 70.3 ± 0.0 (n = 3) |
| Mini-Gs352 | Mini-Gs199 + L63Y, I372A | 34.5 (n = 1) | 64.7 ± 0.1 (n = 3) | |
| Mini-Gs345 | Mini-Gs199 + I372A, V375I | 35.0 (n = 1) | 65.4 ± 0.1 (n = 3) | |
| Mini-Gs393f | Final mini-Gs construct | 34.1 ± 0.5 (n = 3) | 65.3 ± 0.0 (n = 3) |
Thermostability data for either detergent-solubilised β1AR∆NC complexes or mini-Gs mutants in the GDP-bound state. Apparent Tm values represent the mean ± SEM from the number (n) of independent experiments performed in duplicate. Some apparent Tm values were determined from a single experiment, with an assumed error of ±0.5°C. Apparent Tm values for mini-Gs in the GDP-bound state were determined by differential scanning fluorimetry (Supplementary Fig. S6).
aCommon Gα numbering (CGN) system (Flock et al., 2015).
bNot applicable.
cNot determined.
dDeletion of switch III residues 255–264 is referred to as SIII.
eMini-Gs199 contains the same mutations as mini-Gs183, but has a redesigned linker region (Supplementary Table SIII), and was used as the parental construct for screening detergent-stabilising mutations.
fMini-Gs393 contains the same mutations as mini-Gs345, but has an additional truncation of the N-terminus and redesigned linker region (Supplementary Table SIII), and was used as the starting construct for crystallisation trials.
A new parental construct (mini-Gs161), was used to test combinations of the mutations; mini-Gs161 contained a larger deletion encompassing GαAH with a slightly longer linker than in mini-Gs77 (Supplementary Table SIII). The A249D mutant (mini-Gs162) produced the largest shift in the agonist binding affinity of membrane-embedded β1AR-84 in the competition binding assay, however, in detergent the β1AR∆NC–mini-Gs162 complex had an apparent Tm of 25.1°C (Table II), which was lower than that of uncoupled of β1AR∆NC (25.9°C). Addition of the switch III deletion, which induced the second largest shift in the agonist binding affinity of membrane-embedded β1AR-84, to make a double mutant (mini-Gs164), increased the apparent Tm of the complex to 28.6°C (Table II). However, this was still lower than that of either β1AR∆NC–Nb80 or β1AR∆NC–Gs–Nb35, by 3.4 and 7.2°C, respectively (Table II). Addition of other mutations that were classed as positive in the competition binding assay failed to further increase the apparent Tm of the β1AR∆NC–mini-Gs complex (examples shown in Table II), indicating that the complex was particularly unstable in detergent. This was confirmed by the observation that mini-Gs mutants were unable to shift the agonist binding affinity of detergent-solubilised β1AR-84 to the same degree as that of membrane-embedded β1AR-84 in competition binding assays (results not shown). Despite the fact that they did not further stabilise the β1AR∆NC–mini-Gs complex in detergent, four additional mutations (G49D, E50N, S252D and L272D) were added to mini-Gs164 to produce the construct mini-Gs183 (Table II). These mutations were utilised because they all individually increased the isoprenaline Ki of membrane-embedded β1AR-84 (Table I). They also increased the stability of the basal GDP-bound state mini-Gs183 by 6°C compared to mini-Gs164 (Table II and Supplementary Fig. S6), as assessed by differential scanning fluorimetry.
Five of the six mutations that were combined in mini-Gs183 were clustered around the nucleotide-binding pocket and phosphate-binding loop (P-loop; Fig. 2a). The A249D mutation was designed to interact with Lys293 and Ser251, in order to stabilise the base of the nucleotide-binding pocket. Deletion of switch III was intended to stabilise mini-Gs, by replacing this flexible loop with the defined secondary structure elements (α-helix, 310-helix and β-turn) found in Arl2 (Hanzal-Bayer et al., 2002). The S252D mutation was also designed to stabilise the region around switch III, through a potential interaction with Arg265. The G49D and E50N mutations, which are located in the P-loop, were designed to reduce flexibility and conformationally constrain this region, through potential interactions with Arg265 and Lys293, respectively. The sixth mutation (L272D) was designed to conformationally constrain switch II, through potential interactions with a cluster of charged and polar residues (227–233) within its N-terminal region (Fig. 2b).
Thermostabilisation of the β1AR∆NC–mini-Gs complex in detergent
The majority of mutations from the first mutagenic screen did not stabilise the β1AR–mini-Gs complex in detergent. We hypothesised that this was because they did not specifically stabilise mini-Gs in it receptor-bound conformation, therefore, a second panel of approximately 150 mutants were designed with the intention of stabilising the receptor-bound conformation of mini-Gs. This mutagenic screen was based on the structure of the β2AR–Gs complex (Rasmussen et al., 2011b), and focused on regions of Gαs that undergo large conformational changes upon receptor binding. Many of the mutations tested during this second screen were destabilising to mini-Gs in its basal GDP-bound state, therefore they could not be tested individually (i.e. in the mini-Gs161 parental construct). Instead, they were added to mini-Gs199, which was identical to mini-Gs183, except that it contained a modified linker region (Supplementary Table SIII). This construct was very stable in its basal GDP-bound state (72.5°C) and could thus negate the destabilising effects of the additional mutations. Mini-Gs mutants were screened in complex with detergent-solubilised β1AR∆NC using the 3H-norepinephrine Tm assay. Four stabilising mutations were identified (Table II), the best of which (I372A) increased the apparent Tm of the complex from 29.2 to 34.0°C and it combined additively with V375I, to give an apparent Tm of 35.0°C. This was 3.0°C higher than the β1AR∆NC–Nb80 complex and only 0.8°C lower than the β1AR∆NC–Gs–Nb35 complex. The other mutations did not combine additively with I372A and V375I and were rejected (results not shown).
Both of the positive detergent-stabilising mutations were located within the α1 or α5 helices. Alignment of Gαs in its receptor-bound conformation (Rasmussen et al., 2011b) with the GTP-bound structure (Fig. 2c; Sunahara et al., 1997) identified an unfavourable steric clash across the α1–α5 helix interface, involving residues Met60, His64 (from the α1 helix) and Ile372 (from the α5 helix). This clash was predicted to prevent close packing of the C-terminal region of the α1 helix against the α5 helix and the core of the GαGTPase domain, thus exposing the core of the protein to the solvent. The I372A mutation was designed to eliminate this clash and facilitate better packing in this region. Similarly, the V375I mutation was designed to improve packing between the α5 helix and the core of the protein in its receptor-bound conformation (Fig. 2d).
Validation of mini-Gs
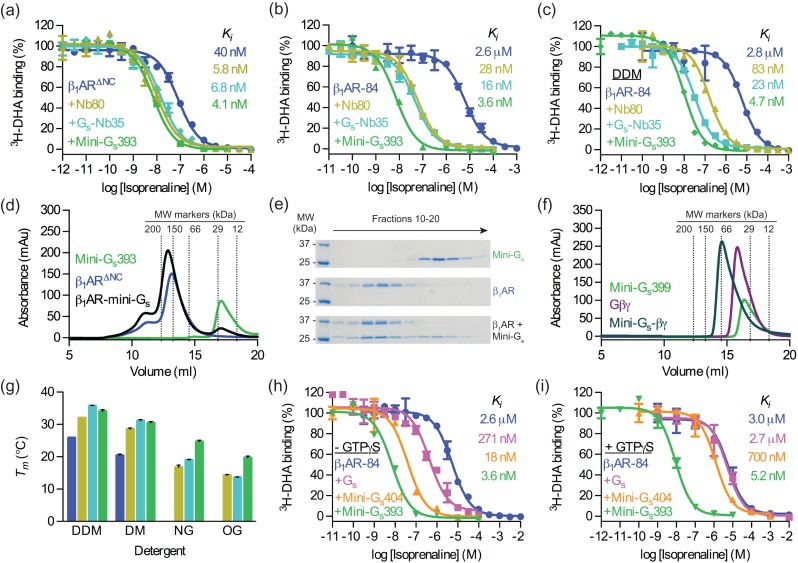
The detergent-stabilised construct (mini-Gs345) was modified for crystallographic applications by changing the linker and shortening the N-terminus (Supplementary Table SIII). The final stabilised construct, mini-Gs393 (Supplementary Fig. S7 and S8), was able to elicit an equal or greater shift in isoprenaline affinity compared to either Nb80 or Gs–Nb35 (Fig. 3a–c) whether the experiments were performed using membrane-embedded β1AR∆NC (Ki of 4.1 ± 1.1 nM compared to 5.8 ± 0.8 nM and 6.8 ± 0.6 nM, respectively), membrane-embedded β1AR-84 (Ki of 3.6 ± 0.0 nM compared to 28 ± 1 nM and 16 ± 4 nM, respectively) or detergent-solubilised β1AR-84 (Ki of 4.7 ± 0.4 nM compared to 83 ± 2 nM and 23 ± 7 nM, respectively). Crucially, for the mini-Gs393 complex, high-affinity isoprenaline binding was maintained when β1AR-84 was solubilised in detergent, and was 17-fold higher than that of the Nb80 complex and 5-fold higher than that of the Gs–Nb35 complex. Thus mini-Gs393 is an ideal protein for the formation of stable GPCR complexes in detergent solution.
Fig. 3.
Validation of mini-Gs (a–c) A competition binding assay was used to measure the change in affinity (Ki) of isoprenaline induced by Nb80, Gs–Nb35, or mini-Gs393 coupling to: (a) membrane-embedded β1AR∆NC, (b) membrane-embedded β1AR-84 and (c) DDM-solubilised β1AR-84. (d) Analytical gel filtration analysis of β1AR∆NC binding to mini-Gs393. The apparent molecular weight of mini-Gs393 was 23 kDa (17.1 ml), which compares well with the theoretical value of 27 kDa. The apparent molecular weight of β1AR∆NC was 139 kDa (13.2 ml), which is consistent with the 45 kDa receptor being associated with a large detergent micelle; the shoulder at 11 ml (> 300 kDa) probably represents aggregated receptor. A mixture of β1AR∆NC and mini-Gs393 (1.2-fold molar excess) resolved as a predominant peak with an apparent molecular weight of 160 kDa (12.9 ml). The 21 kDa increase in the apparent molecular weight of the β1AR∆NC–mini-Gs393 complex compared to uncoupled β1AR∆NC is consistent with mini-Gs393 binding with 1:1 stoichiometry. (e) SDS-PAGE analysis of the gel filtration eluate confirmed the presence of both β1AR∆NC and mini-Gs393 in the peak fractions. (f) Analytical gel filtration analysis of Gβγ binding to mini-Gs399. The apparent molecular weights of mini-Gs399 (Supplementary Table SIII) and Gβγ were 32 kDa (16.4 ml) and 42 kDa (15.8 ml), respectively, which is in close agreement with the theoretical values of 29 kDa and 46 kDa, respectively. An equimolar mixture of mini-Gs399 and Gβγ resolved as a single peak with an apparent molecular weight of 73 kDa (14.6 ml). The 31 kDa increase in the apparent molecular weight of the mini-Gs399–Gβγ complex compared to Gβγ is consistent with mini-Gs399 binding with 1:1 stoichiometry. (g) Thermostability of detergent-solubilised β1AR∆NC alone or in complex with Nb80, Gs–Nb35, or mini-Gs393, in different detergents (Supplementary Fig. S10). Uncoupled β1AR∆NC did not survive solubilisation in NG or OG. Colours correspond to those used in (a). (h–i) GTP-mediated dissociation of β1AR-84 complexes, measured by competition binding assay. The response in isoprenaline Ki induced by Gs, mini-Gs404 (Supplementary Table SIII), or mini-Gs393 coupling to β1AR-84 was measured in the presence or absence of GTPγS (250 μM). (a–c,h,i) Data are representative of at least two independent experiments, each performed in duplicate, with error bars ± SEM. (g) Data represent mean ± SEM of at least two independent experiments, each performed in duplicate.
The biochemical properties of mini-Gs393 also make it ideal for structural studies of GPCR complexes. Mini-Gs393 was readily purified to homogeneity with a yield of 100 mg of purified protein per litre of E. coli culture, and it could be concentrated to over 100 mg/ml (Supplementary Fig. S9). Analytical gel filtration showed that mini-Gs393 bound to purified β1AR∆NC in LMNG (Fig. 3d and e), demonstrating that the complex could be purified in detergent. Recent innovations in electron cryo-microscopy (cryo-EM; Bai et al., 2015) suggest that large GPCR–G protein complexes could be amenable for structure determination by single particle imaging. It is therefore useful to note that mini-Gs399, a construct in which the N-terminal residues 6–25 were replaced and the L272D mutation was reverted to wild type (Supplementary Table SIII), retained its ability to form a heterotrimer with Gβγ (Fig. 3f). The main advantage of the mini-Gs399–Gβγ complex over heterotrimeric Gs, is that it lacks the GαAH domain, which is highly dynamic in the GPCR-bound conformation (Westfield et al., 2011). Therefore GPCR complexes composed of mini-Gs399–Gβγ are predicted to be more conformationally homogenous than those involving heterotrimeric Gs, and thus better suited to cryo-EM applications.
Crystallisation of GPCRs often requires the use of short chain detergents, so the thermostability of the β1AR∆NC–mini-Gs393 complex was tested in four different detergents using the 3H-norepinephrine Tm assay and compared to the analogous complexes with Nb80 and Gs–Nb35 (Fig. 3g and Supplementary Fig. S10). Under all conditions tested uncoupled β1AR∆NC was significantly less stable than when bound to either mini-Gs393, Nb80 or Gs–Nb35. Similarly, β1AR∆NC was always more stable bound to mini-Gs393 compared to Nb80. β1AR∆NC–mini-Gs393 complexes were also considerably more stable than β1AR∆NC–Gs–Nb35 complexes in short chain detergents, although the later was marginally more stable in DDM. The striking improvement in the thermostability by 5–8°C of the β1AR∆NC–mini-Gs393 complex in NG and OG in comparison to other complexes suggests it has significant advantages for the structure determination of receptors in the active state.
The nucleotide-binding properties of the mutants were not extensively studied in this work, but one interesting observation was that the β1AR-84–mini-Gs393 complex was resistant to dissociation by physiological concentrations of GTP (Fig. 3h and i). GTPγS fully reversed the shift in Ki induced by Gs binding to β1AR-84 from 271 ± 54 nM to 2.7 ± 0.1 μM. The shift in isoprenaline Ki induced by mini-Gs404 (an identical construct to mini-Gs393, except that the I372A and V375I mutations were reverted to wild type; Supplementary Table SIII) binding to β1AR-84 was almost fully reversed by GTPγS (from 18 ± 2 nM to 700 ± 60 nM). However, there was no significant difference in the isoprenaline Ki of the β1AR-84–mini-Gs393 complex in either the presence or absence of GTPγS (3.6 ± 0.0 nM compared to 5.2 ± 0.7 nM; P = 0.137). This unresponsiveness to GTPγS was caused by the I372A mutation (see Discussion), because mini-Gs391 (an identical construct to mini-Gs393, except that only the V375I mutation was reverted to wild type; Supplementary Table SIII), behaved in a similar fashion to mini-Gs393 (Supplementary Fig. S11). Unresponsiveness to GTP is a useful property that should allow the formation of stable GPCR–mini-Gs complexes in vivo, which may be a novel method to improve expression and purification of unstable GPCRs.
Discussion
Several novel approaches have been developed to stabilise and crystallise GPCRs in their active conformation, including complexation with G protein-derived peptides (Scheerer et al., 2008), G protein-mimicking nanobodies (Huang et al., 2015; Kruse et al., 2013; Rasmussen et al., 2011a; Ring et al., 2013) and a nanobody-stabilised heterotrimeric G protein (Rasmussen et al., 2011b). All of these complexes appear to stabilize the receptor in its active state, which is characterised by an outward movement of helix 6 and conserved conformational changes of residues within the core of the receptor, particularly R3.50, Y5.58 and Y7.53 (Huang et al., 2015). The β2AR–Gs complex provided the first insight into the organisation of the native GPCR–G protein interface, which is something that other binding proteins cannot recreate, but frustratingly, complexes involving heterotrimeric Gs are also the most difficult to crystallise, due to their large size and dynamic nature. Therefore, we designed a minimal G protein that offers significant advantages to the crystallisation of native-like GPCR–G protein complexes, specifically mini-Gs is a small, soluble, highly expressed protein, which readily forms a detergent-stable complex with GPCRs.
Our lab has previously determined the structures of both β1AR and the adenosine A2A receptor (A2AR) bound to agonists (Lebon et al., 2011b; Warne et al., 2011), and therefore crystallisation trials were conducted for both β1AR and A2AR in complex with mini-Gs, employing a parallel approach of lipidic cubic phase (LCP) and vapour diffusion. Crystals were obtained quickly for wild type A2AR in complex with mini-Gs by vapour diffusion in the detergent octylthioglucoside (OTG), and we were able to solve the structure to 3.4 Å resolution (Carpenter et al., 2016). β1AR crystallisation trials are still at an early stage, and have not yet yielded crystals that diffract to sufficient resolution for structure determination. The molecular organisation of A2AR–mini-Gs is remarkably similar to that of the β2AR–Gs complex (Rasmussen et al., 2011b), with A2AR adopting a conformation that closely resembles Gs-bound β2AR. In addition, mini-Gs bound to A2AR is very similar to the analogous region in Gs bound to β2AR (Rmsd 0.9 Å; Carpenter et al., 2016). Thus mini G proteins are useful surrogates for heterotrimeric G proteins to stabilise and determine structures of GPCRs in their active conformation. However, it must be appreciated that GPCRs have not evolved to be stable in the activated state bound to a G protein, but instead have evolved to be unstable so that signalling lasts for short, defined, periods of time before being terminated. Therefore, additional approaches, such as thermostabilisation of the GPCR (Tate, 2012) bound to mini-Gs, may be required before the structures of many complexes can be determined. The high-level expression and stability of mini-Gs makes this a trivial undertaking compared to using the wild type heterotrimeric G protein. It is also important to note that further modifications of mini-Gs, such as deletions and mutations, may be required to facilitate crystallisation of different GPCRs. The crystal structure of the A2AR–mini-Gs complex was solved using mini-Gs414, which is identical to mini-Gs393 except that it contains the additional mutation L63Y (Table II). Well diffracting crystals of the A2AR–mini-Gs complex were grown using either mini-Gs393 or mini-Gs414, however, the A2AR–mini-Gs414 complex produced a different crystal form that diffracted to slightly better resolution, and was thus used for structure determination. There was no discernible difference between these two complexes in either the competition binding assay or thermostability assay (results not shown), suggesting that the main effect of the L63Y mutation was on crystallogenesis.
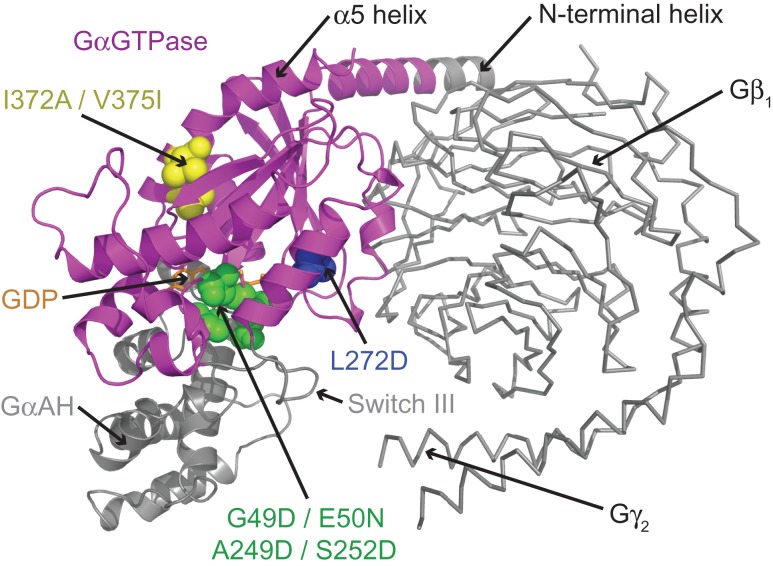
The development of mini-Gs involved extensive screening to identify key deletions and point mutations that improved both the conformational homogeneity and thermostability of the β1AR–mini-Gs complex (Fig. 4). Deletion of the GαAH domain, which is the most dynamic region of Gs in its GPCR-bound conformation, significantly reduced the conformational heterogeneity in the β1AR–mini-Gs complex. This deletion also eliminated the requirement of Gβγ subunits for GPCR coupling, removing the need for exogenous components, such as Nb35, to stabilise the Gα–Gβγ interface. In the absence of Gβγ subunits the N-terminus of mini-Gs could be partially deleted, resulting in a more compact protein. The switch III deletion removed a dynamic region of mini-Gs, replacing it with defined secondary structure components, and resulted in a significant improvement in the thermostability of the complex. A total of seven point mutations were required to fully stabilise mini-Gs in complex with β1AR (Fig. 4). Extensive mutagenic screens were employed that targeted most regions of mini-Gs, however, all of the mutations utilised in the final construct were clustered around three regions of the protein (nucleotide-binding pocket, switch II, and α5 helix). The G49D, E50N, A249D and S252D mutations were designed to stabilise the nucleotide-binding pocket and P-loop (Fig. 2a), and all of these mutations improved the thermostability of both the basal GDP-bound state of mini-Gs and the β1AR–mini-Gs complex. The L272D mutation, which was located adjacent to switch II, was designed to conformationally constrain this flexible region (Fig. 2b), and resulted in improved thermostability of the β1AR–mini-Gs complex. The I372A and V375I mutations, which were located in the α5 helix, were designed to improve packing between the core of protein and the α1 or α5 helices, respectively (Fig. 2c and d). These two mutations specifically improved the thermostability of the β1AR–mini-Gs complex in detergent. None of these mutations were located within the receptor-binding site, ensuring that the native GPCR–G protein interface was maintained, and allowing mini-Gs to be co-crystallised with other Gs-coupled receptors. During the course of this work the I372A mutation was also independently reported to stabilise a complex between rhodopsin and the adenylate cyclase inhibiting G protein Gi1 (Sun et al., 2015).
Fig. 4.
A model of heterotrimeric Gs highlighting the region that corresponds to mini-Gs (magenta). The model of heterotrimeric Gs was constructed by superposition of the crystal structures of Gαs (PDB code 1AZT; Sunahara et al., 1997) and heterotrimeric Gαt/i1 (PDB code 1GOT; Lambright et al., 1996). Residues that were mutated in mini-Gs (shown as spheres) were clustered in three regions of the protein: the nucleotide-binding pocket (green), switch II (blue), and the α5 helix (yellow). Regions of Gαs that were deleted in mini-Gs (GαAH, switch III and half of the N-terminal helix) are coloured grey. The Gβγ subunits, which are not required for mini-Gs coupling to GPCRs are shown as ribbons and coloured grey. GDP is shown as sticks and coloured orange.
The G protein engineering work has also provided insight into the mechanism of G protein activation. Binding of Gαs to β2AR triggers displacement of the G protein α5 helix to a position that is predicted to sterically clash with the α1 helix. This unfavourable clash appears to prevent close packing of the C-terminal region of the α1 helix against the α5 helix and the core of the GTPase domain (Fig. 2c), and this region is indeed disordered in the β2AR–Gs complex (Rasmussen et al., 2011b). The α1 helix forms part of the nucleotide-binding pocket and directly connects to the P-loop (Fig. 2c), which is the main determinant of nucleotide binding affinity in G proteins (John et al., 1990). Destabilisation of the α1 helix has previously been suggested to be a key event in receptor-mediated nucleotide exchange (Flock et al., 2015; Kaya et al., 2014; Sun et al., 2015), however the mechanism of this destabilisation was unclear. Here, we identified a potential steric clash between Ile372 from the α5 helix and residues from the α1 helix (in particular Met60), which appears to play an important role in this nucleotide exchange. Mutation of Ile372 to alanine, which was predicted to eliminate the steric clash, was shown to inhibit GTP-mediated dissociation of the β1AR–mini-Gs complex. These data indicate that, in Gs, Ile372 acts as a relay between the GPCR-binding site (α5 helix) and the key regions of the nucleotide-binding pocket (α1 helix and P-loop), allowing the receptor to allosterically destabilise the nucleotide-binding pocket and modulate nucleotide exchange. We suggest that the I372A mutation uncouples GPCR binding from occupancy of the nucleotide-binding pocket, a hypothesis that was supported by the presence of GDP in one of the two copies of mini-Gs in the asymmetric unit of the A2AR–mini-Gs structure (Carpenter et al., 2016).
Mini G proteins are novel tools that have many potential applications, including characterisation of receptor pharmacology in response to different classes of agonists (full, partial and weak), binding affinity and kinetic studies, thermostabilisation of GPCRs in their active conformation, drug discovery, and structure determination of native-like GPCR–G protein complexes. Furthermore, all of the mutations reported here are located within conserved regions of the Gα subunit. Therefore, the concept is potentially transferable to all classes of heterotrimeric G proteins, which would allow the production of a panel of mini G proteins capable of coupling any GPCR.
Supplementary Material
Acknowledgement
We thank R. Nehmé, T. Warne and A.G.W. Leslie for comments on the article.
Supplementary data
Funding
This work was funded by a grant from Heptares Therapeutics Ltd and core funding from the Medical Research Council [MRC U105197215].
References
- Alexander N.S., Preininger A.M., Kaya A.I., Stein R.A., Hamm H.E. and Meiler J. (2014) Nat. Struct. Mol. Biol., 21, 56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X.C., McMullan G. and Scheres S.H. (2015) Trends Biochem. Sci., 40, 49–57. [DOI] [PubMed] [Google Scholar]
- Baker J.G., Proudman R.G. and Tate C.G. (2011) Naunyn Schmiedebergs Arch. Pharmacol., 384, 71–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornancin F., Pfister C. and Chabre M. (1989) Eur. J. Biochem., 184, 687–698. [DOI] [PubMed] [Google Scholar]
- Carpenter B., Nehme R., Warne T., Leslie A.G. and Tate C.G. (2016) Nature, 536, 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delean A., Stadel J.M. and Lefkowitz R.J. (1980) J. Biol. Chem., 255, 7108–7117. [PubMed] [Google Scholar]
- Dror R.O., Mildorf T.J., Hilger D. et al. (2015) Science, 348, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock T., Ravarani C.N., Sun D., Venkatakrishnan A.J., Kayikci M., Tate C.G., Veprintsev D.B. and Babu M.M. (2015) Nature, 524, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung B.K., Hurley J.B. and Stryer L. (1981) Proc. Natl. Acad. Sci. USA, 78, 152–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm H.E., Deretic D., Arendt A., Hargrave P.A., Koenig B. and Hofmann K.P. (1988) Science, 241, 832–835. [DOI] [PubMed] [Google Scholar]
- Hanzal-Bayer M., Renault L., Roversi P., Wittinghofer A. and Hillig R.C. (2002) EMBO J., 21, 2095–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann R., Heck M., Henklein P., Hofmann K.P. and Ernst O.P. (2006) J. Biol. Chem., 281, 30234–30241. [DOI] [PubMed] [Google Scholar]
- Higashijima T., Ferguson K.M., Sternweis P.C., Smigel M.D. and Gilman A.G. (1987) J. Biol. Chem., 262, 762–766. [PubMed] [Google Scholar]
- Holm L. and Rosenstrom P. (2010) Nucleic Acids Res., 38, W545–W549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W., Manglik A., Venkatakrishnan A.J. et al. (2015) Nature, 524, 315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J., Sohmen R., Feuerstein J., Linke R., Wittinghofer A. and Goody R.S. (1990) Biochemistry, 29, 6058–6065. [DOI] [PubMed] [Google Scholar]
- Kaya A.I., Lokits A.D., Gilbert J.A., Iverson T.M., Meiler J. and Hamm H.E. (2014) J. Biol. Chem., 289, 24475–24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse A.C., Ring A.M., Manglik A. et al. (2013) Nature, 504, 101–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn H. (1981) Curr. Top. Membr. Trans., 15, 171–201. [Google Scholar]
- Lagerstrom M.C. and Schioth H.B. (2008) Nat. Rev. Drug Discov., 7, 339–357. [DOI] [PubMed] [Google Scholar]
- Lambright D.G., Sondek J., Bohm A., Skiba N.P., Hamm H.E. and Sigler P.B. (1996) Nature, 379, 311–319. [DOI] [PubMed] [Google Scholar]
- Lebon G., Bennett K., Jazayeri A. and Tate C.G. (2011. a) J. Mol. Biol., 409, 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebon G., Warne T., Edwards P.C., Bennett K., Langmead C.J., Leslie A.G. and Tate C.G. (2011. b) Nature, 474, 521–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markby D.W., Onrust R. and Bourne H.R. (1993) Science, 262, 1895–1901. [DOI] [PubMed] [Google Scholar]
- Miller J.L. and Tate C.G. (2011) J. Mol. Biol., 413, 628–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Gallacher J.L., Nehme R., Warne T., Edwards P.C., Schertler G.F., Leslie A.G. and Tate C.G. (2014) PLoS One, 9, e92727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesen F.H., Berglund H. and Vedadi M. (2007) Nat. Protoc., 2, 2212–2221. [DOI] [PubMed] [Google Scholar]
- Oldham W.M., Van Eps N., Preininger A.M., Hubbell W.L. and Hamm H.E. (2006) Nat. Struct. Mol. Biol., 13, 772–777. [DOI] [PubMed] [Google Scholar]
- Phillips W.J., Wong S.C. and Cerione R.A. (1992) J. Biol. Chem., 267, 17040–17046. [PubMed] [Google Scholar]
- Rasmussen S.G., Choi H.J., Fung J.J. et al. (2011. a) Nature, 469, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S.G., DeVree B.T., Zou Y. et al. (2011. b) Nature, 477, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring A.M., Manglik A., Kruse A.C., Enos M.D., Weis W.I., Garcia K.C. and Kobilka B.K. (2013) Nature, 502, 575–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D.M., Rasmussen S.G. and Kobilka B.K. (2009) Nature, 459, 356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum D.M., Zhang C., Lyons J.A. et al. (2011) Nature, 469, 236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheerer P., Park J.H., Hildebrand P.W., Kim Y.J., Krauss N., Choe H.W., Hofmann K.P. and Ernst O.P. (2008) Nature, 455, 497–502. [DOI] [PubMed] [Google Scholar]
- Serrano-Vega M.J., Magnani F., Shibata Y. and Tate C.G. (2008) Proc. Natl. Acad. Sci. U S A, 105, 877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel A.M., Backlund P.S. Jr, Butrynski J.E., Jones T.L. and Simonds W.F. (1991) Trends Biochem. Sci., 16, 338–341. [DOI] [PubMed] [Google Scholar]
- Sprang S.R. (1997) Annu. Rev. Biochem., 66, 639–678. [DOI] [PubMed] [Google Scholar]
- Sun D., Flock T., Deupi X. et al. (2015) Nat. Struct. Mol. Biol., 22, 686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunahara R.K., Tesmer J.J., Gilman A.G. and Sprang S.R. (1997) Science, 278, 1943–1947. [DOI] [PubMed] [Google Scholar]
- Tate C.G. (2012) Trends Biochem. Sci., 37, 343–352. [DOI] [PubMed] [Google Scholar]
- Van Eps N., Preininger A.M., Alexander N., Kaya A.I., Meier S., Meiler J., Hamm H.E. and Hubbell W.L. (2011) Proc. Natl. Acad. Sci. U S A, 108, 9420–9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuong T.M., Chabre M. and Stryer L. (1984) Nature, 311, 659–661. [DOI] [PubMed] [Google Scholar]
- Warne T., Chirnside J. and Schertler G.F. (2003) Biochim. Biophys. Acta, 1610, 133–140. [DOI] [PubMed] [Google Scholar]
- Warne T., Moukhametzianov R., Baker J.G., Nehme R., Edwards P.C., Leslie A.G., Schertler G.F. and Tate C.G. (2011) Nature, 469, 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warne T., Serrano-Vega M.J., Tate C.G. and Schertler G.F. (2009) Protein Expr. Purif., 65, 204–213. [DOI] [PubMed] [Google Scholar]
- Westfield G.H., Rasmussen S.G., Su M. et al. (2011) Proc. Natl. Acad. Sci. U S A, 108, 16086–16091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F., Wu H., Katritch V., Han G.W., Jacobson K.A., Gao Z.G., Cherezov V. and Stevens R.C. (2011) Science, 332, 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.