Abstract
First reported in 2003, mosaic tetracycline resistance genes are a subgroup of the genes encoding ribosomal protection proteins (RPPs). They are formed when two or more RPP-encoding genes recombine resulting in a functional chimera. To date, the majority of mosaic genes are derived from sections of three RPP genes, tet(O), tet(W) and tet(32), with others comprising tet(M) and tet(S). In this first review of mosaic genes, we report on their structure, diversity and prevalence, and suggest that these genes may be responsible for an under-reported contribution to tetracycline resistance in bacteria.
Introduction
Tetracyclines bind to the A-site on the bacterial ribosome, resulting in steric blocking of the aminoacyl-tRNA binding site, which prevents protein synthesis.1 They are effective against both Gram-positive and Gram-negative bacteria and, due to the relative lack of major side effects and cheap cost, have been used extensively in the treatment of infections2 as well as growth promoters in animal husbandry.3
Bacterial resistance to tetracycline is often mediated through the acquisition of DNA encoding proteins that confer resistance by one of three main mechanisms: ATP-dependent efflux, enzymatic inactivation of tetracycline, or ribosomal protection.2 To date, a total of 60 different classes of tetracycline resistance gene, including oxytetracycline resistance genes, have been reported. These include 33 predicted or proven to encode active efflux pumps, 12 encoding ribosomal protection proteins (RPPs), 13 encoding inactivating enzymes and 1 reported to confer resistance via an as yet undetermined mechanism, designated tet(U) (a full list is periodically updated by Roberts4). Although it has yet to be assigned a mechanistic class, tet(U) has been identified in Enterococcus and Staphylococcus isolates.5,6 However, a study by Caryl et al.7 reported that when tet(U) was cloned and expressed in Escherichia coli, the transformants were not resistant to tetracycline.
To be considered a new class of tetracycline resistance gene, it must encode a protein <80% identical to known tetracycline resistance proteins.8 Determinants representing new classes were originally assigned a letter from the English alphabet.9 However, as all letters are used, they are now assigned an Arabic numeral,8 with new determinants referred to the Levy group (bonnie.marshall@tufts.edu) in order to obtain a designation prior to publication to avoid duplication and ensure taxonomic consistency.
RPPs
RPPs are a related group of proteins that, when bound to the ribosome, result in the release of tetracycline from the ribosome, enabling protein synthesis to proceed10 (reviewed by Thaker et al.11). Of the 12 classes of RPP gene currently reported [tet(M), (O), (Q), (S), (T), (W), (32), (36), (44), B(P), otr(A) and tet], tet(M) is considered the most prevalent due to its association with the broad host range Tn916/Tn1545 family of conjugative transposons.12 However, a subgroup of RPP genes has been identified that consist of regions of different, already characterized RPP genes that appear to have undergone recombination forming a mosaic gene. It must be stressed here that the progenitors of mosaic genes are assumed based purely on the order in which they were discovered and we cannot be sure of the directionality of mosaic gene formation.
Mosaic RPP genes
In 2003, Stanton and Humphery13 reported two RPP genes in Megasphaera elsdenii that encoded predicted proteins showing 89.1% and 91.9% identity to Tet(W) (accession number AJ222769) from Butyrivibrio fibrisolvens. As this was above the <80% cut-off, they did not qualify as a new resistance class under the nomenclature system. However, further analysis of the amino acid sequence revealed variability in the percentage identity to Tet(W) across its length. The large central section in both sequences showed 98.1% identity to Tet(W), while small sections at the N- and C-terminal ends were found to have a lower amino acid sequence identity to Tet(W) [between 66.6% and 75.3%]. However, these same N- and C-terminal sections were shown to have between 99.3% and 100% amino acid identity to Tet(O) (accession number M18896), despite the central section showing identity to Tet(W). Given the evidence, this suggested recombination had occurred, creating a mosaic determinant with a central Tet(W) region flanked by two Tet(O) regions. Although never before observed between two different RPP classes, recombination resulting in functional genes has previously been reported between different phylotypes of tet(M)14 as well as in other antibiotic resistance genes, such as penA and pbp2x, which confer resistance to penicillin.15,16 Furthermore, in vitro experiments have successfully recombined tet(A) and tet(C) to create mosaics that confer resistance to tetracycline at levels comparable to the non-mosaic tet(C).17
The guideline for determining a new resistance gene class was established prior to the discovery of these mosaic RPP genes and none of the mosaic genes qualified as a new class when analysed as one single continuous sequence. It was clear, however, that these mosaic genes were different from their non-mosaic counterparts and that the current classification did not adequately reflect the true evolutionary background of these genes. Therefore, an expansion of the nomenclature system was suggested whereby the mosaic gene would receive a designation that reflected the structural order and class of the genes they comprised, better reflecting their variable nature.18,19 For example, the two resistance genes reported in M. elsdenii, which comprised a central tet(W) region flanked by two tet(O) regions, were designated tet(O/W/O).13
Although Stanton and Humphrey13 were the first to report mosaic RPP genes, Melville et al.20 had unknowingly reported a mosaic gene 2 years previously. This resistance gene, found in Clostridium saccharolyticum K10, encoded a predicted protein that showed 76% amino acid identity to Tet(O) (accession number Y07780). As per the original nomenclature guidelines, it was given the new designation Tet(32). However, subsequent re-examination of the sequence found that only the central section showed <80% identity to known proteins, while the N- and C-terminal regions flanking the central section shared 100% and 97.7% identity, respectively, to Tet(O) (accession number M18896). The central region was still thought to represent a section of a new Tet(32) class and therefore the determinant was reclassified Tet(O/32/O).18 Subsequently, the proposed full, non-mosaic sequences of Tet(32) have been reported in several isolates identified from the human oral cavity,21,22 with the Tet(O/32/O) mosaic determinant now showing 89% amino acid identity to these.
Similarly, the previously reported tet(S) allele (accession number AY534326) on the conjugative transposon Tn916S23 has subsequently been reclassified as a result of in silico analysis. The amino acid sequence shows identity to Tet(S) across 595 amino acids (1–595 inclusive), with the final 61 amino acids at the C-terminus end identical to Tet(M) (accession number U09422), resulting in it being reclassified as Tet(S/M).24
Mosaic gene diversity
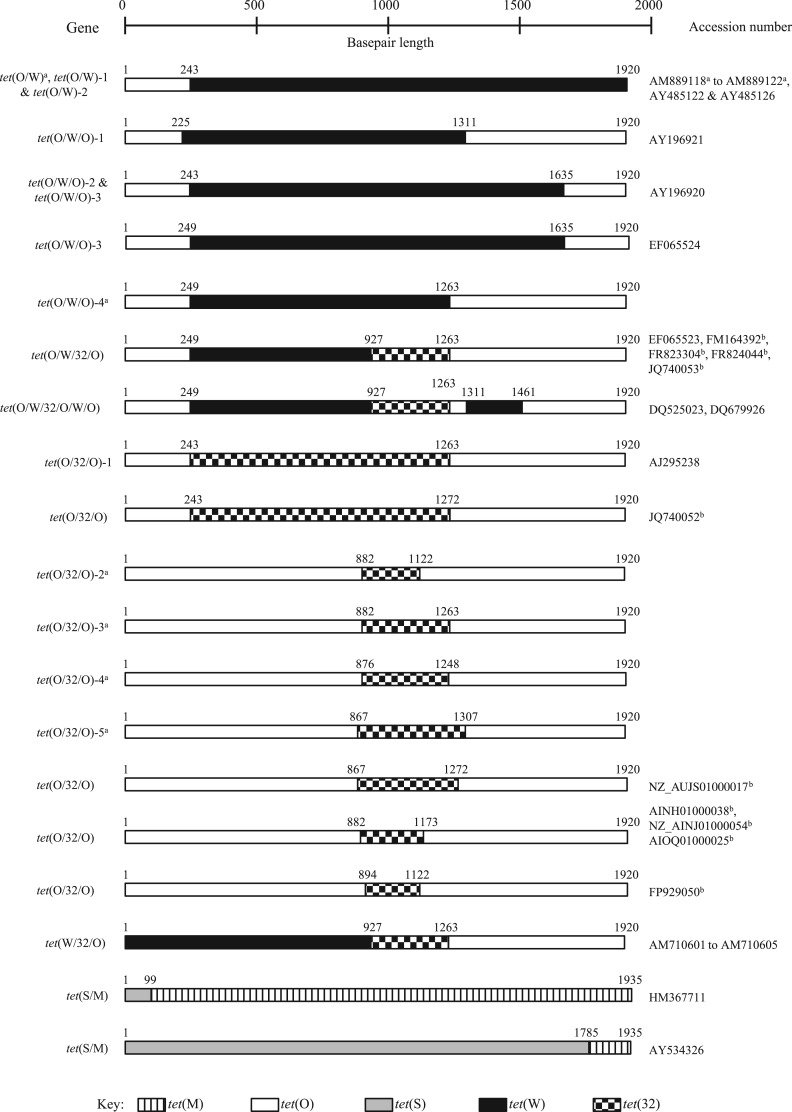
To date, a total of 30 mosaic genes have been reported in the literature, of which 26 currently have sequences deposited in GenBank (Table 1). Some studies have reported multiple occurrences of known genes; however, many of these have been characterized by PCR amplification only. Structurally, these chimeric genes currently comprise either two [e.g. tet(O/W)], three [e.g. tet(O/W/O)], four [e.g. tet(O/W/32/O)] or six [e.g. tet(O/W/32/O/W/O)] different regions (Figure 1), with tet(O), tet(W) and tet(32) being the predominant RPP genes reported to form mosaic genes, comprising all but two of the reported variants, and tet(M) and tet(S) forming the remaining two.24,25 Given the prevalence of tet(M) in certain samples, and the previous reports of self-recombination,14,26 it is surprising that there are so few reports of mosaic genes containing tet(M). Furthermore, alignment of 12 representative RPP gene sequences shows tet(M) sharing 75% and 70% identity, respectively, to tet(O) and tet(44), which is higher than the percentage identity observed between the more commonly reported RPP mosaic genes comprising tet(O), (W) and (32) (Table 2). However, mosaic genes comprising tet(M) and any other gene, with the exception of tet(S), have yet to be reported. It is entirely possible that this may be due to a lack of investigation rather than an absence of recombination followed by fixation of the recombinant allele in the bacterial population. Alternatively, it is possible that there is little selective pressure for tet(M)-based mosaic genes if the resultant protein is no more efficient than Tet(M) itself and/or there is no indirect selective pressure for mosaicism. A similar situation may exist for other proteins, such as Tet(S). Stanton et al.27 reported that the protein encoded by the tet(O/W/O) mosaic genes in M. elsdenii conferred a higher level of resistance to tetracycline than their non-mosaic counterparts, but similar investigations are still to be reported for other RPP genes. Therefore, the prevalence of certain mosaic gene variants could suggest that they are in some way more beneficial to the host than the non-mosaic genes they comprise.
Table 1.
A summary of the mosaic tetracycline genes reported to date
| Gene | Organism | Source(s) | Accession number | Reference(s) |
|---|---|---|---|---|
| tet(O/W) | Bifidobacterium thermophilum B0219 | environmental (pig slaughterhouse) sample | AM889118 | 32 |
| tet(O/W) | B. thermophilum B0241 | pig faeces | AM889119 | 32 |
| tet(O/W) | B. thermophilum B0242 | pig faeces | AM889120 | 32 |
| tet(O/W) | B. thermophilum B0253 | pig faeces | AM889121 | 32 |
| tet(O/W) | B. thermophilum B0256 | pig faeces | AM889122 | 32 |
| tet(O/W)-2 | Megasphaera elsdenii 25-51 | swine faeces | AY485122 | 18,27 |
| tet(O/W)-1 [n = 15a] | M. elsdenii 27-51 | swine faeces | AY485126 | 27,33 |
| tet(O/W/O)-4 | uncultured bacterial clone | pig faeces | no accession number | 21 |
| tet(O/W/O)-3 [n = 9] | uncultured bacterial clone | pig faeces | EF065524 | 21 |
| tet(O/W/O)-2 [n = 28b] | M. elsdenii 14-14 | swine caecum | AY196920 | 13,18,27,33 |
| tet(O/W/O)-1 [n = 2] | M. elsdenii 7-11 | swine caecum | AY196921 | 13,18,27 |
| tet(O/W/32/O) [n = 32] | uncultured bacterial clone | pig faeces | EF065523 | 21 |
| tet(O/W/32/O) [n = 7c] | Streptococcus suis Ss1303 | pig (brain, lung and spleen) and human (CSF) samples | FM164392 | 34 |
| tet(O/W/32/O) | S. suis 32 457 | diseased pig lung | FR823304 | 34,35 |
| tet(O/W/32/O) | Streptococcus gallolyticus subsp. gallolyticus ATCC 2069 plasmid pSGG1 | human blood | FR824044 | 36 |
| tet(O/W/32/O) | S. suis | diseased pig (blood, brain, heart, joint and lung) samples | JQ740053 | 28 |
| tet(O/W/32/O/W/O) | Lactobacillus johnsonii G41 | human faeces | DQ525023 | 32 |
| tet(O/W/32/O/W/O) | uncultured bacterial clone | pig faeces | DQ679926 | 21 |
| tet(O/32/O) | S. suis | diseased pig (blood, brain, heart, joint and lung) samples | JQ740052 | 28 |
| tet(O/32/O) | Clostridium saccharolyticum K10 | human colon | AJ295238 | 18 |
| tet(O/32/O)-2 [n = 3] | uncultured bacterial clone | human and animal faecal samples | no accession number | 21 |
| tet(O/32/O)-3 | uncultured bacterial clone | human and animal faecal samples | no accession number | 21 |
| tet(O/32/O)-4 | uncultured bacterial clone | human and animal faecal samples | no accession number | 21 |
| tet(O/32/O)-5 | uncultured bacterial clone | human and animal faecal samples | no accession number | 21 |
| tet(O/32/O) | Dorea longicatena AGR2136 | rumen microbiome | NZ_AUJS01000017 (41 626–43 545 bp) | direct submission, analysed in this study |
| tet(O/32/O) | Campylobacter coli 202/04 | human faeces | AINH01000038 (2361–4280 bp) | direct submission, analysed in this study |
| tet(O/32/O) | C. coli 317/04 | human faeces | NZ_AINJ01000054 (2094–4013 bp) | direct submission, analysed in this study |
| tet(O/32/O) | Campylobacter jejuni subspecies jejuni 2008-894 | human | AIOQ01000025 (14 515–16 434 bp) | direct submission, analysed in this study |
| tet(O/32/O) | Roseburia intestinalis XB6B4 | human intestinal tract | FP929050 (2 873 814–2 875 733 bp) | direct submission, analysed in this study |
| tet(S/M) | Streptococcus equinus 1357 | food | HM367711 | 25 |
| tet(S/M) | Streptococcus intermedius | human isolate | AY534326 | 23,24 |
| tet(W/32/O) | B. thermophilum B0219 | environmental (pig slaughterhouse) sample | AM710601 | 32 |
| tet(W/32/O) | B. thermophilum B0241 | pig faeces | AM710602 | 32 |
| tet(W/32/O) | B. thermophilum B0242 | pig faeces | AM710603 | 32 |
| tet(W/32/O) | B. thermophilum B0253 | pig faeces | AM710604 | 32 |
| tet(W/32/O) | B. thermophilum B0256 | pig faeces | AM710605 | 32 |
The number given in square brackets indicates the instances of that mosaic gene variant reported, if more than one.
aFourteen of the 15 tet(O/W)-1 variants were only determined by PCR analysis and so could be either tet(O/W)-1 or tet(O/W)-2.
bEleven of the 28 tet(O/W/O)-2 variants were only determined by PCR analysis and so could be either tet(O/W/O)-2 or tet(O/W/O)-1.
cAll S. suis isolates, but not the same strain.
Figure 1.
Schematic representation of reported mosaic tetracycline RPP genes. The coded bars indicate sequences of high identity to specific RPP genes: vertical line bars for tet(M), white bars for tet(O), grey bars for tet(S), black bars for tet(W) and checked bars for tet(32). The number above the bar indicates the reported crossover point. aIndicates those sequences that are incomplete or absent in GenBank, with the crossover points taken from the publication. bIndicates sequences that have been analysed in this study due to no specific crossover point(s) reported.
Table 2.
Sequence identity matrix showing the percentage nucleotide identity between representatives of all 12 RPP gene classes, in descending order, compared with tet(M)
| RPP gene | tet(M) | tet(S) | tet(O) | tet(44) | tet(32) | tet(W) | tet(T) | tet(36) | tet(Q) | tetB(P) | otr(A) | tet |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| tet(M) | 100 | 78 | 75 | 70 | 69 | 64 | 57 | 49 | 46 | 23 | 11 | 11 |
| tet(S) | 100 | 70 | 69 | 67 | 62 | 56 | 56 | 48 | 11 | 11 | 10 | |
| tet(O) | 100 | 69 | 69 | 65 | 56 | 49 | 48 | 15 | 12 | 11 | ||
| tet(44) | 100 | 71 | 64 | 50 | 58 | 46 | 15 | 11 | 10 | |||
| tet(32) | 100 | 67 | 55 | 49 | 47 | 11 | 12 | 10 | ||||
| tet(W) | 100 | 12 | 45 | 15 | 5 | 14 | 12 | |||||
| tet(T) | 100 | 57 | 56 | 18 | 8 | 2 | ||||||
| tet(36) | 100 | 64 | 9 | 11 | 11 | |||||||
| tet(Q) | 100 | 13 | 12 | 12 | ||||||||
| tetB(P) | 100 | 1 | 1 | |||||||||
| otr(A) | 100 | 63 | ||||||||||
| tet | 100 |
Accession numbers of representative genes included in the matrix: tet(M), U09422; tet(O), Y07780; tetB(P), AE001437; tet(Q), X58717; tet(S), X92946; tet(T), L42544; tet(W), AJ222769; tet(32), DQ647324; tet(36), AJ514254; tet(44), FN594949; otr(A), X53401; tet, AL939106.
Shaded boxes represent those genes currently reported to comprise mosaic genes.
PCR-based analysis
PCR-based assays have been developed to help researchers detect specific mosaic genes. Stanton and Humphrey13 describe an assay that distinguished between the non-mosaic genes tet(O) and tet(W) and the mosaic tet(O/W/O) from Megasphaera strains, enabling them to detect tet(O/W/O) variants in six additional M. elsdenii strains. Patterson et al.21 investigated the presence of mosaic genes using various specific oligonucleotide sets that either bound within the resistance genes or flanked them. Amplicons specific to tet(O/W), tet(O/32) and tet(W/32) were detected in faecal samples, with tet(O/32) being the most common of these mosaic amplicons; it was amplified in all 12 pig faecal samples and 6 of 7 human faecal samples tested. In contrast, the faecal samples from cows and sheep, as well as human saliva samples, failed to produce any amplicons for these mosaic genes, suggesting they were not present at detectable levels.
Chen et al.28 also used an oligonucleotide primer set that annealed outside tet(O) to determine the presence of tetracycline resistance genes in two Streptococcus suis isolates. Although no amplicon was produced using internal, tet(O)-specific primers, the primers binding to flanking DNA yielded an amplicon, indicating the presence of mosaic genes [identified as tet(O/32/O) and tet(O/W/32/O)]. This full-length oligonucleotide primer set does aid the identification of mosaic genes; however, it is only specific for those with regions homologous to tet(O) flanking sequences. Since PCR strategies aimed at identifying resistance genes require knowledge of the sequence of the target, mosaic RPP genes are likely to be largely undetected and under-reported by PCR-based studies.
Reflecting the findings by Patterson et al.,21 almost all the mosaic genes reported to date have originated from faecal samples, with the majority identified from a porcine origin and less commonly from humans (Table 1). The gut houses a complex and diverse bacterial community with potential for widespread horizontal gene transfer, and the mosaic genes found in faecal samples are likely to reflect the pool of non-mosaic genes present within the gut microbiota. Genes such as tet(W) and tet(O) are commonly reported from these types of samples,29 but the prevalence of tet(32)-containing mosaic genes suggests that tet(32) may be more common than initially thought. In fact, tet(O/32/O) was found to be the most common mosaic gene in both the human and pig faecal samples tested and was present in almost as many samples tested as the non-mosaic tet(O) and tet(W) genes.21 In contrast, mosaic genes have not yet been reported in faecal samples from bovine and ovine origin or in human saliva.21 Why they are predominantly found in pigs while as yet unreported in other animals is not immediately clear, though the extensive use of tetracyclines in the swine industry3,30,31 may have contributed to their selection.
Draft genome analysis
The advent of high-throughput genomic sequencing has led to an increase in the number of genomes being deposited in sequence databases. Many contain tetracycline resistance genes that are generically labelled simply as ‘tetracycline resistance protein’ or as ‘tet(M)-like’, the designation of which may be a result of automated annotation pipelines. A preliminary search of the NCBI nucleotide database, using tet(O) (accession number Y07780) as the query, found that some of these generically labelled tetracycline resistance genes gave a partial match to tet(O). Further examination indicates that some are as yet uncharacterized and unreported mosaic genes, which have been further defined for this review using the nucleotide sequence to determine the crossover points. For example, the tet(M)-like gene (accession number NZ_AUJS01000017, location 41 626–43 545 bp) in the draft genome of Dorea longicatena AGR2136 from a human faecal sample appears to be a previously unreported variant of tet(O/32/O) (Figure 1).
Furthermore, the tetracycline resistance genes present in Campylobacter jejuni subspecies jejuni 2008-894, Campylobacter coli 202/04, C. coli 317/04 (accession numbers AIOQ01000025, AINH01000038 and NZ_AINJ01000054, respectively) and Roseburia intestinalis XB6B4 (accession number FP929050) are also structurally novel variants of tet(O/32/O) (Figure 1). The three mosaic genes present in the Campylobacter spp. are identical to each other, while that in R. intestinalis is different. Taking into account these newly defined genes, the total number of mosaic genes reported increases from 30 to 35 (not including those identified via PCR amplification only; Table 1) and suggests that other generically labelled tetracycline resistance genes present in the database [e.g. those labelled as tet(M)-like] could be further classified, helping to understand mosaic gene proliferation and diversity.
Conclusions
Our knowledge of the mosaic RPP gene group is steadily increasing since their discovery in 2003, with the majority derived from tet(O), tet(W) and tet(32) and others deriving from tet(M) and tet(S). It is clear that these genes are being under-reported both in terms of experimental detection and also within genomic data. Further work and increased attention on mosaic RPP genes is important if we are to understand the evolutionary selective pressures driving their fixation in bacterial populations and the subsequent effects on resistance and mobile genetic element evolution within their host.
Transparency declarations
None to declare.
Acknowledgements
P. J. W. initiated this work whilst at Anglia Ruskin University.
References
- 1.Brodersen DE, Clemons WM Jr, Carter AP et al. . The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 2000; 103: 1143–54. [DOI] [PubMed] [Google Scholar]
- 2.Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev 2001; 65: 232–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Landers TF, Cohen B, Wittum TE et al. . A review of antibiotic use in food animals: perspective, policy, and potential. Public Health Rep 2012; 127: 4–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts MC. Mechanism of Resistance for Characterized tet and otr Genes. http://faculty.washington.edu/marilynr/tetweb1.pdf.
- 5.Ridenhour MB, Fletcher HM, Mortensen JE et al. . A novel tetracycline-resistant determinant, tet(U), is encoded on the plasmid pKq10 in Enterococcus faecium. Plasmid 1996; 35: 71–80. [DOI] [PubMed] [Google Scholar]
- 6.Weigel LM, Donlan RM, Shin DH et al. . High-level vancomycin-resistant Staphylococcus aureus isolates associated with a polymicrobial biofilm. Antimicrob Agents Chemother 2007; 51: 231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caryl JA, Cox G, Trimble S et al. . ‘tet(U)’ is not a tetracycline resistance determinant. Antimicrob Agents Chemother 2012; 56: 3378–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levy SB, McMurry LM, Barbosa TM et al. . Nomenclature for new tetracycline resistance determinants. Antimicrob Agents Chemother 1999; 43: 1523–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy SB, McMurry LM, Burdett V et al. . Nomenclature for tetracycline resistance determinants. Antimicrob Agents Chemother 1989; 33: 1373–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connell SR, Tracz DM, Nierhaus KH et al. . Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob Agents Chemother 2003; 47: 3675–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thaker M, Spanogiannopoulos P, Wright GD. The tetracycline resistome. Cell Mol Life Sci 2010; 67: 419–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts AP, Mullany P. Tn916-like genetic elements: a diverse group of modular mobile elements conferring antibiotic resistance. FEMS Microbiol Rev 2011; 35: 856–71. [DOI] [PubMed] [Google Scholar]
- 13.Stanton TB, Humphrey SB. Isolation of tetracycline-resistant Megasphaera elsdenii strains with novel mosaic gene combinations of tet(O) and tet(W) from swine. Appl Environ Microbiol 2003; 69: 3874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oggioni MR, Dowson CG, Smith JM et al. . The tetracycline resistance gene tet(M) exhibits mosaic structure. Plasmid 1996; 35: 156–63. [DOI] [PubMed] [Google Scholar]
- 15.Spratt BG, Zhang QY, Jones DM et al. . Recruitment of a penicillin-binding protein gene from Neisseria flavescens during the emergence of penicillin resistance in Neisseria meningitidis. Proc Natl Acad Sci USA 1989; 86: 8988–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hakenbeck R. Mosaic genes and their role in penicillin-resistant Streptococcus pneumoniae. Electrophoresis 1998; 19: 597–601. [DOI] [PubMed] [Google Scholar]
- 17.Rubin RA, Levy SB. Interdomain hybrid Tet proteins confer tetracycline resistance only when they are derived from closely related members of the tet gene family. J Bacteriol 1990; 172: 2303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanton TB, Humphrey SB, Scott KP et al. . Hybrid tet genes and tet gene nomenclature: request for opinion. Antimicrob Agents Chemother 2005; 49: 1265–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy SB, McMurry LM, Roberts MC. Tet protein hybrids. Antimicrob Agents Chemother 2005; 49: 3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melville CM, Scott PS, Mercer DK et al. . Novel tetracycline resistance gene, tet(32), in the Clostridium-related human colonic anaerobe K10 and its transmission in vitro to the rumen anaerobe Butyrivibrio fibrisolvens. Antimicrob Agents Chemother 2001; 45: 3246–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patterson AJ, Rincon MT, Flint HJ et al. . Mosaic tetracycline resistance genes are widespread in human and animal fecal samples. Antimicrob Agents Chemother 2007; 51: 1115–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warburton PJ, Roberts AP, Allan E et al. . Characterisation of tet(32) genes from the oral metagenome. Antimicrob Agents Chemother 2009; 53: 273–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lancaster H, Roberts AP, Bedi R et al. . Characterization of Tn916S, a Tn916-like element containing the tetracycline resistance determinant tet(S). J Bacteriol 2004; 186: 4395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novais C, Freitas A, Silveira E et al. . 2012. A tet(S/M) hybrid from CTn6000 and CTn916 recombination. Microbiology 2012; 158: 2710–1. [DOI] [PubMed] [Google Scholar]
- 25.Barile S, Devirgiliis C, Perozzi G. Molecular characterization of a novel mosaic tet(S/M) gene encoding tetracycline resistance in foodborne strains of Streptococcus bovis. Microbiology 2012; 158: 2353–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huys G, D'Haene K, Collard JM et al. . Prevalence and molecular characterization of tetracycline resistance in Enterococcus isolates from food. Appl Environ Microbiol 2004; 70: 1555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanton TB, McDowall JS, Rasmussen MA. Diverse tetracycline resistance genotypes of Megasphaera elsdenii strains selectively cultured from swine feces. Appl Environ Microbiol 2004; 70: 3754–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Song Y, Wei Z et al. . Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J Vet Med Sci 2013; 75: 583–7. [DOI] [PubMed] [Google Scholar]
- 29.Patterson AJ, Colangeli R, Spigaglia P et al. . Distribution of specific tetracycline and erythromycin resistance genes in environmental samples assessed by macroarray detection. Environ Microbiol 2007; 9: 703–15. [DOI] [PubMed] [Google Scholar]
- 30.Dewey CE, Cox BD, Straw BE et al. . Use of antimicrobials in swine feeds in the United States. Swine Health Prod 1999; 7: 19–25. [Google Scholar]
- 31.Rajić A, Reid-Smith R, Deckert AE et al. . Reported antibiotic use in 90 swine farms in Alberta. Can Vet J 2006; 47: 446–52. [PMC free article] [PubMed] [Google Scholar]
- 32.van Hoek AH, Mayrhofer S, Domig KJ et al. . Mosaic tetracycline resistance genes and their flanking regions in Bifidobacterium thermophilum and Lactobacillus johnsonii. Antimicrob Agents Chemother 2008; 52: 248–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton TB, Humphrey SB, Stoffregen WC. Chlortetracycline-resistant intestinal bacteria in organically raised and feral swine. Appl Environ Microbiol 2011; 77: 7167–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Princivalli MS, Palmieri C, Magi G et al. . Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003–2007). Euro Surveill 2009; 14: pii=19310. [DOI] [PubMed] [Google Scholar]
- 35.Palmieri C, Magi G, Mingoia M et al. . Characterization of a Streptococcus suis tet(O/W/32/O)-carrying element transferable to major streptococcal pathogens. Antimicrob Agents Chemother 2012; 56: 4697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hinse D, Vollmer T, Rückert C et al. . Complete genome and comparative analysis of Streptococcus gallolyticus subsp. gallolyticus, an emerging pathogen of infective endocarditis. BMC Genomics 2011; 12: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]