Abstract
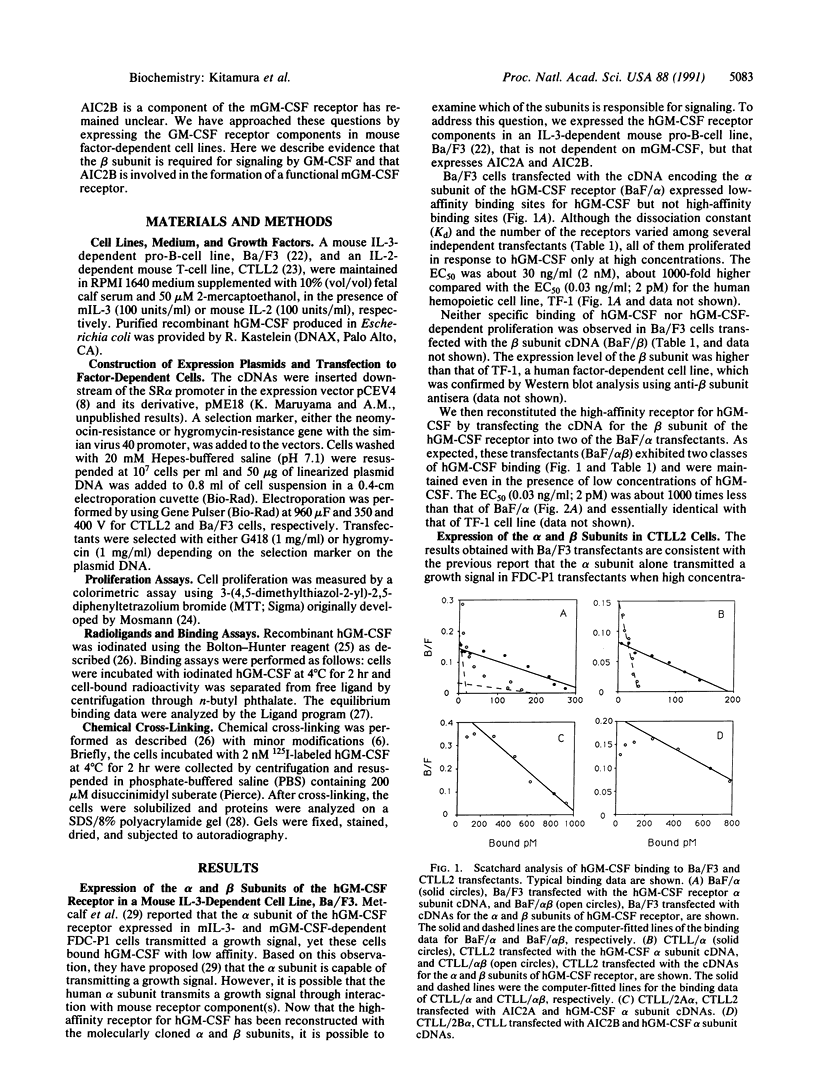
The high-affinity receptor for human granulocyte/macrophage colony-stimulating factor (hGM-CSF) is composed of two subunits, alpha and beta. The alpha subunit binds GM-CSF with low affinity, whereas the beta subunit does not bind GM-CSF by itself. The alpha and beta subunits together form the high-affinity GM-CSF receptor. The beta subunit has extensive sequence homology with the mouse interleukin 3 (IL-3) receptor (AIC2A) and its homologue (AIC2B) that does not bind IL-3 or other cytokines including GM-CSF. To examine the function of these receptor components, we expressed the alpha subunit of the hGM-CSF receptor with the human beta subunit or the mouse AIC2A or AIC2B in a mouse IL-3-dependent pro-B-cell line, Ba/F3, and in a mouse IL-2-dependent T-cell line, CTLL2. Coexpression of the alpha and beta subunits in Ba/F3 and CTLL2 cells resulted in high-affinity hGM-CSF binding and growth response to low concentrations of hGM-CSF. Whereas Ba/F3 cells expressing the alpha subunit alone proliferated in response to high concentrations of hGM-CSF, CTLL2 cells expressing the alpha subunit alone did not respond to hGM-CSF at all. Since Ba/F3 cells express endogenous AIC2A and AIC2B whereas CTLL2 expresses neither of them, we examined the possibility that either AIC2A or AIC2B is involved in the formation of a functional GM-CSF receptor. The expression of the human alpha subunit with AIC2B, but not with AIC2A, in CTLL2 cells conferred a growth response to hGM-CSF. These results indicate that the beta subunit of the GM-CSF receptor is required for generation of growth signals and that AIC2B is likely the beta subunit of the mouse GM-CSF receptor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budel L. M., Elbaz O., Hoogerbrugge H., Delwel R., Mahmoud L. A., Löwenberg B., Touw I. P. Common binding structure for granulocyte macrophage colony-stimulating factor and interleukin-3 on human acute myeloid leukemia cells and monocytes. Blood. 1990 Apr 1;75(7):1439–1445. [PubMed] [Google Scholar]
- Carroll M. P., Clark-Lewis I., Rapp U. R., May W. S. Interleukin-3 and granulocyte-macrophage colony-stimulating factor mediate rapid phosphorylation and activation of cytosolic c-raf. J Biol Chem. 1990 Nov 15;265(32):19812–19817. [PubMed] [Google Scholar]
- Cerottini J. C., Engers H. D., Macdonald H. R., Brunner T. Generation of cytotoxic T lymphocytes in vitro. I. Response of normal and immune mouse spleen cells in mixed leukocyte cultures. J Exp Med. 1974 Sep 1;140(3):703–717. doi: 10.1084/jem.140.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Shibuya K., Piao Y. F., Tojo A., Sasaki N., Matsuki S., Miyagawa K., Miyazono K., Takaku F. Identification and cellular distribution of distinct proteins forming human GM-CSF receptor. Cell Regul. 1990 Mar;1(4):327–335. doi: 10.1091/mbc.1.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba S., Tojo A., Kitamura T., Urabe A., Miyazono K., Takaku F. Characterization and molecular features of the cell surface receptor for human granulocyte-macrophage colony-stimulating factor. Leukemia. 1990 Jan;4(1):29–36. [PubMed] [Google Scholar]
- Clark S. C., Kamen R. The human hematopoietic colony-stimulating factors. Science. 1987 Jun 5;236(4806):1229–1237. doi: 10.1126/science.3296190. [DOI] [PubMed] [Google Scholar]
- Gearing D. P., King J. A., Gough N. M., Nicola N. A. Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 1989 Dec 1;8(12):3667–3676. doi: 10.1002/j.1460-2075.1989.tb08541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. M., Itoh N., Kitamura T., Schreurs J., Yonehara S., Yahara I., Arai K., Miyajima A. Cloning and expression of a gene encoding an interleukin 3 receptor-like protein: identification of another member of the cytokine receptor gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5459–5463. doi: 10.1073/pnas.87.14.5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF): reconstitution of a high-affinity GM-CSF receptor. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9655–9659. doi: 10.1073/pnas.87.24.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isfort R. J., Ihle J. N. Multiple hematopoietic growth factors signal through tyrosine phosphorylation. Growth Factors. 1990;2(2-3):213–220. doi: 10.3109/08977199009071507. [DOI] [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Schreurs J., Gorman D. M., Maruyama K., Ishii A., Yahara I., Arai K., Miyajima A. Cloning of an interleukin-3 receptor gene: a member of a distinct receptor gene family. Science. 1990 Jan 19;247(4940):324–327. doi: 10.1126/science.2404337. [DOI] [PubMed] [Google Scholar]
- Kanakura Y., Druker B., Cannistra S. A., Furukawa Y., Torimoto Y., Griffin J. D. Signal transduction of the human granulocyte-macrophage colony-stimulating factor and interleukin-3 receptors involves tyrosine phosphorylation of a common set of cytoplasmic proteins. Blood. 1990 Aug 15;76(4):706–715. [PubMed] [Google Scholar]
- Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J Cell Physiol. 1989 Aug;140(2):323–334. doi: 10.1002/jcp.1041400219. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linnekin D., Farrar W. L. Signal transduction of human interleukin 3 and granulocyte-macrophage colony-stimulating factor through serine and tyrosine phosphorylation. Biochem J. 1990 Oct 15;271(2):317–324. doi: 10.1042/bj2710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez A. F., Eglinton J. M., Lyons A. B., Tapley P. M., To L. B., Park L. S., Clark S. C., Vadas M. A. Human interleukin-3 inhibits the binding of granulocyte-macrophage colony-stimulating factor and interleukin-5 to basophils and strongly enhances their functional activity. J Cell Physiol. 1990 Oct;145(1):69–77. doi: 10.1002/jcp.1041450111. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P., Gough N. M. Low-affinity placenta-derived receptors for human granulocyte-macrophage colony-stimulating factor can deliver a proliferative signal to murine hemopoietic cells. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4670–4674. doi: 10.1073/pnas.87.12.4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. The molecular biology and functions of the granulocyte-macrophage colony-stimulating factors. Blood. 1986 Feb;67(2):257–267. [PubMed] [Google Scholar]
- Miyajima A., Miyatake S., Schreurs J., De Vries J., Arai N., Yokota T., Arai K. Coordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988 Jun;2(9):2462–2473. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Park L. S., Friend D., Price V., Anderson D., Singer J., Prickett K. S., Urdal D. L. Heterogeneity in human interleukin-3 receptors. A subclass that binds human granulocyte/macrophage colony stimulating factor. J Biol Chem. 1989 Apr 5;264(10):5420–5427. [PubMed] [Google Scholar]
- Park L. S., Waldron P. E., Friend D., Sassenfeld H. M., Price V., Anderson D., Cosman D., Andrews R. G., Bernstein I. D., Urdal D. L. Interleukin-3, GM-CSF, and G-CSF receptor expression on cell lines and primary leukemia cells: receptor heterogeneity and relationship to growth factor responsiveness. Blood. 1989 Jul;74(1):56–65. [PubMed] [Google Scholar]
- Schrader J. W. The panspecific hemopoietin of activated T lymphocytes (interleukin-3). Annu Rev Immunol. 1986;4:205–230. doi: 10.1146/annurev.iy.04.040186.001225. [DOI] [PubMed] [Google Scholar]
- Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Takaki S., Tominaga A., Hitoshi Y., Mita S., Sonoda E., Yamaguchi N., Takatsu K. Molecular cloning and expression of the murine interleukin-5 receptor. EMBO J. 1990 Dec;9(13):4367–4374. doi: 10.1002/j.1460-2075.1990.tb07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



