Summary
Background
Eosinophilic granulomatosis with polyangiitis (EGPA) is a rare systemic vasculitis with a prevalence rate of seven per million. Cardiac involvement was reported in 20–50%, yet with improved diagnostic methods, the frequency of cardiac involvement is expected to be even higher. It can result in significant morbidity and mortality, accounting for about 50% of death. Cardiac magnetic resonance (CMR) imaging is used to evaluate the myocardium, valves, coronary arteries, pericardium, also to assess cardiac structure and function. Perfusion study allows tissue characterisation with a suggestive pattern of late gadolinium enhancement.
Case Report
We report a rare case of EGPA in a 54-year-old male patient who presented with fever, sore throat and dizziness. Echocardiography showed a filling defect at the apex of the right ventricle (RV). CMR findings suggested the diagnosis of EGPA by demonstrating an impressive lesion at RV apex with the typical 3-layer appearance and thrombus formation. Post-gadolinium subendocardial hyperenhancement suggested focal involvement at the inferolateral wall of the left ventricle. Computed Tomography (CT) was done to rule out calcific or soft plaques of the coronary arteries, small vessel vasculitis and small aneurysm. CT scan showed a low-attenuation lesion at the inner wall of the right ventricle. In the lungs, bilateral interstitial changes and bilateral cystic bronchiectases were found. Under appropriate treatment, the patient improved clinically.
Conclusions
It is of crucial importance to perform full cardiac imaging that includes CMR even in asymptomatic patients with suspected EGPA, since early identification of cardiac involvement may allow to apply appropriate therapy and full recovery of the patient.
MeSH Keywords: Churg-Strauss Syndrome; Eosinophilic Granuloma; Heart Ventricles; Magnetic Resonance Imaging; Magnetic Resonance Imaging, Cine
Background
Eosinophilic granulomatosis with polyangiitis (EGPA), formerly known as Churg-Strauss syndrome (CSS), is a rare multi-systemic disease that affects both small and medium-sized blood vessels of nearly all organs. It involves the respiratory tract, skin, nervous system, gastrointestinal tract, kidneys and heart [1]. The prevalence rate is seven per million but it can be significantly greater in patients with bronchial asthma (64 per million) [2]. The exact aetiology of EGPA is unknown; it is considered most likely to be an autoimmune disorder with antineutrophil cytoplasmic antibodies (ANCA). However, it was shown that different phenotype could be observed, depending on the ANCA status, suggesting various pathogenic mechanisms [3].
The disease is characterised by necrotising vasculitis, extravascular granuloma formation, and eosinophilic infiltration of various organs [4]. The involvement of the myocardium represents a bad prognostic factor [5]. Cardiac involvement was reported in 20–50%, yet with improved diagnostic methods, the frequency of cardiac involvement is expected to be even higher and is the major cause of morbidity and mortality [6]. Cardiomyopathy can result from vasculitis-related ischemia affecting small myocardial vessels and coronary arteries [7] and from eosinophilic myocardial infiltration [8]. Cardiac EGPA tends to affect mainly the left myocardium, valves and pericardium [9]. Other presentations can be found in these patients including pericarditis (25%), pericardial effusion (up to 22% of patients), heart failure (18%), ventricular and supraventricular arrhythmias and sudden cardiac death [10,11]. Orthotopic heart transplantation is feasible in case of a severe disease and optimal post transplantation immunosuppressive therapy has yet to be defined [12].
We report a case of EGPA associated with an unusual prominent right cardiac involvement.
Case Report
A 54-year-old male, with bronchial asthma, presented with a one-month history of dry cough and fever. The fever was associated with chills and rigors but without sweats. There was a ten-day history of tiredness and lethargy, which was progressively increasing and was associated with shortness of breath on exertion. The patient was free from any gastrointestinal tract or urinary symptoms.
On admission, he was afebrile with normal blood pressure (120/70 mmHg), pulse rate 70 bpm, respiratory rate 20/min, and oxygen saturation 98% at room air. Generally he was cachetic, with bluish discoloration at the tip of his fingers and intact peripheral pulses. Head and neck examination showed tenderness over the left maxillary sinus and healing ulcers on the lateral side of the tongue. Chest examinations revealed bibasal fine crepitations, more pronounced on the left side. The vascular system examination was normal. The patient had no neurological abnormalities.
Blood tests were as follows: white blood cells 42.7 G/L with a total blood eosinophil count of 34.2 G/L, sodium 134 mmol/L, potassium 4.7 mmol/L, creatinine 92 μmol/L, and glucose 4.48 mmol/L. Liver chemistry was normal. The complete immunology evaluation showed IgE of 258 IU/mL (0–114), antinuclear antibodies positive (1:640) with positive anti-myeloperoxidase antibody and positive perinuclear-ANCA. Urine microscopy was normal.
The 12-lead ECG was normal.
Chest X-ray showed interstitial changes predominantly in the lower parts of both lungs.
Computed tomography (CT) of the brain and sinuses revealed normal brain parenchyma with no infarction or intracranial bleed. Mucosal thickening of the left maxillary sinus, posterior ethmoidal air cells and right sphenoid sinus compartment were also noted.
Echocardiography revealed an echogenic 40×19-mm mass lesion occupying the right ventricle (RV) apex. The volume of the left atrium indicated its mild dilatation. The sinuses of Valsalva were dilated. The ejection fraction of the left ventricle (LV) was 55–60% with no regional motion abnormalities.
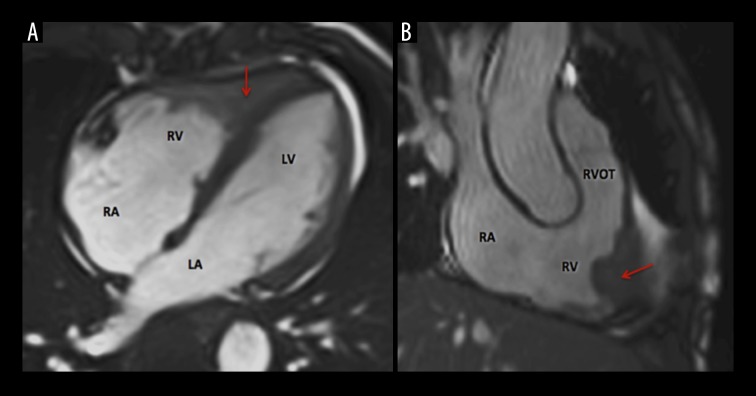
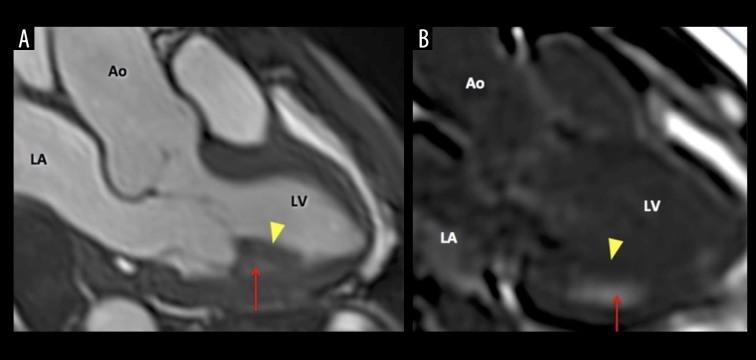
Cardiac magnetic resonance (CMR, 1.5 Tesla Avanto, Siemens) showed mild dilation of the aortic root (Valsalva sinuses − 43 mm) with a normal ascending aorta. Cine sequences were acquired during breath-hold with the use of fast-gradient echo steady-state free precession sequences. They showed an enlarged basal RV with a diameter of 46 mm. There was a focal lesion involving the RV apex (Figure 1A) extending anteriorly through just above the RV outflow tract (Figure 1B). The mass lesion measured 70×30 mm. On T2-weighted and T2-weighted fat-suppression sequences, the mass noted to be iso- to hyper-intense (Figure 2). On T1-weighted dark blood sequence, the signal was iso-intense. The first pass study using T1-weighted sequence immediately after dynamic intravenous infusion of 20 mL of gadolinium (0.1 mmol/kg body weight) at a flow rate of 5mL/sec, demonstrated a three-layered appearance (V-Sign) in the apex of the RV in a four-chamber view with an inner layer showing no perfusional enhancement, suggestive of a mural-based thrombus. The middle layer revealed mildly hyper-intense signal intensity and the outer layer of normal myocardium with iso-intense signal (Figure 3). Ten minutes after contrast injection, late gadolinium enhancement (LGE) imaging was performed using a T1-weighted segmented inversion-recovery pulse sequence. The inversion time was set to null normal myocardium. The delayed phase showed heterogeneous hyper-enhancement of the apical mass of the RV, while there was no abnormal enhancement of the right myocardial wall. It also showed diffuse subendocardial delayed hyper-enhancement with involvement of the papillary muscles that were also seen in Cine sequences (Figure 4). The type of gadolinium distribution indicates either fibrosis or inflammation of this part of the myocardium with subendocardial hyper-enhancement suggestive of focal involvement of the inferolateral wall as coronary arteritis and acute infarction is increasingly reported in these patients [13], CT coronary angiography did not reveal any calcific or soft plaques of the coronary arteries and no aneurysms were detected. There was a thin band of low attenuation within the endomyocardium of the RV on contrast-enhanced CT scan, which corresponded with the areas of MR heterogeneous signals at the RV apex, extending anteriorly with predominantly subendocardial location.
Figure 1.
Cine MRI sequence of a 54-year-old male with eosinophilic granulomatosis. (A) 4-chamber view: focal lesion (arrow) involving the RV apex; (B) right 3-chamber view: lesion extending anteriorly just above the right ventricle outflow tract (RVOT). LA – left atrium; LV – left ventricle; RA – right atrium; RV – right ventricle.
Figure 2.
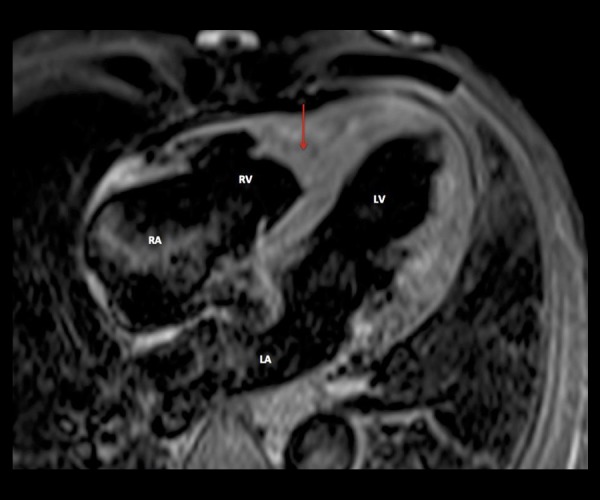
T2-weighted MRI sequence in a 54-year-old male with eosinophilic granulomatosis. Focal lesion filling the apex of the RV appearing as a heterogeneous signal ranging from iso- to hyper-intense (arrow). LA – left atrium; LV – left ventricle; RA – right atrium; RV – right ventricle.
Figure 3.
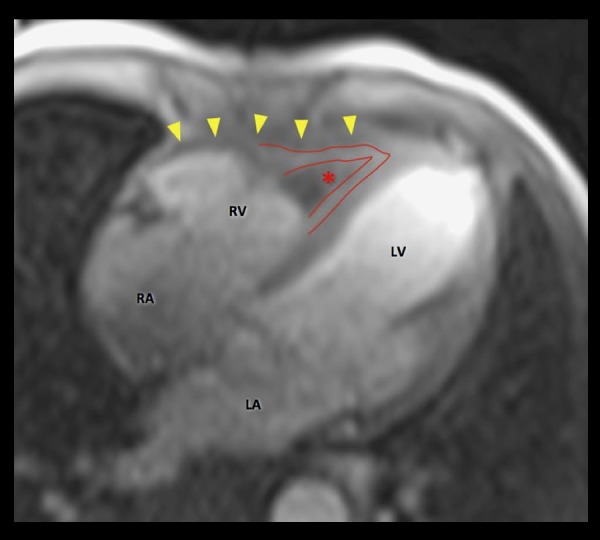
First pass MRI gadolinium perfusion sequence in a 54-year-old male with eosinophilic granulomatosis. Red lines delimit the three layers with an inner layer showing no perfusional enhancement suggestive of mural-based thrombus (asterisk). Middle layer revealed mildly hyper-intense signal intensity and outer layers of normal RV myocardium wall (arrowheads) of iso-intense signal. LA – left atrium; LV – left ventricle; RA – right atrium; RV – right ventricle.
Figure 4.
MRI Cine sequence of a 54-year-old male with eosinophilic granulomatosis showing involvement of the inferolateral wall beside the papillary muscle. (A) A hypertrophied papillary muscle (arrowhead) with subpapillary involvement (arrow). (B) Contrast-enhanced image 10 minutes after iv administration of 0.1 mmol/kg body weight of gadolinium: diffuse subendocardial delayed hyper-enhancement (arrow) with involvement of the papillary muscles (arrowhead). LA – left atrium; LV – left ventricle; RA – right atrium; RV – right ventricle.
The high-resolution CT scan of the lungs demonstrated bilateral interstitial changes and bilateral cystic bronchiectases.
Based on CMR and echocardiographic findings, considering the patient’s medical history and laboratory data, the diagnosis of EGPA with cardiac involvement was established. However, the patient refused tissue biopsy.
Initially, the patient was treated with intravenous methylprednisolone 60 mg, followed by oral prednisolone 60 mg once daily. The patient clinically improved dramatically and his eosinophil count dropped to zero.
Discussion
Eosinophilic granulomatosis with polyangiitis (EGPA) formerly known as Churg-Strauss syndrome, is a rare multi-systemic disease that affects both small and medium-sized blood vessels of nearly all organs. Cardiac involvement is known to be associated with very poor prognosis, accounting for approximately one-half of deaths [14]. However, there has been limited information on the proper diagnosis and management of cardiac involvement of EGPA.
In a series of 383 patients with EGPA, 16 percent had cardiomyopathy and 15 percent had pericarditis [15]. In a separate series of 22 patients with evidence of cardiac involvement, cardiac abnormalities included an abnormal ECG in all patients; valvular insufficiency 73%, pericardial effusion 50%, and heart failure 41% [16]. Endomyocardial involvement was found in 12 patients, based on cardiac MRI findings of mural thrombus and a positive endomyocardial biopsy [17]. The majority of patients improved with treatment.
This case shows an unusual prominence of the RV associated with papillary muscle involvement of the LV as described above [17]. This uncommon case shows the complementary value of non-invasive cardiac imaging modalities in the diagnostics of myocardial involvement in a patient with suspected EGPA. CMR findings suggested the diagnosis of EGPA by demonstrating an impressive lesion at the RV apex with a typical 3-layer appearance and thrombus formation. Subendocardial hyper-enhancement was suggestive of focal involvement at the inferolateral wall of the left ventricle. CT was done to evaluate the status of the coronary arteries and to rule out calcific or soft plaques, small vessel vasculitis and small aneurysms. CT scan showed a low-attenuation lesion at the inner wall of the right ventricle.
Conclusions
As cardiac involvement is one of the main predictors of mortality in EGPA [18] and an important prognostic factor in the 5-factor score [5], it is suggested that cardiac involvement of EGPA may be considered as a reversible condition. Therefore, it is of crucial importance to perform full cardiac imaging that includes CMR even in asymptomatic patients with suspected EGPA, since the early identification of cardiac involvement may lead to the application of appropriate therapy and full recovery of the patient.
References
- 1.Pagnoux C, Guilpain P, Guillevin L. Churg-Strauss syndrome. Curr Opin Rheumatol. 2007;19:25–32. doi: 10.1097/BOR.0b013e3280119854. [DOI] [PubMed] [Google Scholar]
- 2.Martin RM, Wilton LV, Mann RD. Prevalence of Churg-Strauss syndrome, vasculitis, eosinophilia and associated conditions: retrospective analysis of 58 prescription-event monitoring cohort studies. Pharmacoepidemiol Drug Sat. 1999;8:179–89. doi: 10.1002/(SICI)1099-1557(199905/06)8:3<179::AID-PDS414>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.Sinico RA, Di Toma L, Maggiore U, et al. Prevalence and clinical significance of antineutrophil cytoplasmic antibodies in Churg-Strauss syndrome. Arthritis Rheum. 2005;52:2926–35. doi: 10.1002/art.21250. [DOI] [PubMed] [Google Scholar]
- 4.Lee T, Lee YS, Yoon SY, et al. Clinical characteristics that distinguish eosinophilic organ infiltration from metastatic nodule development in cancer patients with eosinophilia. World J Surg Oncol. 2012;10:175. doi: 10.1186/1477-7819-10-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guillevin L, Lhote F, Casassus P, et al. Prognostic factors in polyarteritis nodosa and Churg-Strauss syndrome. A prospective study in 342 patients. Medicine. 1996;75:17–28. doi: 10.1097/00005792-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Merten C, Beurich H, Zachow D, et al. Cardiac involvement in hypereosinophilic syndromes detected by cardiac magnetic resonance imaging. J Cardiovasc Magn Resonan. 2015;17(Suppl 1):Q75. [Google Scholar]
- 7.Hellemans S, Dens J, Knockaert D. Coronary involvement in the Churg-Strauss syndrome. Heart. 1997;77:576–78. doi: 10.1136/hrt.77.6.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pagnoux C, Guillevin L. Cardiac involvement in small and medium sized vessel vasculitides. Lupus. 2005;14:718–22. doi: 10.1191/0961203305lu2207oa. [DOI] [PubMed] [Google Scholar]
- 9.Smedema JP, van Paassen P, van Kroonenburgh MJ, et al. Cardiac involvement of Churg Strauss syndrome demonstrated by magnetic resonance imaging. Clin Exp Rheumatol. 2004;22( 6 Suppl 36):S75–78. [PubMed] [Google Scholar]
- 10.Sable-Fourtassou R, Cohen P, Mahr A, et al. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med. 2005;143:632–38. doi: 10.7326/0003-4819-143-9-200511010-00006. [DOI] [PubMed] [Google Scholar]
- 11.Setoguchi M, Okishige K, Sugiyama K, et al. Sudden cardiac death associated with Churg-Strauss syndrome. Circ J. 2009;73:2355–59. doi: 10.1253/circj.cj-08-0926. [DOI] [PubMed] [Google Scholar]
- 12.Brucato A, Maestroni S, Masciocco G, et al. Cardiac involvement in the syndrome Churg- Strauss. G Ital Cardiol. 2015;16(9):493–500. doi: 10.1714/1988.21524. [DOI] [PubMed] [Google Scholar]
- 13.Sakadakis EA, Papafilippaki A, Katsimbri P, et al. Churg-Strauss syndrome masquerading as an acute coronary syndrome. Am J Emerg Med. 2015;33(2):313.e5–6. doi: 10.1016/j.ajem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Grau RG. Churg-Strauss syndrome: 2005–2008 update. Curr Rheumatol Rep. 2008;10:453–58. doi: 10.1007/s11926-008-0074-x. [DOI] [PubMed] [Google Scholar]
- 15.Comarmond C, Pagnoux C, Khellaf M, et al. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): Clinical characteristics and long-term follow up of the 383 patients enrolled in the French Vasculitis Study Group cohort. Arthritis Rheum. 2013;65:270–81. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 16.Neumann T, Manger B, Schmid M, et al. Cardiac involvement in Churg-Strauss syndrome: impact of endomyocarditis. Medicine (Baltimore) 2009;88:236–43. doi: 10.1097/MD.0b013e3181af35a5. [DOI] [PubMed] [Google Scholar]
- 17.Peterson SE, Kardos A, Neubauer S. Subendocardial and papillary muscle involvement in a patient with Churg-Strauss syndrome, detected by contrast enhanced cardiovascular magnetic resonance. Heart. 2005;91:e9. doi: 10.1136/hrt.2004.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knockaert DC. Cardiac involvement in systemic inflammatory diseases. Eur Heart J. 2007;28:1797–804. doi: 10.1093/eurheartj/ehm193. [DOI] [PubMed] [Google Scholar]