Abstract
Background
Language impairment (LI) risk is increased for perinatally acquired human immunodeficiency virus-infected (PHIV) and perinatally exposed to HIV but uninfected (PHEU) youth. This study evaluates the persistence of LI in these groups.
Methods
The Clinical Evaluation of Language Fundamentals was repeated on participants of the Pediatric HIV/AIDS Cohort Study Adolescent Master Protocol 18 months postbaseline. Regression models identified factors associated with change in standardized score (SC) and the resolution or development of LI.
Results
Of 319 participants, 112 had LI at baseline. Upon re-evaluation, SCs were highly stable and changes were similar in PHIV (n = 212) and PHEU (n = 107) participants. Those with family history of language delays had a 2.39 point lower mean increase in SCs than those without, after controlling for demographic and socioeconomic factors and baseline LI status. Among PHIV participants, CD4 count <350 cells/mm3 was associated with lower mean SC change (4.32 points), and exposure to combination antiretroviral therapy (cART) or protease inhibitors (PIs) was associated with a higher mean SC change (5.93 and 4.19 points, respectively). Initial LI was persistent in most cases (78%); 20 new cases occurred (10%). Female sex was associated with higher odds of LI resolution. Among PHIV, duration and baseline cART and history of PI use were associated with LI resolution; higher percentage of detectable viral loads before baseline was associated with lower odds of resolution.
Conclusions
The PHIV and PHEU youth are at risk for persistent LI, and family history of language delays was a risk factor for persistence of problems. Measures of successful HIV treatment predicted more favorable outcomes among PHIV youth.
Keywords: antiretroviral therapy, language development, language impairment, perinatal HIV, youth
Youth with perinatally acquired human immunodeficiency virus (PHIV) are at increased risk for a variety of developmental and behavioral problems. A recent systematic review implicated language development as a particular vulnerability [1]. Furthermore, studies of school-age children and adolescents indicate that elevated language risk applies equally to children with PHIV and to children who were perinatally exposed to HIV but uninfected (PHEU), which suggests significant contributions from factors other than infection status [2, 3]. Although consistent in findings, the evidence base is limited and comes primarily from cross-sectional studies, leaving unknown the longitudinal course of language impairment (LI) within PHIV and PHEU populations and the potential contributions of demographic, antiretroviral treatment (ART), comorbidity of hearing and cognitive impairments, and other key factors that might inform intervention efforts. One early precombination antiretroviral therapy (cART) era study [4] observed 17 pediatric patients with symptomatic HIV for 24 months (age range at baseline, 1–15 years) and found that participants' scores across infant, preschool, and school-age language scales—but not intelligence quotient (IQ) scales—declined significantly over the course of the study. The 17 participants in the sample were survivors from an original cohort of 38 children. No significant associations were found between changes in survivors' language scores and their disease severity or treatment parameters. The sample was too small to examine potential contributing factors, leaving mechanisms behind observed declines undetermined. The extent to which the results of this singular longitudinal study sample could be applied to pediatric patients in the current context is unknown, and updated information is critically needed to determine risk for language development associated with modern HIV treatments.
The neurodevelopmental mechanisms and environmental contexts supporting language development vary for different ages and stages of development, and the impact of perinatal HIV exposure and/or infection and associated factors might depend on maturational level. An important prerequisite to the task of untangling effects would be to limit examination to delineated developmental stages rather than combining data from infant, preschool, and school-age groups. Adolescence marks a particularly important transition from preceding stages of language development into adulthood and corresponds to increasing facility with the advanced semantic and syntactic skills instrumental in literacy, critical thinking, and intensive social interactions [5]. Language impairments that persist through adolescence contribute to individual long-standing academic, interpersonal, and ultimately vocational difficulties [6–8]. For youth with HIV, persistent LIs may compromise their transition to adult care.
Rice et al [3] cross-sectionally examined the language and cognitive profiles of 284 PHIV and 153 PHEU adolescents (median age, 12 years; range, 7–17 years) from the Adolescent Master Protocol (AMP), a component of the Pediatric HIV/AIDS Cohort Study. Results indicated that both PHIV and PHEU groups had compromised language performance relative to general population norms. Group means on a comprehensive standardized language test, the Clinical Evaluation of Language Fundamentals-Fourth Edition (CELF-4), were depressed to similar levels in the PHIV and PHEU groups (core language standard scores of 88.5 and 87.5, respectively, where 100 is the population mean). The PHIV and PHEU groups were also similar in their proportions of LI (34% and 37%, respectively), where “impairment” was defined as performance 1 standard deviation (SD) below the population mean on the CELF-4. These results were comparable to PHIV and PHEU group mean vocabulary test scores provided by Brackis-Cott et al [2] and to baseline estimates of expressive language standard scores provided in the 1 longitudinal study of children with PHIV from the pre-cART era [4]. Rice et al [3] also reported that among the AMP PHIV group, those with poorly controlled HIV had 3 times the odds for LIs concomitant with cognitive or hearing impairment compared with those with well controlled HIV.
Rice et al [3] was a cross-sectional analysis of language functioning among the AMP study sample at a single time point. Longitudinal follow up is needed to determine the persistence of LI in this group and to improve our understanding of the long-term risks associated with disease status, treatment, and other factors. This report provides a follow-up longitudinal assessment of the language abilities of PHIV and PHEU youth from the AMP study sample. We were interested in whether declines in language performance would be observed in this cohort. We evaluated and compared changes in participants' CELF-4 standard score and initial LI status across 2 times of assessment, 18 months apart, and examined potential predictors of each of these outcomes. For the PHIV participants, we evaluated the association between changes in language scores or LI status with disease severity and antiretroviral (ARV) drug exposure.
STUDY COHORT AND METHODS
The AMP study details are presented in the introduction to this issue and have been published elsewhere [3]. In brief, participants were children and adolescents born to women with HIV infection and initially enrolled in AMP at ages 7 to 16 years. Results of neurodevelopmental testing, clinical interviews, and medical record reviews collected over the course of the AMP study were used. The AMP study protocol was reviewed and approved by the Institutional Review Boards at the Harvard T. H. Chan School of Public Health and at each of the 15 participating sites. Each participant provided assent and their legal guardian provided informed consent.
Administrations of the CELF-4 were conducted by trained psychologists or other staff personnel specifically trained in the administration of the CELF-4 at 6 months after enrollment to the study (which we will consider as baseline) and again after 18 months (follow up). The CELF-4 was selected as our main language outcome because it has demonstrated strong psychometric properties and is widely used by speech-language clinicians to identify LIs in school-age children and adolescents [9]. To be included in the current study sample, AMP participants needed to be assessed in their primary language at both time points (either English or Spanish) and needed to be monolingual (English or Spanish) at baseline. Analysis was restricted to monolingual individuals to prevent misclassifying differences due to bilingual development as LIs.
Change in participants' CELF-4 core language score was assessed in 2 ways. First, numerical change in the core language standard score was analyzed by subtracting participants' baseline standard score from their follow-up standard score. Standard scores adjust for expected changes associated with age, thus large discrepancies between baseline and follow-up would be indicative of development “off-course”, either in the direction of less language growth than expected over time or an accelerated rate of growth.
Second, to reflect the clinical relevance of observed changes in participants' language performance, CELF-4 standard scores were used to classify participants into groups either with LI or with typical language skills (non-LI) at baseline and follow up. Standard scores lower than 1 SD below the general population estimate (less than 85) were considered indicative of LI. Those participants with LI at baseline who had LI at follow up were classified as “persistent LI”. whereas those without LI at follow up were classified as “resolved LI”. Those with non-LI at baseline who did not have LI at follow up were considered “persistent non-LI”, whereas those with LI at follow up were classified as “LI developed”.
To investigate the association between HIV infection and the change in language ability, other potential contributing factors representing participant, family, or caregiver characteristics were considered. These covariates included demographic variables (age, sex, race, and ethnicity), the presence or absence of concomitant nonverbal cognitive or hearing impairments at baseline, the presence/absence of a family history of language delays (“was there anyone in the study participant's biological family [mother, father, siblings] who was slow to talk, or had a problem with reading or language in school?”), whether or not participants' caregivers were their biological parent, high school graduates, had performance IQ levels below 70 (as measured by the Wechsler Abbreviated Scale of Intelligence), or had income levels at or below $20,000 annually.
Nonverbal cognitive impairment was defined as a standard score less than 85 on the Perceptual Reasoning Index of the Wechsler Intelligence Scale for Children-Fourth Edition (if age <17 years) or of the Wechsler Adult Intelligence Scale (if age ≥17 years). The cognitive assessment closest to the baseline language assessment was selected from among those that were administered within 6 months before the baseline or 1 year after the follow-up CELF-4. Hearing impairment was defined as the worse ear pure tone average of thresholds at 500, 1000, 2000, and 4000 Hz ≥20 dB hearing level from the audiometric examination taken before a cutoff date 6 months after the baseline CELF-4 assessment.
After our earlier analysis, the LI group was divided based on baseline status into a subgroup of Primary Language Impairment (Primary LI), or 44 cases of LI in the absence of concomitant cognitive/hearing impairment, and a Concomitant Language Impairment (Concomitant LI) subgroup, which consisted of 68 cases of LI with concomitant cognitive/hearing impairment. The non-LI group included 175 cases of typical language and cognition/hearing (No Impairment) as well as a subgroup of 31 cases of typical (or “spared”) language with cognitive/hearing impairment (CHI).
For participants with PHIV, disease severity indices considered included the following: (1) lifetime (assessed at baseline) CD4 nadir <200 cells/mm3, (2) CD4 <350 cells/ mm3 at baseline, (3) nadir CD4 <350 cells/mm3 over the study interval, (4) percentage of plasma HIV ribonucleic acid (viral load) measures at or before baseline language assessment that were >400 copies/mL, (5) viral load >400 copies/mL at baseline, (6) peak viral load >400 copies/mL over the study interval, and (7) Centers for Disease Control Prevention classification of class C (history of AIDS-defining illness) by baseline. Exposures to ART as potential predictors of the PHIV language outcomes included the following: (1) whether or not a participant was on cART at baseline, (2) duration in years on cART, (3) duration in years on nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs), (4) duration in years on nonnucleoside reverse-transcriptase inhibitors (NNRTIs), and (5) duration in years on protease inhibitors (PIs). Whether or not a participant was ever exposed to cART, NNRTIs, or PIs and if less than 6 months of age at first exposure to any ART, cART, NNRTIs, or PIs were also considered. Combination ART was defined here as any ART that contained at least 3 drugs from at least 2 different ARV drug classes.
Statistical Analysis
Characteristics were summarized by HIV infection status and compared with a Wilcoxon rank-sum test or a Fisher's exact test as appropriate. Pearson correlation between the language standard score at baseline and at follow up was calculated by HIV infection status to assess stability in language functioning. Mean change in language standard score was calculated by HIV infection status and concomitant impairment status with 95% confidence intervals (CIs). General linear regression analysis (GLM) was used to model the association between the change in core language standard score with HIV infection status, demographics, and other covariates, adjusting for baseline language status (LI [Primary LI and Concomitant LI] or non-LI [No Impairment and CHI]). An interaction term between baseline language status and baseline cognitive/hearing impairment status was also included for all GLM. The proportion with LI resolution among participants with LI at baseline (combining cases of primary LI and concomitant LI) and the proportion with LI development among participants with non-LI at baseline (combining cases of typically developing participants and those with cognitive/hearing impairment and spared language) were calculated by HIV infection status and compared with a Fisher's exact test. Two separate logistic regression models were fit to identify predictors for LI development (among those with baseline non-LI) and for LI resolution (among those with baseline LI), adjusting for baseline language standard score. The final multivariable models retained HIV infection status, cognition or hearing impairment status, demographics, and other factors with P < .1 through backward selection. Among the PHIV youth, a multivariable linear regression model was fit to evaluate the association of all 7 indices for disease severity with change in language standard score, adjusted for the predictors identified above. Due to the smaller sample sizes, only 3 disease severity indices were included in the multivariable logistic regression models among PHIV youth: (1) the most significant CD4 variable, (2) the most significant viral load variable, and (3) history of AIDS-defining illness. The association of historical and baseline ARV exposures were then analyzed individually, adjusting for predictors and disease severity indicators. All analyses were performed using SAS version 9.4 (SAS Institute, Cary, North Carolina) and were based on data collected as of March 1, 2015.
RESULTS
Baseline and follow-up CELF-4 were available for 445 youth (282 PHIV and 163 PHEU). Among these, 319 (212 PHIV and 107 PHEU) were monolingual at baseline and tested in their primary language and included in our analysis. The youth with PHIV were 2 years older than the youth with PHEU on average, but the 2 groups were similar in their sex, race, and ethnicity distributions (Supplemental Table). Cognitive assessments were available from within 6 months of the baseline language assessment in 32% of participants and from within 1 year in 96%; only 1 participant with complete language data had no cognitive assessment within the window. A marginal difference between PHIV and PHEU groups was found in their rates of concomitant impairment (either nonverbal cognitive impairment or hearing impairment) (PHIV 34% vs PHEU 24%, P = .07). The majority of caregivers for both groups had completed high school (79%). Group differences in the proportion of caregivers with performance IQ scores below 70 were not statistically significant (PHIV = 3%, PHEU = 8%). A significantly higher proportion of caregivers in the PHIV group were not the participant's biological parent, and a higher proportion in the PHEU group had reported incomes less than or equal to $20,000.
Change in Language Standard Score
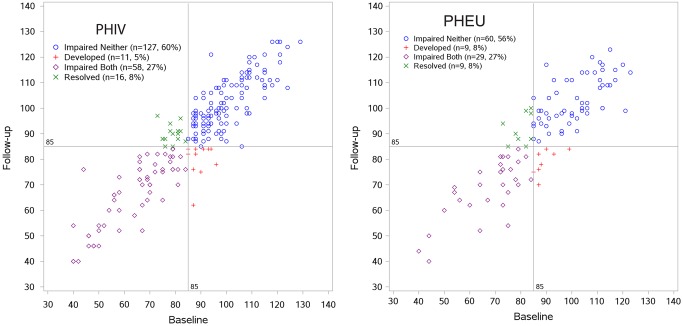
Overall, baseline and follow-up CELF-4 standard scores for the study sample were highly stable in both the PHIV and PHEU groups (correlation coefficient, 0.91 [95% CI, .88–.93] and 0.86 [95% CI, .80–.90], respectively) (Figure 1). Both the baseline mean CELF-4 SC (88.1 [95% CI, 85.6–90.7] and 89.2 [95% CI, 85.8–92.6], respectively) and mean change from baseline over the 18-month follow up (2.2 [95% CI, 1.2–3.3] and 1.5 [95% CI, −.3 to 3.3], respectively; P = 0.46) for the PHIV and PHEU groups were very similar. Stability in mean CELF-4 standard score was also present within the non-LI and LI subgroups: No Impairment (baseline 100.4, follow up 100.8), CHI (baseline 93.6, follow up 96.3), Primary LI (baseline 74.5, follow up 79.0), Concomitant LI (baseline 64.3, follow up 68.3). As expected, in unadjusted models, the mean baseline standard score was higher for participants without cognitive or hearing impairment (100.4 [SD = 10.8] vs 93.6 [SD = 7.1]) for No Impairment vs CHI and 74.5 (SD = 9.1) vs 64.3 (SD = 13.0) for Primary LI vs Concomitant LI; however, the mean change in language scores was similar without or with cognition/hearing impairment (0.4 [SD = 8.4] vs 2.7 [SD = 8.2] for non-LI and 4.5 [SD = 8.0] vs 4.0 [SD = 8.6] for LI). After adjusting for other covariates, the mean change remained similar without and with cognitive/hearing impairment (adjusted mean change = −0.9 vs 1.1 for No Impairment vs CHI and 3.5 vs 2.6 for Primary LI vs Concomitant LI). Black race was associated with an increase in language standard score with marginal statistical significance; family history of language delays/learning difficulties was associated with a smaller increase after controlling for demographic and socioeconomic factors and baseline LI status (Table 1).
Figure 1.
Clinical Evaluation of Language Fundamentals-Fourth Edition (CELF-4) core language standards scores at baseline and follow up, by human immunodeficiency virus (HIV) infection group. The baseline mean was 88.1 (95% confidence interval [CI], 85.6–90.7) for perinatally acquired HIV (PHIV) and 89.2 (95% CI, 85.8–92.6) for perinatally exposed to HIV but uninfected (PHEU); the mean change was 2.2 (95% CI, 1.2–3.3) for PHIV and 1.5 (95% CI, −.3 to 3.3) for PHEU. The Pearson correlation coefficient was 0.91 (95% CI, .88–.93) for PHIV and 0.86 (95% CI, .80–.90) for PHEU.
Table 1.
Adjusted Mean Changes* in Core Language Standard Score by Demographic and HIV Disease Severity Characteristics
| Covariate | n | LS Mean** With, Without | Estimated Effect*** | 95% CI | P Value |
|---|---|---|---|---|---|
| HIV infected | 318 | 1.95, 1.22 | 0.72 | (−1.34 to 2.79) | .49 |
| Language impaired at baseline | n/a& | 4.39 | (1.54–7.24) | <.01 | |
| Nonverbal cognitive/hearing impairment | n/a& | 1.94 | (−1.32 to 5.20) | .24 | |
| Interaction: Lang*concomitant impairment | n/a& | −2.92 | (−7.44 to 1.60) | .20 | |
| Age (per year) | n/a | −0.10 | (−.46 to .26) | .60 | |
| Female sex | 1.65, 1.52 | 0.14 | (−1.72 to 1.99) | .89 | |
| Black race | 2.77, 0.40 | 2.38 | (−.27 to 5.02) | .08 | |
| Hispanic ethnicity | 1.83, 1.34 | 0.49 | (−3.13 to 4.11) | .79 | |
| Family history of language delay | 0.39, 2.78 | −2.39 | (−4.69 to −.09) | .04 | |
| Disease Severity Among PHIV# | |||||
| CD4 nadir <200 cells/mm3 | 211 | 1.46, −1.07 | 2.53 | (−.22 to 5.27) | .07 |
| CD4 <350 cells/mm3 | −1.96, 2.36 | −4.32 | (−8.69 to .05) | .05 | |
| CD4 nadir <350 cells/mm3 (study window) | −0.20, 0.59 | −0.79 | (−4.38 to 2.80) | .66 | |
| VL % lifetime measures >400 copies/mL | n/a | −0.03 | (−.08 to .01) | .17 | |
| VL >400 copies/mL | 0.24, 0.16 | 0.08 | (−3.21 to 3.36) | .96 | |
| VL peak >400 copies/mL (study window) | 0.84, −0.44 | 1.28 | (−1.58 to 4.13) | .38 | |
| History of AIDS-defining illness | 0.95, −0.56 | 1.51 | (−1.07 to 4.10) | .25 | |
| Adjusted ART Models Among PHIV# | |||||
| Ever cART | 211 | 0.13, −5.80 | 5.93 | (.33–11.53) | .04 |
| Ever NNRTI | 211 | 0.22, 0.15 | 0.07 | (−2.23 to 2.36) | .96 |
| Ever PI | 211 | 0.05, −4.13 | 4.19 | (.40 to 7.97) | .03 |
| cART duration (per year) | 211 | n/a | 0.13 | (−.22, .48) | .47 |
| NRTI duration (per year) | 211 | n/a | 0.10 | (−.45 to .65) | .73 |
| NNRTI duration (per year) | 211 | n/a | −0.04 | (−.39 to .30) | .80 |
| PI duration (per year) | 211 | n/a | 0.12 | (−.20 to .44) | .47 |
| cART at baseline | 211 | 0.41, −0.88 | 1.29 | (−2.70 to 5.29) | .52 |
Abbreviations: ART, antiretroviral therapy; cART, combination ART; CI, confidence interval; HIV, human immunodeficiency virus; LS, least squares; n/a, not applicable; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; PHIV, perinatally HIV-infected; PI, protease inhibitor; VL, viral load (plasma HIV ribonucleic acid concentration).
*The adjusted mean change was based on fitting linear regression models to the mean change in standard score between baseline and follow-up dependent on the covariates. All characteristics reflect baseline value unless otherwise indicated.
**The adjusted mean change for those with versus without the characteristic (reference group) for dichotomous measures, adjusted for all other covariates (evaluated at mean values).
***Estimated effect indicates the additional mean change for those with the characteristic (for dichotomous measures) or for each 1-unit increase (for continuous measures).
&The adjusted mean change for the 4 groups accounting for the interaction term was −0.85 for those without language impairment or nonverbal cognitive/hearing impairment, 3.54 with language impairment only, 1.09 with nonverbal cognitive/hearing impairment only, and 2.56 with both.
#One multivariable linear regression model was fit for the overall sample (N = 318), and another for the PHIV sample (N = 211), which included all above covariates and disease severity indices; models for ART exposures were fit separately for each measure adjusting for all covariates in the overall and PHIV models above.
Among youth with PHIV, low CD4 count (<350 cells/mm3) at baseline was associated with a decrease in mean standard score, whereas nadir CD4 <200 cells/mm3 was associated with an increase in mean standard score, both with marginal statistical significance. After adjusting for HIV disease severity and other covariates, ever being on cART or ever being on a PI was associated with an increase in mean standard score compared with never exposed participants (Table 1). None of the duration of ARV exposure measures or age at first exposure or current ART were associated with change in language ability.
Change in Language Impairment Status
The proportion of PHIV and PHEU participants presenting with LI at baseline was 35% and 36%, respectively. Initial LI status was persistent in the majority of cases (78%). No significant differences between PHIV and PHEU groups in their proportions of LI development (11 of 138 [8.0%] vs 9 of 69 [13.0%]; P = .32) or resolved LI (16 of 74 [21.6%] vs 9 of 38 [23.7%]; P = .81) were found. Among the demographic factors, female sex was associated with higher odds of LI resolution, and caregiver performance IQ <70 and family history of language delays were associated with higher odds of LI development with marginal statistical significance (Table 2).
Table 2.
Adjusted Odds of Resolution or Development of Language Impairment for Demographic and HIV Disease Severity Characteristics
| Language Impairment Resolution |
Language Impairment Development |
|||||||
|---|---|---|---|---|---|---|---|---|
| Covariate | n | Adjusted OR* | 95% CI | P Value | n | Adjusted OR* | 95% CI | P Value |
| HIV infected | 112 | 0.78 | (.20–3.06) | .72 | 176 | 0.75 | (.21–2.64) | .66 |
| Core language standard score at baseline | 1.36 | (1.16–1.59) | <.01 | 0.82 | (.72–.93) | <.01 | ||
| Nonverbal cognitive/hearing impairment | 0.90 | (.24–3.28) | .87 | 0.55 | (.09–3.34) | .52 | ||
| Age (per year) | 1.14 | (.89–1.46) | .31 | 0.99 | (.76–1.30) | .96 | ||
| Female sex | 4.14 | (1.07–15.99) | .04 | 1.09 | (.32–3.63) | .89 | ||
| Black race | 0.25 | (.02–3.47) | .30 | 0.38 | (.08–1.75) | .22 | ||
| Hispanic ethnicity | 1.58 | (.09–26.90) | .75 | 0.99 | (.13–7.55) | .99 | ||
| Caregiver performance IQ <70 | 6.64 | (.74–59.86) | .09 | |||||
| Family history of language delay | 3.44 | (.86–13.80) | .08 | |||||
| Disease Severity Among PHIV# | ||||||||
| CD4 <350 cells/mm3 | 74 | 0.13 | (<.01–4.29) | .25 | ||||
| CD4 nadir <350 cells/mm3 (study window) | 114 | 7.86 | (.46 to >99.99) | .16 | ||||
| VL % lifetime measures >400 copies/mL | 0.97 | (.93–1.00) | .07 | 1.03 | (.99–1.08) | .16 | ||
| History of AIDS-defining illness | 0.70 | (.08–6.26) | .75 | 1.08 | (.05–22.07) | .96 | ||
| Adjusted ART Models Among PHIV# | ||||||||
| cART duration (per year) | 74 | 1.46 | (.99–2.15) | .05 | 114 | 1.42 | (.79–2.53) | .24 |
| NRTI duration (per year) | 74 | 1.46 | (.78–2.76) | .24 | 114 | 1.30 | (.78–2.16) | .31 |
| NNRTI duration (per year) | 74 | 1.44 | (.94–2.20) | .09 | 114 | 1.31 | (.87–1.97) | .19 |
| PI duration (per year) | 74 | 1.19 | (.91–1.55) | .20 | 114 | 1.22 | (.75–1.97) | .42 |
Abbreviations: ART, antiretroviral therapy; cART, combination ART; CI, confidence interval; HIV, human immunodeficiency virus; IQ, intelligence quotient; NNRTI, nonnucleoside reverse-transcriptase inhibitor; NRTI, nucleoside/nucleotide reverse-transcriptase inhibitor; OR, odds ratio; PHIV, perinatally HIV-infected; PI, protease inhibitor; VL, viral load (plasma HIV ribonucleic acid concentration).
*The adjusted OR represents the odds of resolution/development of language impairment for those with versus without the characteristic (for dichotomous measures) or for each 1-unit increase in continuous measures (age, ART duration). All characteristics reflect baseline value unless otherwise indicated.
#One multivariable model was fit for the overall sample (N = 112 for resolve, 176 for develop) and another for the PHIV sample (N = 74 for resolve, 114 for develop), which included all above covariates and disease severity indices; models for ART exposures were fit separately for each measure adjusting for all covariates in the overall and PHIV models above.
Within the PHIV group, HIV disease severity indices and treatment parameters were significantly associated with individual language outcomes. Higher percentage of detectable viral loads up to baseline was associated with lower odds of resolving LI with marginal statistical significance. Longer lifetime duration on cART at baseline was associated with higher odds of LI resolution over the duration of the study (Table 2). Being on cART at baseline, ever exposed to NNRTI, and ever exposed to PI were also associated with higher odds of LI resolution with strong statistical evidence (all P values ≤.05); however, the small sample size resulted in imprecise estimates with very wide CIs, and the estimates are therefore not presented in Table 2. None of the HIV disease severity indices and the ART exposure measures were associated with LI development during the 18-month follow-up period (Table 2).
DISCUSSION
Language performance within youth with PHIV and PHEU from the AMP cohort was highly stable across baseline and follow-up measurements, collected 18 months apart. Observed group means at both times were below average and consistent with results of previous cross-sectional reports of PHIV and PHEU school-age children and adolescents, confirming that perinatally HIV exposed youth from similar communities represent a population at risk for LI irrespective of infection status. In particular, an estimated one third of children born to women with HIV infection present with LIs that persist into adolescence.
Language abilities in our PHIV youth were stable, whereas an earlier longitudinal report documented significant declines in the performance of a pre-cART group with PHIV over a comparable duration of follow-up [4]. Potential explanations for the discrepancy between PHIV groups include age differences between the 2 studies, differences in the measurement systems used, and differences in the ARV treatments patients received. However, the observation that group means were highly similar at baseline even though they were based on different instruments suggests that the PHIV study samples were nearly comparable with regard to the their initial language functioning. Thus, differences between samples in terms of either stable or declining language performance over time were more likely due to efficacy of ART regimens prescribed to or used by the participants in the studies. This interpretation is further supported by significant associations found within our PHIV group between better language outcomes and ever being on cART or being on cART at baseline, longer cART durations, ever exposed to a PI, better virologic control, and higher CD4 counts.
This study has limitations that could guide future investigations. For example, a study with larger samples of youth with HIV and HIV exposure or a study with a longer period of observation might detect additional associations. Our outcome measure, the CELF-4 core language score, represents a widely used omnibus measure of verbal ability. As such, it has psychometric stability and provides a reliable and valid method for identifying children performing at the low end of age expectations, but it does not provide enough detailed information to allow differentiation across language abilities, such as vocabulary, grammar, or narratives, each of which could be more sensitive to the predictor variables of interest in youth with HIV or HIV exposure. Nonetheless, our results expand on our earlier work and those provided by other study samples and add further support that, as a group, perinatally HIV-exposed adolescents are at risk for persistent LIs, regardless of infection status. A significant portion of the risk associated with poor language outcomes for perinatally exposed HIV children is probably related to the concomitant psychosocial stressors present in the lives of women living with HIV.
CONCLUSIONS
Our findings have important implications for clinical practice. Duration of cART and viral control provided protection against degradation of language growth. Our results highlight the value of routinely collecting family history of language delays and learning difficulties during pediatric intake to identify and monitor those most at risk for developing LIs and to encourage families to seek speech-language services when delays are detected. The presence of limited language skills among a significant portion of PHIV adolescents (as well as their caregivers) may also be indirectly contributing to patients' health through difficulties in understanding health information and adhering to ARV regimens. Variables uniquely impacting language growth among youth with PHIV included the extent to which their HIV was controlled, ever being exposed to cART or PIs, on cART at baseline, and longer cART duration. Additional observations of the linguistic abilities of the AMP cohort are needed to further determine the role of these risk and protective factors and possibly uncover new ones.
Supplementary Data
Acknowledgments
We thank the children, youth, and their families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS) and the sites and site staff who conducted the studies (see www.phacsstudy.org/About-Us/AMP.Acknowledgements).
Financial support. The PHACS was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with co-funding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T.H. Chan School of Public Health (HD052102) and the Tulane University School of Medicine (HD052104). Data management services were provided by Frontier Science and Technology Research Foundation, and regulatory services and logistical support were provided by Westat, Inc.
Supplement sponsorship. This article appears as part of the supplement “Brain and Cognitive Development Among US Youth With Perinatally Acquired HIV Infection,” sponsored by the PHACS.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Sherr L, Croome N, Parra Castaneda KP et al. Developmental challenges in HIV infected children: an updated systematic review. Child Youth Serv Rev 2014; 45:74–89. [Google Scholar]
- 2.Brackis-Cott E, Kang E, Dolezal C et al. The impact of perinatal HIV infection on older school-aged children and adolescents’ receptive language and word recognition skills. AIDS Patient Care STDS 2009; 23:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rice ML, Buchanan AL, Siberry GK et al. Language impairment in children perinatally infected with HIV compared to children who were HIV-exposed and uninfected. J Dev Behav Pediatr 2012; 33:112–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters PL, Brouwers P, Civitello L, Moss H. Receptive and expressive language function in children with symptomatic HIV infection and relationship with disease parameters: a longitudinal 24-month follow-up study. AIDS 1997; 11:1135–44. [DOI] [PubMed] [Google Scholar]
- 5.Paul R, Norbury CF. Language Disorders from Infancy through Adolescence. 4th ed. St. Louis, MO: Elsevier, 2012. [Google Scholar]
- 6.Tomblin JB, Nipplod MA. Understanding Individual Differences in Language Development Across the School Years. New York, NY: Taylor Francis, 2014. [Google Scholar]
- 7.Young AR, Beitchman JH, Johnson C et al. Young adult academic outcomes in a longitudinal samples of early identified language impaired and control children. J Child Psychol Psychiatry 2002; 43:635–45. [DOI] [PubMed] [Google Scholar]
- 8.Johnson CJ, Beitchman JH, Brownlie EB. Twenty-year follow-up of children with and without speech-language impairment: family, educational, occupational, and quality of life outcomes. Am J Speech Lang Pathol 2010; 19:51–65. [DOI] [PubMed] [Google Scholar]
- 9.Betz SK, Eickhoff JR, Sullivan SF. Factors influencing the selection of standardized tests for the diagnosis of specific language impairment. Lang Speech Hear Serv Sch 2013; 44:133–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.