Abstract
Background
Executive functions (EFs) are critical for management of life activities, but few studies have evaluated EFs in children and adolescents with perinatally acquired HIV (PHIV), who are at risk for problems in academics, behavior, and medication adherence. We compared EFs in youth with PHIV and in perinatally HIV-exposed but uninfected (PHEU) youth.
Methods
Four Delis-Kaplan Executive Function System (D-KEFS) subtests were administered to 173 youth with PHIV and 85 PHEU youth, aged 9 to <19 years, who were enrolled in the Pediatric HIV/AIDS Cohort Study (PHACS) Memory and Executive Functioning Study. Youth with PHIV, with or without history of a Centers for Disease Control and Prevention Class C (AIDS-defining) condition (PHIV/C [n = 45] and PHIV/non-C [n = 128], respectively), were compared with each other and with PHEU youth. Among youth with PHIV, associations with measures of current and past disease severity were evaluated using adjusted linear regression models.
Results
The PHIV/C group (mean age, 15.5 years), compared with the PHIV/non-C and PHEU groups (mean ages, 14.5 and 12.9 years, respectively), were significantly slower on the Inhibition and Color Naming/Reading Combined conditions of the Color–Word Interference subtest and made more errors on Inhibition; differences between the PHIV/C and PHEU groups persisted in adjusted models. No differences in adjusted means for fluency or problem-solving were found. The PHIV/non-C and PHEU groups did not differ on any measure. Associations of specific EF measures with HIV RNA viral load, CD4-positive T-lymphocyte percentage, and age at greatest disease severity were observed.
Conclusions
Youth with PHIV and previous AIDS-defining conditions performed more poorly on some EF measures. Relationships of EF development with the degree and timing of disease severity require further study. Implications for long-term outcomes and interventions are important avenues for follow-up.
Keywords: children, executive functions, perinatal HIV, youth
INTRODUCTION
With the development of effective antiretroviral therapy (ART) and clinical monitoring, HIV has been transformed into a chronic but manageable disease [1]. For no population has this transformation been more life altering than for children and adolescents with perinatally acquired HIV (PHIV). Rather than growing up with illness, disability, and the possibility of early death, they now enjoy the likelihood of independent adulthood and the need to prepare for its challenges and responsibilities.
The risks of major neurological sequelae once associated with HIV can be minimized with early diagnosis and effective ART and disease management [2–5]. Nevertheless, there is evidence that subtle effects on the central nervous system continue to occur in adults and children, particularly in the context of significant immunocompromise [6, 7]. Among the cognitive domains that appear to be at risk in adults with HIV are executive functions (EFs) [8], including problem solving, planning, inhibition, monitoring, fluency, and cognitive flexibility, which are essential for complex behaviors related to individual goal setting, activities of daily living, and coping with diverse environmental demands [9]. The prevalence of EF impairment in adults with HIV is approximately 50% [6]; the rates of EF deficits might have risen slightly among persons with otherwise well-controlled disease during the era of combination ART [8]. EF deficits are reliably and strongly associated with adverse real-world outcomes, including lower academic achievement and higher rates of unemployment, automobile accidents, and medication nonadherence [10–12].
The results of earlier studies of relatively small groups of children with PHIV have varied regarding the existence of EF deficits [13–15] and their relationship to other cognitive processes such as attention, which are also required for performance of higher-level goal-directed behaviors. In a study of 161 children aged 8 to 12 years [16], EF differences were observed between those with PHIV and those who were perinatally HIV exposed but uninfected (PHEU); however, the differences were not significant after adjustment for psychosocial risk factors such as parental education, which suggests that the observed EF differences were not wholly attributable to HIV infection. However, this cohort was relatively young, which leaves open the possibility that differences might emerge in adolescence or young adulthood as EF skills continue to develop. Similarly, in a study of 554 youth aged 7 to 16 years [14], EF differences between youth with PHIV and PHEU youth were attributed to risk factors other than HIV for the majority of the sample; however, a subgroup with PHIV and previous encephalopathy had significantly slower performance and more self-reported problems in metacognition even after adjustment for demographic factors. In addition, EF measures differed from age-referenced norms for the PHIV and PHEU groups, which suggests a risk for EF dysfunction in both populations.
EF continues to develop throughout childhood and adolescence [17] and is amenable to intervention [18, 19]; thus, identifying EF problems in youth with PHIV might allow for preventive and therapeutic interventions to support EF development as youth prepare for daily living, educational and occupational independence. The purpose of this study was to address limitations of previous pediatric HIV studies by extending both the age range of participants and the EF domains assessed. We hypothesized that youth with PHIV and a previous AIDS-defining (Centers for Disease Control and Prevention [CDC] Class C) diagnosis would show significantly lower performance across domains of EF that is not accounted for by differences in demographics or other cognitive and affective domains. Associations with measures of current and past disease severity and timing of most severe disease were also examined.
METHODS
Participants and Procedures
Participants for this study were enrolled in the Memory and Executive Functioning Study (Memory/EF Study) of the Adolescent Master Protocol (AMP) of the Pediatric HIV/AIDS Cohort Study (PHACS) network (see https://phacsstudy.org). Participants were enrolled at 8 of 15 AMP sites in the United States. The Memory/EF Study was a longitudinal assessment of retrospective and prospective memory and EF following perinatal HIV exposure and/or infection. Eligibility criteria included perinatal HIV infection or exposure, an age of 9 to <19 years, enrollment in AMP, ability to participate in testing procedures, and fluency in English (because some study measures were available only in English). Baseline data from the retrospective memory component of the study were published earlier [20]; this article presents results of baseline EF data analyses.
The institutional review boards (IRBs) at each site and at the Harvard T. H. Chan School of Public Health approved the AMP and the Memory/EF studies. All participants, and the parents or legal guardians of participants younger than 18 years, provided informed consent according to local IRB guidelines. The baseline assessment for the Memory/EF Study was timed to coincide with specific AMP study visits to maximize the coordination of data collection.
Measures
Subtests of the Delis-Kaplan Executive Function System (D-KEFS [21]) were used as measures of EF because the D-KEFS includes the aspects of EF relevant to this study, has substantial literature supporting its validity and reliability, is the only EF battery with a large, nationally representative normative sample, and is standardized for the entire age range of study participants, which prevents the need for multiple test versions.
Verbal Fluency includes the Letter Fluency, Category Fluency, and Category Switching (generating words while alternating between 2 different semantic categories) conditions; each involves generating words as quickly as possible for 60 seconds. Measures include total correct words generated in each condition and Switching Accuracy (number of correct switches between categories). Responses that deviate from the response set (eg, words not in the designated category) were designated as set-loss errors in all D-KEFS subtests.
Design Fluency includes Filled Dots, Empty Dots, and Switching conditions, each of which requires participants to connect dots presented within rows of boxes to create as many different designs as possible in 60 seconds. In the Filled Dots condition, the boxes contain only solid dots. In the Empty Dots condition, boxes contain filled and unfilled dots; participants are instructed to connect only empty dots, which requires inhibition. In the Switching condition, the participant must create designs by alternating between filled and unfilled dots.
Color–Word Interference measures inhibition and interference control with 4 timed conditions: Color Naming (naming color patches), Word Reading (reading color words), Inhibition (naming the ink color in which color words are printed), and Inhibition/Switching (naming ink colors or reading words depending on specific visual cues). The Inhibition condition typically results in slower responses than the first 2 conditions.
Twenty Questions is an untimed measure of problem solving and concept formation. On 4 trials, participants discern which of 30 pictured objects the examiner has selected by asking the fewest number of yes/no questions possible. For analyses, we used the Weighted Achievement score, which accounts for serendipitous guessing.
Covariates
For the analyses, we used demographic and health data collected by the AMP protocol closest in time to the EF data. Child information included age, sex, race/ethnicity, and primary language. Caregiver information included relationship to child, household income, and education. For youth with PHIV, historical and current health indicators included current HIV-1 RNA viral load; peak viral load and age at peak viral load; current and nadir CD4-positive T-lymphocyte percent (CD4%) and age at nadir CD4%; CDC classification of HIV disease [22] and age at first CDC Class C classification; diagnosis of encephalopathy and age at diagnosis; and use of ART.
The following age-standardized scores from measures of behavioral and cognitive functioning administered in the AMP protocol were used as covariates to examine whether their inclusion altered observed relationships between EF and HIV disease and severity: parent- and child-reported depression symptoms from the Behavior Assessment System for Children–Second Edition (BASC-2) Depression Scale [23]; Digit Span and Coding subtests of the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV), for ages 9 through 16 years [24] or the Wechsler Adult Intelligence Scale, Fourth Edition (WAIS-IV), for ages 17 through 19 years [25], as measures of auditory attention and processing speed, respectively; the Word Reading subtest of the Wechsler Individual Achievement Test, Second Edition, Abbreviated (WIAT-II-A) [26]; and the Core Language Score of the Clinical Evaluation of Language Fundamentals, Fourth Edition (CELF-4) [27]. The WISC-IV/WAIS-IV subtests were administered during the same visit or within 2 weeks of EF data collection; the other measures were administered during AMP visits within 1 year of the Memory/EF Study visit.
Statistical Methods
Univariate and multivariable linear regression models were used to analyze group differences among youth with PHIV with a previous CDC Class C diagnosis (PHIV/C), youth with PHIV without a Class C diagnosis (PHIV/non-C), and PHEU youth. Multiple regression models were used to control for the following potential confounders: participant sex, age at Memory/EF Study enrollment, race (Black vs non-Black), ethnicity (Hispanic vs non-Hispanic), primary language (English vs another language), caregiver relationship to child (biological parent vs other relationship), annual household income ($20 000 or less vs more than $20 000), and caregiver education (high school graduate vs nongraduate). Generalized estimating equation (GEE) linear regression models, used for their robustness regarding possible departures from assumptions of normality [28], were used to compare the 3 groups with respect to EF outcomes, both unadjusted and adjusted for covariates with a P value of <.15. Covariates retained in the adjusted model were selected by first considering those with an unadjusted P value of <.20 and using a modified stepwise algorithm to reduce to a multivariable model including covariates with a P value of <.15. GEE linear regression models were also used to compare pairs of test results among the 3 groups. Among youth with PHIV, GEE models were used to evaluate associations between EF outcomes and markers of disease severity after adjusting for base models developed for each outcome using personal and family characteristics. Last, the influence of other cognitive functions and depression symptoms on observed group differences was evaluated by refitting models that had shown significant pairwise-comparison results, adding each of the WISC-IV/WAIS-IV, CELF-4, WIAT-II-A, and BASC-2 measures separately to assess their effects on estimated group differences. A final GEE regression sensitivity analysis adjusted for clustered site differences and compared pairwise comparisons before and after adjustment. A 2-sided significance level of P < .05 was used for the analyses; correction for multiple comparisons was not performed because of the exploratory nature of the study. Analyses were performed with SAS 9.2 (SAS Institute, Cary, North Carolina).
RESULTS
Participant Characteristics
Participants were youth with PHIV (PHIV/C, n = 45; PHIV/non-C, n = 128) and PHEU youth (n = 85). The majority of the participants were Black (75%), 18% were Hispanic, and 54% were female (Table 1). Youth with PHIV/C were significantly older than those with PHIV/non-C (mean age, 15.5 vs 14.5 years, respectively), and both groups with PHIV were significantly older than the PHEU youth (mean, 12.9 years). PHEU youth were more likely to live in a home with a lower annual income, with a biological parent, and with a caregiver with HIV. Among youth with PHIV, approximately three-quarters entered the Memory/EF Study with a suppressed viral load (≤400 copies/mL) and a CD4% of ≥25; 11 (6.4%) participants were not on ART. Youth with PHIV/C were more likely to have a lower current CD4%, lower nadir CD4%, higher peak HIV RNA viral load, and previous diagnosis of encephalopathy.
Table 1.
Demographic and Disease Characteristics of 258 Youth Participating in the PHACS Memory/EF Study According to HIV Status and Previous AIDS-Defining Conditiona
| Characteristic | PHEU (N = 85) | PHIV/Non-C (N = 128) | PHIV/C (N = 45) | Pb |
|---|---|---|---|---|
| Participant age (y) | 12.9 (2.6) | 14.5 (2.7) | 15.5 (2.4) | <.001 |
| Sex | ||||
| Female | 41 (48.2) | 69 (53.9) | 29 (64.4) | .21 |
| Male | 44 (51.8) | 59 (46.1) | 16 (35.6) | |
| Black racec | 65 (76.5) | 95 (74.2) | 34 (75.6) | .93 |
| Hispanic ethnicityc | 19 (22.4) | 19 (15.0) | 8 (17.8) | .37 |
| Child's primary language not English | 10 (11.8) | 7 (5.5) | 3 (6.7) | .23 |
| Primary caregiver is biological parent | 66 (77.6) | 61 (47.7) | 21 (46.7) | <.001 |
| Primary caregiver positive HIV statusd | 62 (72.9) | 50 (39.1) | 17 (37.8) | <.001 |
| Caregiver is high school graduate | 64 (75.3) | 99 (77.3) | 32 (71.1) | .70 |
| Annual household income $20 000 or lower | 56 (65.9) | 50 (39.1) | 20 (44.4) | .002 |
| CD4% | 32.8 (11.3) | 27.5 (13.1) | .01 | |
| HIV viral load of ≤400 copies/mL | 96 (75.0) | 29 (64.4) | .17 | |
| Nadir CD4% < CD15% | 43 (33.6) | 31 (68.9) | <.001 | |
| Age at nadir CD4%, ≤3 y | 55 (43.0) | 16 (35.6) | .63 | |
| Peak HIV viral load (log copies/mL) | 5.50 (0.70) | 5.76 (0.59) | .03 | |
| Age at peak HIV viral load, ≤3 y | 83 (64.8) | 26 (57.8) | .40 | |
| Encephalopathy | 1 (0.8) | 15 (33.3) | <.001 | |
| Age at worst CDC classification | 4.31 (4.20) | 3.37 (4.57) | .10 | |
| ARV regimen | .85 | |||
| HAART and PI | 79 (64.8) | 31 (70.5) | ||
| HAART and no PI | 27 (22.1) | 9 (20.5) | ||
| Non-HAART ARV | 6 (4.9) | 2 (4.5) | ||
| Not on ARV | 10 (8.2) | 2 (4.5) |
Abbreviations: ARV, antiretroviral; CD4%, CD4-positive T-lymphocyte percentage; CDC, Centers for Disease Control and Prevention; HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; Memory/EF Study, PHACS Memory and Executive Functioning Study; PHACS, Pediatric HIV/AIDS Cohort Study; PHEU, perinatally HIV-exposed but uninfected; PHIV, perinatally acquired HIV; PHIV/C, PHIV with a CDC class C diagnosis; PHIV/non-C, PHIV without a CDC class C diagnosis; PI, protease inhibitor.
aValues are mean (SD) or number (percent).
bP values were determined by Kruskal–Wallis analysis for continuous covariates and the chi-square test for categorical covariates.
cRace was unknown or not reported for 7 participants.
dCaregiver HIV status was unknown for 32 participants.
Associations of EF With Demographic Covariates
Although specific associations with demographic variables varied across outcomes, in general, better performance on verbal tasks (Verbal Fluency, Color–Word Interference, Twenty Questions) was consistently associated with higher caregiver education and non-Hispanic ethnicity; some measures from these tasks were also associated with female sex, younger age, non-Black race, higher family income, and a biological parent as primary caregiver. Better performance on Design Fluency was associated with younger age, having a biological parent as caregiver, and higher family income; those who were older and of Hispanic ethnicity made fewer set-loss errors.
Comparisons of EF Across Groups
Fluency. Group means for Verbal Fluency were in the low-average to average range relative to the performance of the D-KEFS normative sample; means for Design Fluency were in the average range (Table 2). The PHIV/C group had the lowest scores across all measures except for set losses. Pairwise contrasts between groups showed that the PHIV/C group had significantly lower performance than the PHEU group in Verbal Category Switching (mean difference, –1.28 scaled score points; P = .02); this difference persisted after adjustment for covariates, reflecting a 13.1% lower mean. Similarly, youth with PHIV/C had significantly lower performance than PHEU youth on Design Switching, with some attenuation after adjustment (estimate, –0.81; P = .06), reflecting a 9.1% mean difference. The PHIV/C group had lower mean performance on Verbal Letter Fluency than the PHIV/non-C group, although this effect was attenuated after adjustment (estimate, –0.98; P = .06).
Table 2.
Unadjusted and Adjusted Mean D-KEFS Subtest Scaled Scores for All Groups With Pairwise Group Comparisons
| Outcomea | PHEU (Mean [95% CI]) |
PHIV/Non-C (Mean [95% CI]) |
PHIV/C (Mean [95% CI]) |
Significant Pairwise Differences in Adjusted Means | |||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Verbal Letter Fluencyb,c,d | 8.36 (7.75–8.98) | 7.52 (6.78–8.27) | 8.74 (8.19–9.29) | 8.01 (7.41–8.60) | 7.66 (6.75–8.57) | 7.03 (6.12–7.94) | PHIV/C < PHIV/non-Ci |
| Verbal Category Fluencyd | 9.62 (8.94–10.31) | 9.31 (8.61–10.02) | 9.16 (8.63–9.70) | 8.83 (8.26–9.40) | 8.70 (7.92–9.49) | 8.45 (7.66–9.25) | None |
| Verbal Category Switchingb,d,e,f | 8.80 (8.11–9.49) | 8.16 (7.43–8.89) | 8.39 (7.87–8.91) | 7.79 (7.20–8.39) | 7.52 (6.70–8.34) | 7.09 (6.19–7.99) | PHIV/C < PHEUi |
| Verbal Set-loss Errorse,g | 9.86 (9.32–10.40) | 9.97 (9.44–10.50) | 10.41 (10.03–10.78) | 10.36 (9.97–10.75) | 10.23 (9.56–10.90) | 10.06 (9.38–10.74) | None |
| Design Fluency Composite c,f,g | 8.59 (8.15–9.03) | 8.35 (7.87–8.83) | 8.60 (8.23–8.97) | 8.52 (8.16–8.87) | 8.09 (7.36–8.82) | 8.24 (7.50–8.98) | None |
| Design Fluency Switchingc | 9.14 (8.61–9.67) | 8.98 (8.41–9.54) | 8.88 (8.47–9.30) | 8.89 (8.48–9.30) | 8.14 (7.49–8.78) | 8.16 (7.53–8.80) | PHIV/C < PHEUi |
| Design Fluency Set-loss Errors b,f | 11.34 (10.87–11.81) | 11.86 (11.38–12.34) | 11.46 (11.00–11.92) | 11.70 (11.20–12.21) | 11.27 (10.52–12.03) | 11.29 (10.50–12.08) | None |
| CWI Reading/Color Naming Combined b,d,g | 8.74 (8.02–9.46) | 8.24 (7.44–9.05) | 9.04 (8.53–9.55) | 8.20 (7.59–8.81) | 7.33 (6.28–8.39) | 6.49 (5.39–7.59) | Groupsj PHIV/C < PHEUj PHIV/C < PHIV/non-Cj |
| CWI Inhibitiond,h | 8.24 (7.57–8.91) | 8.29 (7.59–8.99) | 7.99 (7.38–8.60) | 7.88 (7.22–8.54) | 6.56 (5.53–7.58) | 6.80 (5.73–7.88) | Groupsi PHIV/C < PHEUi PHIV/C < PHIV/non-Ci |
| CWI Inhibition–Switchingb,d,h | 8.25 (7.57–8.93) | 8.48 (7.78–9.18) | 7.86 (7.26–8.46) | 7.99 (7.34–8.63) | 7.18 (6.19–8.16) | 7.64 (6.65–8.63) | None |
| CWI Total Errors–Inhibitione,h | 8.29 (7.57–9.01) | 8.83 (8.08–9.58) | 7.87 (7.21–8.52) | 8.27 (7.61–8.93) | 6.91 (5.77–8.05) | 7.20 (5.99–8.41) | PHIV/C < PHEUi |
| CWI Total Errors–Switchingf,h | 7.86 (7.15–8.57) | 8.68 (7.91–9.44) | 7.67 (7.03–8.32) | 8.10 (7.48–8.73) | 7.64 (6.72–8.57) | 7.83 (6.82–8.85) | None |
| 20 Questions–Achievementd,f,h | 8.06 (7.15–8.97) | 8.45 (7.43–9.48) | 8.83 (8.18–9.49) | 8.77 (8.01–9.53) | 9.18 (8.10–10.26) | 8.98 (7.86–10.09) | None |
Abbreviations: CDC, Centers for Disease Control and Prevention; CI, confidence interval; CWI, Color-Word Interference; D-KEFS, Delis-Kaplan Executive Function System; HIV, human immunodeficiency virus; PHEU, perinatally HIV-exposed but uninfected; PHIV, perinatally acquired HIV; PHIV/C, PHIV with a CDC class C diagnosis; PHIV/non-C, PHIV without a CDC class C diagnosis.
aReported results are scaled scores with a mean of 10 (SD, 3). Outcomes were adjusted for the following covariates: bHispanic ethnicity, ccaregiver identity, dcaregiver education, esex, fage, ghousehold income, and hrace.
iP < .05 for unadjusted mean scores only.
jP < .05 for unadjusted and adjusted mean scores.
Pairwise comparisons of Verbal Letter versus Category Fluency, Category versus Category-Switching Fluency, and Design Fluency versus Design-Switching Fluency revealed lower Letter than Category Fluency across groups (Table 2) and a larger score difference among the PHEU youth than among the youth with PHIV/non-C in both unadjusted and adjusted analyses. There were no other significant pairwise comparison results (data not shown).
Color–Word Interference. Mean scores were in the borderline to low-average range for the PHIV/C group across conditions and in the low-average to average range for the PHEU and PHIV/non-C groups (Table 2). Pairwise group comparisons revealed significantly lower performance by the PHIV/C group than by the PHIV/non-C and PHEU groups on the primary combined score (adjusted mean differences, –1.71 [P = .003] and –1.76 [P = .004], respectively), reflecting decreases of 21.2% and 17.9%, respectively. Pairwise differences also revealed lower performance for the PHIV/C group than for the PHIV/non-C and PHEU groups for Inhibition scores in unadjusted analyses; the significant difference between the PHIV/C and PHEU groups persisted after adjustment (mean difference, –1.49; P = .02). Youth with PHIV/C had significantly more total errors on the Inhibition condition than PHEU youth (adjusted mean difference, –1.63; P = .02).
Twenty Questions. There were no group or pairwise differences for the Twenty Questions subtest; performance was in the low-average to average range across groups in both the unadjusted and adjusted analyses.
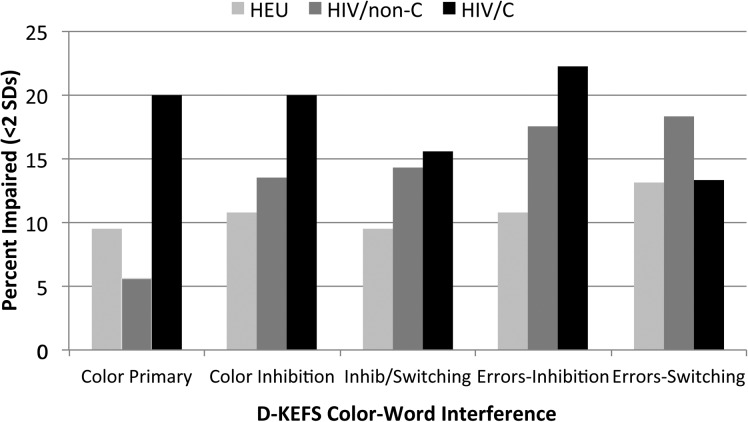
Impairment Rates. The percentage of participants with a score in the clinically impaired range (more than 2 standard deviations [SDs] below the normative mean, with 1 SD also shown [see Supplementary Table 1]) ranged from 0% to as high as 22.2% for the Color-Word Interference total Inhibition condition errors for the PHIV/C group. For all groups, the highest percentages of impaired performance were for the Color-Word Interference and Twenty Questions tasks. The percentage of participants in each group with a score more than 2 SDs below the normative mean on the Color-Word Interference conditions is shown in Figure 1.
Figure. 1.
Percentage of participants with D-KEFS color–word interference scores more than 2 standard deviations below the published normative mean, according to group. Abbreviations: D-KEFS, Delis-Kaplan Executive Function System; HIV, human immunodeficiency virus.
Associations of EF With HIV Disease Severity
Historical Disease Severity. Significant associations of EF with markers of disease severity are listed in Supplementary Table 2. Youth with a previous encephalopathy diagnosis had significantly lower adjusted mean scores than those without encephalopathy in Verbal Letter Fluency, Category Fluency, and Category-Switching Fluency and higher Design Fluency set-loss error scaled scores (fewer errors) and lower Color–Word Interference scores. Peak viral loads >100 000 copies/mL were associated with lower Design Fluency and Color–Word Interference scores, and higher peak viral loads were associated with a lower Design Switching score when viral load was analyzed as a continuous variable. Associations with age at worst disease severity depended on the severity measure being used. Age of <3 years at earliest CDC Class C diagnosis was associated with a lower Color–Word Interference combined score, but age of <3 years at peak viral load was associated with higher Verbal Category Fluency, and age of <3 years at nadir CD4% was associated with fewer Design Fluency set losses and, thus, higher scaled scores.
Current Disease Severity. Significant associations between disease severity and task performance were few and not in consistent directions. Higher CD4% at the time of the closest AMP visit was associated with fewer Design Fluency set-loss errors. However, youth with suppressed viral load (<400 copies/mL) at the closest AMP visit had significantly lower Color–Word Interference combined scores than those with a viral load of >400 copies/mL.
Adjustment for Associations With Other Cognitive and Behavioral Measures and Site Differences
The inclusion of Word Reading scores in models for the combined Color–Word and Inhibition conditions, including Inhibition errors, attenuated the observed contrasts between the PHIV/C and PHEU groups. Including Digit Span and Coding scores in these models attenuated the effects similarly, and they became nonsignificant. Including Word Reading scores attenuated the borderline-significant contrast effects for Verbal Letter Fluency, Design Fluency, and total correct Switching. Sensitivity analyses revealed that effect sizes for reported significant pairwise contrasts were not changed after adjustment for site differences, although the 95% confidence intervals were narrowed (data not shown).
DISCUSSION
The results of this study show that youth with PHIV infection who have not had significant immunocompromise (PHIV/non-C) performed as well on measures of EF as PHEU youth. This was true across all domains of EF assessed, including verbal and design fluency, cognitive flexibility, inhibition, and problem solving. Consistent with the results of past studies of global cognitive functioning [29, 30] and specific domains such as memory and processing speed [20, 31], youth with previous AIDS-defining diagnoses (PHIV/C) performed significantly worse than PHEU youth on some measures and, in some cases, worse than youth with PHIV/non-C. Our results contrast with those of Llorente et al [16] in that some group differences persisted after adjustment for demographic variables, possibly as a result of the inclusion in our study of an older sample and/or a broader range of EF tasks. Thus, our findings add to a burgeoning literature supporting the importance of preventing immunosuppression to optimize cognitive as well as physical health outcomes for children with PHIV.
In general, youth performed in the low-average to average range on most EF measures, with the exception that youth with PHIV/C performed in the borderline range on 2 timed measures from the Color–Word Interference subtest; however, a relatively high percentage of all participants also obtained scores in the impaired range on some EF tasks. This pattern, wherein mean performance is within the average range but is shifted downward compared with that of the general population and a subset has a relatively high risk of impairment, is consistent with the literature on global cognitive functioning in perinatal HIV exposure and infection [1, 29]. The associations with demographic factors such as caregiver education, race/ethnicity, and family income suggest a contribution of socioeconomic disadvantage to the risk for poorer EF functioning in youth with perinatal HIV exposure as a group.
The potential impact of problems in EF as youth transition to adult independence, seek employment, and assume responsibility for managing tasks of daily living, such as managing finances, parenting, and avoiding behaviors that place their health at risk, is concerning. For those with HIV infection, a reduced ability to perform healthcare-management tasks such as adhering to medication regimens and navigating healthcare and pharmacy systems may complicate their efforts to maintain viral suppression. In addition to effects on longevity and quality of life, reduced EF abilities may increase the potential for secondary HIV transmission through sexual and drug-use risk behaviors; coupled with nonadherence and elevated viral load, this possibility has public health implications. Additional research on the contribution of EF and other cognitive abilities to successful transition to adulthood for youth with PHIV is needed.
No differences between groups were observed for the Twenty Questions task, and few were found for measures of task errors, both of which reflect EF without a processing-speed component. In addition, the inclusion of measures of processing speed, attention, and word reading in the models significantly attenuated most of the observed group differences. This result suggests that EF impairments observed in the PHIV/C group might be related to deficits in cognitive functions or reading achievement that underlie the performance of complex EF tasks. The pattern of group differences on speeded measures was observed previously in a more limited set of EF measures for the entire AMP cohort [31]. It is also consistent with a larger literature that shows that processing speed is a cognitive domain at particular risk for those with HIV infection [6, 13, 32–35]. Processing speed has demonstrated associations with markers of both past [29] and ongoing disease processes, such as functional connectivity using functional magnetic resonance imaging [36], brain metabolites [14], and biomarkers of vascular functioning [37], in youth with PHIV. Longitudinal studies regarding cognitive functions such as processing speed or academic skills such as reading that might underlie EF problems in this population might identify targets for prevention and intervention and early indicators of a need for EF intervention.
A previous diagnosis of encephalopathy was associated with poorer performance on some measures, as was higher peak viral load. It is surprising that the findings regarding the effect of age at the time of greatest disease severity did not consistently follow a pattern of earlier disease impact producing the greatest impairments [38, 39]. However, previous studies used measures of global rather than specific domains of functioning, which might explain the differences. An association of poorer performance on EF tasks with more severe disease after age 3 years might relate to the developmental timing of EF and its neural substrates [17, 18]. The isolated finding of significantly lower performance on 1 timed task among participants with PHIV and current viral suppression might be spurious; however, concerns regarding ART neurotoxicity and continued central nervous system replication despite viral suppression support monitoring functions, such as processing speed, among youth regardless of whether they are virally suppressed.
This study had several limitations. Because the measures were available only in English, children without English fluency were not included. Youth with significant motor, sensory, or intellectual impairment that precluded participation in testing were excluded. A comparison group of youth without perinatal HIV exposure was not available, which impeded evaluation of the effect of perinatal HIV and ART exposure. The D-KEFS normative sample was representative of the entire US population instead of matching demographic characteristics of our sample. Participation required substantial time commitments; thus, the study participants might not represent all youth with PHIV. Last, our analyses were performed without correction for multiple comparisons.
In summary, our findings suggest that, on average, youth with perinatal HIV exposure have developed age-appropriate executive functioning, but a subset of youth with history of an AIDS-defining diagnosis is at risk for greater impairment. This finding emphasizes the importance of preventing significant immunocompromise in this population. Furthermore, a relatively high percentage of youth across groups have difficulty with some EF tasks that might be related in part to sociodemographic influences. The results of research with adults suggest that EF deficits place people with HIV at risk for problems with daily functioning, including healthcare and medication management. The implications of EF for functional outcomes and intervention needs before, during, and after transition to adult independence are important areas for future research as more youth with PHIV achieve that milestone.
Supplementary Data
Supplementary materials are available at the Journal of the Pediatric Infectious Diseases Society online (http://jpids.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Acknowledgments
We thank the children and youth and their families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS), and we thank the sites and site staff who conducted both the Adolescent Master Protocol study and the PHACS Memory and Executive Functioning Study (see www.phacsstudy.org/About-Us/AMP-Memory.Acknowledgements).
Author contributions. All of the authors were involved in 1 or more of the following activities, essential to the completion of this work: study design, data collection, data analysis, initial preparation of the manuscript, and critical review and editing of the paper.
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services.
Financial support. The Memory and Executive Functioning Study conducted within the PHACS was funded by the National Institute of Mental Health (Grant MH084794). Additional support for this study was received through the PHACS, which is funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism, through cooperative agreements with the Harvard T. H. Chan School of Public Health (Grant HD052102) and the Tulane University School of Medicine (Grant HD052104). Data-management services were provided by Frontier Science and Technology Research Foundation, and regulatory services and logistical support were provided by Westat, Inc.
Supplement sponsorship. This article appears as part of the supplement “Brain and Cognitive Development Among US Youth With Perinatally Acquired HIV Infection,” sponsored by the PHACS.
Potential conflicts of interest. Dr. Delis is a coauthor of the D-KEFS and receives royalties from the test. All other authors: no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Smith R, Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol 2015; 21:234–68. [DOI] [PubMed] [Google Scholar]
- 2.Sherr L, Mueller J, Varrall R. A systematic review of cognitive development and child human immunodeficiency virus infection. Psychol Health Med 2009; 14:387–404. [DOI] [PubMed] [Google Scholar]
- 3.Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART). J Pediatr 2005; 146:402–7. [DOI] [PubMed] [Google Scholar]
- 4.Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol 2007; 11:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Patel K, Ming X, Williams PL, Robertson KR, Oleske JM, Seage GR 3rd.. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS 2009; 23:1893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton RK, Clifford DB, Franklin DR Jr et al. . HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75:2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol 2014; 24:316–31. [DOI] [PubMed] [Google Scholar]
- 8.Heaton RK, Franklin DR, Ellis RJ et al. . HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 2011; 17:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 2006. [Google Scholar]
- 10.Thames AD, Arentoft A, Rivera-Mindt M, Hinkin CH. Functional disability in medication management and driving among individuals with HIV: a 1-year follow-up study. J Clin Exp Neuropsychol 2013; 35:49–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobson LA, Mahone EM. Educational implications of executive dysfunction. In: Hunter SJ, Sparrow EP, eds. Executive Function and Dysfunction. New York: Cambridge University Press; 2012:232–46. [Google Scholar]
- 12.Gorman AA, Foley JM, Ettenhofer ML, Hinkin CH, van Gorp WG. Functional consequences of HIV-associated neuropsychological impairment. Neuropsychol Rev 2009; 19:186–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin SC, Wolters PL, Toledo-Tamula MA, Zeichner SL, Hazra R, Civitello L. Cognitive functioning in school-aged children with vertically acquired HIV infection being treated with highly active antiretroviral therapy (HAART). Dev Neuropsychol 2006; 30:633–57. [DOI] [PubMed] [Google Scholar]
- 14.Nagarajan R, Sarma MK, Thomas MA et al. . Neuropsychological function and cerebral metabolites in HIV-infected youth. J Neuroimmune Pharmacol 2012; 7:981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bisiacchi PS, Suppiej A, Laverda A. Neuropsychological evaluation of neurologically asymptomatic HIV-infected children. Brain Cogn 2000; 43:49–52. [PubMed] [Google Scholar]
- 16.Llorente AM, Brouwers P, Leighty R et al. . An analysis of select emerging executive skills in perinatally HIV-1-infected children. Appl Neuropsychol Child 2014; 3:10–25. [DOI] [PubMed] [Google Scholar]
- 17.Best JR, Miller PH. A developmental perspective on executive function. Child Dev 2010; 81:1641–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diamond A. Executive functions. Annu Rev Psychol 2013; 64:135–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diamond A, Lee K. Interventions shown to aid executive function development in children 4 to 12 years old. Science 2011; 333:959–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols SL, Chernoff MC, Malee K et al. . Learning and memory in children and adolescents with perinatal HIV infection and perinatal HIV exposure. Pediatr Infect Dis J 2016; 35:649–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 22.Centers for Disease Control. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age. MMWR Morb Mortal Wkly Rep 1994; 43(RR-12):1–10. [Google Scholar]
- 23.Reynolds NR, Testa MA, Marc LG et al. . Factors influencing medication adherence beliefs and self-efficacy in persons naive to antiretroviral therapy: a multicenter, cross-sectional study. AIDS Behav 2004; 8:141–50. [DOI] [PubMed] [Google Scholar]
- 24.Wechsler D. Manual for the Wechsler Intelligence Scale for Children, 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 25.Wechsler D. Manual for the Wechsler Adult Intelligence Scale, 4th ed. San Antonio, TX: The Psychological Corporation; 2008. [Google Scholar]
- 26.Wechsler D. Wechsler Individual Achievement Test, 2nd ed., Abbreviated. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- 27.Semel E, Wiig E, Secord W. Manual for the Clinical Evaluation of Language Fundamentals, 4th ed. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- 28.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: Wiley; 2004. [Google Scholar]
- 29.Smith R, Chernoff M, Williams PL et al. . Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012; 31:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood SM, Shah SS, Steenhoff AP, Rutstein RM. The impact of AIDS diagnosis on long-term neurocognitive and psychiatric outcomes of surviving adolescents with perinatally acquired HIV. AIDS 2009; 23:1859–65. [DOI] [PubMed] [Google Scholar]
- 31.Nichols SL, Brummel SS, Smith RA et al. . Executive functioning in children and adolescents with perinatal HIV infection. Pediatr Infect Dis J 2015; 34:969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. J Int Neuropsychol Soc 2002; 8:410–24. [DOI] [PubMed] [Google Scholar]
- 33.Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol 2002; 21:223–41. [DOI] [PubMed] [Google Scholar]
- 34.Nachman S, Chernoff M, Williams P, Hodge J, Heston J, Gadow KD. Human immunodeficiency virus disease severity, psychiatric symptoms, and functional outcomes in perinatally infected youth. Arch Pediatr Adolesc Med 2012; 166:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruel TD, Boivin MJ, Boal HE et al. . Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis 2012; 54:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Herting MM, Uban KA, Williams PL et al. . Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore) 2015; 94:e1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapetanovic S, Griner R, Zeldow B et al. . Biomarkers and neurodevelopment in perinatally HIV-infected or exposed youth: a structural equation model analysis. AIDS 2014; 28:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crowell CS, Huo Y, Tassiopoulos K et al. . Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS 2015; 29:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lazarus JR, Rutstein RM, Lowenthal ED. Treatment initiation factors and cognitive outcome in youth with perinatally acquired HIV infection. HIV Med 2015; 16:355–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.