Abstract
Background
Combination antiretroviral therapy has led to increased survival among youth with perinatally acquired HIV (PHIV). However, cognitive deficits continue to be common. Histopathological studies in adults have found HIV concentrated in subcortical structures, which are involved in sensory processing, movement, and higher-order cognition that emerges with development.
Methods
We conducted magnetic resonance imaging and cognitive testing in 40 youth with PHIV at one site of the Adolescent Master Protocol of the Pediatric HIV/AIDS Cohort Study. We collected HIV disease-severity measures and substance-use reports. Subcortical volume and shape deformation were generated with FreeSurfer-Initiated Large Deformation Diffeomorphic Metric Mapping. Inward shape deformation was defined as negative displacement. We evaluated associations of subcortical shape deformation with past HIV severity after adjustment for sex, age at neuroimaging, age at HIV severity marker, and substance use. We examined associations between subcortical deformation and cognitive function.
Results
Negative correlations between shape deformation and peak HIV viral load (VL) were found in clusters in the caudate tail, globus pallidus, lateral putamen, and anterior and medial thalamus. Positive correlations between shape deformation and nadir CD4-positive T-lymphocyte percentage (CD4%) were found in clusters in the medial and posterior thalamus. Inward deformation in caudate and thalamic clusters correlated with worse cognition.
Conclusions
Youth with PHIV have demonstrable subcortical shape deformation related to past HIV severity and cognition; inward deformation was associated with higher peak VL, lower nadir CD4%, and worse cognition. Identifying subcortical deformation may inform clinical practice for early intervention to help improve cognitive outcomes and assess the neuroefficacy of combination antiretroviral therapy in youth with PHIV.
Keywords: adolescence, neuroimaging, perinatally acquired HIV, subcortical, viral load
INTRODUCTION
Perinatally acquired HIV (PHIV) is the most common cause of HIV in children, and it affects approximately 3 million children worldwide [1, 2]. With the development of combination antiretroviral therapy (cART), infants born with HIV are surviving into young adulthood. Despite increased survival rates, HIV infection can have long-term effects on the brain and cognitive and functional outcomes. Youth with PHIV may have neurological symptoms and neurodevelopmental problems at increased rates across infancy, childhood, and adolescence [3] related to HIV disease severity and/or timing and efficacy of ART [4–6]. Mild or global neurocognitive deficits across multiple domains are observed in children and adolescents with PHIV despite ART adherence and current peripheral virologic control and immunologic reconstitution, which places them at risk for poor academic development and challenging transitions to adulthood [7–11].
Cognitive impairment in youth with PHIV may be a result of damage to or aberrantly developed underlying brain structures. Youth with PHIV are particularly vulnerable to structural brain differences, because they are infected with HIV during a critical period of brain development with possible ongoing exposure to the virus and neurotoxic effects of chronic inflammation as the brain grows. In addition, the central nervous system remains less accessible to cART because of the variable penetrance across treatment regimens due to poor blood–brain barrier penetration and thus might act as a reservoir for latent virus [12, 13]. In addition, youth may have suboptimal exposure to cART because of poor adherence influenced by complex drug regimens, family factors, developmental disability, or stigma, among others [14, 15]. These structural brain differences may underlie cognitive dysfunction and reflect the effectiveness of cART in the brain.
Studies on the underlying pathophysiology of subtle and prominent cognitive deficits in youth with PHIV have been limited [16]. However, there is emerging evidence for brain changes associated with ongoing exposure to latent virus and chronic inflammation despite treatment with cART. Recent neuroimaging studies have found that youth with PHIV with worse HIV disease severity have altered cortical gray matter, white matter, and functional connectivity in the brain and that these measures correlate with cognitive performance [17–22]. Cortical gray matter studies in which youth with PHIV were compared with HIV-unexposed and uninfected youth have had various results, with some finding global and regional atrophy, possibly caused by aberrant development or tissue loss, whereas others found regional volume increases, which possibly resulted from inflammation [19, 20].
It is notable that studies focused on subcortical structures in youth with PHIV remain limited, despite their central role in facilitating motor control, sensory processing, and higher-order cognitive function that emerges developmentally [23] and the location of high concentrations of virus [24–26]. Studies in adults with horizontally transmitted HIV have highlighted the importance of subcortical structures in disease pathogenesis and cognitive outcomes. Chronic HIV infection causes neurological sequelae in up to 50% of adults with HIV [27]. Neurological outcomes include HIV-associated neurocognitive disorder, which presents with a triad of cognitive, motor, and behavior dysfunction, and the basal ganglia and subcortical regions are often affected early in the disease course. These cognitive deficits and dementia with subcortical features can persist even in those treated with cART and despite evidence of viral suppression and reconstituted CD4+ count [28, 29]. In addition, histopathological studies of HIV have identified a differential distribution of HIV RNA load in the brain in adults, and subcortical brain structures often have the highest levels of the virus [24, 25]. Autopsy studies on infant brains have also found a presence of the virus in subcortical astrocytes [26]. In general, neuroimaging studies in adults have implicated subcortical structures, finding smaller subcortical structures among those with a worse prognosis [30–34]. Thus, understanding differences of subcortical structures in youth with PHIV who are reaching young adulthood, who have the additional vulnerability of exposure to the virus during critical periods of brain development, is essential.
How subcortical brain regions are affected in youth with PHIV who are reaching adolescence and adulthood is not yet well characterized. Similarly, the neurocognitive effects of variably timed ART initiation, viral suppression, and immune activation across developmental stages are not fully understood. In this study, we examined structural shape deformation of subcortical structures in youth with PHIV and their associations with past HIV disease severity and cognitive performance; we hypothesized that the youth with PHIV and worse past disease severity would have localized inward deformation in their subcortical regions and that this difference is associated with worse cognitive performance.
METHODS
Study Population
We recruited and evaluated 40 youth with PHIV who were participating in the Adolescent Master Protocol (AMP) study of the National Institutes of Health Pediatric HIV/AIDS Cohort Study (PHACS) network. Each participant in the neuroimaging substudy of the AMP was enrolled at a single PHACS site, the Ann and Robert H. Lurie Children's Hospital of Chicago. Institutional review boards at the participating site and the Harvard T. H. Chan School of Public Health approved the study. Parents, legal guardians, and youth who were 18 years or older provided written informed consent for research participation; participating minor adolescents provided assent.
HIV Disease Markers, Substance Use, and Cognitive Functioning in Youth With PHIV
Adolescent Master Protocol visits were conducted semiannually from 2007 through 2010 and annually thereafter, as described previously [11]. At each AMP visit, clinical diagnosis and laboratory results, including CD4-positive T-lymphocyte percentage (CD4%), plasma HIV RNA concentration (viral load [VL]), and Centers for Disease Control and Prevention HIV classification [35] were abstracted from each patient's medical chart. At study entry, historical HIV disease severity was determined by collecting the lowest known CD4% (nadir CD4%) and highest known lifetime HIV VL (peak VL) before entry.
In the AMP study, information on use of alcohol, tobacco, marijuana, and other illicit drugs was collected starting at 10 years of age by an Audio Computer-Assisted Self-Interview (ACASI) [36]. ACASIs were completed at the 6-month AMP visit, the 2.5-year visit, the 4-year visit, and then annually. Lifetime prevalence of alcohol, marijuana, and cigarette use was defined as any self-reported use on the ACASI performed within 1 year before neuroimaging. This lifetime prevalence was coded as a binary variable (having or not having a history of any substance use).
Subtests of the Wechsler Intelligence Scale for Children, 4th Edition (WISC-IV) (for 6- to 16-year-olds) [37], and the Wechsler Adult Intelligence Scale, 4th Edition (WAIS-IV) [38] (for ≥17-year-olds), were used to evaluate working memory and processing speed in youth with PHIV. The Working Memory Index (WMI) and Processing Speed Index (PSI), standardized with a mean score of 100 (standard deviation [SD], 15) in the general population, were used to calculate the Cognitive Proficiency Index (CPI), an estimate of the efficiency with which information is processed for learning, problem solving, and higher-order reasoning [37].
Image Acquisition and Processing
Magnetic resonance imaging (MRI) scans were performed using a standardized acquisition protocol [17, 39] on a single 3.0-Tesla Magnetom Tim Trio scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head coil. Structural MRI used a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (sagittal; repetition/echo/inversion times, 2170/4.37/1100 milliseconds; field of view, 256 × 256 mm; flip angle, 7°; matrix, 256 × 256 × 160; voxel resolution, 1 × 1 × 1.2 mm3; scan time, 8 minutes 8 seconds). Subcortical segmentations were generated using multiatlas FreeSurfer-initiated large-deformation diffeomorphic metric mapping (ma-FSLDDMM) [40–43], which is an atlas-based mapping algorithm that combines FreeSurfer [44] and high-dimensional large-deformation diffeomorphic metric mapping (LDDMM) [45] with multiple-atlas libraries [43] and multistructure extension [42]. FreeSurfer analysis provided a set of noisy labels for the structures, which was used to provide initial alignment between atlas and participant scans, which was followed by LDDMM for mapping the smooth atlas structure boundaries onto each participant. This process generated smooth subcortical surfaces necessary for subsequent shape analysis. Structures included the caudate, putamen, nucleus accumbens, globus pallidus, thalamus, hippocampus, and amygdala. FreeSurfer also provided an estimated measure of intracranial volume.
Surface-Based Shape Analysis
The final surface was adjusted for individual head size by multiplying vertex coordinates by a scaling factor, defined as the cubic root of the following quotient: population average intracranial volume divided by the individual intracranial volume. Adjusted subcortical structural volume was calculated as volume enclosed within the surface. Adjusted surfaces were used in subsequent shape analysis.
A population average surface for youth with PHIV was generated. Perpendicular displacements were computed between corresponding vertices from the population average to each subject to determine deformation at each vertex; inward deformation was defined as negative displacements, and outward deformation was defined as positive displacements.
Statistical Analyses
Descriptive statistics and graphical methods were used to confirm the normality assumption, and when appropriate, transformations were applied. Specifically, a log-transformation was applied to peak VL and age of peak VL to more closely approximate a normal distribution. For each subcortical structure, a repeated-measure general linear model with hemisphere as a repeated measure was performed with volume as the dependent variable and past HIV disease severity (peak HIV RNA load or nadir CD4%) as the independent predictor, adjusting for age at scan, age at HIV disease-severity marker, sex, and history of substance use.
General linear models were performed on each vertex; deformation, a measure of localized volume loss, was the dependent variable, and past HIV disease severity (peak HIV RNA load or nadir CD4%) was the independent predictor, adjusting for age at scan, age at HIV disease-severity marker, sex, and history of substance use. Using SurfStat (see http://www.math.mcgill.ca/keith/surfstat/), random field theory [46, 47] was applied to determine clusters of significant associations with familywise-error (FWE) correction (P < .05, FWE corrected). T values of clusters with significant associations were visualized on the surface. The mean deformation of significant clusters within a structure was correlated with volume for that structure. Last, for each subcortical structure for which significant associations were found, a mean deformation value was calculated across the clusters. Pearson correlation coefficients were calculated between the mean deformation values and measures of cognitive performance for the participants with PHIV.
RESULTS
Study Population
Among the 40 youth with PHIV, the mean age at MRI scan was 16.7 years (range, 11–20 years); 53% were female, 73% were black, and 13% were Hispanic (Table 1). Cognitive performance in youth with PHIV was significantly lower than the general population mean of 100, particularly for working memory (mean, 87.6; P < .001), and 43% had a CPI of <85 (more than 1 SD below the norm). The median log peak VL was 5.7 copies/mL (interquartile range [IQR], 5.2–5.9 copies/mL), and the median age at peak VL was 2.5 years (IQR, 0.6–5.3 years). The median nadir CD4% was 16.5 (IQR, 8.0–23.8), and median age at nadir CD4% was 5.7 years (IQR, 2.2–10.9 years).
Table 1.
Demographic Characteristics, Disease Severity, Substance Use, and Cognitive Functioning Measures Among Youth With PHIV
| Characteristic | Youth With PHIV (N = 40) |
|---|---|
| Age at scan (mean [SD]) (y) | 16.7 (2.4) |
| Female sex (n [%]) | 21 (53) |
| Race (n [%])a | |
| Black | 29 (73) |
| Ethnicity (n [%])a | |
| Hispanic or Latino | 5 (13) |
| HIV disease-severity measures | |
| Nadir CD4% (median [IQR]) | 16.5 (8.0–23.8) |
| Nadir CD4% < 15% (n [%]) | 17 (43) |
| Age at nadir CD4% (median [IQR]) (y) | 5.7 (2.2–10.9) |
| Most recent CD4% (median [IQR]) | 35.9 (27.6–42.9) |
| Age at most recent CD4% (y) | 17.0 (14.9–18.2) |
| Log peak HIV RNA VL (median [IQR]) (copies/mL) | 5.7 (5.2–5.9) |
| Age at peak RNA (median [IQR]) (y) | 2.5 (0.6–5.3) |
| Most recent RNA count > 400 copies/mL (n [%]) | 6 (15) |
| Age at most recent RNA (median [IQR]) (y) | 17.0 (14.9–18.2) |
| % of VL > 1000 copies/mL in past 5 y (median [IQR]) | 6.5 (0.0–29.0) |
| Substance use, according to ACASI (n [%])b | |
| Alcohol | 14 (35) |
| Tobacco use | 10 (25) |
| Marijuana use | 14 (35) |
| Illicit drug use, excluding marijuanac | 3 (8) |
| Use of alcohol, tobacco, or marijuana, excluding other illicit drugs | 15 (38) |
| Age at start of cART (median [IQR]) (y) | 3.5 (1.4–6.3) |
| ARV regimen at the time of scan (n [%]) | |
| cART | 37 (92) |
| Not on cART | 1 (3) |
| Not on any ARVs | 2 (5) |
| Wechsler cognitive indicesd (mean [SD]) | |
| Cognitive Proficiency Index | 90.3 (16.0) |
| Working Memory Index | 87.6 (16.8) |
| Processing Speed Index | 95.4 (14.2) |
Abbreviations: ACASI, audio computer-assisted self-interview; ARV, antiretroviral; cART, combination antiretroviral therapy (regimen including at least 3 drugs from at least 2 drug classes); CD4%, CD4-positive T-lymphocyte percentage; HIV, human immunodeficiency virus; IQR, interquartile range; PHIV, perinatally acquired HIV; SD, standard deviation; VL, viral load.
aOne youth with PHIV did not report race.
bTwo youth with PHIV were missing information on substance use.
cIllicit drugs include inhalants, amphetamines, cocaine, methamphetamine, crack, sedatives/barbiturates, ecstasy, hallucinogens, and heroin.
dAge-appropriate Wechsler tests included the Wechsler Intelligence Scale for Children, 4th Edition, and Wechsler Adult Intelligence Scale, 4th Edition.
The mean interval between MRI scanning and cognitive testing was 3.8 months (SD, 5.9 months). One-fourth of the youth with PHIV reported tobacco use, 35% reported alcohol use, and 35% reported marijuana use. Thirteen (33%) youth reported both alcohol and marijuana use, and 8 (20%) youth reported use of tobacco, alcohol, and marijuana. Fifteen (38%) youth reported any use of alcohol, tobacco, or marijuana, excluding the use of other illicit drugs. A small number of youth reported illicit drug use other than marijuana (8%). Substance use was unknown for 2 participants who were excluded from further analyses; the remaining 38 subjects with complete information on HIV disease severity and substance use were used for all further analyses.
Association of Brain-Volume Measures With HIV Disease Severity
In the volume analysis, in which a repeated-measure model with hemisphere as a repeated measure was used, no relationship between peak VL and whole volume for any of the subcortical structures was found after controlling for age at scan, age at peak VL, sex, and substance use. No relationship was found between nadir CD4% and volume for any of the subcortical structures (Supplementary Table 1).
Association of Local Deformation With HIV Disease Severity
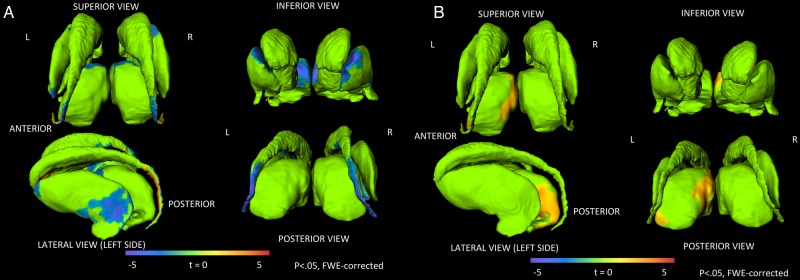
In the vertex-wise deformation analysis, negative associations between shape deformation and peak HIV RNA VL were found in clusters in the dorsal tail of the caudate, the lateral putamen, and the anterior and medial thalamus after controlling for age at scan, age at peak HIV RNA VL, sex, and substance use (P < .05, FWE corrected [Table 2; Figure 1A]); that is, inward deformation was associated with higher peak VL. A cluster of positive association (shape deformation and peak VL) was also found in the ventral tail of the caudate (P < .05, FWE corrected [Table 2; Figure 1A]). Positive associations between shape deformation and nadir CD4% were found in clusters in the medial dorsal and posterior thalamus after controlling for age at scan, age at nadir CD4%, sex, and substance use (P < .05, FWE corrected [Table 2; Figure 1B]); that is, inward deformation was associated with lower nadir CD4%. For each of these structures, the mean deformation in significant clusters correlated with volume (r = 0.54 to r = 0.87; all P < 5 × 10–6).
Table 2.
Clusters of Local Deformation in Subcortical Structures Correlated With Peak HIV RNA Load and With Nadir CD4%
| Structure | Cluster no. | Direction of Deformation | No. of Vertices | Resolution Elementsa | Pb |
|---|---|---|---|---|---|
| Local deformation associated with peak HIV RNA load | |||||
| Thalamus | 1 | Inward | 500 | 6.11 | .0078 |
| 2 | Inward | 457 | 4.59 | .0297 | |
| Caudate | 1 | Inward | 578 | 10.87 | .0006 |
| 2 | Inward | 648 | 10.65 | .0006 | |
| 3 | Inward | 550 | 7.72 | .0048 | |
| 4 | Outward | 652 | 15.15 | 5.36 × 10–5 | |
| 5 | Outward | 526 | 13.34 | .0001 | |
| 6 | Outward | 558 | 5.72 | .0235 | |
| Globus pallidus | 1 | Inward | 227 | 4.81 | .0158 |
| 2 | Inward | 239 | 3.67 | .0478 | |
| Putamen | 1 | Inward | 866 | 9.00 | .0010 |
| 2 | Inward | 656 | 5.80 | .0122 | |
| 3 | Inward | 515 | 4.83 | .0289 | |
| 4 | Outward | 274 | 6.60 | .0062 | |
| Local deformation associated with nadir CD4% | |||||
| Thalamus | 1 | Outward | 780 | 10.41 | .0003 |
| 2 | Outward | 535 | 6.43 | .006 | |
Abbreviations: CD4%, CD4-positive T-lymphocyte percentage; HIV, human immunodeficiency virus.
aResolution elements were defined by random field theory and estimate the spatial smoothness of the data and the number of independent statistical tests that should be performed.
bAll P values (familywise error corrected) were <.05.
Figure 1.
Human immunodeficiency virus (HIV) disease severity is correlated with local deformation in subcortical structures. (A) Clusters of significant negative correlations between peak HIV viral load (VL) and deformation in subcortical structures. Higher peak VLs are correlated with inward deformation. (B) Clusters of significant positive association between nadir CD4-positive T-lymphocyte percentage (CD4%) and deformation in the subcortical structures. Lower nadir CD4%s are associated with inward deformation.
Association of HIV Disease Severity and Deformation With Cognitive Performance
Peak HIV RNA VLs were negatively correlated with working memory (WMI), processing speed (PSI), and a composite score of cognitive proficiency (CPI) (WMI, r = –0.41, P = .01; PSI, r = –0.43, P = .006; CPI, r = –0.44, P = .005). In the 4 structures in which shape deformation was significantly related to peak HIV VL, the mean deformation was correlated with cognitive proficiency for the caudate and the thalamus. Inward deformation in the caudate and thalamus was associated with lower CPI scores (r = 0.35, P = .03, and r = 0.34, P = .04, respectively) (Supplementary Figure 1). Inward deformation in the caudate and thalamus trended toward lower WMI scores (r = 0.32, P = .05, and r = 0.32, P = .05, respectively) and lower PSI scores (r = 0.30, P = .07, and r = 0.31, P = .06, respectively). No other associations between average participant deformation and cognitive performance were found.
Nadir CD4% was positively correlated with CPI and WMI (CPI, r = 0.29, P = .08; WMI, r = 0.31, P = .06). In the thalamus, where deformation was significantly related to nadir CD4%, the mean deformation in the structure correlated with CPI. Inward deformation in the thalamus was associated with lower CPI scores (r = 0.40; P = .01) (Supplementary Figure 2), lower WMI scores (r = 0.38; P = .02), and lower PSI scores (r = 0.34; P = .03).
DISCUSSION
To our knowledge, this is the first study to investigate subcortical gray matter deformation in adolescents with PHIV in the cART era, a region previously identified as vulnerable in histopathological and neuroimaging studies in adults with HIV [30–34, 48]. We found clusters of significant and primarily negative associations between local deformation and peak VL in the caudate, putamen, globus pallidus, and thalamus (i.e., those with a higher peak VL had more inward deformation in these clusters). We also found clusters of significant positive association between local deformation and nadir CD4%s in the thalamus (i.e., those who a had lower nadir CD4% had more inward deformation in these clusters). Youth with inward deformation in the caudate and thalamus had worse cognitive performance in several domains. Previous research in HIV-unexposed youth and adults showed that subcortical structures, including the caudate and thalamus, are involved in higher-order cognitive function that emerges with development as well as attention and affective control, in addition to the well-studied roles of sensory processing and movement [23]. In neuroimaging studies in adults, decreased subcortical brain volume in the caudate and thalamus were associated with higher detectable HIV RNA VLs and poorer neurocognitive performance and dementia in adults with HIV [49–52]. In a study that compared adults with HIV to uninfected controls, those with HIV had a significantly smaller thalamus, globus pallidus, and putamen and shape variations that correlated with HIV status and cognitive performance [53]. Impairment in the domains of cognition related to these brain regions is more common in youth with PHIV [7–11]. This similar pattern of structural associations related to HIV disease severity in youth with PHIV and adults with HIV suggests that the structural effect of HIV infection in the brain might be similar for youth with PHIV and adults with horizontally transmitted HIV, despite differences in the routes and timing of HIV infection.
Although many studies have identified smaller volumes in the subcortical structures, including the caudate and thalamus, in adults with HIV [30–34, 48–51, 54], we did not find volume differences for whole subcortical structures. Instead, in our cohort of youth with PHIV, we discovered shape deformation consistent with localized volume loss within structures that was related to past HIV disease severity. In addition, we found that local inward deformation was associated with smaller whole subcortical structure volumes. Shape deformation analyses can be used to localize more subtle structural differences in the surfaces of each structure, which enables detection of vertex-level differences in the absence of differences on volume analyses, which examine only a single value for the whole structure. Perhaps the absence of global subcortical structure-volume differences related to past HIV disease severity despite the presence of clusters of local deformation is a result of our smaller sample size or lack of sensitivity in the global volume measurement relative to local alterations or because the scans were performed before HIV-related subcortical volume reductions, so only local deformation was evident. It is also possible that our finding of localized subcortical deformation in the absence of overt volume loss is a result of delayed subcortical volume loss compared with cortical loss over the developmental and aging trajectories [55]. In addition, our analyses focused on past HIV disease severity because, at the time of neuroimaging, the participants had generally high CD4 counts, and almost all of them were virologically suppressed.
The etiology of vulnerability of subcortical structures in youth with PHIV is not yet understood. One possible explanation is that subcortical structures are affected because of increased blood–brain barrier damage in these structures. In one study of HIV-infected adults in which contrast-enhanced MRI was used, participants with a higher HIV VL had more severe blood–brain barrier breakdown in the basal ganglia, and the severity of cognitive dysfunction was associated with the degree of blood–brain barrier leakage [56]. Another hypothesis is that subcortical structures have a greater density of N-methyl-d-aspartate receptors and, thus, are more likely to undergo cell death via excitotoxicity [57–59].
Local subcortical deformation was related to HIV disease severity in our cohort of youth with PHIV despite treatment with cART. Although cART has improved survival rates in this population, HIV may persist in the brain during periods of rapid, critical brain development, so these youth remain at risk for long-term structural brain abnormalities. Our study has provided preliminary evidence that patterns of brain dysmorphology in youth with PHIV on cART share similarities with those observed in adults with horizontally acquired HIV. It is unclear if the subtle deficits in youth with PHIV are a result of differences in the effects of HIV during brain development (compared to its effects on adult brains) and/or if treatment with cART might be partially neuroprotective.
In studies that used other imaging modalities, regional and overall structural and functional brain differences were found in our cohort of youth with PHIV despite treatment with cART [17, 18]. In a diffusion tensor imaging study, white matter tracts in these youth had reduced whole-brain fractional anisotropy and increased radial and mean diffusivity compared with those in HIV-unexposed youth, with regional differences partially mediating the relationship between peak VL and working memory [17]. Another study of this cohort in which resting-state functional MRI was used to study functional connectivity found that, compared with HIV-unexposed youth, youth with PHIV had global and regional alterations within and between default-mode network connectivity related to disease severity and cognition. A cortical volume study using structural MRI showed that, compared with HIV-unexposed youth, participants in this cohort had smaller regional gray matter volumes in regions of higher-order cognition and smaller volume in the whole brain [22]. Within the group of youth with PHIV, these regional reductions in cortical gray matter volume were related to HIV disease severity, cognition, and substance use. Taken together, these neuroimaging studies of our cohort of youth with PHIV have found underlying brain differences despite evidence of viral suppression in the majority of the participants.
Future longitudinal studies of youth with PHIV should assess subcortical gray matter structure and changes during childhood and adolescence while accounting for the timing of ART initiation, lifetime virologic control, and immune activation, each of which might have unique effects. Moreover, although our study focused on within-group analyses of youth with PHIV, future studies should include HIV-unexposed and HIV-exposed-but-uninfected comparison groups and should aim to identify the relationships between subcortical and cortical changes and cognitive function. These studies might improve our understanding of structural brain differences in youth with PHIV and help clinicians identify youth at risk for long-term subcortical brain changes, cognitive sequelae, and functional deficits. Our results suggest that effective virologic control can lead to improved neurological outcomes as youth with PHIV transition into adulthood. The global epidemic of PHIV demands novel approaches to exploring the pathophysiology of cognitive dysfunction in patients with PHIV to better inform clinical practice for early monitoring and intervention, neuroeffectiveness of cART, and prediction of cognitive outcomes.
Supplementary Data
Acknowledgments
We thank the children and youth and their families for their participation in the Pediatric HIV/AIDS Cohort Study (PHACS), and we thank the sites and site staff who conducted the studies (see www.phacsstudy.org/About-Us/AMP.Acknowledgements).
Author contributions. C. P. L.-d. l. A., P. L. W., J. G. C., R. Y., R. B. V. D., E. R. S., and L. W. designed the study; K. M., Y. H., J. G. C., R. Y., R. V. B. D., and L. W. collected the data; C. P. L.-d. l. A., K. I. A., P. L. W., and L. W. performed the analysis; C. P. L.-d. l. A. and L. W. wrote the manuscript; and all authors helped revise the manuscript and provide critical feedback.
Financial support. The PHACS was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development with cofunding from the National Institute on Drug Abuse, the National Institute of Allergy and Infectious Diseases, the Office of AIDS Research, the National Institute of Mental Health, the National Institute of Neurological Disorders and Stroke, the National Institute on Deafness and Other Communication Disorders, the National Heart Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, and the National Institute on Alcohol Abuse and Alcoholism through cooperative agreements with the Harvard T. H. Chan School of Public Health (Grant HD052102) and the Tulane University School of Medicine (Grant HD052104). Data-management services were provided by Frontier Science and Technology Research Foundation and regulatory services and logistical support were provided by Westat, Inc. The neuroimaging study was also supported by the Northwestern University Training Program in the Neuroscience of Human Cognition (Grant T32 NS047987) and the Northwestern University Medical Scientist Training Program (Grant T32 GM008152).
Disclaimer. The conclusions and opinions expressed in this article are those of the authors and do not necessarily reflect those of the National Institutes of Health or US Department of Health and Human Services. Supplement sponsorship. This article appears as part of the supplement “Brain and Cognitive Development Among US Youth With Perinatally Acquired HIV Infection,” sponsored by the PHACS.
Potential conflicts of interest. All authors: no reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Joint United Nations Programme on HIV/AIDS. The gap report. Geneva, Switzerland: UNAIDS, 2014. [Google Scholar]
- 2.Hazra R, Siberry GK, Mofenson LM. Growing up with HIV: children, adolescents, and young adults with perinatally acquired HIV infection. Annu Rev Med 2010; 61:169–85. [DOI] [PubMed] [Google Scholar]
- 3.Smith R, Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol 2015; 21:234–68. [DOI] [PubMed] [Google Scholar]
- 4.Chase C, Ware J, Hittelman J et al. . Early cognitive and motor development among infants born to women infected with human immunodeficiency virus. Pediatrics 2000; 106:e25. [DOI] [PubMed] [Google Scholar]
- 5.Lindsey JC, Malee KM, Brouwers P, Hughes MD; PACTG 219C Study Team. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics 2007; 119:e681–93. [DOI] [PubMed] [Google Scholar]
- 6.Smith R, Malee K, Charurat M et al. . Timing of perinatal human immunodeficiency virus type 1 infection and rate of neurodevelopment. The Women and Infant Transmission Study Group. Pediatr Infect Dis J 2000; 19:862–71. [DOI] [PubMed] [Google Scholar]
- 7.Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol 2014; 24:316–31. [DOI] [PubMed] [Google Scholar]
- 8.Garvie PA, Zeldow B, Malee K et al. . Discordance of cognitive and academic achievement outcomes in youth with perinatal HIV exposure. Pediatr Infect Dis J 2014; 33:e232–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols SL, Montepiedra G, Farley JJ et al. . Cognitive, academic and behavioral correlates of medication adherence in children and adolescents with perinatally acquired HIV infection. J Dev Behav Pediatr 2012; 33:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puthanakit T, Ananworanich J, Vonthanak S et al. . Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy. Pediatr Infect Dis J 2013; 32:501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith R, Chernoff M, Williams PL et al. . Impact of HIV severity on cognitive and adaptive functioning during childhood and adolescence. Pediatr Infect Dis J 2012; 31:592–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Churchill MJ, Deeks SG, Margolis DM, Siliciano RF, Swanstrom R. HIV reservoirs: what, where and how to target them. Nat Rev Microbiol 2016; 14:55–60. [DOI] [PubMed] [Google Scholar]
- 13.Ene L, Duiculescu D, Ruta S. How much do antiretroviral drugs penetrate into the central nervous system? J Med Life 2011; 4:432–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Haberer J, Mellins C. Pediatric adherence to HIV antiretroviral therapy. Curr HIV/AIDS Rep 2009; 6:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malee K, Williams P, Montepiedra G et al. . Medication adherence in children and adolescents with HIV infection: associations with behavioral impairment. AIDS Patient Care STDS 2011; 25:191–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musielak KA, Fine JG. An updated systematic review of neuroimaging studies of children and adolescents with perinatally acquired HIV. J Pediatr Neuropsychol June 2016; 2:34–49. [Google Scholar]
- 17.Uban KA, Herting MM, Williams PL et al. . White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. AIDS 2015; 29:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herting MM, Uban KA, Williams PL et al. . Default mode connectivity in youth with perinatally acquired HIV. Medicine (Baltimore) 2015; 94:e1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen S, Caan M, Mutsaerts H et al. . Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology 2015;November:1–10. [DOI] [PubMed] [Google Scholar]
- 20.Sarma MK, Nagarajan R, Keller MA et al. . Regional brain gray and white matter changes in perinatally HIV-infected adolescents. Neuroimage Clin 2014; 4:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoare J, Fouche J-P, Phillips N et al. . White matter micro-structural changes in ART-naive and ART-treated children and adolescents infected with HIV in South Africa. AIDS 2015; 29:1793–801. [DOI] [PubMed] [Google Scholar]
- 22.Williams P, Huo Y, Wang S et al. . Brain volumes, HIV disease severity, and substance use in perinatally infected youth [abstract 822]. In: Conference on Retroviruses and Opportunistic Infections. Boston, 2016. [Google Scholar]
- 23.Giedd J, Shaw P, Wallace G, Gogtay N, Lenroot R. Anatomic brain imaging studies of normal and abnormal brain development in children and adolescents. In: Cicchetti D, Cohen D, eds. Developmental Psychopathology, 2nd ed Hoboken, NJ: John Wiley and Sons, 2006; 127–94. [Google Scholar]
- 24.Wiley C, Soontornniyomkij V, Radhakrishnan L et al. . Distribution of brain HIV load in AIDS. Brain Pathol 1998; 8:277–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar AM, Borodowsky I, Fernandez B, Gonzalez L, Kumar M. Human immunodeficiency virus type 1 RNA levels in different regions of human brain: quantification using real-time reverse transcriptase–polymerase chain reaction. J Neurovirol 2007; 13:210–24. [DOI] [PubMed] [Google Scholar]
- 26.Tornatore C, Chandra R, Berger JR, Major EO. HIV-1 infection of subcortical astrocytes in the pediatric central nervous system. Neurology 1994; 44(3 pt 1):481–7. [DOI] [PubMed] [Google Scholar]
- 27.Ances BM, Clifford DB. HIV-associated neurocognitive disorders and the impact of combination antiretroviral therapies. Curr Neurol Neurosci Rep 2008; 8:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol 2000; 14:214–21. [DOI] [PubMed] [Google Scholar]
- 29.Bonnet F, Amieva H, Marquant F et al. . Cognitive disorders in HIV-infected patients. AIDS 2013; 27:391–400. [DOI] [PubMed] [Google Scholar]
- 30.Ances BM, Ortega M, Vaida F, Heaps J, Paul R. Independent effects of HIV, aging, and HAART on brain volumetric measures. J Acquir Immune Defic Syndr 2012; 59:469–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jernigan TL, Archibald SL, Fennema-Notestine C et al. . Clinical factors related to brain structure in HIV: the CHARTER study. J Neurovirol 2011; 17:248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holt JL, Kraft-Terry SD, Chang L. Neuroimaging studies of the aging HIV-1-infected brain. J Neurovirol 2012; 18:291–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker JT, Sanders J, Madsen SK et al. . Subcortical brain atrophy persists even in HAART-regulated HIV disease. Brain Imaging Behav 2011; 5:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masters M, Ances B. Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol 2014; 34:89–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selik RM, Mokotoff ED, Branson B, Owen SM, Whitmore S, Hall HI. Revised surveillance case definition for HIV infection—United States, 2014. MMWR Recomm Rep 2014; 63(RR-03):1–10. [PubMed] [Google Scholar]
- 36.Alperen J, Brummel S, Tassiopoulos K et al. . Prevalence of and risk factors for substance use among perinatally human immunodeficiency virus–infected and perinatally exposed but uninfected youth. J Adolesc Health 2014; 54:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss LG, Saklofski DH, Schwartz DM, Prifitera A, Courville T. Advanced clinical interpretation of the WISC-IV index scores. In: Weiss LG, Saklofske A, Prifitera A, Holdnac J, eds. WISC-IV Advanced Clinical Interpretation. New York: Academic Press, 2006: 140–76. [Google Scholar]
- 38.Weschler D. Wechsler Adult Intelligence Scale, 4th ed San Antonio, TX: The Psychological Corp, 2008. [Google Scholar]
- 39.Jernigan TL, Brown TT, Hagler DJ et al. . The Pediatric Imaging, Neurocognition, and Genetics (PING) Data Repository. Neuroimage 2015; 124:1149–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using large deformation diffeomorphic metric mapping. Neuroimage 2008; 41:735–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang L, Khan A, Csernansky JG et al. . Fully-automated, multi-stage hippocampus mapping in very mild Alzheimer disease. Hippocampus 2009; 19:541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan AR, Wang L, Beg MF. Multistructure large deformation diffeomorphic brain registration. IEEE Trans Biomed Eng 2013; 60:544–53. [DOI] [PubMed] [Google Scholar]
- 43.Christensen A, Alpert K, Rogalski E et al. . Hippocampal subfield surface deformity in nonsemantic primary progressive aphasia. Alzheimers Dement (Amst) 2015; 1:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desikan RS, Ségonne F, Fischl B et al. . An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006; 31:968–80. [DOI] [PubMed] [Google Scholar]
- 45.Beg MF, Miller MI, Trouvé A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. Int J Comput Vis 2005; 61:139–57. [Google Scholar]
- 46.Chung MK, Worsley KJ, Nacewicz BM, Dalton KM, Davidson RJ. General multivariate linear modeling of surface shapes using SurfStat. Neuroimage 2010; 53:491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp 1999; 8:98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jernigan TL, Archibald S, Hesselink JR et al. . Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Arch Neurol 1993; 50:250–5. [DOI] [PubMed] [Google Scholar]
- 49.Kallianpur KJ, Shikuma C, Kirk GR et al. . Peripheral blood HIV DNA is associated with atrophy of cerebellar and subcortical gray matter. Neurology 2013; 80:1792–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ances BM, Roc AC, Wang J et al. . Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology 2006; 66:862–6. [DOI] [PubMed] [Google Scholar]
- 51.Aylward EH, Henderer JD, McArthur JC et al. . Reduced basal ganglia volume in HIV-1-associated dementia: results from quantitative neuroimaging. Neurology 1993; 43:2099–104. [DOI] [PubMed] [Google Scholar]
- 52.Archibald SL, Masliah E, Fennema-Notestine C et al. . Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 2004; 61:369–76. [DOI] [PubMed] [Google Scholar]
- 53.Wade BSC, Valcour VG, Wendelken-Riegelhaupt L et al. . Mapping abnormal subcortical brain morphometry in an elderly HIV+ cohort. Neuroimage Clin 2015; 9:564–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Archibald SL, Masliah E, Fennema-Notestine C et al. . Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch Neurol 2004; 61:369–76. [DOI] [PubMed] [Google Scholar]
- 55.Giedd JN, Raznahan A, Alexander-Bloch A, Schmitt E, Gogtay N, Rapoport JL. Child psychiatry branch of the National Institute of Mental Health longitudinal structural magnetic resonance imaging study of human brain development. Neuropsychopharmacology 2014; 40:43–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Avison MJ, Nath A, Greene-Avison R, Schmitt FA, Greenberg RN, Berger JR. Neuroimaging correlates of HIV-associated BBB compromise. J Neuroimmunol 2004; 157:140–6. [DOI] [PubMed] [Google Scholar]
- 57.Lipton SA. Models of neuronal injury in AIDS: another role for the NMDA receptor? Trends Neurosci 1992; 15:75–9. [DOI] [PubMed] [Google Scholar]
- 58.Lipton SA, Sucher NJ, Kaiser PK, Dreyer EB. Synergistic effects of HIV coat protein and NMDA receptor-mediated neurotoxicity. Neuron 1991; 7:111–8. [DOI] [PubMed] [Google Scholar]
- 59.Martin A. HIV, cognition, and the basal ganglia. In: Grant I, Martin A, eds. Neuropsychology of HIV Infection. New York: Oxford University Press; 1994: 234–60. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.