Highlight
Brassinosteroids alone promote stomatal closure, and in combination with ABA, they positively and negatively modulate ABA-induced stomatal closure in Arabidopsis.
Key words: ABA sensitivity, ABA-induced stomatal closure, abscisic acid (ABA), brassinosteroid (BR), ROS production, stomatal movement.
Abstract
Stomatal movement in response to water availability is an important physiological process in the survival of land plants. The plant hormone abscisic acid (ABA) and brassinosteroids (BRs) regulate stomatal closure. The physiological functions of ABA and BRs, including germination, cell elongation and stomatal movement, are generally known to be antagonistic. Here, we investigated how BRs affect stomatal movement alone and in combination with ABA. We demonstrate that brassinoslide (BL), the most active BR, promotes stomatal closure in an ABA-independent manner. Interestingly, BL also inhibited ABA-induced stomatal closure when a high concentration of BL was added to ABA. Furthermore, we found that the induction of some genes for reactive oxygen species (ROS) generation by ABA (AtrbohD, NIA1 and NIA2) and subsequent ROS production were repressed by BL treatment. The BR signaling mutant bri1-301 failed to inhibit ABA-induced stomatal closure upon BL treatment. However, BRI1-overexpressing transgenic plants were hypersensitive to ABA during stomatal closure, and BL reversed ABA-induced stomatal closure more completely than in wild type plants. Taken together, these results suggest that BRs can positively and negatively modulate ABA-induced stomatal closure. Therefore, interactions between ABA and BR signaling are important for the regulation of stomatal closure.
Introduction
Stomatal closure under water-deficient conditions is a cellular process common to most land plants. The proper development of stomata and regulation of stomatal movements are critical for the regulation of water levels and for facilitating the productivity of plants. Stomatal movement is regulated not only by various environmental conditions, such as light, CO2 (Hubbard et al., 2012), nitric oxide (NO) (Sugiyama et al., 2012), and ozone (Vahisalu et al., 2010), but also by multiple plant hormones, such as abscisic acid (ABA), ethylene, methyl jasmonate, and brassinosteroids (BRs) (Suhita et al., 2004; Desikan et al., 2006; Haubrick et al., 2006; An et al., 2008).
ABA is a major plant hormone that is increased under a variety of abiotic stresses (Chinnusamy et al., 2004; Nakashima et al., 2009). It is detected by the soluble receptor PYRABACTIN RESISTANCE1 (PYR1)/PYR1-LIKE (PYL)/REGULATORY COMPONENT OF ABA RECEPTORS (RCAR) (Park et al., 2009; Klingler et al., 2010). This inhibits ABA-insensitive 1 (ABI1), a clade A-type protein phosphatase 2C (PP2C) (Vlad et al., 2009), which releases major positive regulators for ABA signaling, including the cytoplasmic protein kinases, sucrose non-fermenting 1-related subfamily 2 (SnRK2.6)/OPEN STOMATA1 (OST1), SnRK2.2 and SnRK2.3, from inhibition by PP2Cs (Ng et al., 2011). Free activated OST1 activates the S-type and R-type anion channels SLAC1 and QUAC1, respectively (Lee et al., 2009; Vahisalu et al., 2010; Imes et al., 2013), and the K+ channel KAT1 (Mori et al., 2000; Sato et al., 2009) in the plasma membrane of guard cells, resulting in stomatal closure. OST1 also activates AtrbohF, a subunit of NADPH oxidase, resulting in the production of reactive oxygen species (ROS) (Sirichandra et al., 2009). Further downstream signaling through the activation of OST1 activates the transcription factors ABI3, ABI4, and ABI5 (Feng et al., 2014), leading to changes in the expression of genes involved in the regulation of seed germination and root and hypocotyl growth (Finkelstein et al., 2002; Nakashima et al., 2009; Hayashi et al., 2014).
The cellular physiological functions of ABA are thought to be antagonistic to those of BRs. In seed maturation and germination, embryonic ABA maintained seed dormancy, preventing precocious germination (Seo et al., 2009) and exogenous ABA delayed seed germination, while several ABA-deficient mutants, such as nced6, nced9, aba2 and aao3, showed a higher percentage of germination than wild type plants (Seo et al., 2006; Preston et al., 2009). In comparison, 24-epibrassinolide (EBR) treatment rescued germination of gibberellic acid (GA)-deficient and GA response mutants (Steber et al., 1998; Steber and McCourt, 2001; Ullah et al., 2002). Furthermore, germination of the BR-deficient mutant det2 and the BR signaling mutant bri1-1 was more delayed in response to ABA than it was in wild type plants (Steber and McCourt, 2001), indicating that BRs promote seed germination. Hypocotyl and root elongation were more severely inhibited in the det2 and bri1-9 mutants in response to ABA than in the wild type (Xue et al., 2009).
However, the functional relation of BRs with ABA in stomatal closure, which is an ABA-induced phenotype, seems to be more complex. On one hand, the BR-deficient mutants sax1 and det2 and BR signaling mutant bri1-9 showed enhanced ABA-induced stomatal closure (Ephritikhine et al., 1999; Xue et al., 2009), supporting the hypothesis that ABA sensitivity is inversely related to BR level, as suggested previously (Steber and McCourt, 2001). On the other hand, a BR and ABA cooperated to reduce stomatal transpiration in Vicia faba guard cells in response to drought (Haubrick et al., 2006). In maize leaves, BR-induced NO production and subsequent NO-activated ABA biosynthesis were reported to exert water stress tolerance (Zhang et al., 2011). Recently, EBR was reported to induce stomatal closure in Arabidopsis leaves (Shi et al., 2015). Taken together, these findings support the hypothesis that BRs promote stomatal closure. Moreover, Xia et al (2014) reported a dose-dependent effect of EBR on stomatal closure and opening in tomato leaves (Xia et al., 2014). A low EBR concentration (<0.1 μM) induced stomatal opening, whereas stomatal closure was observed under higher concentrations of EBR.
BR signaling is triggered by BR binding to BRI1, a leucine rich-repeat (LRR) serine/threonine receptor-like kinase (RLK) located in the plasma membrane (Li and Chory, 1997; Wang et al., 2005). BR-bound BRI1 recruits another type of LRR-RLK, BAK1, to form a functional receptor complex (Li et al., 2002; Nam and Li, 2002). Recently, we reported the reduced ABA sensitivity of bak1 in stomatal closure and showed that BAK1 interacts with OST1 near the plasma membrane. Moreover, brassinoslide (BL) negatively affected the ability of BAK1 to form a complex with OST1 in the presence of ABA (Shang et al., 2016). Taken together, these results make it difficult to assess the function of BRs in the regulation of stomatal movement.
We have noted that, thus far, many experiments performed to measure stomatal apertures have involved single applications of ABA or BR to BR-related or ABA-related mutants, respectively. In this study, we further investigated how BR affects stomatal movement on its own or in combination with ABA. BR promotes stomatal closing in Arabidopsis, as does ABA. However, in combination with ABA, BR concentrations exceeding a threshold level antagonized the effects of ABA on stomatal closure. Co-treatment of BR and ABA repressed the expression of genes responsible for ABA-induced ROS production, resulting in decreased ROS signaling. Using BR-deficient and signaling mutants, and a BR-hypersensitive BRI1-overexpressing transgenic plant, we further investigated how BR affects the movement of stomata following closure in response to ABA treatment. We found that BR signaling is required for the plants to maintain ABA sensitivity and to inhibit ABA-induced stomatal closure by BR. Taken together, these results suggest that interactions between ABA and BR signaling are important for the regulation of stomatal closure.
Materials and methods
Plant material and growth conditions
Arabidopsis thaliana Columbia-0 (Col-0) was used as the wild type plant in all experiments, except when aba2-1 [Landsberg (Ler) background] and dwf4-1 [Wassilewskija-2 (Ws-2) background] were examined. Other mutants and the BRI1-GFP transgenic plants used in this study were from our own lab stocks. Brassinazole (BRZ) was provided by Prof. Seong-Ki Kim from Chung-Ang University. Seeds were sterilized with 75% ethanol containing 0.05% Tween-20 for 15 min, washed twice in 95% ethanol and once in 100% ethanol, and then placed onto 1/2 Murashige and Skoog (MS) (Duchefa Biochemie) plates containing 0.8% phytoagar. All plants were grown at 22 °C under long-light conditions (16 h light–8 h dark).
Stomatal aperture measurement
We followed the methods described by Shang et al. (2016) to measure stomatal apertures. Abaxial surfaces of cotyledons from 10-d-old seedlings were incubated in stomatal opening solution (50 mM KCl, 10 μM CaCl2, 10 mM MES, pH 6.15) in a growth chamber with light intensity of 130 μmol m–2 s–1 at 22 °C. Various reagents, such as ABA (Sigma-Aldrich), BL (Sigma-Aldrich), BRZ, H2O2 and sodium nitroprusside (SNP) (Sigma-Aldrich) were added to the opening solution for the indicated period. Stomatal opening was evaluated by measuring the width and length of the stomata observed under a microscope (Leica, DM2500) and was calculated by determining the width/length ratio.
Quantitative RT-PCR
To analyse the quantitative expression of genes involved in H2O2 and NO production in response to ABA or BL, RNA was isolated from 10-d-old seedlings using the TRIzol reagent (Sigma-Aldrich) after the seedlings were treated with 1 μM ABA or BL, or together for 1 h. First-strand cDNA was synthesized using M-MLV reverse transcriptase (Promega) and the oligo(dT) 15 primer using 1 μg RNA. The same aliquot of first-strand cDNA was used as a template in the second PCR with gene-specific primers. Quantitative RT-PCR was performed with UBQ5 as an internal control and analysed with the Step-one Plus Real Time PCR system using the same cDNA and SYBR Green PCR Master Mix as described previously (Applied Biosystems, Foster City, CA, USA). Data were normalized to UBQ5 expression. Primer sequences used in this experiment are provided in Supplementary Table S1 at JXB online.
Detection of H2O2 and NO production
Abaxial leaf epidermal peels from 4-week-old plants were used to determine H2O2 and NO production. Epidermis was incubated in the stomatal opening solution containing ABA or BL alone, or in combination, and were then incubated in 100 µM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA) (Fisher Scientific) for 15 min to detect H2O2 production. To detect NO production, epidermis was incubated with 200 µM 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FMDA) (Thermo Fisher Scientific) for 20 min. Dyes were washed off with distilled water. H2O2 and NO production in guard cells was observed under a fluorescence microscope (Leica, DM2500 with a fluorescence module; Fluo Illuminator L4/23) and the L5 filter system (excitation BP480/40, emission BP527/30). To prevent photo-oxidation of H2DCF-DA, all fluorescence images were collected with a single rapid capture (150.8 ms frame–1) at ×400 magnification, as described by Shang et al. (2016).
Results
Treatment with ABA or BR induces stomatal closure
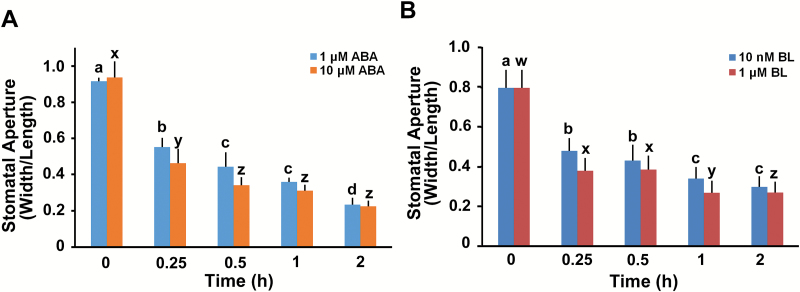
To determine the roles of BRs and ABA in stomatal closure, we examined ABA- or BR-induced stomatal movements for various time periods. Stomatal closure in cotyledons of 10-d-old seedlings was detected 15 min after ABA treatment. Two-hour ABA treatment almost completely closed the stomata of the cotyledons (Fig. 1A). Treatment with brassinolide (BL), which is the most bioactive BR, also induced stomatal closure over the duration of treatment similarly to ABA (Fig. 1B). We observed that stomata were closed proportionally to the concentration of BL when treated for a short period (15 min). However, the degree of stomatal closure by BL showed little difference at concentrations higher than 1 nM for longer period (2 h) (Supplementary Fig. S1). These results are consistent with reports that BL causes stomatal closure (Haubrick et al., 2006; Shi et al., 2015), as observed in response to ABA. Furthermore, BR-induced stomatal closure is consistent with results reported by Xia et al. (2014), in which high concentrations of EBR (more than 1 μM) induced stomatal closure of tomato in the light (Xia et al., 2014).
Fig. 1.
Abscisic acid (ABA) and brassinolide (BL) induced stomatal closure. ABA (1 or 10 μM; A) or BL (10 nM or 1 μM; B) was added to the opening solution and the seedlings were incubated for the indicated times. Stomatal closure was measured as the width/length ratio of the cotyledon stomata. Experiments were independently repeated three times (n=30 each time). Error bars indicate standard errors. Values labeled with different letters are statistically different analysed by one-way ANOVA (P<0.05). (This figure is available in color at JXB online.)
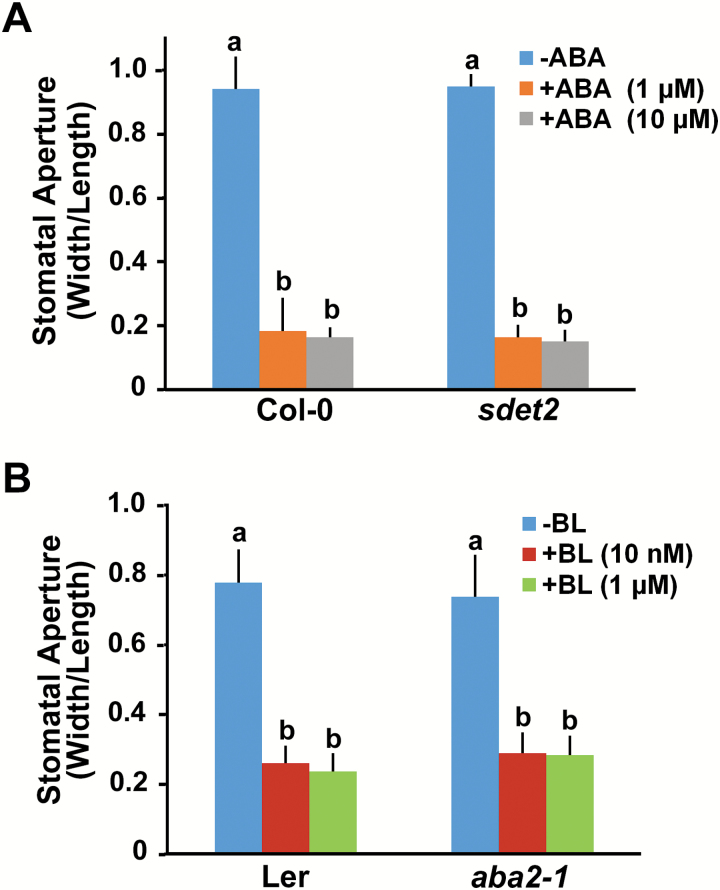
To further examine whether stomatal closure in response to BL or ABA is independent of each hormone, we measured the stomatal aperture from sdet2 (Park et al., 2014), a BR biosynthetic mutant, and aba2-1, an ABA biosynthetic mutant (Schwartz et al., 1997) under ABA or BL treatment, respectively. In this study, we used the sdet2 mutant instead of the original det2 mutant, because sdet2 was newly segregated from the unknown additional mutation that led to severe inhibition of root elongation in det2 plants (Park et al., 2014). The sdet2 mutant still carries the mutation in the DET2 gene, resulting in a rounded and dark-green aerial rosette and dwarfed stature. We also confirmed that treatment with BL partially rescued sdet2 shoot phenotypes (Supplementary Fig. S2). Then, we determined stomatal aperture of sdet2 and aba2-1 in response to low and high concentrations of ABA and BL, respectively. The sdet2 mutant remained sensitive to ABA-induced stomatal closure under both low (1 μM) and high (10 μM) concentrations of ABA treatment (Fig. 2A). It showed the same sensitivity to ABA even at concentrations lower than 1 μM compared with wild type plant (Supplementary Fig. S3). Stomata of the aba2-1 plants were also closed in response to both low (10 nM) and high (1 μM) and concentrations of BL treatment (Fig. 2B). Another BR-biosynthetic mutant, dwf4-1, and ABA biosynthetic mutant, aao3-4, showed similar trends in stomatal closure in response to BL and ABA, respectively (Supplementary Fig. S4A, B). In addition, the ABA signaling mutant abi1-1 (Wu et al., 2003) showed similar stomatal aperture closing to that of wild type plants in response to BL, while ABA-induced stomatal closure was suppressed (Supplementary Fig. S4C). These results suggested that BL and ABA can cause stomatal closure independently.
Fig. 2.
BR- or ABA-deficient mutants showed normal sensitivity to ABA or BL, respectively, in stomatal closure. ABA-induced stomatal closure in the sdet2 mutant (A) and BL-induced stomatal closure in the aba2-1 mutant (B) were measured after 2 h incubation with each hormone and compared with the corresponding wild type plants. ABA or BL with indicated concentrations was added to the opening solution and the seedlings were incubated for an additional 2 h. The stomatal aperture was determined as the width/length ratio of the cotyledon stomata. Experiments were independently repeated twice (n=30 each time). Error bars indicate standard errors. Values labeled with different letters are statistically different analysed by two-way ANOVA (P<0.05). (This figure is available in color at JXB online.)
ABA-induced stomatal closure was reversed by a high concentration of BR
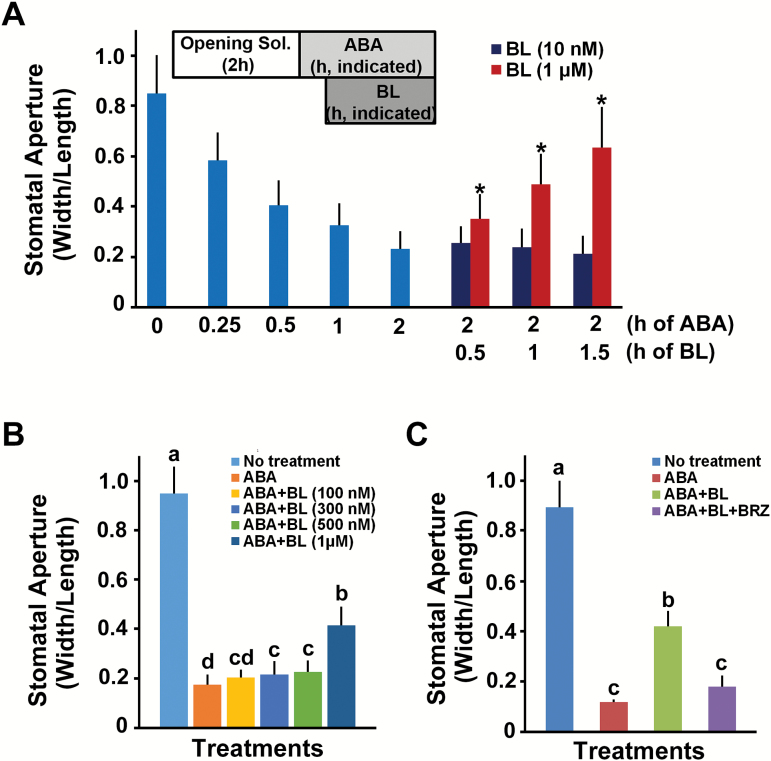
As ABA is a major hormone that affects stomatal movement in various plants (Du et al., 2014; Qu et al., 2014; Tombesi et al., 2015), and both BL and ABA induced stomatal closure independently as shown in Fig. 1, we questioned whether BL acts additively on ABA-induced stomatal closure. Therefore, we examined stomatal closure induced by ABA following the addition of BL for various time periods. Stomata were almost completely closed in 2 h in the presence of 1 μM ABA alone. Addition of 10 nM of BL for different time periods did not affect ABA-induced stomatal closure (Fig. 3A). However, surprisingly, we observed that ABA-induced stomatal closure was reversed proportional to the BL exposure time when a high concentration of BL (1 μM) was added to ABA (Fig. 3A). This reversal of ABA-induced stomatal closure by a high concentration of BL was also observed when the higher concentration of ABA (10 μM) was used (Supplementary Fig. S5). To further examine the effects of BL concentration on ABA-induced stomatal closure, we monitored the effect of increasing concentrations of BL, and the effects of BRZ, a biosynthetic inhibitor of BRs (Asami et al., 2000). We observed that ABA-induced stomatal closure was inhibited by BL in a dose-dependent manner. At concentrations higher than 500 nM, BL treatment opened the stomata that had closed in response to ABA (Fig. 3B). To reverse this pattern, co-treatment of BRZ with BL partially recovered ABA sensitivity in stomatal movement when compared with the effect of the BL treatment alone (Fig. 3C). Taken together, these results suggest that exogenously applied BL antagonizes ABA-induced stomatal movements dose-dependently.
Fig. 3.
High concentrations of BL inhibited the ABA-induced stomatal closure. (A) ABA-induced stomatal closure was measured with or without BL treatment for indicated times. Low (10 nM) or high (1 μM) concentrations of BL were added to the ABA-containing solution for the indicated times. Experiments were independently repeated three times (n=50 each time). Error bars indicate standard errors (*P<0.0001, compared with the corresponding samples treated with ABA only). (B) Effects of BL concentrations on the inhibition of ABA-induced stomatal closure. Different concentrations of BL were added to the ABA-containing solution for 1.5 h. Experiments were independently repeated twice (n=30 each time). (C) Effect of BRZ on BL inhibition of ABA-induced stomatal closure. BRZ (1 μM) was applied 30 min after BL (1 μM) to the ABA-containing solution. Experiments were independently repeated twice (n=30 each time). In all experiments, 1 μM ABA was used. Error bars indicate standard errors. In (B) and (C), values labeled with different letters are statistically different analysed by one-way ANOVA (P<0.05). (This figure is available in color at JXB online.)
BR negatively affects ABA-induced H2O2 and NO production
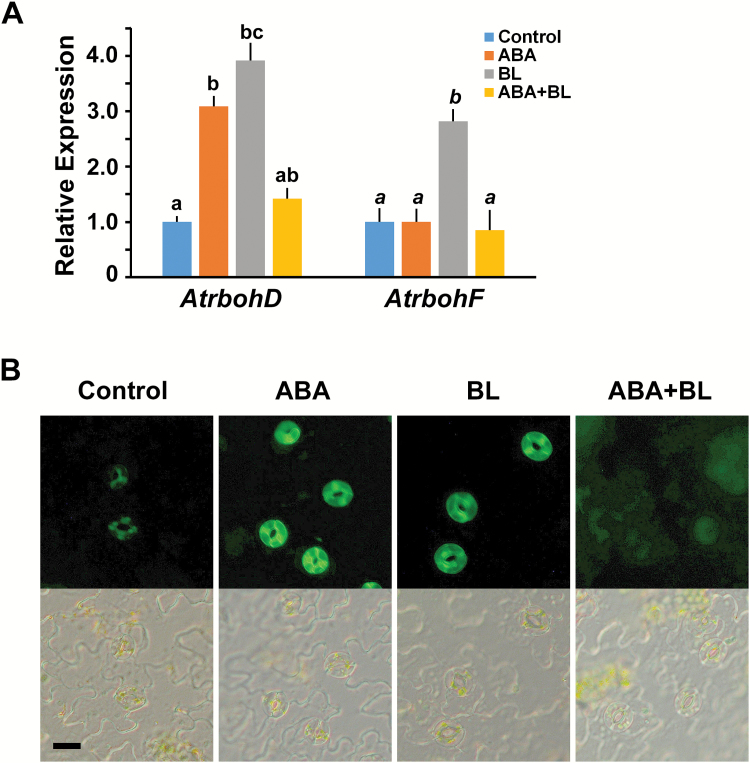
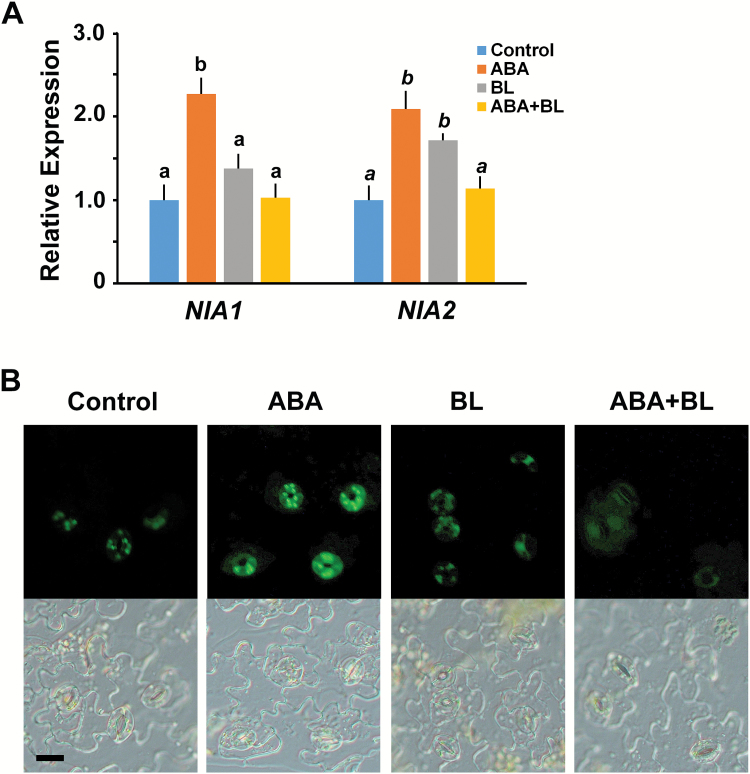
ROS, H2O2, and NO are signaling intermediates in stomatal closure in response to plant hormones as well as environmental stimuli (Pei et al., 2000; Desikan et al., 2002; Kolla et al., 2007). ABA-induced ROS or NO production is a pre-requisite for stomatal closure (Kolla et al., 2007; Gayatri et al., 2013). Therefore, we wanted to determine whether ROS production was affected following co-treatment with ABA and BL, leading to the BL-induced inhibition of ABA on stomatal closure. First we examined the expression of genes encoding the proteins required for H2O2 and NO biosynthesis in wild type plants. Expressions of AtrbohD and AtrbohF, which encode NADPH oxidase catalytic subunits required for ROS production, were induced by ABA in guard cells (Kwak et al., 2003). Consistent with a previous report, the expression of AtrbohD was increased around three-fold by ABA. However, the expression of AtrbohF was not changed by ABA. We also found that the expression of AtrbohD and AtrbohF was up-regulated in response to BL treatment. However, the transcript levels of AtrbohD and AtrbohF were lower upon ABA and BL co-treatment than with single hormone treatment (Fig. 4A). These gene expression patterns for ROS-producing enzymes correlated well with ROS production. Using a H2DCF-DA fluorescent dye, we examined ROS production in guard cells. We observed that upon ABA or BL treatment, ROS were produced specifically in the guard cells, and that ROS production was lower with the co-treatment of ABA and BL than with single hormone treatment, although the ROS level was still slightly above the basal level (Fig. 4B). Similar results were obtained in experiments investigating NO production. The expression of NIA1 and NIA2, which encode two nitrate reductases in Arabidopsis (Desikan et al., 2002), was also up-regulated following ABA or BL treatment. However, the expression of these genes decreased almost to basal levels under ABA and BL co-treatment (Fig. 5A). The patterns of NO production detected using DAF fluorescence were correlated with the patterns of NIA1 and NIA2 expression (Fig. 5B). These results indicate that BR negatively affects ABA-induced H2O2 and NO production, through the down-regulation of genes involved in ROS production.
Fig. 4.
High concentrations of BL inhibited ABA-induced ROS production. (A) Relative expression of AtrbohD and AtrbohF in response to ABA and BL alone, and as a co-treatment. qRT-PCR analyses were performed in triplicate using RNA isolated from 10-d-old wild type Col-0 seedlings. Data were normalized to the expression of ubiquitin. Experiments were independently repeated twice. Error bars indicate standard error. Values labeled with different letters (roman and italic, respectively) are statistically different analysed by one-way ANOVA (P<0.05). (B) ROS production in response to each condition was detected by fluorescent H2DCF-DA in the guard cells (upper panels). Lower panels show the bright field images corresponding to the same region of the epidermal tissues. Scale bar indicates 20 μm.
Fig. 5.
High concentrations of BL inhibited ABA-induced NO production. (A) Relative expression of NIA1 and NIA2 in response to ABA and BL alone, and in combination. qRT-PCR analyses were performed in triplicate using RNA isolated from 10-d-old wild type Col-0 seedlings. Data were normalized to the expression of ubiquitin. Experiments were independently repeated twice. Error bars indicate standard error. Values labeled with different letters (roman and italic, respectively) are statistically different analysed by one-way ANOVA (P<0.05). (B) NO production in response to each condition was detected by fluorescent DAF in the guard cells (upper panels). Lower panels show the bright field images corresponding to the same region of the epidermal tissues. Scale bar indicates 20 μm.
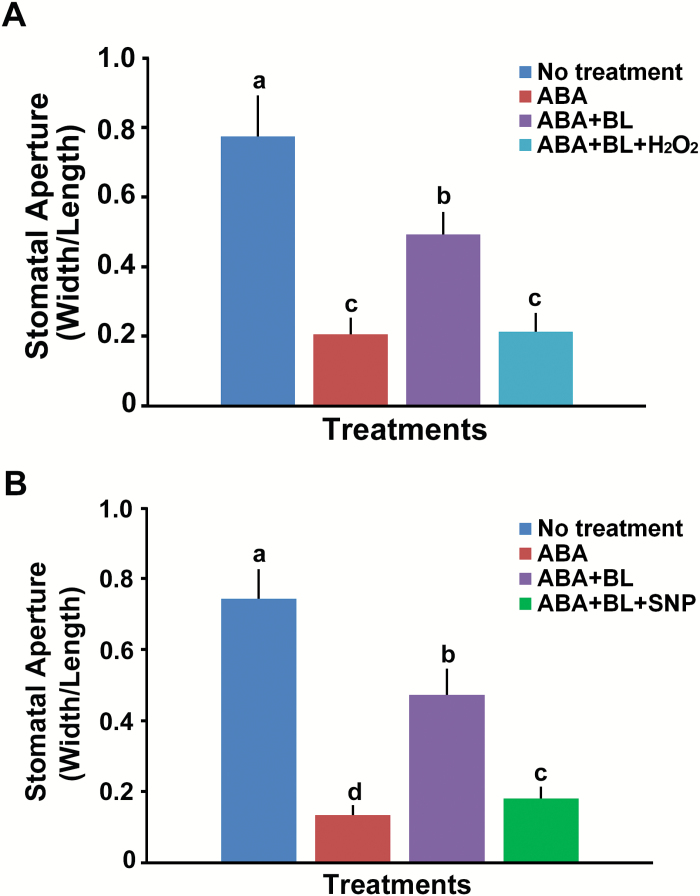
To test this hypothesis, we determined whether application of H2O2 and the NO donor sodium nitroprusside (SNP) could suppress the inhibiting effect of BR on ABA-induced stomatal closure. We sequentially added ABA, BL, and H2O2 or SNP to the solution at 30 min intervals and measured the stomatal aperture after 1 h of treatment with H2O2. We observed that 1 h treatment of H2O2 or SNP was sufficient to inhibit the effect of BR on stomatal opening in the presence of ABA (Fig. 6A, B).
Fig. 6.
H2O2 or SNP treatment repressed the effect of BL on the inhibition of ABA-induced stomatal closure. (A) H2O2 (100 μM) or (B) SNP (100 μM) was applied 30 min after BL (1 μM) to the ABA-containing solution, and the cotyledons were incubated for a further 1 h before apertures were measured. Experiments were independently repeated twice (n=30 each time). Error bars indicate standard errors. Values labeled with different letters are statistically different analysed by one-way ANOVA (P<0.05). (This figure is available in color at JXB online.)
BR signaling capacity is required for ABA-induced stomatal closure
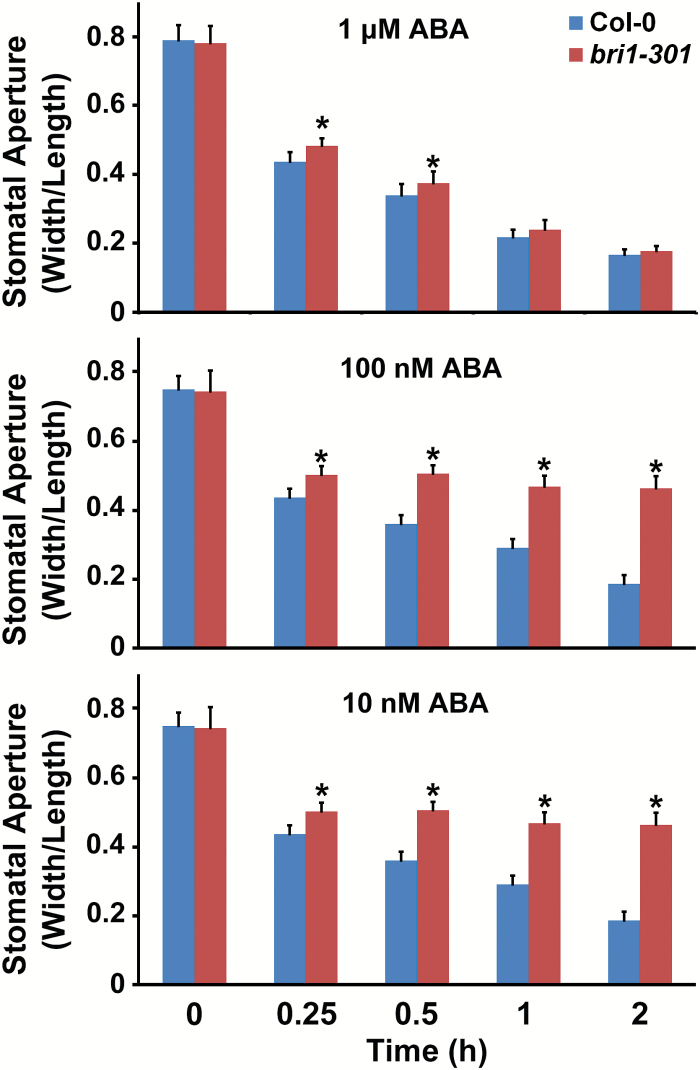
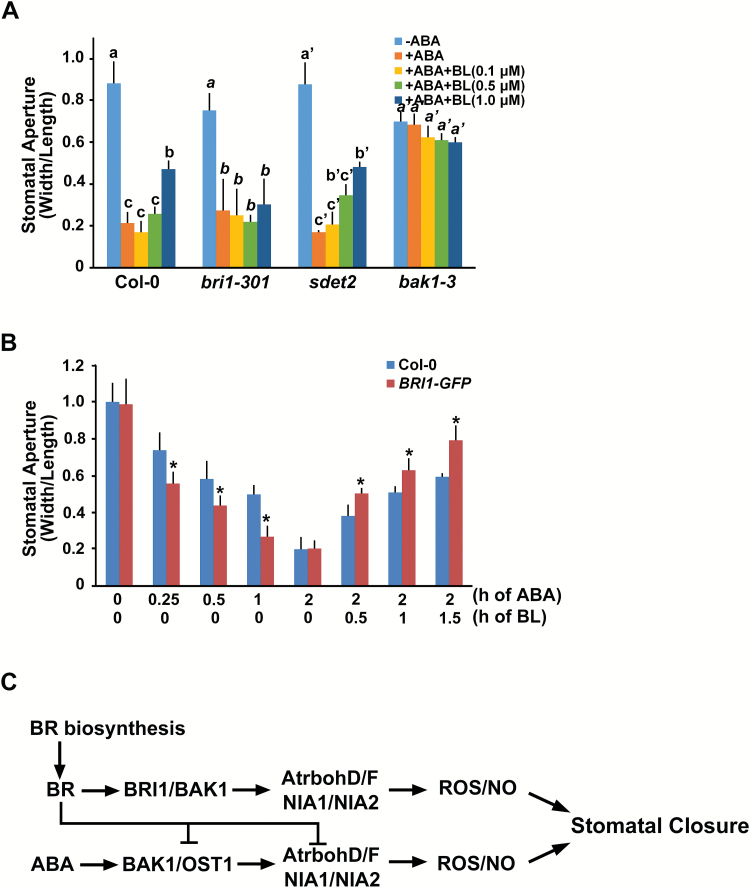
To examine whether the inhibition of ABA-induced stomatal closure by BL occurs through the activation of BR signaling, we examined stomatal movement in the bri1-301 mutant, which is a weak bri1 mutant allele (Xu et al., 2008). First, to confirm the ABA sensitivity of bri1-301 mutant in stomatal closure, we measured the stomatal aperture of the bri1-301 plants in response to various durations of ABA exposure with different ranges of ABA concentrations (Desikan et al., 2002). Stomatal closing occurred later following short period of ABA treatment in the bri1-301 mutant than in the wild type, although a 2 h treatment period was sufficient to close the stomata of bri1-301 mutant to a similar degree to that observed in the wild type. Furthermore, decreased stomatal closure in the bri1-301 mutant was more distinct upon treatment with lower concentrations of ABA (Fig. 7). These results indicate that ABA sensitivity was reduced in the bri1-301 mutant. Upon treatment of BL alone, as expected, BL-induced stomatal closure was not observed in the bri1-301 mutant and the bak1-3 mutant (Supplementary Fig. S6). Based on these results, next, we monitored whether the inhibition of ABA-induced stomatal closure by BL was affected in the bri1-301 mutant. We also confirmed the stomatal movements of the bak1-3 and sdet2 mutants in response to BL and ABA together for comparison. When 10-d-old cotyledons of each plant were incubated in an ABA-containing solution, the stomata of the bri1-301 and sdet2 mutants were closed within 2 h, consistent with the response in wild type plants. When plants were co-treated with BL and ABA, ABA-induced stomatal closure of the sdet2 mutant was inhibited proportionally to the concentration of BL used, which was similar to the response observed in the wild type plant. However, ABA-induced stomatal closure of the bri1-301 mutant was not inhibited by BL treatment (Fig. 8A). These results suggest that functional BRI1 is required for the regulation of stomatal movement: closure of stomata in response to BL and inhibition of ABA-induced stomatal closure. In comparison to the bri1-301 mutant, the stomata of the bak1-3 mutant were less sensitive to ABA (Fig. 8A), consistent with findings from a previous report (Shang et al., 2016). BAK1 does not directly bind BL (Wang et al., 2008), although it is important for BR signaling as a co-receptor of BRI1. Moreover, as BAK1 can function in guard cells by forming a complex with OST1 under ABA treatment (Shang et al., 2016), ABA insensitivity in the bak1-3 mutant may not be caused solely by the failure of BR signaling due to a lack of BAK1. When BL and ABA are both present, BAK1 can function as a versatile component in both signaling pathways. ROS production in the bak1-3 mutant was not induced by ABA (Shang et al., 2016). In this study, we also found that BL-induced ROS production did not occur in the bak1-3 mutant, either, whereas ROS levels increased dramatically in the guard cells of wild type plants (Supplementary Fig. S7). Therefore, the stomatal aperture in the bak1-3 mutant seemed to be the result of disturbances in both BR and ABA signaling pathways.
Fig. 7.
The bri1-301 mutant showed reduced sensitivity to ABA for stomatal closure. Different concentrations of ABA (1 μM, 100 nM, and 10 nM) were added to the opening solution and the seedlings were incubated for the indicated times. Stomatal closure was measured as the width/length ratio of the cotyledon stomata. Experiments were independently repeated three times (n=25 each time). Error bars indicate standard errors (*P<0.0001, compared with the Col-0 plants under the same treatment, t-test). (This figure is available in color at JXB online.)
Fig. 8.
BRI1 is required for the BL inhibition of ABA-induced stomatal closure. (A) Comparison of the effect of BL on the inhibition of ABA-induced stomatal closure in various BR-related mutants. Indicated concentrations of BL were added to the 1 μM ABA-containing solution in which each mutant seedling was incubated. Stomatal closure was measured as a width/length ratio of the cotyledon stomata. Experiments were independently repeated three times (n=30 each time). Error bars indicate standard errors. Values labeled with different letters (roman, italic, primed letters, respectively) are statistically different analysed by one-way ANOVA (P<0.05). (B) ABA-induced stomatal closure of the BRI1-GFP plants was measured with or without BL treatment for the indicated times compared with wild type. Experiments were independently repeated three times (n=50 each time). Error bars indicate standard errors (*P<0.001, compared with the wild type plant under the same treatment). (C) Proposed model showing how BR induces stomatal closure and affects ABA-induced stomatal closure as well. (This figure is available in color at JXB online.)
As reduced BR signaling led to the failure of BL to inhibit ABA-induced stomatal closure, we attempted to further validate the relationship between BR signaling capacity and stomatal movement using the BRI1-GFP transgenic plant, in which BR signaling capacity is increased by increasing the number of BRI1 receptors (Wang et al., 2001; Kinoshita et al., 2005). We observed that the BRI1-GFP plants were hyper-sensitive to ABA for stomatal closure when compared with wild type plants even in the absence of exogenous BL. Furthermore, the degree of inhibition of ABA-induced stomatal closure by BL was greater in BRI1-GFP transgenic plants than in wild type plants (Fig. 8B). Moreover, even in the presence of low concentration of BL, which did not affect stomatal movement in wild type plants, inhibition of ABA-induced stomatal closure was detected in the BRI1-GFP plants (Supplementary Fig. S8). These results show that BRI1-GFP transgenic plants were more sensitive than wild type plants to ABA and BL, such that they exhibited enhanced stomatal closure upon ABA treatment and enhanced inhibition of ABA-induced stomatal closure in the presence of BL. Therefore, BR signaling capacity affects ABA-induced stomatal closure positively and negatively through BRI1.
Discussion
BR alone promotes stomatal closure, but in concert with ABA, BR modulates ABA-induced stomatal closure both positively and negatively
Plants open stomata by regulating the turgor pressure of the two guard cells in order to obtain CO2 for photosynthesis, which results in over 95% of their water loss occurring through the stomatal pores (Buckley, 2005; Kim et al., 2010). Therefore, plants have developed several multi-layered methods to regulate stomatal closure in an appropriate and timely manner for their survival (Carvalho et al., 2015; Murata et al., 2015; Scuffi et al., 2016). We investigated how BR affects stomatal movement by itself and in combination with ABA. Although low concentrations of EBR (<100 nM) induced stomatal opening in tomato leaves, high concentrations (>1 μM) induced stomatal closure (Xia et al., 2014), as in Arabidopsis thaliana (Shi et al., 2015). In the present study, we describe the detailed kinetics of BL-induced stomatal closure in the presence of low and high concentrations of BL in wild type plants (Fig. 1B). Our result that even low concentration of BL induced stomatal closure differs from findings in tomato leaves treated with low concentrations of EBR (Xia et al., 2014). However, it is difficult to directly compare relative effective doses of EBR and BL, and the possibility of different sensitivities to BRs in different tissues or different plant species cannot be ruled out.
In addition to the BR-induced stomatal closure, we also show that BRs can modulate ABA-induced stomatal closure both positively and negatively, depending on the BR concentration applied. Previous studies addressing the function of BRs in stomatal movements have examined stomatal responses of BR-biosynthetic, or signaling mutants in the presence of ABA (Ephritikhine et al., 1999; Xue et al., 2009). These studies showed that stomatal closure of the det2 or bri1-9 mutants was more sensitive to ABA, leading to the conclusion that BR functions negatively in stomatal closure. However, in the present study, we demonstrated that ABA sensitivity of the bri1-301 mutant was partially defective when ABA was applied for shorter periods or when the concentration of ABA was low (Fig. 7), whereas ABA sensitivity of the sdet2 mutant in the stomatal closure was not changed compared with wild type plant (Supplementary Fig. S3). When ABA was applied for longer periods or was followed by BL treatment (over 0.5 μM), ABA led to stomatal closure in the sdet2 and bri1-301 mutants to a similar degree as was observed in wild type plant (Figs 2A and 8A). However, inhibition of ABA-induced stomatal closure by BL occurred in the sdet2 mutant, not in the bri1-301 mutant (Fig. 8A), and the BR-biosynthetic inhibitor BRZ alleviated the BR repression on ABA-induced stomatal closure (Fig. 3C). These results strongly suggest that BRI1 needs to be functional in order to maintain ABA sensitivity and to transduce BR signaling to inhibit ABA-induced stomatal closure. These assumptions were strengthened by results in the BRI1-overexpressing transgenic plant BRI1-GFP, which displayed increased sensitivity to ABA in stomatal closure. Even low concentration of BL inhibited ABA-induced stomatal closure in the BRI1-GFP plants. Furthermore, BL inhibition of ABA-induced stomatal closure was enhanced in the BRI1-GFP plants (Fig. 8B). Therefore, these results suggest that under endogenous levels of BR, proper BRI1 function seems to be required for ABA-induced stomatal closure by maintaining ABA sensitivity. However, when the BR concentration exceeds a threshold level in the presence of ABA, BR inhibits ABA-induced stomatal closure by activating BR signaling. In this way, BR acts both positively and negatively to regulate stomatal movement, which is primarily governed by ABA.
BL suppressed ABA-induced ROS and NO production, thereby inhibiting ABA-induced stomatal closure
ROS, including H2O2, and NO are important downstream signaling intermediates in various processes regulated by plant hormones (Simontacchi et al., 2013; Sanz et al., 2015; Xia et al., 2015). Many studies have demonstrated that ABA (Pei et al., 2000; Kwak et al., 2003; Zhou et al., 2014) and BR (Zhou et al., 2014; Kang and Nam, 2016) induce ROS production. Usually during ABA-induced stomatal closure, OST1 promotes H2O2 production by activating ROS-producing enzymes (Kwak et al., 2003). The resulting increase in H2O2 further induces an increase in the cytosolic Ca2+ level (Pei et al., 2000). Therefore, our data showing that ROS production and the induction of genes involved in H2O2 and NO productions following treatment of ABA or BL alone are consistent with the findings of previous studies (Figs 4 and 5). When BR was applied to maize leaves, BR-induced NO production was observed, which subsequently activated ABA synthesis, resulting in enhanced water stress tolerance (Zhang et al., 2011). In addition, we also found that ABA-induced H2O2 and NO production was repressed by co-treatment with BL (Figs 4 and 5). These observations were consistent with the observation that stomatal aperture was affected by ABA with or without BL treatment (Fig. 3), providing a possible explanation for why BL inhibited ABA-induced stomatal closure upon co-treatment. These results implied that, although ROS production can be induced in various ways, such as by ABA or BR treatment as shown in the present study, it is not cumulative. Simultaneous ROS production by various stimuli should be regulated in order to maintain homeostasis to prevent the excessive accumulation of ROS, which may be harmful for cellular processes. For example, ethylene played a positive role in ROS production but also induced synthesis of flavonols, which function as antioxidants, leading to slower stomatal closure (Watkins et al., 2014). Xia et al. detected dynamic changes of ROS production depending on the concentrations of EBR (Xia et al., 2014). Transient increases in ROS production induced by low concentrations of EBR are required for EBR-induced stomatal opening in tomato leaves. However, prolonged ROS production was observed in guard cells upon application of high concentrations of EBR, resulting in stomatal closure. These results suggest that high levels of ROS function downstream of ABA and BL signaling in the process of stomatal closure. The requirement of ROS and NO production for stomatal closure was confirmed by direct treatment of H2O2 or SNP in the ABA- and BL-containing solution. Both H2O2 and SNP suppressed the inhibitory effect of BL on ABA-induced stomatal closure (Fig. 6A, B). Taken together, these results indicate that BRs negatively affect ABA-induced H2O2 and NO production, through the down-regulation of genes involved in ROS production.
Interactions of ABA and BR signaling are important for the regulation of stomatal closure
Based on these and our previous results showing that BAK1 acts in ABA signaling through the interaction with OST1 in the regulation of stomatal closure triggered by ABA (Shang et al., 2016), we propose that BR inhibition of ABA-induced stomatal closure occurs in two ways. One is through the activation of BR signaling. As discussed, application of BR to stomata closed in response to ABA did not affect stomatal movement in the bri1-301 mutant (Fig. 8A). However, application of BR to stomata closed in response to ABA caused the stomata to open to a greater degree in the BRI1-GFP plants than in wild type plants (Fig. 8B). In addition, even when ABA synthesis is disturbed, closed stomata were normally observed in aba2-1 in response to BL, as the BR-signaling capacity in this mutant is intact (Fig. 2B). A second way is through BAK1, which was originally identified as a co-receptor of BRI1 (Li et al., 2002; Nam and Li, 2002). BAK1 was subsequently shown to be a co-receptor of FLS2, which is a flagellin-binding receptor involved in plant immunity (Chinchilla et al., 2007; Sun et al., 2013; Koller and Bent, 2014), and of ER and ERL1, which are EPF2 and EPF1 receptors for stomatal development and patterning (Meng et al., 2015). These results suggest that BAK1 can act in multiple pathways to regulate plant development. Here, we demonstrated that the bak1-3 mutant also showed defects in BL-induced stomatal closure and BL-induced ROS production (Supplementary Figs S6 and S7). We previously showed that ABA-induced stomatal closure was abolished in the bak1 mutant. Therefore, as shown in Fig. 8A, in the presence of both BR and ABA, the stomatal movement of the bak1 mutant was prevented. ABA-induced OST1 expression and ROS production were also impaired in the bak1 mutant. These results suggest that BAK1 has a dual function in ABA and BR signaling. Therefore, in addition to the inhibition of ABA-induced ROS and NO productions by BL, it is possible that BL can affect ABA-induced stomatal closure by inhibiting the interaction between BAK1 and OST1 (Shang et al., 2016), which lead to recruitment of further BAK1 to BRI1, leading to stomatal reopening (Fig. 8C). Taken together, these results suggest that interactions between ABA and BR signaling are important for fine-tuning the regulation of stomatal closure throughout the plant lifespan.
Supplementary data
Supplemental data are available at JXB online.
Figure S1. Brassinolide (BL) induced stomatal closure.
Figure S2. Phenotypes of the sdet2 mutants.
Figure S3. Stomatal aperture in sdet2 mutant in response to various concentration of ABA.
Figure S4. Stomatal aperture in dwf4-1, aao3-4 and abi1-1 mutants in response to ABA or BL.
Figure S5. ABA-induced stomatal closure was inhibited by BL even in the presence of high concentration of ABA.
Figure S6. BL-induced stomatal closure was not observed in bri1-301 and bak1-3 mutants.
Figure S7. ROS productions in bak1-3.
Figure S8. BRI1-GFP plants showed higher sensitivity to BL in the inhibition of ABA-induced stomatal closure.
Table S1. List of primers used in this study.
Acknowledgements
This work was supported by grants from the Next-Generation BioGreen 21 Program (SSAC, PJ011059 to K.H.N), Rural Development Administration, Republic of Korea and from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2014R1A1A3050921 to K.H.N). The authors would like to thank Prof. Tae-Wuk Kim from Hanyang University for providing sdet2 seeds and for critical discussion on the manuscript. We would also like to express our appreciation for Prof. Giltsu Choi from KAIST for providing aba2-1, aao3-4 and abi1-1 mutant seeds. Dwf4-1 seeds were kindly provided by Prof. Sunghwa Choe from Seoul National University. We thank Prof. Yang Jin Kim from Sookmyung Women’s university for statistical analyses and Prof. Myeong Min Lee from Yonsei University for helpful suggestions and for proofreading the manuscript.
References
- An Z, Jing W, Liu Y, Zhang W. 2008. Hydrogen peroxide generated by copper amine oxidase is involved in abscisic acid-induced stomatal closure in Vicia faba . Journal of Experimental Botany 59, 815–825. [DOI] [PubMed] [Google Scholar]
- Asami T, Min YK, Nagata N, Yamagishi K, Takatsuto S, Fujioka S, Murofushi N, Yamaguchi I, Yoshida S. 2000. Characterization of brassinazole, a triazole-type brassinosteroid biosynthesis inhibitor. Plant Physiology 123, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN. 2005. The control of stomata by water balance. New Phytologist 168, 275–292. [DOI] [PubMed] [Google Scholar]
- Carvalho DR, Torre S, Kraniotis D, Almeida DP, Heuvelink E, Carvalho SM. 2015. Elevated air movement enhances stomatal sensitivity to abscisic acid in leaves developed at high relative air humidity. Frontiers in Plant Science 6, 383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nurnberger T, Jones JD, Felix G, Boller T. 2007. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448, 497–500. [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. 2004. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants. Journal of Experimental Botany 55, 225–236. [DOI] [PubMed] [Google Scholar]
- Desikan R, Griffiths R, Hancock J, Neill S. 2002. A new role for an old enzyme: nitrate reductase-mediated nitric oxide generation is required for abscisic acid-induced stomatal closure in Arabidopsis thaliana . Proceedings of the National Academy of Sciences of the United States of America 99, 16314–16318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan R, Last K, Harrett-Williams R, Tagliavia C, Harter K, Hooley R, Hancock JT, Neill SJ. 2006. Ethylene-induced stomatal closure in Arabidopsis occurs via AtrbohF-mediated hydrogen peroxide synthesis. The Plant Journal 47, 907–916. [DOI] [PubMed] [Google Scholar]
- Du M, Zhai Q, Deng L, et al. 2014. Closely related NAC transcription factors of tomato differentially regulate stomatal closure and reopening during pathogen attack. The Plant Cell 26, 3167–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ephritikhine G, Fellner M, Vannini C, Lapous D, Barbier-Brygoo H. 1999. The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. The Plant Journal 18, 303–314. [DOI] [PubMed] [Google Scholar]
- Feng CZ, Chen Y, Wang C, Kong YH, Wu WH, Chen YF. 2014. Arabidopsis RAV1 transcription factor, phosphorylated by SnRK2 kinases, regulates the expression of ABI3, ABI4, and ABI5 during seed germination and early seedling development. The Plant Journal 80, 654–668. [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SS, Rock CD. 2002. Abscisic acid signaling in seeds and seedlings. The Plant Cell 14 Suppl, S15–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayatri G, Agurla S, Raghavendra AS. 2013. Nitric oxide in guard cells as an important secondary messenger during stomatal closure. Frontiers in Plant Science 4, 425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haubrick LL, Torsethaugen G, Assmann SM. 2006. Effect of brassinolide, alone and in concert with abscisic acid, on control of stomatal aperture and potassium currents of Vicia faba guard cell protoplasts. Physiologia Plantarum 128, 134–143. [Google Scholar]
- Hayashi Y, Takahashi K, Inoue S, Kinoshita T. 2014. Abscisic acid suppresses hypocotyl elongation by dephosphorylating plasma membrane H+-ATPase in Arabidopsis thaliana. Plant and Cell Physiology 55, 845–853. [DOI] [PubMed] [Google Scholar]
- Hubbard KE, Siegel RS, Valerio G, Brandt B, Schroeder JI. 2012. Abscisic acid and CO2 signalling via calcium sensitivity priming in guard cells, new CDPK mutant phenotypes and a method for improved resolution of stomatal stimulus-response analyses. Annals of Botany 109, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imes D, Mumm P, Bohm J, Al-Rasheid KA, Marten I, Geiger D, Hedrich R. 2013. Open stomata 1 (OST1) kinase controls R-type anion channel QUAC1 in Arabidopsis guard cells. The Plant Journal 74, 372–382. [DOI] [PubMed] [Google Scholar]
- Kang HK, Nam KH. 2016. Reverse function of ROS-induced CBL10 during salt and drought stress responses. Plant Science 243, 49–55. [DOI] [PubMed] [Google Scholar]
- Kim TH, Bohmer M, Hu H, Nishimura N, Schroeder JI. 2010. Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annual Review of Plant Biology 61, 561–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Cano-Delgado A, Seto H, Hiranuma S, Fujioka S, Yoshida S, Chory J. 2005. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature 433, 167–171. [DOI] [PubMed] [Google Scholar]
- Klingler JP, Batelli G, Zhu JK. 2010. ABA receptors: the START of a new paradigm in phytohormone signalling. Journal of Experimental Botany 61, 3199–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla VA, Vavasseur A, Raghavendra AS. 2007. Hydrogen peroxide production is an early event during bicarbonate induced stomatal closure in abaxial epidermis of Arabidopsis. Planta 225, 1421–1429. [DOI] [PubMed] [Google Scholar]
- Koller T, Bent AF. 2014. FLS2-BAK1 extracellular domain interaction sites required for defense signaling activation. PLoS ONE 9, e111185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI. 2003. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. The EMBO Journal 22, 2623–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. 2009. A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proceedings of the National Academy of Sciences of the United States of America 106, 21419–21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Chory J. 1997. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell 90, 929–938. [DOI] [PubMed] [Google Scholar]
- Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. 2002. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell 110, 213–222. [DOI] [PubMed] [Google Scholar]
- Meng X, Chen X, Mang H, Liu C, Yu X, Gao X, Torii KU, He P, Shan L. 2015. Differential function of Arabidopsis SERK family receptor-like kinases in stomatal patterning. Current Biology 25, 2361–2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori IC, Uozumi N, Muto S. 2000. Phosphorylation of the inward-rectifying potassium channel KAT1 by ABR kinase in Vicia guard cells. Plant and Cell Physiology 41, 850–856. [DOI] [PubMed] [Google Scholar]
- Murata Y, Mori IC, Munemasa S. 2015. Diverse stomatal signaling and the signal integration mechanism. Annual Review of Plant Biology 66, 369–392. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Fujita Y, Kanamori N, et al. 2009. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant and Cell Physiology 50, 1345–1363. [DOI] [PubMed] [Google Scholar]
- Nam KH, Li J. 2002. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell 110, 203–212. [DOI] [PubMed] [Google Scholar]
- Ng LM, Soon FF, Zhou XE, et al. 2011. Structural basis for basal activity and autoactivation of abscisic acid (ABA) signaling SnRK2 kinases. Proceedings of the National Academy of Sciences of the United States of America 108, 21259–21264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CH, Jang M-S, Yun JH, Lee JE, Kim MK, Park S-C, Kim S-K. 2014. Effects of secondary mutation in det2-1 on root growth and development in Arabidopsis. Journal of Plant Biology 57, 255–263. [Google Scholar]
- Park SY, Fung P, Nishimura N, et al. 2009. Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324, 1068–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. 2000. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406, 731–734. [DOI] [PubMed] [Google Scholar]
- Preston J, Tatematsu K, Kanno Y, Hobo T, Kimura M, Jikumaru Y, Yano R, Kamiya Y, Nambara E. 2009. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: a comparative study on dormant and non-dormant accessions. Plant and Cell Physiology 50, 1786–1800. [DOI] [PubMed] [Google Scholar]
- Qu Y, An Z, Zhuang B, Jing W, Zhang Q, Zhang W. 2014. Copper amine oxidase and phospholipase D act independently in abscisic acid (ABA)-induced stomatal closure in Vicia faba and Arabidopsis. Journal of Plant Research 127, 533–544. [DOI] [PubMed] [Google Scholar]
- Sanz L, Albertos P, Mateos I, Sanchez-Vicente I, Lechon T, Fernandez-Marcos M, Lorenzo O. 2015. Nitric oxide (NO) and phytohormones crosstalk during early plant development. Journal of Experimental Botany 66, 2857–2868. [DOI] [PubMed] [Google Scholar]
- Sato A, Sato Y, Fukao Y, et al. 2009. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochemical Journal 424, 439–448. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Leon-Kloosterziel KM, Koornneef M, Zeevaart JA. 1997. Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiology 114, 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scuffi D, Lamattina L, Garcia-Mata C. 2016. Gasotransmitters and stomatal closure: Is there redundancy, concerted action, or both? Frontiers in Plant Science 7, 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Hanada A, Kuwahara A, et al. 2006. Regulation of hormone metabolism in Arabidopsis seeds: phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. The Plant Journal 48, 354–366. [DOI] [PubMed] [Google Scholar]
- Seo M, Nambara E, Choi G, Yamaguchi S. 2009. Interaction of light and hormone signals in germinating seeds. Plant Molecular Biology 69, 463–472. [DOI] [PubMed] [Google Scholar]
- Shang Y, Dai C, Lee MM, Kwak JM, Nam KH. 2016. BRI1-associated receptor kinase 1 regulates guard cell ABA signaling mediated by open stomata 1 in Arabidopsis. Molecular Plant 9, 447–460. [DOI] [PubMed] [Google Scholar]
- Shi C, Qi C, Ren H, Huang A, Hei S, She X. 2015. Ethylene mediates brassinosteroid-induced stomatal closure via Galpha protein-activated hydrogen peroxide and nitric oxide production in Arabidopsis. The Plant Journal 82, 280–301. [DOI] [PubMed] [Google Scholar]
- Simontacchi M, Garcia-Mata C, Bartoli CG, Santa-Maria GE, Lamattina L. 2013. Nitric oxide as a key component in hormone-regulated processes. Plant Cell Reports 32, 853–866. [DOI] [PubMed] [Google Scholar]
- Sirichandra C, Gu D, Hu HC, et al. 2009. Phosphorylation of the Arabidopsis AtrbohF NADPH oxidase by OST1 protein kinase. FEBS Letters 583, 2982–2986. [DOI] [PubMed] [Google Scholar]
- Steber CM, Cooney SE, McCourt P. 1998. Isolation of the GA-response mutant sly1 as a suppressor of ABI1-1 in Arabidopsis thaliana. Genetics 149, 509–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steber CM, McCourt P. 2001. A role for brassinosteroids in germination in Arabidopsis. Plant Physiology 125, 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama Y, Uraji M, Watanabe-Sugimoto M, Okuma E, Munemasa S, Shimoishi Y, Nakamura Y, Mori IC, Iwai S, Murata Y. 2012. FIA functions as an early signal component of abscisic acid signal cascade in Vicia faba guard cells. Journal of Experimental Botany 63, 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhita D, Raghavendra AS, Kwak JM, Vavasseur A. 2004. Cytoplasmic alkalization precedes reactive oxygen species production during methyl jasmonate- and abscisic acid-induced stomatal closure. Plant Physiology 134, 1536–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Li L, Macho AP, Han Z, Hu Z, Zipfel C, Zhou JM, Chai J. 2013. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342, 624–628. [DOI] [PubMed] [Google Scholar]
- Tombesi S, Nardini A, Frioni T, Soccolini M, Zadra C, Farinelli D, Poni S, Palliotti A. 2015. Stomatal closure is induced by hydraulic signals and maintained by ABA in drought-stressed grapevine. Scientific Reports 5, 12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Wang S, Jones AM. 2002. Role of a heterotrimeric G protein in regulation of Arabidopsis seed germination. Plant Physiology 129, 897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzorjova I, Brosche M, et al. 2010. Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. The Plant Journal 62, 442–453. [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Lauriere C, Merlot S. 2009. Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. The Plant Cell 21, 3170–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Kota U, He K, Blackburn K, Li J, Goshe MB, Huber SC, Clouse SD. 2008. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Developmental Cell 15, 220–235. [DOI] [PubMed] [Google Scholar]
- Wang X, Li X, Meisenhelder J, Hunter T, Yoshida S, Asami T, Chory J. 2005. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Developmental Cell 8, 855–865. [DOI] [PubMed] [Google Scholar]
- Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. 2001. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature 410, 380–383. [DOI] [PubMed] [Google Scholar]
- Watkins JM, Hechler PJ, Muday GK. 2014. Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiology 164, 1707–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sanchez JP, Lopez-Molina L, Himmelbach A, Grill E, Chua NH. 2003. The abi1-1 mutation blocks ABA signaling downstream of cADPR action. The Plant Journal 34, 307–315. [DOI] [PubMed] [Google Scholar]
- Xia XJ, Gao CJ, Song LX, Zhou YH, Shi K, Yu JQ. 2014. Role of H2O2 dynamics in brassinosteroid-induced stomatal closure and opening in Solanum lycopersicum. Plant, Cell and Environment 37, 2036–2050. [DOI] [PubMed] [Google Scholar]
- Xia XJ, Zhou YH, Shi K, Zhou J, Foyer CH, Yu JQ. 2015. Interplay between reactive oxygen species and hormones in the control of plant development and stress tolerance. Journal of Experimental Botany 66, 2839–2856. [DOI] [PubMed] [Google Scholar]
- Xu W, Huang J, Li B, Li J, Wang Y. 2008. Is kinase activity essential for biological functions of BRI1? Cell Research 18, 472–478. [DOI] [PubMed] [Google Scholar]
- Xue LW, Du JB, Yang H, Xu F, Yuan S, Lin HH. 2009. Brassinosteroids counteract abscisic acid in germination and growth of Arabidopsis. Zeitschrift für Naturforschung C 64, 225–230. [DOI] [PubMed] [Google Scholar]
- Zhang A, Zhang J, Zhang J, Ye N, Zhang H, Tan M, Jiang M. 2011. Nitric oxide mediates brassinosteroid-induced ABA biosynthesis involved in oxidative stress tolerance in maize leaves. Plant and Cell Physiology 52, 181–192. [DOI] [PubMed] [Google Scholar]
- Zhou J, Wang J, Li X, Xia XJ, Zhou YH, Shi K, Chen Z, Yu JQ. 2014. H2O2 mediates the crosstalk of brassinosteroid and abscisic acid in tomato responses to heat and oxidative stresses. Journal of Experimental Botany 65, 4371–4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.