Abstract
Study Objectives:
Our group and others have reported a high rate of vitamin D deficiency in obstructive sleep apnea (OSA), where vitamin D levels (25(OH) D) correlate negatively with OSA severity and some of its associated metabolic alterations. Data regarding vitamin D supplementation in OSA are lacking. We wanted to evaluate the effect of vitamin D3 supplementation on OSA symptoms and metabolic parameters.
Methods:
We conducted a pilot, double-blind, randomized, placebo-controlled trial of daily supplementation with 4,000 IU vitamin D3 (D3) or placebo (PL). We studied 19 Caucasian adults (14 male, mean age 55 y, mean body mass index [BMI] 30.4 kg/m2) with OSA. Fifteen patients were stable on continuous positive airways pressure (CPAP) therapy, whereas four were CPAP naïve. Assessments were completed at baseline and after 15 weeks of supplementation. Outcomes included sleepiness (Epworth Sleepiness Scale), quality of life (Sleep Apnea Quality of Life Inventory), fatigue (fatigue severity scale) and neuropsychological function (trail making test and Connor's Continuous Performance Test II). In addition, we assessed biochemical indices of vitamin D status (25(OH)D, calcium), inflammation (high sensitivity C-reactive protein, and lipoprotein-associated phospholipase A2), lipids (total cholesterol [low-density and high-density lipoprotein]) and glycemic indices (fasting glucose, oral glucose tolerance test).
Results:
There was no change in BMI, medication, or CPAP usage. Although there was no change in neuropsychological or quality of life indices, we observed a significant increase in 25(OH)D (p = 0.00001) and significant decreases in both low-density lipoprotein (p = 0.04) and lipoprotein-associated phospholipase A2 (p = 0.037) as well as trends toward decreased fasting glucose (p = 0.09) and increased high-density lipoprotein (p = 0.07) in the D3 group compared to PL.
Conclusions:
Vitamin D3 supplementation increased vitamin D levels and decreased metabolic markers compared to placebo. Larger trials are required.
Citation:
Kerley CP, Hutchinson K, Bramham J, McGowan A, Faul J, Cormican L. Vitamin D Improves selected metabolic parameters but not neuropsychological or quality of life indices in OSA: a pilot study. J Clin Sleep Med. 2017;13(1):19–26.
Keywords: inflammation, lipids, obstructive sleep apnea, vitamin D
INTRODUCTION
Obstructive sleep apnea (OSA) is strongly associated with obesity, which is reported in up to 70% of cases. OSA incidence and/or severity is also related to ethnicity,1 winter season,2,3 and lack of physical activity.4 Additionally, OSA has been associated with multiple metabolic disturbances including excess systemic inflammation, hyperglycemia, hyperlipidemia, cardiovascular disease, and increased bone loss.5
Vitamin D could, at least partially, mediate these relationships. Indeed, recently there has been interest in the idea that vitamin D could be important for sleep disorders.6–8 A recent study suggested that the association between lower 25(OH) D and OSA was largely confounded by larger BMI and neck circumference.9 In contrast, our group10 and others11–14 have reported a high rate of vitamin D deficiency in OSA, where vitamin D levels (25(OH)D) are lower in severe OSA10,11,13 and inversely correlate with some of its associated metabolic alterations, including abnormal glucose metabolism11,12 as well as nocturnal heart rate.10 Vitamin D receptors are located systemically and recent advances have demonstrated the potential of vitamin D to influence a wide variety of organs.15 There is preliminary evidence that vitamin D supplementation may attenuate some metabolic disturbances through anti-inflammatory, antihyperglycemic, and antihyperlipidemic roles, although these potential effects are inconsistent and controversial.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Low vitamin D levels are common in subjects with obstructive sleep apnea and correlate with multiple cardiometabolic parameters. There is evidence the supplementing with vitamin D may improve several physiological, biochemical and subjective features of obstructive sleep apnea.
Study Impact: We present the first report of vitamin D supplementation in obstructive sleep apnea where 15 weeks of daily vitamin D supplementation at 4,000 IU/day decreased low-density lipoprotein cholesterol and systemic inflammation (lipoprotein-associated phospholipase A2). Our pilot study is limited by small sample size and heterogeneous study sample, but may serve to increase knowledge and work in this area.
A recent comprehensive review regarding the link between vitamin D metabolism and sleep medicine concluded that controlled studies are needed to further explore the relationship between inadequate vitamin D and daytime neurocognitive impairment, OSA and associated morbidity, particularly cardiovascular disease.16 Further, vitamin D supplementation may have roles regarding sleep quality,17 sleepiness,18,19 fatigue,20 poor mood,21 and neuropsychological function.22 In this context, it is noteworthy that a 2010 case study reported resolution of hypersomnia following vitamin D supplementation in a vitamin D deficient, African American woman.18
To our knowledge, there are no reports regarding the effect of vitamin D supplementation in OSA. In this pilot study, we examined the effect of short-term vitamin D supplementation among urban adults with OSA, in terms of quality of life, neuropsychological function, and cardiovascular biomarkers.
METHODS
Subjects/Study Population
This pilot intervention study was conducted at Connolly Hospital, Blanchardstown (Dublin 15, Ireland, latitude, 53°N) after institutional review board approval.
Inclusion criteria included previous OSA diagnosis by polysomnography (PSG), Caucasian race, and stable medical regimen including with or without CPAP for 6 mo or longer. Exclusion criteria included mixed sleep apnea, history of coronary artery disease or diabetes or hypoglycemic agents and syndromes/therapy known to interfere with vitamin D metabolism, including inflammatory bowel disease, as well as renal/ liver diseases and use of anticonvulsants, multivitamins, or vitamin D. At baseline, trial information was provided and written informed consent was obtained.
Study Design
This pilot study was designed as a parallel, randomized, double-blind, placebo-controlled trial and involved two clinic visits (Figure 1). Recruitment occurred between November 2013 and January 2014, with follow-up 15 w later. All assessments were conducted at both baseline and follow-up in an identical manner at the same time of morning with the same researcher (CPK). Further, all assessments were conducted in the same windowless room with identical lighting and temperature conditions. Following completion of day 1 presupplementation assessments, subjects were randomized to vitamin D3 (D3) or placebo (PL) groups using an online randomization program.
Figure 1. Trial design.
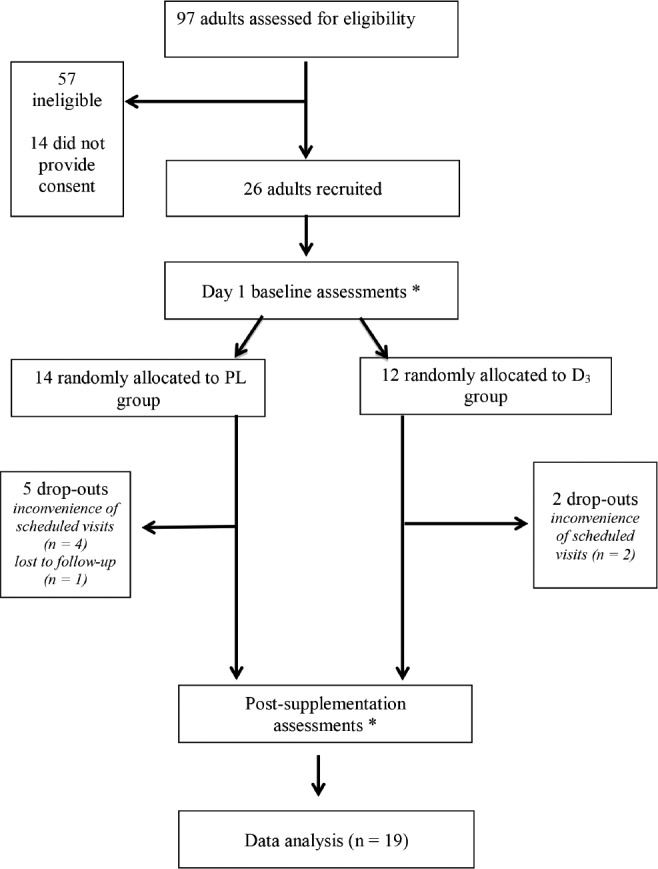
*Assessments included: fasting blood drawn, oral glucose tolerance test, Epworth Sleepiness Scale, Fatigue Severity Scale, Beck Depression Inventory and neuropsychological testing (trail making forms A and B, three subscales of Repeatable Battery for the Assessment of Neuropsychological Status and Conner's Continuous Performance test. PL = placebo; D3 = vitamin D3
Assessments
PSG was conducted on all study participants as previously described.10 At each time point, subjects reported to our research facility after an overnight fast, had blood drawn, and consumed 75 g of glucose. Next, a battery of self-report questionnaires was completed in the same order followed by neuropsychological testing and a second blood sample was taken 2 h after glucose ingestion (Figure 1). For those established on CPAP, compliance data were downloaded at baseline and endpoint. For those not established on CPAP, therapy was not initiated during this study.
The battery of self-report questionnaires included validated questionnaires regarding sleep quality sleepiness (Epworth Sleepiness Scale), quality of life (Sleep Apnea Quality of Life Inventory), fatigue (Fatigue Severity Scale), and mood (Beck Depression Inventory).
The neuropsychological testing included trail-making forms A and B and two subscales of the Repeatable Battery for the Assessment of Neuropsychological Status (R-BANS) (semantic fluency and coding). Subjects also completed the computerized Conner's Continuous Performance test. Additionally, all participants completed the Wechsler test of adult reading at baseline only.
Biochemistry
Venous blood was analyzed locally for full blood count, glucose, hemoglobin A1c, and lipids (total cholesterol, low-density lipoprotein [LDL], high-density lipoprotein [HDL], triglycerides). Additional blood was centrifuged with isolated serum aliquoted and frozen to −80°C until required for further analysis.
Circulating levels of total 25-hydroxyvitamin D (25(OH)D) are considered the most reliable measure of overall vitamin D status because they reflect vitamin D2 + D3 contributions from all sources (i.e., diet, supplements, and sun exposure. 25(OH) D was measured using the Architect 25(OH)D chemiluminescent microparticle immunoassay (CMIA) with interassay coefficient of variation of 3.5% and functional sensitivity less than 20 nmol/L 25(OH)D, parathyroid hormone, insulin, high sensitivity C-reactive protein, and lipoprotein-associated phospholipase A2 (Lp-PLA2) were analyzed on Abbott Architect ci8200 instrument (Abbott Laboratories, Abbott Park, IL, USA). The interassay coefficient of variation for these assays ranged between 1% and 5.6%. Lp-PLA2 activity was assessed using an enzyme-linked immunoassay (PLAC test, diaDexus, Inc., San Franciso, CA, USA). The range of detection is 10 – 400 nmol/ min/mL, with a clinical sensitivity less than 10 nmol/min/mL. Low Lp-PLA2 activity is indicated with values of 151 nmol/ min/mL or less, with medium values falling between 152–194 nmol/min/mL and high values indicated by a result of 195 nmol/min/mL or higher.
Supplements
At baseline, each subject was provided with a consecutively numbered bottle containing 120 softgel capsules of vitamin D3 (cholecalciferol) or identical placebo in a double-blind, randomized fashion. A physician not involved in data gathering (JF) generated the allocation sequence, while a dietitian and nutrition researcher enrolled subjects (CPK). Subjects were instructed to ingest one softgel daily with food. The vitamin D dose was 4,000 IU daily, which corresponds to the tolerable upper intake limit in Europe.
Diet, supplement use, and particularly exposure to ultraviolet B radiation contribute to vitamin D status. Therefore, we conducted this trial at high latitude during winter season when skin vitamin D synthesis is minimal. Further, we advised recruits not to change dietary/supplemental behaviors during the trial. We assessed behavior relating to vitamin D status with the VIDSun questionnaire at baseline and endpoint, which contains questions relating to BMI, skin type, sun exposure, and supplement use.23 Supplement compliance was assessed with a diary and by counting the remaining softgels at the follow-up visit.
Statistical Analysis
For this pilot study we did not conduct a sample size calculation. To our knowledge, this pilot study represents the only vitamin D supplementation trial in OSA and therefore we recruited a convenience sample to preliminary assess the effect, if any, of vitamin D3 supplementation in OSA. We used two-tailed, unpaired t-tests to compare baseline demographics between the two groups. We used two-tailed, paired t-tests to compare changes within groups and two-tailed, unpaired t-tests to compare changes between groups. All analyses were performed using a software package (SPSS, version 18; SPSS, Inc., Chicago, IL). Results were expressed as mean ± standard deviation. We defined statistical significance as p < 0.05.
RESULTS
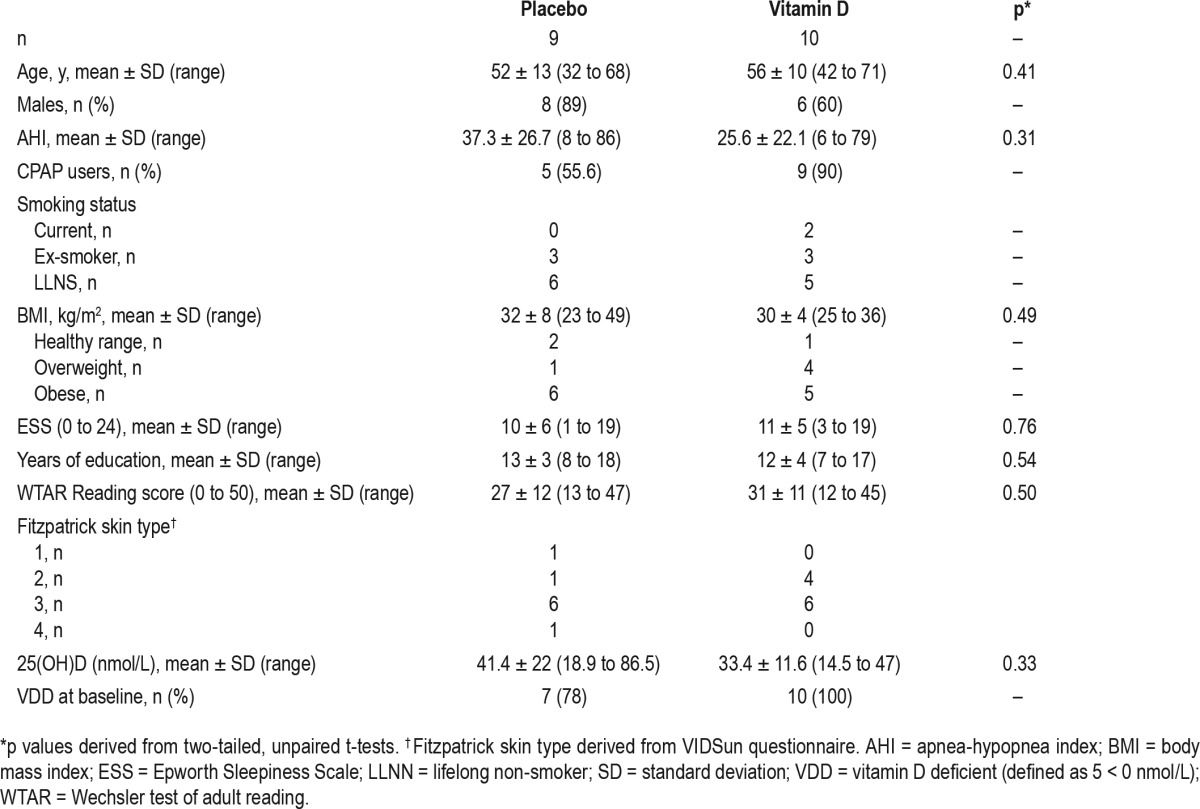
We screened 97 adults attending sleep clinics of Connolly Hospital. Of these 97, 40 were eligible and 26 provided consent. These 26 subjects were recruited and completed baseline assessments. Of the 26, there were 7 dropouts for miscellaneous reasons (Figure 1). Baseline demographics for the 19 subjects who completed the study are presented in Table 1. The mean pretreatment 25(OH)D level for all patients was 37.2 nmol/L (range, 14.5 to 86.5 nmol/L) and there was no significant difference in baseline 25(OH)D levels between the PL and D3 groups. Further, there were no significant differences regarding age, BMI, sleepiness (Epworth Sleepiness Scale score), education level, or reading score between the D3 and PL group; however, there were more males in the PL group and more CPAP users in the D3 group.
Table 1.
Baseline demographics.
Throughout the study, there was no change in medication or CPAP compliance in either group. In addition, there was no change in dietary habit or use of medication, alcohol, tobacco, or caffeine. Compliance with the supplements was high in both groups (93%) and there were no adverse effects.
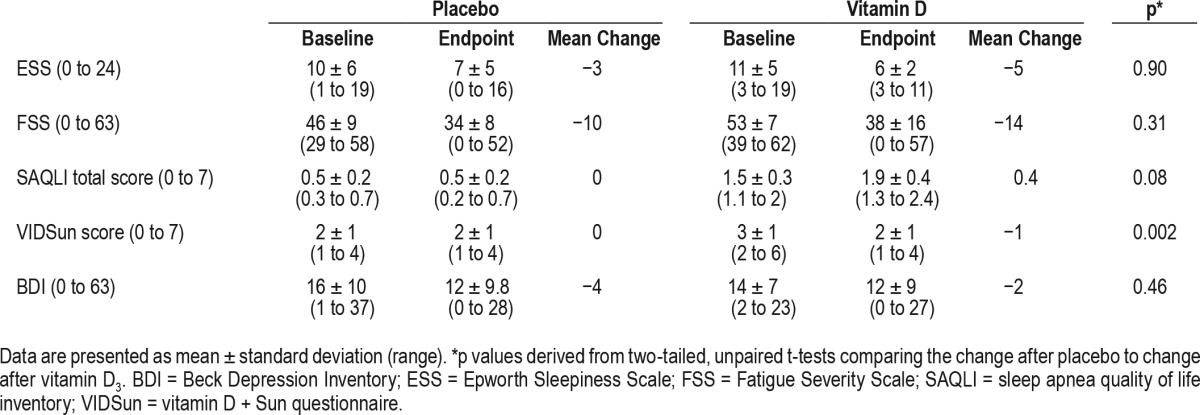
Results before and after supplementation and comparisons between groups are displayed in Table 2 (biochemical indices), Table 3 (neuropsychological indices), and Table 4 (self-reported questionnaire scores).
Table 2.
Changes in biochemical indices.
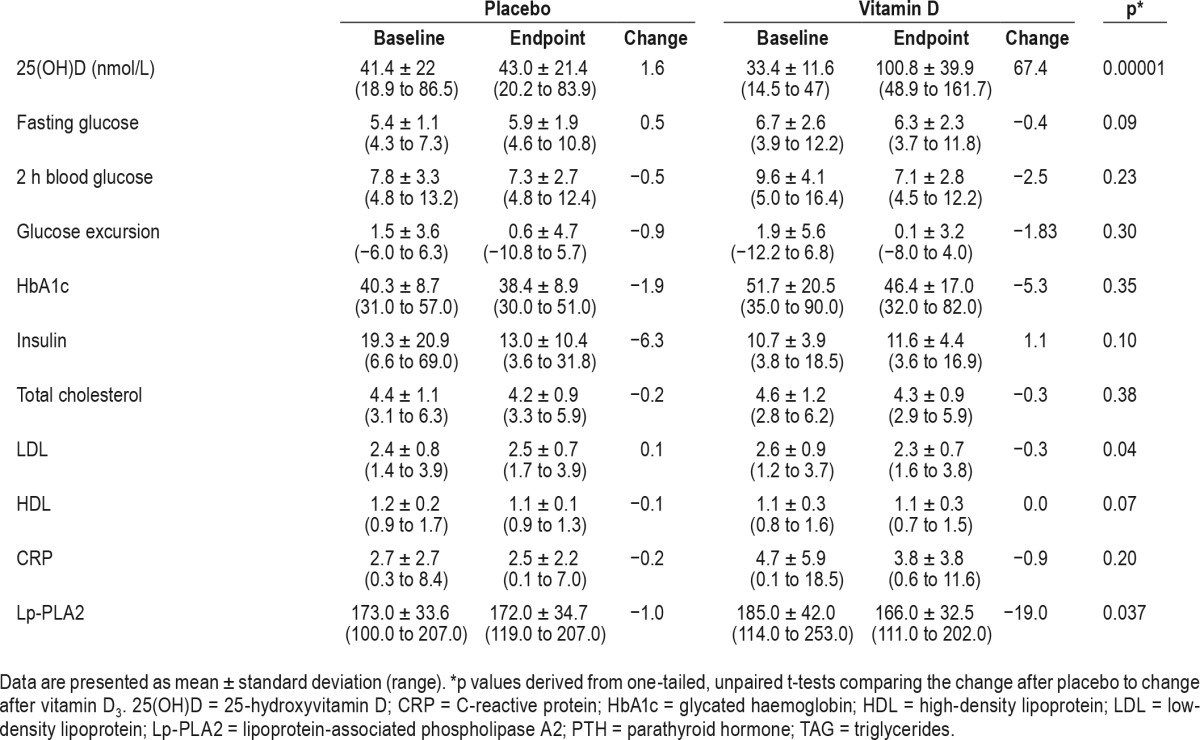
Table 3.
Changes in neuropsychological indices.
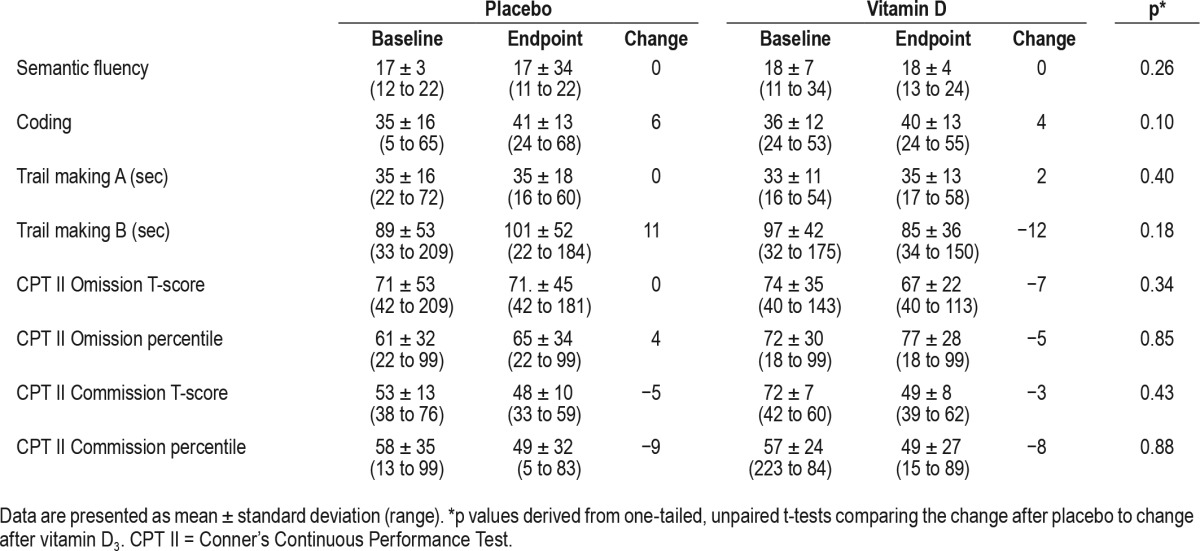
Table 4.
Changes in self-reported questionnaire score.
There was a significant increase in 25(OH)D, which was accompanied by a significant decrease in LDL and Lp-PLA2 in the vitamin D3 group compared to placebo. Additionally, there was a trend toward decreased fasting glucose and increased HDL in the D3 group (Table 2).
We did not detect any differences in neuropsychological indices (Table 3) or quality of life scores (Table 4), except significantly improved fatigue in the vitamin D group.
DISCUSSION
To our knowledge, this is the first report of vitamin D supplementation in OSA. We investigated the effect of vitamin D3 supplementation (4,000 IU/day) for 15 w compared to placebo among a small sample (n = 19) of urban, Caucasian adults with definite OSA as diagnosed by PSG. Consistent with previous studies9–14 we observed a high rate of vitamin D deficiency (89%) in our small OSA cohort. In addition to significantly increased 25(OH)D in the vitamin D supplementation group (n = 10), there were significant reductions in both LDL and Lp-PLA2 compared to placebo. Additionally, there was a trend toward decreased fasting glucose and increased HDL in the D3 group.
Interest in the potential role of vitamin D in sleep disorders has grown recently.6–8 We and others have observed widespread vitamin D OSA.9–14 Interestingly, 25(OH)D levels have been shown to be inversely correlated with OSA severity10,11,13 and with metabolic alterations associated with OSA, including abnormal glucose metabolism11,12 and nocturnal heart rate.10
A 2014 meta-analysis concluded that “Patients with OSA appear to have increased dyslipidemia (high total cholesterol, LDL, TG, and low HDL).”24 Further, a large 2015 cross-sectional study demonstrated an independent association between OSA and elevated LDL.25 The association between OSA and dyslipidemia may mediate some of the excess cardiovascular morbidity and mortality risk associated with OSA. Further, a 2014 mendelian randomization study concluded that interventions that increased HDL may have an effect on coronary artery disease, but that interventions that decrease LDL have a stronger effect on coronary artery disease.26 CPAP represents the current gold standard treatment option for OSA and results in numerous biochemical and physiological benefits. However, the effect of CPAP on lipidemia is controversial. Two 2014 meta-analyses provided conflicting evidence. One meta-analysis concluded that “treatment for OSA seems to improve dyslipidemia (decrease in total cholesterol and LDL, and increase in HDL).”27 However, a second meta-analysis concluded that “CPAP did not alter TG, LDL, or HDL levels, suggesting that CPAP may have no clinically important effect on lipid metabolism.”28 Therefore, non-CPAP approaches to lipid lowering in OSA are warranted. Although the association between vitamin D and cholesterol profiles is controversial, previous reports have demonstrated significantly negative correlations between serum LDL cholesterol and 25(OH)D.29–31 We provide evidence that vitamin D3 supplementation can modestly but significantly decrease serum LDL while maintaining serum HDL concentration in OSA over a 15 w period with no change in lifestyle or dietary intake. There was only a single subject on lipid-lowering therapy (Atorvastatin) in this pilot study. Interestingly, vitamin D supplementation decreased both total and LDL cholesterol in this subject (−0.3 and −0.44 mmol, respectively) despite concurrent lipid-lowering therapy, which can be expected to dilute any benefit.
Lp-PLA2 is a biomarker that may be viewed as a potential link between the pathogenic effects of oxidized LDL cholesterol and plaque vulnerability. Although there is a lack of data regarding Lp-PLA2 and OSA, a recent cross-sectional study of 50 male Turkish subjects with newly diagnosed OSA demonstrated a moderate linear relationship between arousal index and Lp-PLA2 levels.32 Because the arousal index is an important index of sleep fragmentation and the restorative quality of sleep, this association may contribute to the increased cardiovascular risk association with frequent arousal in OSA. Further, several epidemiology studies have shown an association between Lp-PLA2 and both cardiovascular and cerebrovascular events. We are not aware of any reports linking vitamin D and Lp-PLA2. The relationship between vitamin D and inflammation has been controversial. However, in vitro evidence suggests that vitamin D has potent anti-inflammatory properties.33,34 Further, there are reports of decreased production of several proinflammatory markers including tumor necrosis factor-α, interferon γ, and interleukins 2,12,17, and 2,1 but increased production of anti-inflammatory interleukin-10 has been reported with vitamin D supplementation in humans.34 Here, we observed a significant 19-point decrease in Lp-PLA2 after vitamin D3 supplementation compared to placebo.
Patients with OSA demonstrate cognitive dysfunction both subjectively and objectively.35 A recent comprehensive review concluded that controlled studies are needed to further explore the relation between inadequate vitamin D and daytime neurocognitive impairment in OSA.7 There is much interest in the potential of vitamin D regarding cognition, with a recent meta-analysis demonstrating that low 25(OH)D predicts executive dysfunctions, especially on mental shifting, information updating, and processing speed.35 It is has been suggested that hypoxemia contributes more to cognitive dysfunction in OSA than frequent arousals or daytime sleepiness.36 However, this suggestion is controversial. Considering that 90% of the vita-min D supplementation group were CPAP users, any potential benefit of vitamin D supplementation in OSA could have been diluted. Therefore, although we performed very detailed neuropsychological assessments and observed no cognitive benefit here, our small sample and the use of CPAP mean that this pilot study cannot rule out a potential benefit of vitamin D supplementation in OSA.
Our pilot study has several strengths. We utilized the gold standard study design and assessed objective, subjective, and biochemical markers of OSA. We studied a well-characterized group of urban, Caucasian adults with definite OSA. The placebo and vitamin D groups were well matched at baseline with no significant differences in demographics. In the current study, all patients were Caucasian and resided in Dublin, Ireland. All of the 25(OH)D assays were conducted using the same batch of commercial assays and were performed concomitantly by the same biochemist in the same laboratory, thereby reducing biochemical variability. Vitamin D trials can be influenced by a number of factors, including fluctuations in sun exposure and hence 25(OH)D levels, variable quality of vitamin D assays, compliance with the intervention, and provision of inadequate vitamin D supplementation doses. To overcome these factors, we conducted this trial over the winter season at high latitude (53°N) when vitamin D photosynthesis is minimal. Although we purposely did not restrict nonprotocol dietary or supplemental vitamin D intake, we did ask that such behaviors were not altered during the trial. Identical vitamin D behaviors were evident in both groups throughout the trial as confirmed with VIDSun scores. Compliance, as assessed by diary data and capsule counts, was high (93%). We utilized a moderate-high dose of vitamin D3 (4,000 IU/day). In unison with our trial design and high compliance with the supplements, serum 25(OH) D increased significantly in the vitamin D3 group, but did not change in the placebo group.
Our pilot study also has several important limitations. Although there was no difference in 25(OH)D levels at baseline, the mean 25(OH)D was higher in the placebo group. Twenty-two percent of subjects were vitamin D sufficient at baseline, which is of note because supplementation appears most beneficial to those with vitamin D deficiency. Further, as expected all recruits in the intervention arm had increases in 25(OH)D but three of the placebo group (33%) also experienced increases in 25(OH)D (+10 to +22 nmol/L). Although we recruited an exclusively Caucasian sample, the sample was very small (n = 19) and heterogeneous in terms of CPAP usage. However, we ensured that CPAP was neither commenced nor discontinued throughout the trial. Further, we downloaded CPAP compliance data at baseline and endpoint, observing the CPAP usage did not change and ensuring that CPAP was carefully controlled during the study. An additional limitation is the lack of a power calculation and primary endpoint. However, the value of this pilot is to act as a hypothesis-generating basis for future work. It is possible that the statistical significance of our observations would be increased with a large sample size and a more homogeneous population.
CONCLUSIONS
In conjunction with a significant increase in 25(OH)D levels, this pilot study suggests that vitamin D supplementation has the potential to improve relevant biomarkers of cardiometabolic health in OSA. It can be hypothesized that this effect may translate into clinical benefit over the long term due to decreased morbidity and mortality. Vitamin D replenishment warrants further investigation as an adjunct therapeutic strategy in OSA.
DISCLOSURE STATEMENT
This was not an industry supported study. The Irish Thoracic Society, Irish Lung Foundation and the Irish Research Council provided financial support. The sponsors had no role in the design or conducting of this research. The authors have indicated no financial conflicts of interest. This work was completed at the Respiratory and Sleep Diagnostics Department, Connolly Hospital, Blanchardstown, Dublin 15, Ireland.
ABBREVIATIONS
- 25(OH)D
25-hydroxyvitamin D
- AHI
apnea-hypopnea index
- BDI
Beck depression inventory
- BMI
body mass index
- CAD
coronary artery disease
- CPT II
Conner's Continuous Performance Test
- CRP
C-reactive protein
- ESS
Epworth Sleepiness Scale
- HbA1c
glycated haemoglobin
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- LLNS
lifelong non-smoker
- LP-PLA2
lipoprotein-associated phospholipase A2
- OSA
obstructive sleep apnoea
- PSG
polysomnography
- PTH
parathyroid hormone
- SAQLI
sleep apnea quality of life inventory
- TAG
triacylglycerol
- VDD
vitamin D deficiency
- VIDSun
vitamin D + Sun questionnaire
- WTAR
Wechsler test of adult reading
REFERENCES
- 1.Ruiter ME, DeCoster J, Jacobs L, Lichstein KL. Sleep disorders in African Americans and Caucasian Americans: a meta-analysis. Behav Sleep Med. 2010;8(4):246–259. doi: 10.1080/15402002.2010.509251. [DOI] [PubMed] [Google Scholar]
- 2.Cassol CM, Martinez D, da Silva FA, Fischer MK, Lenz Mdo C, Bós ÂJ. Is sleep apnea a winter disease?: meteorologic and sleep laboratory evidence collected over 1 decade. Chest. 2012;142(6):1499–1507. doi: 10.1378/chest.11-0493. [DOI] [PubMed] [Google Scholar]
- 3.Gozal D, Shata A, Nakayama M, Spruyt K. Seasonal variability of sleep-disordered breathing in children. Pediatr Pulmonol. 2011;46(6):581–586. doi: 10.1002/ppul.21408. [DOI] [PubMed] [Google Scholar]
- 4.Peppard PE, Young T. Exercise and sleep-disordered breathing: an association independent of body habitus. Sleep. 2004;27(3):480–484. doi: 10.1093/sleep/27.3.480. [DOI] [PubMed] [Google Scholar]
- 5.Chakhtoura M, Nasrallah M, Chami H. Bone loss in obesity and obstructive sleep apnea: a review of literature. J Clin Sleep Med. 2015;11(5):575–580. doi: 10.5664/jcsm.4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gominak SC, Stumpf WE. The world epidemic of sleep disorders is linked to vitamin D deficiency. Med Hypotheses. 2012;79(2):132–135. doi: 10.1016/j.mehy.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 7.McCarty DE, Chesson AL, Jr, Jain SK, Marino AA. The link between vitamin D metabolism and sleep medicine. Sleep Med Rev. 2014;18(4):311–319. doi: 10.1016/j.smrv.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Evatt ML. Vitamin D associations and sleep physiology-promising rays of information. Sleep. 2015;38(2):171–172. doi: 10.5665/sleep.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goswami U, Ensrud KE, Paudel ML, et al. Osteoporotic Fractures in Men Study Research Group. Vitamin D concentrations and obstructive sleep apnea in a multicenter cohort of older males. Ann Am Thorac Soc. 2016;13(5):712–718. doi: 10.1513/AnnalsATS.201507-440OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerley CP, Hutchinson K, Bolger K, McGowan A, Faul J, Cormican L. Serum vitamin D Is significantly inversely associated with disease severity in caucasian adults with obstructive sleep apnea syndrome: a case control study. Sleep. 2016;39(2):293–300. doi: 10.5665/sleep.5430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bozkurt NC, Cakal E, Sahin M, Ozkaya EC, Firat H, Delibasi T. The relation of serum 25-hydroxyvitamin-D levels with severity of obstructive sleep apnea and glucose metabolism abnormalities. Endocrine. 2012;41(3):518–525. doi: 10.1007/s12020-012-9595-1. [DOI] [PubMed] [Google Scholar]
- 12.Barceló A, Esquinas C, Piérola J, et al. Vitamin D status and parathyroid hormone levels in patients with obstructive sleep apnea. Respiration. 2013;86(4):295–301. doi: 10.1159/000342748. [DOI] [PubMed] [Google Scholar]
- 13.Mete T, Yalcın Y, Berker D, Ciftci B, Guven SF, Topaloğlu O, Yavuz HC, Guler S. Obstructive sleep apnea syndrome and its association with vitamin D deficiency. J Endocrinol Invest. 2013 Oct;36(9):681–685. doi: 10.3275/8923. [DOI] [PubMed] [Google Scholar]
- 14.Erden ES, Genc S, Motor S, et al. Investigation of serum bisphenol A, vitamin D, and parathyroid hormone levels in patients with obstructive sleep apnea syndrome. Endocrine. 2014;45(2):311–318. doi: 10.1007/s12020-013-0022-z. [DOI] [PubMed] [Google Scholar]
- 15.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 16.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8(6):693–697. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Çakır T, Doğan G, Subaşı V, et al. An evaluation of sleep quality and the prevalence of restless leg syndrome in vitamin D deficiency. Acta Neurol Belg. 2015;115(4):623–627. doi: 10.1007/s13760-015-0474-4. [DOI] [PubMed] [Google Scholar]
- 18.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6(6):605–608. [PMC free article] [PubMed] [Google Scholar]
- 19.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8(6):693–697. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roy S, Sherman A, Monari-Sparks MJ, Schweiker O, Hunter K. Correction of low vitamin D improves fatigue: effect of correction of low vitamin D in Fatigue Study (EViDiF Study) N Am J Med Sci. 2014;6(8):396–402. doi: 10.4103/1947-2714.139291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song BM, Kim HC, Rhee Y, Youm Y, Kim CO. Association between serum 25-hydroxyvitamin D concentrations and depressive symptoms in an older Korean population: a cross-sectional study. J Affect Disord. 2016;189:357–364. doi: 10.1016/j.jad.2015.09.043. [DOI] [PubMed] [Google Scholar]
- 22.Menant JC, Close JC, Delbaere K, et al. Relationships between serum vitamin D levels, neuromuscular and neuropsychological function and falls in older men and women. Osteoporos Int. 2012;23(3):981–989. doi: 10.1007/s00198-011-1637-7. [DOI] [PubMed] [Google Scholar]
- 23.Nabak AC, Johnson RE, Keuler NS, Hansen KE. Can a questionnaire predict vitamin D status in postmenopausal women? Public Health Nutr. 2014;17(4):739–746. doi: 10.1017/S1368980013001973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadeem R, Singh M, Nida M, et al. Effect of obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(5):475–489. doi: 10.5664/jcsm.3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Guan J, Yi H, et al. Shanghai Sleep Health Study Research Group. Elevated low-density lipoprotein cholesterol is independently associated with obstructive sleep apnea: evidence from a large-scale cross-sectional study. Sleep Breath. 2016;20(2):627–634. doi: 10.1007/s11325-015-1262-3. [DOI] [PubMed] [Google Scholar]
- 26.Burgess S, Freitag DF, Khan H, Gorman DN, Thompson SG. Using multivariable Mendelian randomization to disentangle the causal effects of lipid fractions. PLoS One. 2014;9(10):e108891. doi: 10.1371/journal.pone.0108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadeem R, Singh M, Nida M, et al. Effect of CPAP treatment for obstructive sleep apnea hypopnea syndrome on lipid profile: a meta-regression analysis. J Clin Sleep Med. 2014;10(12):1295–1302. doi: 10.5664/jcsm.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, Yi H, Guan J, Yin S. Effect of continuous positive airway pressure on lipid profile in patients with obstructive sleep apnea syndrome: a meta-analysis of randomized controlled trials. Atherosclerosis. 2014;234(2):446–453. doi: 10.1016/j.atherosclerosis.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 29.Gagnon C, Lu ZX, Magliano DJ, et al. Low serum 25-hydroxyvitamin D is associated with increased risk of the development of the metabolic syndrome at five years: results from a national, population-based prospective study (The Australian Diabetes, Obesity and Lifestyle Study: AusDiab) J Clin Endocrinol Metab. 2012;97(6):1953–1961. doi: 10.1210/jc.2011-3187. [DOI] [PubMed] [Google Scholar]
- 30.García-Bailo B, Karmali M, Badawi A, El-Sohemy A. Plasma 25-hydroxyvitamin D, hormonal contraceptive use, and cardiometabolic disease risk in an ethnically diverse population of young adults. J Am Coll Nutr. 2013;32(5):296–306. doi: 10.1080/07315724.2013.826112. [DOI] [PubMed] [Google Scholar]
- 31.Zhang MC, Li HX, Liu HM, et al. Serum vitamin D is low and inversely associated with LDL cholesterol in the Kazak ethnic population: a cross-sectional study. Med Sci Monit. 2014;20:1274–1283. doi: 10.12659/MSM.890930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bekci TT, Kayrak M, Kiyici A, Maden E, Ari H, Kaya Z, Teke T, Akilli H. The association among lipoprotein-associated phospholipase A2 levels, total antioxidant capacity and arousal in male patients with OSA. Int J Med Sci. 2011;8(5):369–376. doi: 10.7150/ijms.8.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo J, Ma Z, Ma Q, et al. 1, 25(OH)2D3 inhibits hepatocellular carcinoma development through reducing secretion of inflammatory cytokines from immunocytes. Curr Med Chem. 2013;20(33):4131–4141. doi: 10.2174/09298673113209990248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Korf H, Wenes M, Stijlemans B, et al. 1,25-Dihydroxyvitamin D3 curtails the inflammatory and T cell stimulatory capacity of macrophages through an IL-10-dependent mechanism. Immunobiology. 2012;217(12):1292–1300. doi: 10.1016/j.imbio.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 35.Annweiler C, Montero-Odasso M, Llewellyn DJ, Richard-Devantoy S, Duque G, Beauchet O. Meta-analysis of memory and executive dysfunctions in relation to vitamin D. J Alzheimers Dis. 2013;37(1):147–171. doi: 10.3233/JAD-130452. [DOI] [PubMed] [Google Scholar]
- 36.Shpirer I, Elizur A, Shorer R, Peretz RB, Rabey JM, Khaigrekht M. Hypoxemia correlates with attentional dysfunction in patients with obstructive sleep apnea. Sleep Breath. 2012;16(3):821–827. doi: 10.1007/s11325-011-0582-1. [DOI] [PubMed] [Google Scholar]


