Abstract
Study Objectives:
To elucidate the links between the two most prevalent sleep disorders, insomnia and obstructive sleep apnea (OSA), and mortality.
Methods:
We studied 4,225 subjects who were referred to the Center for Sleep and Chronobiology, Seoul National University Hospital, from January 1994 to December 2008. We divided the subjects into five groups: mild OSA (5 ≤ AHI < 15), moderate OSA (15 ≤ AHI < 30), severe OSA (AHI ≥ 30), insomnia, and a no-sleep-disorder group consisting of subjects without sleep disorders. Standardized mortality ratio (SMR), hazard ratio, and the survival rates of the five groups were calculated and evaluated.
Results:
The SMR of all-cause mortality was significantly higher in the severe OSA group than in the general population (1.52, 95% CI 1.23–1.85, p < 0.05). The SMR of cardiovascular mortality increased progressively with the severity of OSA (no-sleep-disorder: 0.09, mild: 0.40, moderate: 0.52, severe: 1.79, p < 0.05). Statistical analyses of the hazard ratios indicated that severe OSA is a risk factor for all-cause mortality (HR 3.50, 95% CI 1.03–11.91, p = 0.045) and cardiovascular mortality (HR 17.16, 95% CI 2.29–128.83, p = 0.006). Cardiovascular mortality was also significantly elevated in the insomnia group (HR 8.11, 95% CI 1.03–63.58, p = 0.046).
Conclusions:
Severe OSA was associated with increased all-cause mortality and cardiovascular mortality compared to the no-sleep-disorder group. Insomnia was associated with increased cardiovascular mortality compared to the no-sleep-disorder group.
Citation:
Choi JW, Song JS, Lee YJ, Won TB, Jeong DU. Increased mortality in relation to insomnia and obstructive sleep apnea in Korean patients studied with nocturnal polysomnography. J Clin Sleep Med. 2017;13(1):49–56.
Keywords: insomnia, OSA, mortality
INTRODUCTION
Sleep disorders have negative effects on physical and mental health.1–3 Two of the most common sleep disorders are insomnia and obstructive sleep apnea (OSA). The estimated prevalence of insomnia varies according to the criteria used for its definition, ranging from 14.7% for the RDC/ICSD-2 criteria to 22.1% for the DSM-IV-TR criteria.4 The prevalence of insomnia in Korea is 22.8%. The prevalence is higher in women (25.3%) than in men (20.2%).5 OSA is the second most common sleep disorder with a prevalence of about 3% to 7% in men and 2% to 5% in women.6 In Korea, an epidemiologic study found that the prevalence of OSA was 4.5% in men and 3.2% in women.7
Although the mechanistic basis for the association between insomnia and death is unclear, a growing number of studies have suggested that insomnia is related to inflammation, an increase in the activation of the hypothalamic-pituitary-adrenal (HPA) axis, and the sympathetic system, which, in turn, can lead to hypertension, increased heart rate, metabolic dysfunction, and possibly death.8–12 Moreover, insomnia is comorbid with other psychiatric disorders such as mood disorder and anxiety disorder, which are both closely associated with risk factors for death including suicide.13,14
OSA is characterized by the presence of frequent collapses of the upper airway that lead to hypoxemia, repetitive arousal, and increased blood pressure during sleep. These conditions can facilitate the development of cardiovascular diseases, cerebrovascular diseases, and metabolic syndrome.15,16 Negative consequences of OSA extend far beyond its effect on the physical body, as several studies have revealed that OSA is associated with cognitive impairment, excessive daytime sleepiness, and decreased overall quality of life.17–19 Given its link to numerous negative health outcomes, OSA is thought to be an important risk factor for death and needs to be studied extensively.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Most previous mortality studies of OSA and insomnia have been conducted without consideration of other sleep disorders, and reports concerning insomnia have relied heavily on subjective symptoms or questionnaires. In this study, to overcome these shortcomings, we excluded all other sleep disorders and objectively determined insomnia by nocturnal polysomnography.
Study Impact: Severe OSA was associated significantly with all-cause mortality and cardiovascular mortality, and insomnia showed a significant correlation with deaths due to cardiovascular causes. These results suggest that early screening and efficient treatment are needed not only for OSA, but also for insomnia.
Several studies have investigated the association between insomnia and mortality and between OSA and mortality.20–24 The results of previous studies on the association between insomnia and mortality have been inconsistent; some have suggested no association25 and others have indicated that insomnia patients with a short sleep duration (< 6 h of sleep per night) had a higher risk of death than normal sleepers.21 However, these studies relied on subjective symptoms and questionnaires for diagnosis and therefore conflated other sleep disorders with insomnia. In addition, most separated OSA patients from the reference group (with no OSA) based only on apnea-hypopnea index (AHI) or respiratory distress index (RDI) criteria, and did not screen out other sleep disorders.20–24
Although previous studies have evaluated the associations of the two common sleep disorders, OSA and insomnia, with mortality, none has compared the two disorders and mortality rate simultaneously. Moreover, data on the mortality rate of patients with insomnia have been inconsistent. In the current study, we hypothesized that insomnia and OSA contribute to all-cause and disease-specific mortality.
METHODS
Subjects
We retrospectively reviewed the medical records and polysomnographic (PSG) recordings of 4,982 subjects who visited the Center for Sleep and Chronobiology at Seoul National University Hospital due to sleep disturbances, snoring, observed apnea, or abnormal behavior during sleep between January 1994 and December 2008.
All subjects in the study underwent PSG for the following reasons: snoring, observed apnea, abnormal behavior during sleep, and sleep disturbances, among other. Among the patients excluded from the insomnia group, subjects with AHI < 5, periodic limb movement index (PLMI) < 15, and no symptom of any other sleep disorder, such as narcolepsy or REM sleep behavior disorder (RBD), were included in the no-sleep-disorder group. These subjects were referred for suspicion of sleep disorders, but were found to be normal according to PSG. Based on PSG results, we classified the subjects into mild OSA (5 ≤ AHI < 15), moderate OSA (15 ≤ AHI < 30), and severe OSA (AHI ≥ 30) groups. Subjects who requested PSG due to insomnia symptoms and had AHI > 5 on PSG recordings were classified into the OSA group. The following criteria were used to determine that patients had insomnia: difficulty initiating or maintaining sleep for ≥ 3 months; certified psychiatrists' confirmation of insomnia symptoms based on the DSM-IV criteria; maintenance of an AHI < 5 and PLMI < 15; and no sign of any other sleep disorder, such as narcolepsy or RBD, based on PSG. We excluded subjects under 15 years of age. Subsequently, we investigated the deaths of these subjects that occurred up to December 31, 2013. Other than PSG parameters, data on demography, hypertension, diabetes, and body mass index (BMI) were also collected. We obtained the approval of the Seoul National University Institutional Review Board.
Nocturnal Polysomnography
A Grass model 15 or 78 (Grass Instrument, Middleton, WI) was used for PSG. Sleep stage, respiratory events, and movement events were scored according to the standard criteria,25,26 primarily by trained sleep technicians and secondarily by sleep physicians. Apnea was defined as a complete or near-complete (≥ 90%) cessation of airflow ≥ 10 s. Hypopnea was defined as a reduction of respiratory signal by > 30% lasting for at least 10 s and associated with oxygen saturation ≥ 3% from the baseline or an arousal. Periodic limb movements in sleep (PLMS) were defined as at least four consecutive movements at 25% of the amplitude of the resting legs, lasting 0.5 to 5 s and occurring 5 to 90 s apart. Sleep efficiency (SE), sleep latency, and wake after sleep onset time (WASO) were calculated.
Mortality Data
We searched each subject's Korean Identification Number and name in the death records from Statistics of Korea, the national bureau of statistics, to determine the deaths in the cohort that occurred prior to December 31, 2013. Date of death and primary cause of death were noted in these records. We divided the subjects into three groups that reflected the most common causes of death reported in these records (cancer, cardiovascular diseases, and trauma) and calculated each group's mortality rate.
Statistical Analysis
The expected number of deaths was first calculated to determine the standardized mortality ratio (SMR), and was then compared to the death rate in the general population.27 The SMR is the ratio of observed deaths in the study group to the number of expected deaths in the general population. An SMR of 1.0 indicates that the expected number of deaths in the study group equals that in the general population. An SMR > 1.0 indicates a higher number of deaths in the study group than in the general population. The data on the death rate of the general population were collected from Statistics of Korea. Cox proportional hazard regression analysis was employed to estimate the hazard ratios (HRs) against the no-sleep-disorder group and 95% confidence intervals (CIs) adjusted for age, gender, BMI, hypertension, and diabetes. Kaplan-Meier survival analysis was used to compare survival rates among the groups. We used SPSS version 21.0.
RESULTS
Subject Characteristics
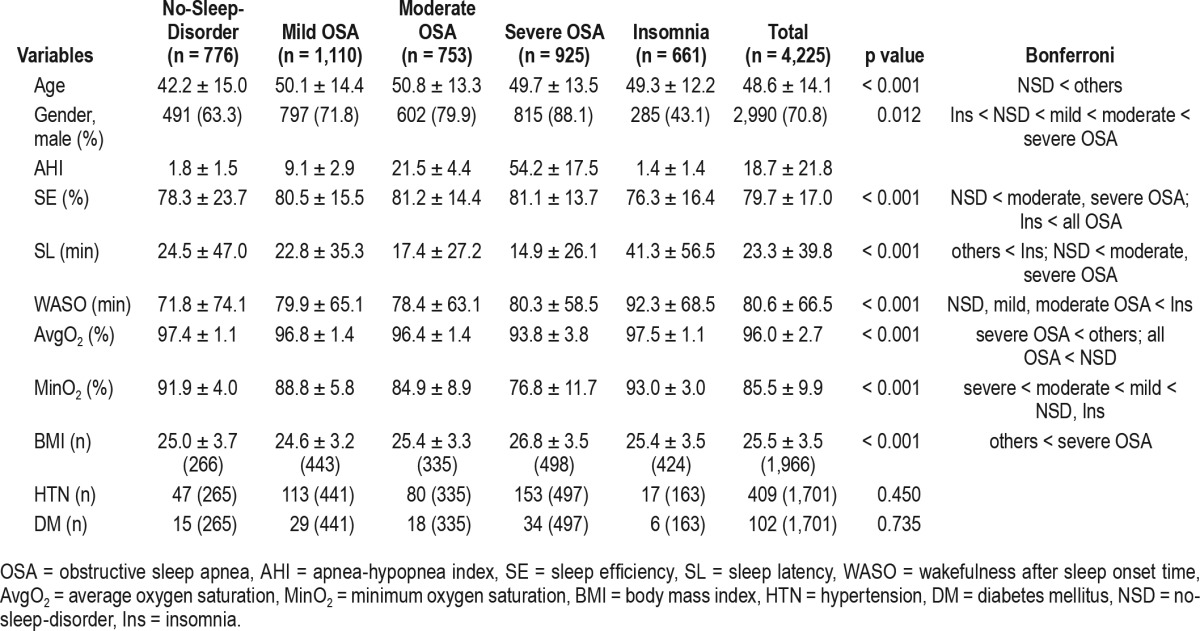
Of 4,982 subjects, 144 subjects who were < 15 years old, 119 subjects with RBD, 130 subjects with narcolepsy, and 274 patients with insomnia symptoms, but PLMIs of > 15 events were excluded. Finally, the study included a total of 4,225 subjects, all of whom underwent complete (> 8 h) PSG. The average age was 48.6 years, male gender was 70.8% and the mean BMI was 25.5 kg/m2. Among the 4,225 subjects, 1,110 (24%) had mild OSA, 753 (16%) had moderate OSA, 925 (20%) had severe OSA, and 661 (14%) had insomnia. The severe OSA group had a significantly higher BMI than the others. The insomnia group showed significantly lower SE than all OSA groups and significantly higher WASO (min) than the mild and moderate OSA groups. All of the OSA subjects had lower average oxygen saturation (SpO2, in percentage) than the no-sleep-disorder group and the severe OSA group had a significantly lower average SpO2 than all other groups (Table 1).
Table 1.
General characteristics of OSA patients, insomnia patients, and subjects without sleep disorders.
Mortality Compared to the General Population
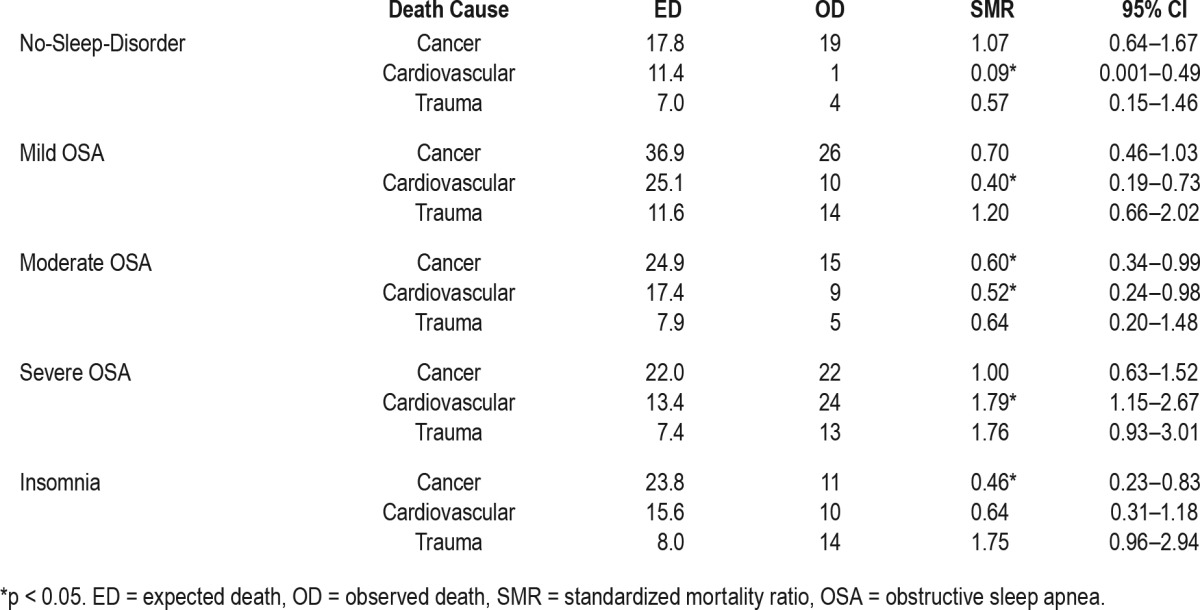
The observation period was 48,763.6 person-years and the average observation period was 10.5 ± 4.1 years. There were 354 deaths during the observation period: 109 died from cancer (30.8%), 59 died from cardiovascular diseases (16.7%), and 55 died from trauma (15.5%). The SMR of the general population in the Republic of Korea was set to 1, and the SMR of all-cause mortality for all of the current subjects was 0.82, which suggests that the subjects had a 12% lower mortality rate than the general population. The SMR of all-cause mortality in the no-sleep-disorder group was 0.57. The severe OSA group had mortality rates 1.52 times higher than those of the general population. The SMR of all-cause mortality in the mild OSA group was 0.77, indicating that they died less than the general population but died 20% more than the no-sleep-disorder group. The SMR of all-cause mortality in the insomnia group was 0.72, which was also lower than that of the general population but higher than that of the no-sleep-disorder group (Table 2). There were no significant differences in the SMR of cancer between the no-sleep-disorder and OSA groups. However, the SMR of cardiovascular diseases increased with the severity of OSA (no-sleep-disorder: 0.09, mild: 0.40, moderate: 0.52, severe: 1.79) (Table 3).
Table 2.
Standardized mortality ratio of all-cause mortality.
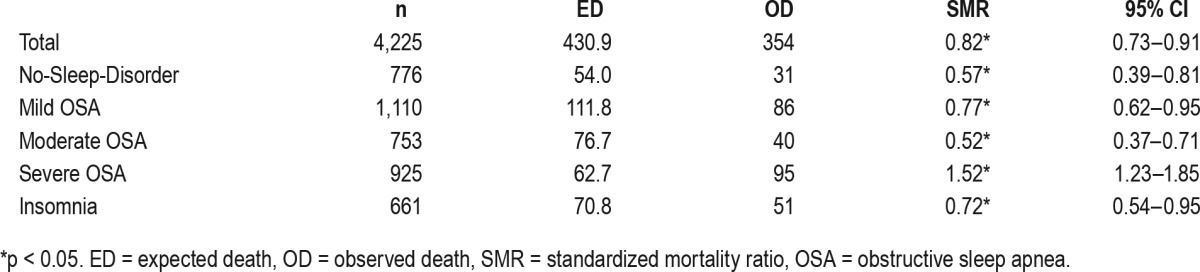
Table 3.
Standardized mortality ratio of each cause of mortality.
Mortality Compared to the No-Sleep-Disorder Group
The HR of all-cause mortality was significantly elevated in the mild OSA group, the severe OSA group, and the insomnia group. However, only the severe OSA group showed significantly increased HR (3.50, 95% CI 1.03–11.91; p = 0.045) after adjusting for age, gender, hypertension, diabetes, and BMI (Table 4). The HR of cardiovascular mortality adjusted for age and gender was significantly elevated in the severe OSA and insomnia groups, compared to the no-sleep-disorder group (17.16, 95% CI 2.29–128.83, p = 0.006; 8.11, 95% CI 1.03–63.58, p = 0.046, respectively; Table 5). The HR of the other general characteristics and sleep-related variables was also calculated. Age was significantly associated with increased all-cause mortality (1.07, 95% CI 1.06–1.08, p < 0.001) and cardiovascular mortality (1.08, 95% CI 1.05–1.11, p < 0.001). Male gender showed a significant association with increased all-cause mortality (1.68, 95% CI 1.24–2.28, p = 0.001). A high AHI was a significant predictor of all-cause mortality (1.01, 95% CI 1.01–1.02, p < 0.001) and cardiovascular mortality (1.02, 95% CI 1.00–1.03, p = 0.020). In addition, the Cox regression analysis indicated that a high PLMI was associated with increased all-cause mortality (1.008, 95% CI 1.00–1.01, p = 0.001) and cardiovascular mortality (1.014, 95% CI 1.01–1.02, p = 0.002). Low SE was associated with increased all-cause mortality and cardiovascular mortality (0.949, 95% CI 0.916–0.984, p = 0.004; 0.912, 95% CI 0.843–0.988, p = 0.023, respectively; Table 6). The HR of cancer and trauma mortality was not statistically significant in the OSA groups or the insomnia group. Kaplan-Meier survival curves for all-cause mortality showed that the survival rate of the severe OSA group was significantly lower than that of all other groups (p = 0.001). The mild OSA and insomnia groups had lower survival rates than the no-sleep-disorder group (p < 0.001, p = 0.02; Figure 1). When cardiovascular mortality was calculated using the Kaplan-Meier survival curve, all of the OSA groups had significantly lower survival rates than the no-sleep-disorder group (mild: p = 0.019; moderate: p = 0.007; severe: p < 0.001). The cardiovascular survival rate of the severe OSA group was significantly lower than those of the other groups (p = 0.005). No significant difference was found between the mild and moderate OSA groups (p = 0.489) for cardiovascular mortality. The cardiovascular survival rate of the insomnia group was significantly lower than that of the control group (p = 0.006; Figure 2).
Table 4.
Hazard ratios for all-cause mortality.
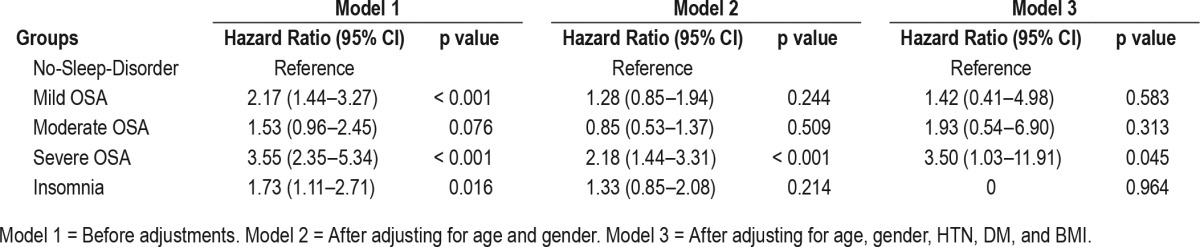
Table 5.
Hazard ratios for cardiovascular mortality.
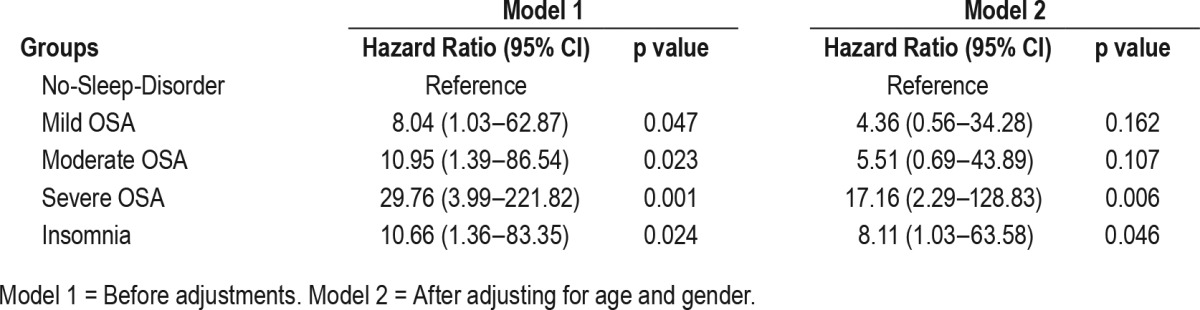
Table 6.
Hazard ratios of other variables.
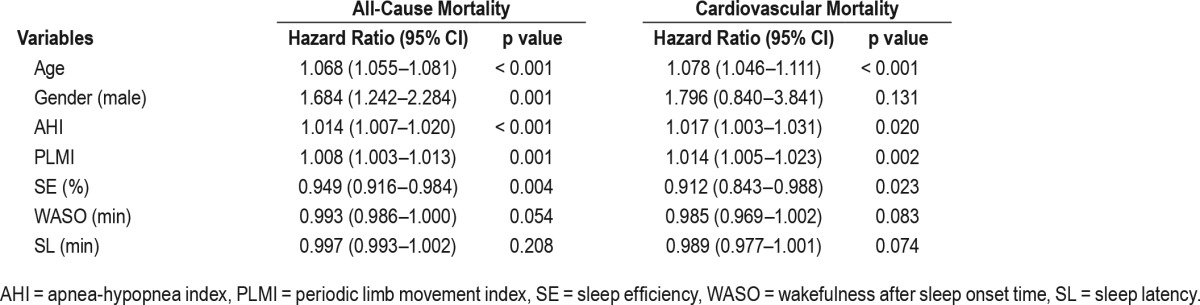
Figure 1. Kaplan-Meier survival curves for all-cause mortality.
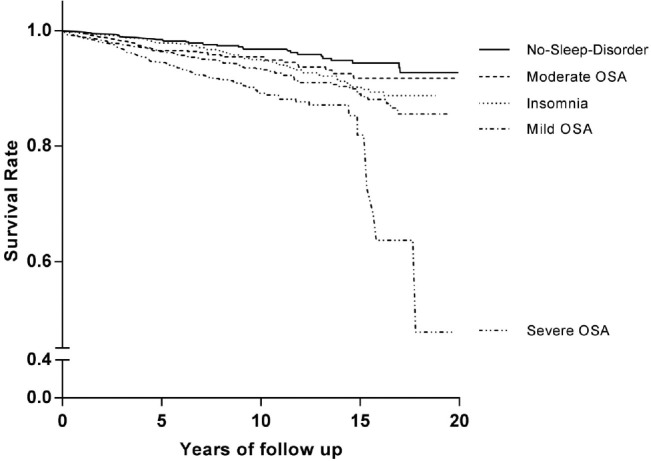
Kaplan-Meier estimated survival rate for all-cause mortality according to OSA severity and insomnia. The survival rate was significantly lower in the severe OSA group that in all other groups (p = 0.001). The mild OSA and insomnia groups had lower survival rates than the no-sleep-disorder group (p < 0.001 and p = 0.02, respectively). The survival rate did not significantly differ between the moderate OSA group and the no-sleep-disorder group. OSA = obstructive sleep apnea.
Figure 2. Kaplan-Meier survival curves for cardiovascular mortality.
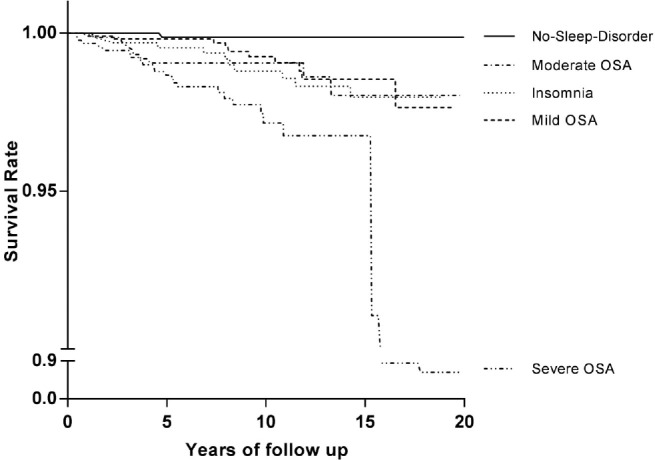
Kaplan-Meier estimated survival rates for cardiovascular mortality according to OSA severity and insomnia. All of the OSA groups and the insomnia group had significantly lower survival rates than the no-sleep-disorder group (p = 0.019, p = 0.007, p < 0.001, and p = 0.006 respectively). The cardiovascular survival rate of the severe OSA group was significantly lower than those of all of the other groups (p = 0.005). The cardiovascular survival rate of the insomnia group was significantly lower than that of the no-sleep-disorder group (p = 0.006). No significant difference was found among the mild OSA group, the moderate OSA group, and the insomnia group for cardiovascular mortality. OSA = obstructive sleep apnea.
DISCUSSION
The severe OSA group exhibited increased all-cause mortality and cardiovascular mortality compared to the no-sleep-disorder group and the general population of the Republic of Korea. Patients with insomnia also had increased cardiovascular mortality compared to the no-sleep-disorder group.
A seminal cross-sectional study of OSA and mortality showed that OSA was associated significantly with stroke or death, based on an AHI ≥ 5.24 In an 18-year follow-up study of sleep apnea patients, Young et al.22 found that only the severe OSA group had significantly high HRs of all-cause mortality and cardiovascular mortality (3.8, 95% CI 1.6–9.0; 5.2, 95% CI 1.4–19.2). In a meta-analysis of prospective cohort studies, Wang et al.23 also reported significant associations of severe OSA with all-cause mortality and cardiovascular disease. Consistent with these findings, the SMR of all-cause mortality in the severe OSA group in our study was 1.52, which is 2.7 times higher than that of the no-sleep-disorder group. The SMRs of cardiovascular mortality increased with the severity of OSA.
Although the SMR of all-cause mortality in insomnia was just 0.72, it was still 15% higher than that of the no-sleep-disorder group. The SMR of all-cause mortality in the no-sleep-disorder group, which consisted of subjects with symptoms of sleep disturbances, snoring, or observed apnea, but who did not meet the criteria for any sleep disorder, was significantly lower than that of the general population (SMR = 0.57, p < 0.05). This finding suggests that the all-cause mortality rate of individuals with no sleep disorder is 43% lower than that of the general population. However, the SMRs of other subjects (patients with mild OSA, moderate OSA, and insomnia) were also lower than those of the general population in the Republic of Korea. These results may be attributable to the potential existence of clinical referral bias in this study, as we utilized only patient data collected from the Center for Sleep and Chronobiology at the Seoul National University Hospital. Because the national insurance system in the Republic of Korea does not cover PSG, it is likely that the patients who could afford PSG have a higher socioeconomic status (SES) and more means to obtain the necessary medical care. Thus, the mortality rate of the no-sleep-disorder group was lower than that of the general population. Although adjustments were made when calculating the SMR, it is possible that unmeasured and overlooked confounding variables may have introduced bias into the study. One possible reason why the SMR of the moderate group was lower than that of the mild group may be because more moderate OSA patients could receive treatment using continuous positive airway pressure (CPAP), according to treatment guidelines.29 These findings suggest that early detection and effective treatment of mild OSA may reduce the mortality rate. To further elucidate whether treatment of OSA reduces the mortality rate, studies that take into consideration CPAP treatment and compliance therewith are required.
Cox regression analyses supported these findings, as the HR of all-cause mortality was significantly higher for the severe OSA group than for the no-sleep-disorder group. The reason for such an elevated mortality rate in the severe OSA group may be due to oxygen desaturation from OSA. The average SpO2 was significantly lower in the severe group than in the other groups, and the minimum SpO2 decreased as the severity of OSA increased. Considering that the mortality of OSA is associated with desaturation,30–32 we can hypothesize that the reason why only the severe group was significantly associated with higher all-cause mortality rate is because the average desaturation level during sleep affects mortality more than the minimum desaturation level. This is because the severity of multiple breathing events must be more relevant to predicting the severity of the disorder than that of a single event.
We identified cardiovascular diseases, cancer, and trauma as the three most common causes of death in the cohort. We categorized subjects into these groups to examine the association between each cause and mortality. Only cardiovascular mortality was significantly associated with OSA and the risk of cardiovascular mortality increased with the severity of OSA. The SMR related to cancer and trauma was not significantly different than that reported in studies conducted on Caucasian populations. Cox proportional hazard regression analyses suggested the same result, as cardiovascular mortality was significantly higher in the severe OSA group than in the no-sleep-disorder group; nonetheless, no significant difference in mortality rate was observed for cancer or trauma between these groups. Our results do not support the results of many studies that found an association between OSA severity and increased cancer mortality.32,33 This may be due to the physiological difference that exists between Asians and Caucasians. Asian OSA patients, compared to their Caucasian counterparts, have lower BMIs and higher central fat distribution. However, Asian OSA patients tend to have higher OSA severity because their upper airway is more severely restricted by their craniofacial bone structure.34 In our research, it is apparent that although severe OSA patients have higher BMIs than other groups, the difference is minimal and their BMI is lower than that of Caucasian OSA patients.35
Until now, there have been inconsistent findings across studies about the relationship between insomnia and mortality.21,25,36,37 The SMRs of the insomnia group and the Kaplan-Meier survival curve analyses showed that insomnia was a risk factor for increased all-cause mortality compared to the no-sleep-disorder group. According to Cox regression analyses, the risk of cardiovascular mortality in the insomnia group was 8.11 times higher even after adjusting for age and sex, and the Kaplan-Meier curve showed that the survival rate of the insomnia group was significantly lower than that of the no-sleep-disorder group. This reduced rate is probably due to inflammation and HPA activation associated with the stress and autonomic systems. Our results support a recent report that persistent insomnia is related to cardiovascular mortality, rather than cancer mortality.8 Unfortunately, we could not statistically adjust for hypertension or diabetes; thus, our results cannot provide a clear explanation for the high mortality rate of the insomnia group.10,11,21 However, a previous study that objectively defined insomnia using PSG indicated that short sleep duration is associated with a higher mortality rate,21 which partially supports our results.
A limitation of this study is that we collected data from clinical samples instead of the general population, which could have led to selection bias. However, we employed SMR to detect selection bias and compared the SMR of insomnia and OSA to not only the SMR of the no-sleep-disorder group but also to that of the general population. Because our statistical analyses were based on medical records and PSG results, data on confounding variables (e.g., treatment status, depression, ordinary sleep duration) were limited. Therefore, an extensive study that compiles statistics on subjects' treatment, scale of depression, and average sleep duration will further clarify the association between the risk of mortality and insomnia or OSA. The lack of knowledge about treatment information for insomnia and OSA, including use of CPAP, also limits the findings. We could not assess information about the effects of sleep apnea and insomnia treatment on mortality because treatment adherence and compliance were not fully evaluated for all patients. However studies have shown inconsistent results on the effectiveness of CPAP treatment in reducing mortality as a number of prior studies have reported that CPAP has protective effect against mortality, while other studies were unable to demonstrate a significant correlation between CPAP treatment and mortality.38 In the case of insomnia, some studies have shown that insomnia may be associated with increased mortality after adjusting for hypnotics use.39 However, other studies have shown that increased mortality was associated with the use of hypnotic medications rather than the insomnia it self.40 Future studies with a prospective design will be needed to assess whether or not treatment for OSA and insomnia is associated with reduced mortality. Another limitation of the study is that its retrospective nature precluded the recruitment of healthy participants for the control group. Instead, subjects with no sleep disorder according to PSG were used as a comparison sample.
In spite of the limitations, the large sample size and the long follow-up period of our research add to the validity of our results. In addition, we analyzed the mortality of sleep-disordered patients using both SMR calculations and Cox regression analysis. We compared not only the SMR of OSA patients to that of patients without the disorder but also went further by comparing it to that of the general population. In addition, because we used PSG to determine insomnia levels, we eliminated the problem of subjectivity and the possibility of confounding insomnia symptoms caused by other sleep disorders, which is often a concern in studies where insomnia is only diagnosed by self-reporting. Most previous studies on the mortality rate of OSA patients collected data from subjects who were older than 40 years. However, we decreased the inclusion criteria by lowering the age limit and including subjects from a more diverse age group to minimize the effects of age on mortality rate.
In conclusion, the results of the current study suggest that increased mortality is associated with insomnia and OSA. In particular, severe OSA increased all-cause mortality and cardiovascular mortality compared to the no-sleep-disorder group and the general population. Patients with insomnia showed increased cardiovascular mortality compared to the no-sleep-disorder group. These findings emphasize the need for more active treatment of OSA and insomnia. As the current study has the limitations inherent to any retrospective review of medical records, a large prospective study is needed to elucidate the causal associations of OSA and insomnia with mortality. Moreover, such studies should also examine whether treatment of OSA and insomnia reduces the mortality rate.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CI
confidence interval
- CPAP
continuous positive airway pressure
- HPA
hypothalamic-pituitary-adrenal
- HR
hazard ratio
- OSA
obstructive sleep apnea
- PLMI
periodic limb movement index
- PLMS
periodic limb movements in sleep
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RDI
respiratory distress index
- SE
sleep efficiency
- SMR
standardized mortality ratio
- SpO2
oxygen saturation
- WASO
wake after sleep onset time
REFERENCES
- 1.Babson KA, Del Re AC, Bonn-Miller MO, Woodward SH. The comorbidity of sleep apnea and mood, anxiety, and substance use disorders among obese military veterans within the Veterans Health Administration. J Clin Sleep Med. 2013;9(12):1253–1258. doi: 10.5664/jcsm.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beneto A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13(4):287–293. doi: 10.1016/j.smrv.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 3.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31(4):473–480. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; International Statistical Classification of Diseases and Related Health Problems, Tenth Revision; and Research Diagnostic Criteria/International Classification of Sleep Disorders, Second Edition criteria: results from the America Insomnia Survey. Biol Psychiatry. 2011;69(6):592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 5.Cho YW, Shin WC, Yun CH, Hong SB, Kim J, Earley CJ. Epidemiology of insomnia in Korean adults: prevalence and associated factors. J Clin Neurol. 2009;5(1):20–23. doi: 10.3988/jcn.2009.5.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):136–143. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J, In K, Kim J, et al. Prevalence of sleep-disordered breathing in middle-aged Korean men and women. Am J Respir Crit Care Med. 2004;170(10):1108–1113. doi: 10.1164/rccm.200404-519OC. [DOI] [PubMed] [Google Scholar]
- 8.Parthasarathy S, Vasquez MM, Halonen M, et al. Persistent insomnia is associated with mortality risk. Am J Med. 2015;128(3):268–275.e2. doi: 10.1016/j.amjmed.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stepanski E GM, Zorick F, Roehrs T, Roth T. Heart rate changes in chronic insomnia. Stress Med. 1994;10(4):261–266. [Google Scholar]
- 10.Vgontzas AN, Bixler EO, Lin HM, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab. 2001;86(8):3787–3794. doi: 10.1210/jcem.86.8.7778. [DOI] [PubMed] [Google Scholar]
- 11.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep. 2009;32(4):491–497. doi: 10.1093/sleep/32.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roehrs T, Gumenyuk V, Drake C, Roth T. Physiological correlates of insomnia. Curr Top Behav Neurosci. 2014;21:277–290. doi: 10.1007/7854_2014_324. [DOI] [PubMed] [Google Scholar]
- 13.Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. doi: 10.4088/JCP.11r07586. [DOI] [PubMed] [Google Scholar]
- 14.Seidel WF, Ball S, Cohen S, Patterson N, Yost D, Dement WC. Daytime alertness in relation to mood, performance, and nocturnal sleep in chronic insomniacs and noncomplaining sleepers. Sleep. 1984;7(3):230–238. doi: 10.1093/sleep/7.3.230. [DOI] [PubMed] [Google Scholar]
- 15.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: a bidirectional relationship. Circulation. 2012;126(12):1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 16.Seicean S, Kirchner HL, Gottlieb DJ, et al. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diabetes Care. 2008;31(5):1001–1006. doi: 10.2337/dc07-2003. [DOI] [PubMed] [Google Scholar]
- 17.Gagnon K, Baril AA, Gagnon JF, et al. Cognitive impairment in obstructive sleep apnea. Pathologie-Biologie. 2014;62(5):233–240. doi: 10.1016/j.patbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 18.Mulgrew AT, Ryan CF, Fleetham JA, et al. The impact of obstructive sleep apnea and daytime sleepiness on work limitation. Sleep Med. 2007;9(1):42–53. doi: 10.1016/j.sleep.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Akashiba T, Kawahara S, Akahoshi T, et al. Relationship between quality of life and mood or depression in patients with severe obstructive sleep apnea syndrome. Chest. 2002;122(3):861–865. doi: 10.1378/chest.122.3.861. [DOI] [PubMed] [Google Scholar]
- 20.Chen HC, Su TP, Chou P. A nine-year follow-up study of sleep patterns and mortality in community-dwelling older adults in Taiwan. Sleep. 2013;36(8):1187–1198. doi: 10.5665/sleep.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with short sleep duration and mortality: the Penn State cohort. Sleep. 2010;33(9):1159–1164. doi: 10.1093/sleep/33.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31(8):1071–1078. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Ouyang Y, Wang Z, Zhao G, Liu L, Bi Y. Obstructive sleep apnea and risk of cardiovascular disease and all-cause mortality: a meta-analysis of prospective cohort studies. Int J Cardiol. 2013;169(3):207–214. doi: 10.1016/j.ijcard.2013.08.088. [DOI] [PubMed] [Google Scholar]
- 24.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 25.Phillips B, Mannino DM. Does insomnia kill? Sleep. 2005;28(8):965–971. doi: 10.1093/sleep/28.8.965. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A Manual of Standardized Terminology Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: US Government Printing Office; 1968. [Google Scholar]
- 27.Iber C, Ancoli-Israel S, Chesson AL, Jr., Quan SF for the American Academy of Sleep Medicine. AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 28.Jones ME, Swerdlow AJ. Bias in the standardized mortality ratio when using general population rates to estimate expected number of deaths. Am J Epidemiol. 1998;148(10):1012–1017. doi: 10.1093/oxfordjournals.aje.a009567. [DOI] [PubMed] [Google Scholar]
- 29.Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263–276. [PMC free article] [PubMed] [Google Scholar]
- 30.Hayashi M, Fujimoto K, Urushibata K, Uchikawa S, Imamura H, Kubo K. Nocturnal oxygen desaturation correlates with the severity of coronary atherosclerosis in coronary artery disease. Chest. 2003;124(3):936–941. doi: 10.1378/chest.124.3.936. [DOI] [PubMed] [Google Scholar]
- 31.Myllymaa S, Myllymaa K, Kupari S, et al. Effect of different oxygen desaturation threshold levels on hypopnea scoring and classification of severity of sleep apnea. Sleep Breath. 2015;19(3):947–954. doi: 10.1007/s11325-015-1118-x. [DOI] [PubMed] [Google Scholar]
- 32.Miller YE, Karoor V, Dempsey EC, Fagan KA. Sleep-disordered breathing, hypoxemia, and cancer mortality. Am J Respir Crit Care Med. 2013;187(3):330–331. doi: 10.1164/ajrccm.187.3.330. [DOI] [PubMed] [Google Scholar]
- 33.Marshall NS, Wong KK, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the Busselton Health Study cohort. J Clin Sleep Med. 2014;10(4):355–362. doi: 10.5664/jcsm.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee RW, Vasudavan S, Hui DS, et al. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep. 2010;33(8):1075–1080. doi: 10.1093/sleep/33.8.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Villaneuva AT, Buchanan PR, Yee BJ, Grunstein RR. Ethnicity and obstructive sleep apnoea. Sleep Med Rev. 2005;9(6):419–436. doi: 10.1016/j.smrv.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR. Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry. 2002;59(2):131–136. doi: 10.1001/archpsyc.59.2.131. [DOI] [PubMed] [Google Scholar]
- 37.Rod NH, Vahtera J, Westerlund H, et al. Sleep disturbances and cause-specific mortality: results from the GAZEL cohort study. Am J Epidemiol. 2011;173(3):300–309. doi: 10.1093/aje/kwq371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yuan X, Fang J, Wang L, et al. Adequate continuous positive airway pressure therapy reduces mortality in Chinese patients with obstructive sleep apnea. Sleep Breath. 2015;19(3):911–920. doi: 10.1007/s11325-014-1091-9. [DOI] [PubMed] [Google Scholar]
- 39.Li Y, Zhang X, Winkelman JW, et al. The association between insomnia symptoms and mortality: a prospective study of US men. Circulation. 2014;129(7):737–746. doi: 10.1161/CIRCULATIONAHA.113.004500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kripke DF, Langer RD, Kline LE. Hypnotics' association with mortality or cancer: a matched cohort study. BMJ Open. 2012;2:e000850. doi: 10.1136/bmjopen-2012-000850. [DOI] [PMC free article] [PubMed] [Google Scholar]


