Abstract
Study Objectives:
Posttraumatic stress disorder (PTSD) is common among veterans of the military, with sleep disturbance as a hallmark manifestation.
A growing body of research has suggested a link between obstructive sleep apnea and PTSD, potentially due to obstructive sleep apnea (OSA) related sleep disruption, or via other mechanisms. We examined the hypothesis that treatment of OSA with positive airway pressure would reduce PTSD symptoms over 6 months.
Methods:
A prospective study of Veterans with confirmed PTSD and new diagnosis of OSA not yet using PAP therapy were recruited from a Veteran's Affairs sleep medicine clinic. All subjects were instructed to use PAP each night. Assessments were performed at 3 and 6 months. The primary outcome was a reduction in PTSD symptoms at 6 months.
Results:
Fifty-nine subjects were enrolled; 32 remained in the study at 6 months. A significant reduction in PTSD symptoms, measured by PCL-S score was observed over the course of the study (60.6 ± 2.7 versus 52.3 ± 3.2 points; p < 0.001). Improvement was also seen in measures of sleepiness, sleep quality, and daytime functioning, as well as depression and quality of life. Percentage of nights in which PAP was used, but not mean hours used per night, was predictive of improvement.
Conclusions:
Treatment of OSA with PAP therapy is associated with improvement in PTSD symptoms, although the mechanism is unclear. Nonetheless, PAP should be considered an important component of PTSD treatment for those with concurrent OSA. Improving PAP compliance is a challenge in this patient population warranting further investigation.
Clinical Trial Registration:
ClinicalTrials.gov, ID: NCT02019914.
Commentary:
A commentary on this article appears in this issue on page 5.
Citation:
Orr JE, Smales C, Alexander TH, Stepnowsky C, Pillar G, Malhotra A, Sarmiento KF. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among veterans. J Clin Sleep Med. 2017;13(1):57–63.
Keywords: obstructive sleep apnea, posttraumatic stress disorder, continuous positive airway pressure, lung
INTRODUCTION
Posttraumatic stress disorder (PTSD) is highly prevalent among veterans of the United States armed forces, afflicting 11% to 22% of those returning from Iraq and Afghanistan.1,2 Among Vietnam veterans, the lifetime prevalence approaches 31%, and despite 40 years having elapsed since combat exposure, PTSD remains in 10% of veterans of this era, reflecting the challenges for recovery.3 Patients with PTSD have a substantially reduced quality of life and increased mortality.4,5 Sleep disturbance is often called the “hallmark” of PTSD,6 and formal DSM-5 criteria includes several sleep-related complaints, including recurrent distressing dreams and sleep disturbances. Sleep disturbance has been recognized as both an early predictor of development of PTSD, and a marker of disease severity.7–9 However, whether sleep disturbance is a reflection of disease, or a pre-disposing factor in development and continuance of PTSD is an ongoing subject of debate. Moreover, treatment of PTSD has been elusive, leading to a search for potential therapeutic targets in this condition.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Recent research has revealed an association between OSA and PTSD, although the significance of this association is unclear. We sought to determine if treatment of OSA with CPAP could improve PTSD symptoms.
Study Impact: CPAP appears to improve a variety of symptoms related to PTSD, with greatest improvements in those with better compliance. Future research is needed to elucidate the mechanistic link between PTSD and OSA, and to optimize patient compliance with CPAP.
A growing body of research has suggested an association between OSA and PTSD. Prevalence estimations for OSA in PTSD patients range from 52% to 69%, with some as high as 95%.10–12 The odds of PTSD have been estimated at 2.7-fold higher in patients with sleep apnea.13 While the nature of this interaction is unclear, it has been hypothesized that OSA-related sleep disruption contributes to PTSD, either by contributing to symptoms or more direct influences on disease pathogenesis. In general, untreated OSA might contribute to symptoms of depression and anxiety.14,15 In PTSD patients, recurrent arousals might potentiate nighttime symptoms, such as recall of nightmares, an assertion supported by the finding that treatment of OSA with CPAP appears to reduce the frequency of nightmares in PTSD patients.16 Poor sleep quality related to sleep apnea may also contribute to daytime PTSD symptoms of irritability, impairment in concentration, fatigue, as well as comorbid psychiatric conditions. Sleep apnea has been associated with worsened scores across a wide range of daytime and nighttime symptoms.17 More fundamentally, there are a number of mechanisms by which OSA has been postulated to contribute to disease development, persistence or progression, primarily relating to the effects of state instability.12,18
Given the possible causal role of sleep apnea in PTSD severity, we hypothesized that for patients with concurrent PTSD and OSA, treatment with CPAP might reduce PTSD symptoms and improve quality of life in Veterans of the United States Armed Services.
METHODS
Subjects
This study was approved by the VA San Diego Healthcare System IRB (#H130095). Veterans were recruited from the sleep clinic and were eligible to participate if they had clinically significant PTSD, a new diagnosis of OSA based on home sleep apnea testing, and a willingness to use PAP therapy. The diagnosis of obstructive sleep apnea was established using the NOX-T3 system (Carefusion, Yorba Linda, CA). As part of routine clinical care, all subjects participated in a group class where they received education about OSA and treatment with PAP therapy from a sleep technologist and were instructed on how to apply the recording device. Comprehensive sleep questionnaires were also completed at that time, which included asking subjects about willingness to try PAP therapy if diagnosed with OSA. A single night of sleep using the T3 was used to assess for the presence of sleep disordered breathing. Events were scored according to American Academy of Sleep Medicine criteria, with an apnea-hypopnea index (AHI) 3% greater than or equal to 5 events per hour considered significant. Subjects were enrolled following interpretation of their sleep test but prior to initiating PAP treatment. Auto-titrating PAP devices (ResMed S9 or Philips Remstar Auto with A-flex) were used for therapy (ResMed, San Diego, CA and Respironics, Murrysille, PA). Minimum pressures were empirically selected by the interpreting sleep physician based on body mass index, overall AHI, and dominant event type (apnea versus hypopnea). Maximum pressures were set at 20 cm H2O.
The diagnosis of PTSD was established by a mental health professional utilizing DSM-IV criteria. Severity of PTSD was based on both clinical history and PTSD checklist specific version (PCL-S) score. The PCL-S was selected for use to capture any stressful experience or trauma, not just military experiences. Exclusion criteria were major medical illness with anticipated survival less than 6 months, or inability to travel to follow up appointments.
Following informed consent, subjects underwent baseline evaluations which included a face-to-face interview with a board-certified sleep physician, completion of the Epworth Sleepiness Scale (ESS), patient health questionnaire (PHQ-9), PCL-S, Functional Outcomes of Sleep Short form (FOSQ-10), Pittsburgh Sleep Quality Index (PSQI), and general quality of life (QOL) scale. Subjects were educated about OSA and potential barriers identified related to PAP as part of standard clinical care in VA sleep clinics. Participants were asked to complete one-week sleep diaries at the beginning of the study. Diary entries included nightmare frequency, sedative hypnotic and alcohol use, bedtime, rise time, number of awakenings (and reasons), and barriers to PAP use.
Subjects were scheduled for follow up visits at 3 and 6 months. All questionnaires were again administered at these visits. As part of standard clinical care, the durable medical equipment company providing PAP therapy to veterans at our center, including the subjects enrolled, performed telephone interviews to assess tolerance of therapy and offer help when needed at days 7 and 30 after PAP initiation.
PAP Data
PAP device downloads were performed at 3- and 6-month follow-up visits. Data abstracted included percentage of nights used, average hours used per night, and percentage of nights used more than 4 hours, and residual device AHI for time periods 0–3 months, 3–6 months, and 0–6 months.
Outcome Measures
The primary outcome of this study was pre-specified as a change in PCL-S score from baseline to 6 months. Secondary outcomes included changes in scores for the other questionnaires (PHQ-9, PSQI, FOSQ-10, QOL, and ESS), as well as PAP compliance and efficacy. Data from the 3-month follow-up were also examined to better understand the timing of any changes. Insomnia, traumatic brain injury (TBI), and medication use were examined for interaction effects.
Statistical Analysis
Baseline characteristics were compared between the initial and follow up cohorts using a t-test, rank sum, or Fisher exact test as appropriate. Change in PCL-S score from baseline through 3 and 6 months was compared using ANOVA. Subjects in whom data were only available up to 3 months were included in the analysis using mixed effects modeling. The association between change in PCL-S score and covariates were analyzed using linear regression. Data were analyzed using SAS (SAS Institute Inc, Cary, NC). A p value of < 0.05 was defined as statistically significant. Based on a reported minimum reliable PCL change of 5 points and an estimated standard deviation of 10 points, enrollment was targeted to 34 subjects to achieve a power of 80%.
RESULTS
Subjects
A total of 172 patients were screened for inclusion. Sixty patients provided informed consent and 1 patient was excluded due to not meeting PTSD diagnostic criteria (erroneous medical record entry of the diagnosis). A total of 59 subjects were therefore enrolled. Forty-one subjects completed the 3-month follow-up, and 32 subjects completed the 6-month follow-up. Subject flow throughout the study is shown in Figure 1. Baseline characteristics of subjects who participated through each study interval are shown in Table 1.
Figure 1. CONSORT diagram of subjects and follow up through the study.
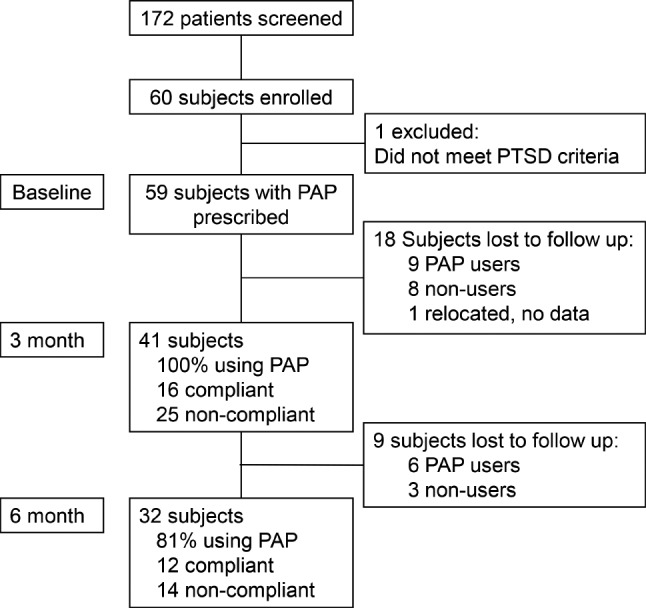
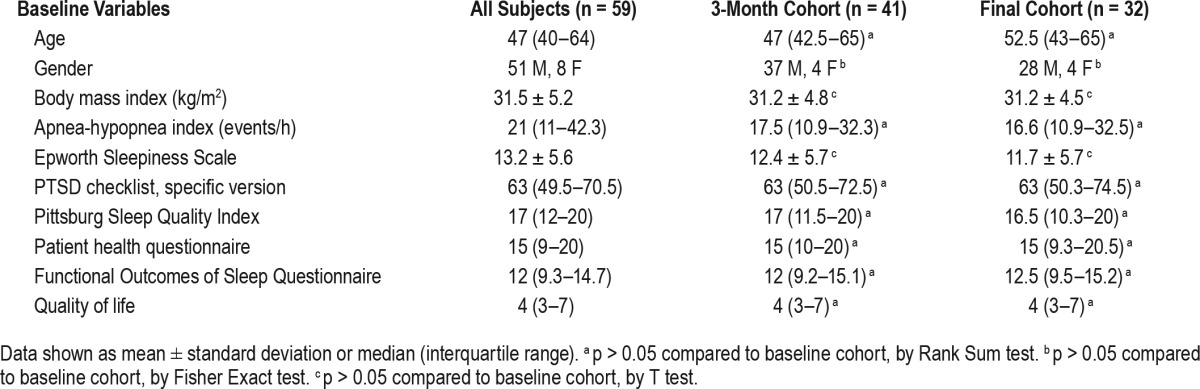
Table 1.
Baseline characteristics of subjects participating in the study.
Change in PTSD Symptoms
For the final cohort of 32 subjects, there was a significant reduction in PCL-S score (Figure 2) between baseline and 3- and 6-month visits. The mean reduction from baseline to 3-month follow-up was 8.3 points and from baseline to 6 months was 8.2 points. No significant change was observed in PCL-S score between 3 and 6 months (difference 0.13; p = 0.951). A mixed model analysis incorporating the 41 subjects with at least 3-month follow-up data revealed similar findings (Table 2).
Figure 2. For the final cohort (n = 32) PCL-S scores decreased significantly across baseline, 3 months, and 6 months follow up (p < 0.001 for the trend by ANOVA).
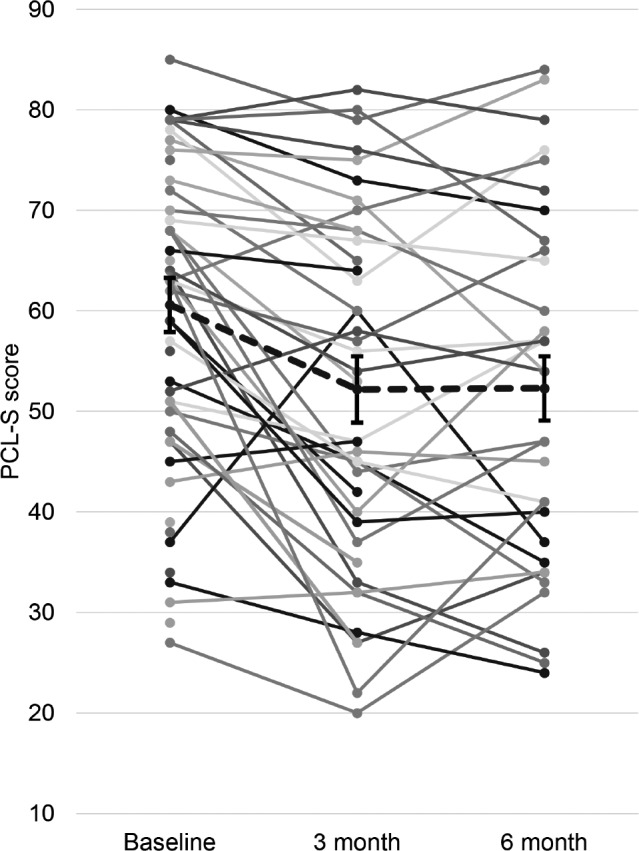
Mean score (dotted line) decreased from baseline to 3 months (60.6 versus 52.2 points; p < 0.001) but did not change significantly from 3 months to 6 months (52.2 versus 52.3; p = 0.95). Graph includes all subjects (n = 59) denoted as solid dots and lines, including those with only baseline (n = 18) or 3 month follow up (n = 9) for illustrative purposes.
Table 2.
Mixed effects model in subject with at least 3-month follow up (n = 41).
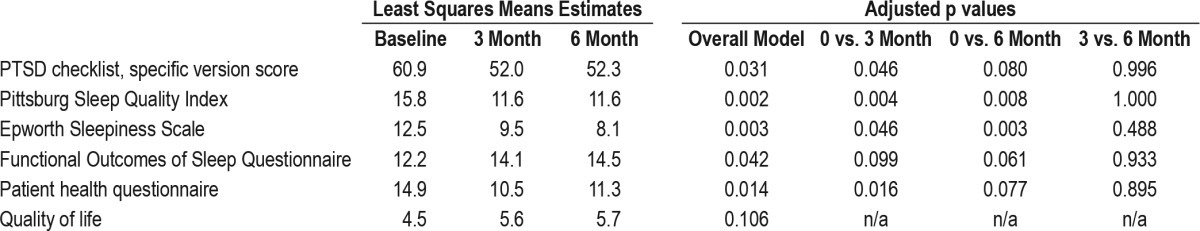
Change in Other Measures
Significant improvements were observed in PSQI, PHQ-9, FOSQ-10, QOL and ESS over the course of the study (Table 3). Improvements in each measure seen throughout the study were established by the 3-month visit; no significant improvement was noted between the 3- and 6-month visits for these measures. A mixed model analysis incorporating the 41 subjects with at least 3-month follow-up data similarly revealed significant improvements in PSQI, PHQ-9, FOSQ-10, QOL, and ESS (Table 2).
Table 3.
Change in symptoms in the final cohort (n = 32) during baseline, 3-month, and 6-month follow-up.
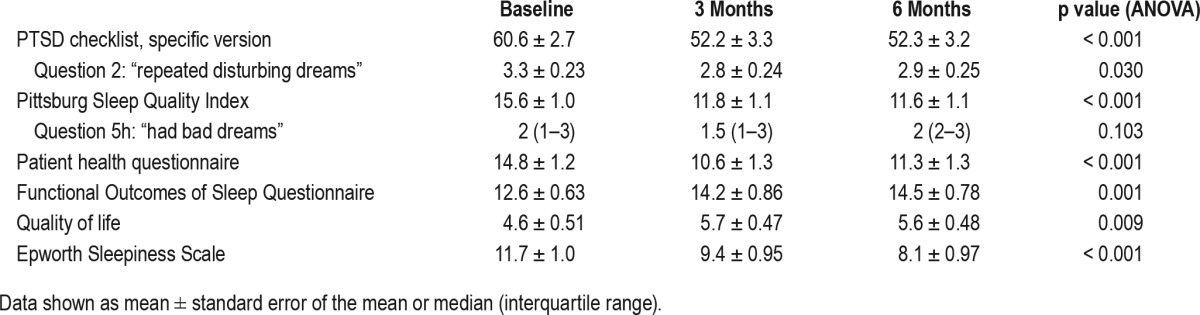
Questions within the PCL-S and PSQI questionnaires were used to assess nightmares. An improvement over the course of the study was observed in PCL-S question 2 (“repeated disturbing dreams”) (Table 3), although the improvement between baseline and 3 month follow up was not statistically significant at 6 months. No improvement was observed in PSQI question 5h (“had bad dreams”).
CPAP Efficacy and Compliance
At 3 months, all 42 subjects were using PAP therapy, with 39% meeting CMS criteria for compliance (> 4h per night for > 70% of nights). At 6 months, 81% of subjects were still using PAP therapy, with 46% of users meeting Medicare compliance criteria. Across the 6 months of follow-up, average hours of PAP use were 3.5 ± 2.6 hours. The mean percentage of nights used was 59% ± 36%, and percentage nights with > 4 h use was 45% ± 37%. Device related AHI information revealed a residual mean AHI of 3.8 ± 7.4 events/h at 3 months and 2.0 ± 1.7 events/h at 6 months. All patients except one (with mild OSA) had at least a 50% reduction in AHI and residual AHI < 5 events/h at 6 months.
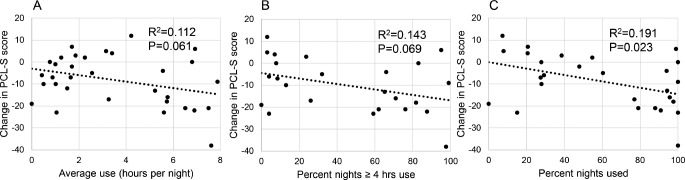
During this time period, there was no significant correlation between average hours of PAP use and change in PCL-S score or for percentage nights with use > 4 hours and PCL-S score (Figure 3). However, increasing percentage nights used was predictive of improvement in PCL-S score (Figure 3).
Figure 3. Association between PAP use (averaged over 6 months) and change in PCL-S score from baseline to 6-month follow-up.
Neither average hours per night of PAP use (A) nor percentage of nights with > 4 h of PAP use (B) significantly predicted a change in PCL-S score (p = 0.061 and p = 0.069, respectively). Increasing percentage of nights with PAP use was found to modestly predict improvement in PCL-S score (C) (p = 0.023; R2 = 0.191; B = −0.146).
Sleep Duration and Insomnia
Self-reported sleep duration did not change from baseline to 3 months and 6 months (6.2 ± 0.37 versus 6.6 ± 0.36 versus 6.6 ± 0.37 hours; p = 0.235 by ANOVA). Using each subject's average hours of PAP use divided by self-reported sleep duration, an estimated percentage of sleep with PAP use over the 6 months was calculated (mean 56% ± 33%). There was no association between this measure and 6-month change in PCL-S score (p = 0.279). In multivariable linear modeling, there was no association or interaction of self-reported hours of sleep and average hours of PAP use on change in PCL-S score at 6 months (p = 0.207 overall).
Those with insomnia did have lower mean hours PAP use compared to those without (1.8 ± 0.6 versus 3.7 ± 0.5 h/night; p = 0.032) but no statistically significant difference in percentage nights used (45% ± 17% versus 61% ± 8%; p = 0.493). However, the presence of insomnia was not predictive of change in PCL-S score at 6 months. There was no significant independent or interactive effect of insomnia with percentage nights of PAP use on the change in PCL-S score at 6 months (p = 0.08 overall, p = 0.477 for insomnia, and p = 0.271 for the interaction).
In addition, a history of traumatic brain injury, use of narcotics, use of sedative-hypnotics, did not predict change in PCL-S score. For each of these variables, multivariable linear modeling including percent of nights of PAP use and an interaction term to predict change in PCL-S score was performed. The only significant model was for prazosin: (p = 0.005 overall, p = 0.011 for percent nights used, p = 0.377 for prazosin, and p = 0.008 for the interaction).
DISCUSSION
The major finding of this study was a modest reduction in PTSD symptoms as determined by PCL-S scores in subjects with concurrent OSA who were treated with CPAP for 6 months. This finding is in line with a prior retrospective study suggesting an improvement in PTSD with use of CPAP for OSA.19 The mean change observed reached statistical significance for the PCL-S survey instrument (decrease of at least 5 points), but was less than the threshold often cited for clinical significance (decrease of at least 10 points).20 Nonetheless, PAP therapy is a therapy with few downsides, and thus the benefit observed in this study is encouraging.
Improvement was also noted in the other assessments measuring sleep quality, daytime functioning, and quality of life. Of note, the improvements occurred within the first 3 months of treatment, suggesting that even short duration of PAP use can be efficacious and strategies to improve compliance early in the treatment period should be emphasized. Conversely, substantial residual PTSD symptoms might not be expected to improve after this early PAP treatment period, and therefore additional PTSD treatments such as pharmacotherapy and prolonged exposure therapy remain highly important.
While the correlation between improvement in PTSD symptoms and duration of PAP use each night did not reach statistical significance, PTSD improvement was associated with higher percentage of nights used. This finding suggests that the improvement in PTSD is mediated by PAP use, although the absence of a clear relationship with hours used per night might indicate that the amount of use needed for benefit may differ among individuals. Of note, the overall PAP compliance was poor, which is consistent with recent literature revealing lower PAP use and greater symptoms among OSA patients with PTSD compared to OSA patients without PTSD.21–23 Our findings are complementary in that benefits—albeit modest— are associated with PAP compliance, while emphasizing that there are unique treatment challenges in this population.
One potential modifier of the effect of PAP is insomnia and resulting short sleep duration. Subjects with relatively low hours of PAP use each night may have had either shorter sleep times, or simply were not compliant with PAP use during sleep. Those with short sleep duration might benefit by PAP compliance—even if absolute hours of use are low—if prevention of OSA-related hypoxemia and fragmentation is of key importance in improving PTSD. On the other hand, if a longer period of consolidated sleep is most important, the presence of insomnia might negate any benefit to PAP use, as overall sleep duration remains inadequate. In examining this relationship, we found no significant modifying effect of insomnia or sleep duration on the benefit of PAP use, but a dedicated study on this topic would be required to make definitive conclusions.
The mechanism by which treatment of OSA might lead to improvement in PTSD is unclear.9 Overlap in the effects of untreated OSA and diagnostic or severity markers of PTSD is considerable, such as daytime fatigue, irritability, and difficulty concentrating. On the other hand, several lines of evidence regarding the pathophysiology of PTSD indicate that OSA treatment might have a more fundamental effect on disease. First, REM abnormalities are common in PTSD.24 OSA might further fragment sleep, worsening a proposed REM deficit, which might be an impediment to emotional processing. The importance of avoiding disrupted REM sleep is exemplified by nightmares, as studies in holocaust survivors have suggested that nightmare recall, but not the presence of nightmares, is associated with poor recovery from PTSD.25 Respiratory events related to OSA might interrupt dreams that, even if disturbing, are important in emotional processing.26 Second, complex effects on the hypothalamic-pituitary-adrenal (HPA) axis leading to dysfunction have been observed in PTSD.27 In untreated OSA, the HPA axis is hyperactive, which may interact with alterations seen in PTSD in maladaptive ways. Finally, hippocampal function and structure is impaired in PTSD, which might account for deficits in fear extinction and neuroplasticity that could help with recovery.28 Reductions in growth hormone are felt to be responsible for these changes,29 and appear to be caused by sleep fragmentation, to which OSA would contribute. On the other hand, PTSD may worsen OSA by promoting sleep fragmentation and recurrent arousals. A low arousal threshold is thought to contribute to OSA in some patients based on state instability and by allowing insufficient time for accumulation of respiratory stimuli to activate upper airway dilator muscles and promote pharyngeal patency.18 Thus, further work is clearly required, but biological plausibility exists that OSA and PTSD are mechanistically linked.
Additional physiological studies examining these putative mechanisms in PTSD patients with and without OSA are warranted. For PTSD patients with OSA, carefully controlled treatment studies utilizing PAP may provide the most definitive evidence as to whether there is a true pathophysiological link between these two disorders.
Limitations
This study was performed in a population referred for sleep studies, rather than pure PTSD cohort, and although OSA screening is common in the VA system, this design may have introduced some referral bias. Despite similar baseline characteristics between those who enrolled in the study and subjects who remained at 6 months, a relatively high attrition rate might have affected the results, with concern that those subjects least likely to improve might not have followed up during the study. We cannot exclude that PAP contributed to the dropout of these subjects, including the possibility that PAP worsened PTSD symptoms in that group. However, our conclusions are limited to the sample studied as we cannot draw conclusions about those who did not follow-up. Due to the clinical indications for PAP use and difficulties providing sham PAP treatment, no control group was examined for comparison. Therefore, it is possible that the effect seen on PTSD symptoms was due to co-interventions of the study or natural history of disease. In addition, the association between PAP use and improvement in PTSD could be due to a “healthy user” effect, whereby those who are compliant with one intervention may be more compliant with non-study interventions, which were not controlled during this study. However, we doubt substantial efficacy of any co-interventions in PTSD based on our clinical experience and the existing literature. Finally, the sample size in this study was small, and a larger study would be needed to confirm these findings, better quantify the magnitude of effect, and investigate important covariates. Despite these limitations, we believe that our new findings have value and that they provide compelling rationale for further investigation.
CONCLUSIONS
In patients with PTSD and concurrent OSA, treatment with PAP is associated with modest improvements in PTSD symptoms, as well as other subjective measures. These benefits are seen within the first 3 months of treatment, but do not appear to diminish at 6 months. PAP compliance appears to be associated with improvement, although comorbid insomnia may complicate this relationship. Future investigations specifically exploring strategies to concurrently treat OSA and PTSD are warranted.
DISCLOSURE STATEMENT
Funding by American Sleep Medicine Foundation 2013 Jr. Faculty Research Award. Dr. Sarmiento was funded by the American Sleep Medicine Foundation. She has received an investigational devices/drugs from Jazz Parmaceuticals and is a consultant for Jazz Pharmaceuticals. Dr. Malhotra is PI on NIH RO1 HL085188, K24 HL132105, and co-investigator on R21 HL121794, RO1 HL 119201, RO1 HL081823. As an Officer of the American Thoracic Society, Dr. Malhotra has relinquished all outside personal income since 2012. ResMed, Inc. provided a philanthropic donation to the UC San Diego in support of a sleep center. Dr. Pillar is on the speakers' bureau of Sanofi, Itamar-Medical, and TEVA; and has received research support, investigational devices/drugs and is a consultant for Itamar-Medical. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- HPA
hypothalamic-pituitary-adrenal
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PCL-S
PTSD checklist, specific version
- PHQ-9
patient health questionnaire
- PSQI
Pittsburg Sleep Quality Index
- PTSD
posttraumatic stress disorder
- QOL
quality of life
- TBI
traumatic brain injury
- VA
Veterans Affairs
REFERENCES
- 1.Seal KH, Metzler TJ, Gima KS, Bertenthal D, Maguen S, Marmar CR. Trends and risk factors for mental health diagnoses among Iraq and Afghanistan veterans using Department of Veterans Affairs health care, 2002-2008. Am J Public Health. 2009;99(9):1651–1658. doi: 10.2105/AJPH.2008.150284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351(1):13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- 3.Koenen KC, Stellman SD, Sommer JF, Stellman JM. Persisting posttraumatic stress disorder symptoms and their relationship to functioning in Vietnam Veterans: a 14-year follow-up. J Trauma Stress. 2008;21(1):49–57. doi: 10.1002/jts.20304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boscarino JA. Posttraumatic stress disorder and mortality among U.S. Army Veterans 30 years after military service. Ann Epidemiol. 2006;16(4):248–256. doi: 10.1016/j.annepidem.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Hoge CW, Terhakopian A, Castro CA, Messer SC, Engel CC. Association of posttraumatic stress disorder with somatic symptoms, health care visits, and absenteeism among Iraq War Veterans. Am J Psychiatry. 2007;164(1):150–153. doi: 10.1176/ajp.2007.164.1.150. [DOI] [PubMed] [Google Scholar]
- 6.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry. 1989;146(6):697–707. doi: 10.1176/ajp.146.6.697. [DOI] [PubMed] [Google Scholar]
- 7.Koren D, Arnon I, Lavie P, Klein E. Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry. 2002;159(5):855–857. doi: 10.1176/appi.ajp.159.5.855. [DOI] [PubMed] [Google Scholar]
- 8.Belleville G, Guay S, Marchand A. Impact of sleep disturbances on PTSD symptoms and perceived health. J Nerv Ment Dis. 2009;197(2):126–132. doi: 10.1097/NMD.0b013e3181961d8e. [DOI] [PubMed] [Google Scholar]
- 9.Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med Rev. 2000;4(2):183–200. doi: 10.1053/smrv.1999.0095. [DOI] [PubMed] [Google Scholar]
- 10.Yesavage JA, Kinoshita LM, Kimball T, et al. Sleep-disordered breathing in Vietnam veterans with posttraumatic stress disorder. Am J Geriatr Psychiatry. 2012;20(3):199–204. doi: 10.1097/JGP.0b013e3181e446ea. [DOI] [PubMed] [Google Scholar]
- 11.Krakow B, Haynes PL, Warner TD, et al. Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress. 2004;17(3):257–268. doi: 10.1023/B:JOTS.0000029269.29098.67. [DOI] [PubMed] [Google Scholar]
- 12.Jaoude P, Vermont LN, Porhomayon J, El-Solh AA. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann Am Thorac Soc. 2015;12(2):259–268. doi: 10.1513/AnnalsATS.201407-299FR. [DOI] [PubMed] [Google Scholar]
- 13.Sharafkhaneh A, Giray N, Richardson P, Young T, Hirshkowitz M. Association of psychiatric disorders and sleep apnea in a large cohort. Sleep. 2005;28(11):1405–1411. doi: 10.1093/sleep/28.11.1405. [DOI] [PubMed] [Google Scholar]
- 14.Pillar G, Lavie P. Psychiatric symptoms in sleep apnea syndrome: effects of gender and respiratory disturbance index. Chest. 1998;114(3):697–703. doi: 10.1378/chest.114.3.697. [DOI] [PubMed] [Google Scholar]
- 15.Gupta MA, Simpson FC, Lyons DC. The effect of treating obstructive sleep apnea with positive airway pressure on depression and other subjective symptoms: a systematic review and meta-analysis. Sleep Med Rev. 2016;28:55–68. doi: 10.1016/j.smrv.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Tamanna S, Parker JD, Lyons J, Ullah MI. The effect of continuous positive air pressure (CPAP) on nightmares in patients with posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) J Clin Sleep Med. 2014;10(6):631–636. doi: 10.5664/jcsm.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krakow B, Melendrez D, Johnston L, et al. Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis. 2002;190(7):442–452. doi: 10.1097/00005053-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383(9918):736–747. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291–298. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 20.Monson CM, Gradus JL, Young-Xu Y, Schnurr PP, Price JL, Schumm JA. Change in posttraumatic stress disorder symptoms: do clinicians and patients agree? Psychol Assess. 2008;20(2):131–138. doi: 10.1037/1040-3590.20.2.131. [DOI] [PubMed] [Google Scholar]
- 21.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495–1500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lettieri CJ, Williams SG, Collen JF. OSA syndrome and posttraumatic stress disorder: clinical outcomes and impact of positive airway pressure therapy. Chest. 2016;149(2):483–490. doi: 10.1378/chest.15-0693. [DOI] [PubMed] [Google Scholar]
- 23.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667–672. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engdahl BE, Eberly RE, Hurwitz TD, Mahowald MW, Blake J. Sleep in a community sample of elderly war veterans with and without posttraumatic stress disorder. Biol Psychiatry. 2000;47(6):520–525. doi: 10.1016/s0006-3223(99)00201-2. [DOI] [PubMed] [Google Scholar]
- 25.Kaminer H, Lavie P. Sleep and dreaming in Holocaust survivors. Dramatic decrease in dream recall in well-adjusted survivors. J Nerv Ment Dis. 1991;179(11):664–669. doi: 10.1097/00005053-199111000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Lavie P, Katz N, Pillar G, Zinger Y. Elevated awaking thresholds during sleep: characteristics of chronic war-related posttraumatic stress disorder patients. Biol Psychiatry. 1998;44(10):1060–1065. doi: 10.1016/s0006-3223(98)00037-7. [DOI] [PubMed] [Google Scholar]
- 27.de Kloet CS, Vermetten E, Geuze E, Kavelaars A, Heijnen CJ, Westenberg HG. Assessment of HPA-axis function in posttraumatic stress disorder: pharmacological and non-pharmacological challenge tests, a review. J Psychiatr Res. 2006;40(6):550–567. doi: 10.1016/j.jpsychires.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152(7):973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E, Grover LM, Bertolotti D, Green TL. Growth hormone rescues hippocampal synaptic function after sleep deprivation. Am J Physiol Regul Integr Comp Physiol. 2010;298(6):R1588–R1596. doi: 10.1152/ajpregu.00580.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]