Abstract
Study Objectives:
Home-based management of sleep-disordered breathing (SDB) generally excludes patients with significant medical comorbidities, but such an approach lacks scientific evidence. The current study examined whether significant medical comorbidities are associated with persistent hypoxia that requires unanticipated nocturnal O2 supplementation to positive airway pressure (PAP) therapy. Conceivably, in such patients, home-based management of SDB may not detect or therefore adequately treat persistent hypoxia.
Methods:
In this retrospective study of 200 patients undergoing laboratory-based polysomnography, we ascertained significant medical comorbidities (chronic obstructive pulmonary disease, congestive heart failure, and morbid obesity) and their association with the need for unanticipated O2 supplementation to PAP therapy. Postural oxygen (SpO2) desaturations between upright and reclining positions were determined during calm wakefulness.
Results:
Postural change in SpO2 during calm wakefulness was greater in patients who eventually needed nocturnal O2 supplementation to PAP therapy than those needing PAP therapy alone (p < 0.0001). The presence of chronic obstructive pulmonary disease (odds ratio [OR] 6.0; 95% confidence interval [CI]; 2.1, 17.5; p = 0.001), morbid obesity (OR 3.6; 95% CI 1.9, 7.0; p < 0.0001), and age older than 50 y (OR 2.8; 95% CI 1.3, 5.9; p = 0.007) but not heart failure were associated with unanticipated need for nocturnal O2 supplementation. A clinical prediction rule of less than two determinants (age older than 50 y, morbid obesity, chronic obstructive pulmonary disease, and postural SpO2 desaturation greater than 5%) had excellent negative predictive value (0.92; 95% CI 0.85, 0.96) and likelihood ratio of negative test (0.08; 95% CI 0.04, 0.16).
Conclusions:
Medical comorbidities can predict persistent hypoxia that requires unanticipated O2 supplementation to PAP therapy. Such findings justify the use of medical comorbidities to exclude home management of SDB.
Commentary:
A commentary on this article appears in this issue on page 7.
Citation:
Shetty S, Fernandes A, Patel S, Combs D, Grandner MA, Parthasarathy S. Unanticipated nocturnal oxygen requirement during positive pressure therapy for sleep apnea and medical comorbidities. J Clin Sleep Med. 2017;13(1):73–79.
Keywords: clinical prediction rule, home sleep apnea testing, obstructive sleep apnea, oxygen, portable testing, positive airway pressure therapy
INTRODUCTION
Home-based management of sleep-disordered breathing (SDB) utilizing home sleep apnea testing (HSAT) and automatic positive airway pressure therapy (autoPAP) is increasingly used as a management pathway for SDB.1–10 Such home-based management of SDB generally excludes patients with significant medical comorbidities because most research studies on which recommendations were based excluded patients with such comorbidities.11 Specifically, significant comorbid medical conditions are thought to degrade the accuracy of HSAT.11 Such comorbid medical conditions include chronic obstructive pulmonary disease (COPD), morbid obesity, and heart failure.11 Whether the existence of such significant comorbid medical conditions contributes to substandard diagnostic or therapeutic management of patients with SDB through home-based management is unclear, and data to support appropriate patient selection for home-based management were recently identified as a research priority.1,12 Moreover, a recent meta-analysis of 59 studies involving 5,026 patients found that none of the studies included patients with unstable medical comorbidities and a small number included mild comorbidities such as hypertension and asthma.13
BRIEF SUMMARY
Current Knowledge/Study Rationale: Home-based management of sleep-disordered breathing (SDB) generally excludes patients with significant medical comorbidities (such as severe chronic obstructive pulmonary disease, congestive heart failure, and morbid obesity), but such an approach lacks scientific evidence. Moreover, patients prescribed home sleep studies and automatic positive airway pressure (PAP) therapies may not be completely assessed for supplemental oxygen therapy needs in addition to PAP therapy.
Study Impact: We tested a clinical prediction rule of less than two determinants (age older than 50 y, morbid obesity, chronic obstructive pulmonary disease, and postural SpO2 desaturation higher than 5%) that had excellent negative predictive value for need for unanticipated oxygen supplementation that could assist in appropriate triage of patients through home-based (portable) over laboratory-based management pathways for their SDB.
Patients with comorbid medical conditions such as morbid obesity or COPD, by virtue of sleep-related nocturnal hypoventilation, may require unanticipated additional treatment for SDB. For example, nocturnal oxygen (O2) supplementation may be required in addition to positive airway pressure (PAP) therapy, due to persistent hypoxemia despite PAP therapy alone. For the purposes of this study, unanticipated O2 supplementation was defined as occurring in patients who were not originally hypoxic or requiring daytime O2 supplementation prior to sleep diagnostic testing, who then required O2 supplementation in addition to PAP therapy after sleep laboratory-based testing. If these individuals had undergone HSAT, such a need for O2 supplementation would not have been discovered, because overnight laboratory-based testing is generally not performed in patients who are initiated on auto-PAP therapy following HSAT. We aimed to determine whether significant medical comorbidities are associated with persistent nocturnal hypoxia that requires unanticipated O2 supplementation to PAP therapy in the setting of a retrospective cohort of patients undergoing laboratory-based polysomnography. This would provide evidence for the recommendation that individuals with these comorbidities should be evaluated in the laboratory, because home testing would not have discovered a need for supplementary O2. To address this aim, we performed a retrospective chart review of patients who underwent laboratory-based sleep testing to determine whether the presence of medical comorbidities (COPD, morbid obesity, and/or heart failure) led to unanticipated O2 supplementation. The hypothesis for the study was that those with medical comorbidities were more likely to require unanticipated oxygen supplementation. If this is the case, then that would support the idea that home-based management of SDB would not detect, or therefore adequately treat, persistent hypoxia in patients with significant comorbid medical conditions who are issued an autoPAP device. The rationale for performing a retrospective chart review of laboratory-based polysomnography patients, as opposed to a prospective study of home-sleep study patients, was to prevent possible and posited harm that is inherent to a prospective study design in at-risk participants. The time spent below oxygen saturation of 90% has been associated with increased risk for all-cause mortality.14 Therefore, knowingly allowing patients to remain hypoxic under the auspices of a prospective research study with informed consent would be opposed to the principle of beneficence.15 Allowing persistent hypoxemia may place research participants under risk, however small, when participating in such a research study. Recently, there have been controversies pertaining to allowing hypoxemia under the auspices of research, even though there may have been perceived clinical equipoise or acceptance at the time of commencing such a study.16
METHODS
We performed a retrospective, case-control study at a single center by reviewing consecutive electronic medical records between January 2012 and December 2014 after receiving appropriate approval from the Institutional Review Board at the University of Arizona (Protocol# 1411574542). Patients who were prescribed PAP therapy with unanticipated entrained O2 were identified (n = 50) and patients who were prescribed PAP therapy alone (n = 150) were identified from an electronic database that was maintained at the sleep center.
Inclusion criteria were age older than 18 y and completion of a laboratory-based overnight polysomnogram. Exclusion criteria included patients who were already on O2 prior to undergoing polysomnography; hypoxemia during wakefulness defined as having oxygen saturation (SpO2) ≤ 88%; lacking SpO2 measurements while in the upright position in the clinic visits within 3 mo prior to the polysomnography; and patients with the head-end of the bed elevated on the night of the sleep study (as documented by the sleep technician and video recordings on the night of the sleep study). Patients without baseline SpO2 measurement in room air within 3 mo of the polysomnography were excluded (n = 28). Titration of PAP therapy was performed according to clinical guidelines for the manual titration of PAP in patients with obstructive sleep apnea (OSA).17 Initiation of entrained oxygen during the manual titration required that OSA be treated adequately (apnea-hypopnea index < 5 events/h) with oxygen saturation ≤ 88% for greater than 5 min.17
We screened patient data of consecutive patients between January 2012 and December 2014 to yield 50 cases and 150 controls. The cases were patients who were not on oxygen supplementation at the time of referral to the sleep laboratory but then were prescribed such therapy in addition to positive airway pressure therapy after the sleep study was performed. Over this period of time, 452 of the 1,536 consecutive sleep studies were duplicates of patients returning for PAP titration. These and other studies yielded the required 150 consecutive controls that met the selection criteria as per a 1:3 case-to-control ratio that was determined a priori. Four hundred eighty-two sleep studies were excluded because those patients met the exclusion criteria.
Determination of Postural Oxygen Desaturation
The rationale for assessing postural oxygen desaturation measured by SpO2 was to quantify the respiratory status that can be compromised in the supine position and was obtained in the following manner. Recumbent SpO2 was derived from the polysomnography data during a period of calm wakefulness in the supine posture (confirmed by electroencephalography and digital time-synchronized video). Such information was available in the time period soon after the performance of bio-calibrations while the patient was in the supine position and breathing at a steady state. In contrast to such measurements obtained in the supine position during the polysomnography, during the clinic visit, patients had their oxygen saturation checked by finger pulse oximetry in sitting posture soon after undergoing measurement of other vital signs such as pulse rate and blood pressure. The postural change in SpO2 was assessed as SpO2 in the sitting position (obtained from electronic medical record chart review that was performed during the clinic visit) minus SpO2 measured in the recumbent calm wakefulness position (derived from polysomnography data).
Patient's age, sex, and medical history for significant comorbid conditions (history of heart failure, COPD, neuromuscular disease) as well other patient characteristics (smoking history) and polysomnography variables were collected by reviewing the medical records. Physician diagnosis of comorbidities that was documented in the electronic medical record was required for such comorbidities to be extracted as being present. Additionally, apnea-hypopnea index, SpO2 during rapid eye movement and nonrapid eye movement sleep were also extracted from the polysomnography reports.
Statistical Analysis
Data were assessed for normality of distribution using one-sample Kolmogorov–Smirnov test. Receiver operating characteristic (ROC) curves were generated for identifying a threshold level with best discriminant function for postural SpO2 desaturation. Sensitivity, specificity, negative predictive value, positive predictive value, and likelihood ratios were calculated by generating 2 × 2 contingency tables. Student t-test or Mann-Whitney U tests were used for making comparisons as appropriate for parametric and nonparametric data. Simple and multiple logistic regressions were performed with dependent variable being the prescription for unanticipated O2 supplementation to PAP therapy. From the list of predictors and potential confounders, simple logistic regression analysis was performed to identify significant factors (independent variables) that influenced the prescription for unanticipated O2 supplementation. Subsequently, we built multivariate logistic regression models with prescription for unanticipated O2 supplementation as the dependent variable using significant independent variables identified by univariate logistic regression analysis (p ≤ 0.10). Multicollinearity between independent variables was verified, and in the event of collinearity, the strongest predictor variable alone was included. Results are presented as mean and standard deviation unless otherwise specified. Values of p < 0.05 were considered significant. All analysis was performed using SPSS version 20.0 (IBM SPSS Inc., Armonk, NY). Unadjusted proportions were compared using Pearson χ2 test.
RESULTS
Patient characteristics by group (PAP with and without unanticipated entrained O2) are provided in Table 1. Patients who eventually required PAP with unanticipated entrained O2 were older, had greater body mass index (BMI), with lower nadirs during polysomnography, and greater postural SpO2 desaturation. Notably, the presence of congestive heart failure was not associated with increased risk for needing unanticipated O2 supplementation (p = 0.25). Degree of postural oxygen desaturation measured by finger pulse oximetry (SpO2) was greater in patients who eventually needed PAP with unanticipated entrained O2 than those needed PAP therapy alone (Figure 1). ROC curve for degree of postural desaturation in SpO2 as a predictor of need for unanticipated O2 supplementation to PAP therapy in patients with SDB is shown in Figure 2 (ROC area under the curve [AUC] 0.73; 95% confidence interval [CI] 0.65, 0.82). A threshold value of 5% SpO2 desaturation was selected with the best test characteristics. Similarly, a threshold of 50 y for age was identified. For BMI the threshold for conventional definition for morbid obesity (35 kg/m2) was used. Analysis with a threshold value for morbid obesity as 40 or 45 kg/m2 yielded weaker associations with unanticipated oxygen prescription (odds ratio [OR] 3.0; 95% CI 1.5, 6.1; p = 0.002 and OR 1.8; 95% CI 0.8, 4.3; p = 0.16) than that for 35 kg/m2 (OR 3.6; 95% CI 1.9, 7.0; p < 0.0001; Table 2).
Table 1.
Patient characteristics.
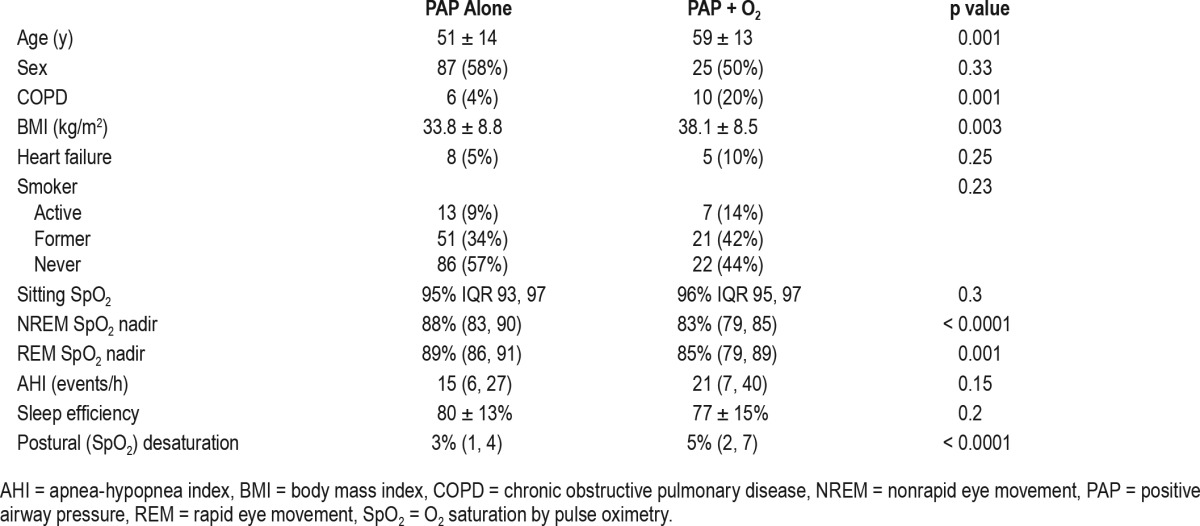
Figure 1. Degree of postural oxygen desaturation in finger pulse oximetry (SpO2) was greater in patients who eventually needed positive airway pressure (PAP) with O2 supplementation than PAP therapy alone.
PAP alone = patients who needed PAP therapy alone, PAP + O2 = patients who needed PAP therapy with unanticipated O2 supplementation for their sleep-disordered breathing (Mann-Whitney U test).
Figure 2. Receiver operating characteristic (ROC) curve for degree of postural desaturation in O2 saturation by pulse oximetry as a predictor of need for O2 supplementation in addition to PAP therapy in patients with sleep-disordered breathing.
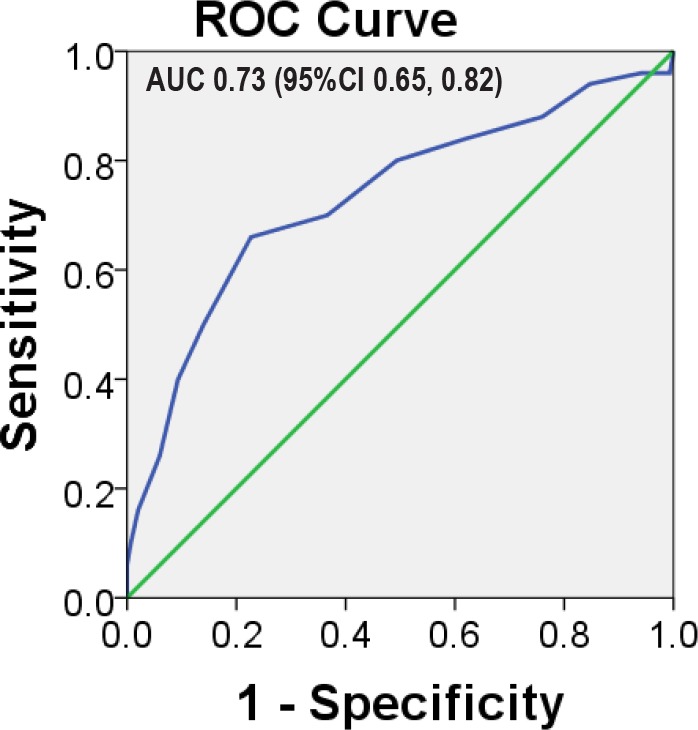
AUC = area under the curve, CI = confidence interval.
Table 2.
Determinants associated with need for unanticipated O2 supplementation to positive airway pressure therapy for sleep-disordered breathing.
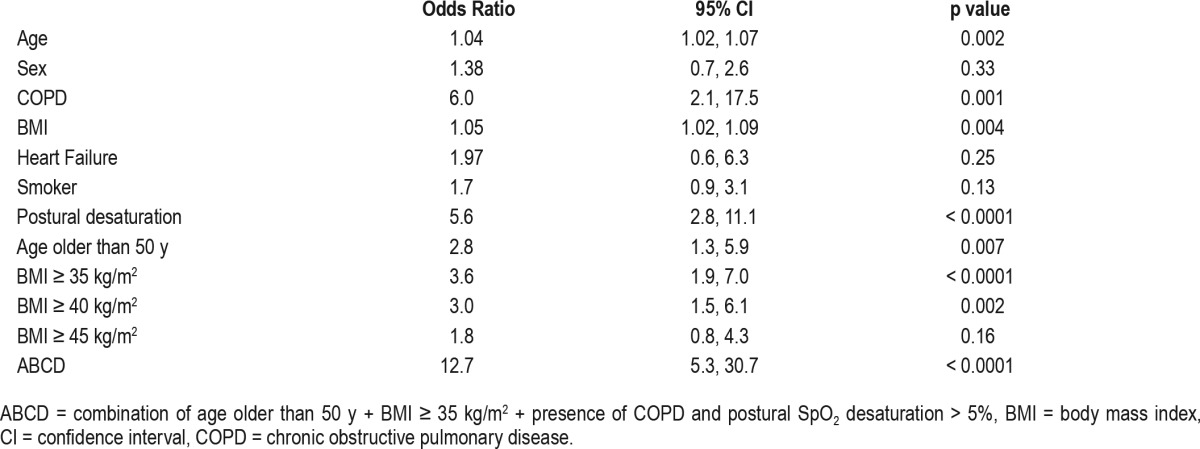
Simple logistic regression identified age older than 50 y, BMI > 35 kg/m2, COPD, and postural SpO2 desaturation > 5% were independently associated with the need to prescribe unanticipated O2 supplementation to PAP therapy (Table 2). A clinical prediction rule of fewer than two of the significant determinants (such as age older than 50 y, morbid obesity, COPD, and postural oxygen desaturation > 5%) had excellent negative predictive value (0.92; 95% CI 0.85, 0.96) and likelihood ratio of negative test (0.08; 95% CI 0.04, 0.16) for unanticipated need for O2 supplementation in addition to PAP therapy.
DISCUSSION
Some general observations can be made about our study. First, a clinical prediction rule of fewer than two of the following determinants—age older than 50 y, morbid obesity, COPD, and postural oxygen desaturation greater than 5%—had excellent negative predictive value and likelihood ratio of negative test for unanticipated need for O2 supplementation in addition to PAP therapy in patients with SDB. Second, our study provides scientific data that support current consensus guidelines that exclude patients with significant comorbid medical conditions from undergoing home-based management of SDB. Last, heart failure was not a determinant that was associated with increased risk for requiring O2 supplementation in addition to PAP therapy in patients with SDB.
HSAT was previously recommended as not appropriate for the diagnosis of OSA in patients with significant comorbid medical conditions such as COPD, morbid obesity, neuromuscular disorders, and heart failure. The stated reason for such exclusion was based primarily on the fact that most studies testing such pathways excluded patients with significant co-morbid medical conditions.11,13,18,19 The cited reason was that such comorbid medical conditions may degrade the accuracy of HSAT. In our study, instead of the accuracy of HSAT as an outcome measure, we chose the requirement for unanticipated need for nocturnal O2 supplementation to PAP therapy as an outcome measure of importance. Such an outcome measure was chosen based on the rationale that patients with comorbid medical conditions such as morbid obesity or COPD, by virtue of sleep-related nocturnal hypoventilation, may require additional treatment for SDB with nocturnal O2 supplementation due to persistent nocturnal hypoxia despite PAP therapy. Such additional need for O2 supplementation would not be apparent immediately because oximetry testing on autoPAP therapy is usually not performed when patients are initiated on autoPAP therapy following HSAT. Our finding that significant medical comorbidities are associated with persistent hypoxia that requires unanticipated nocturnal O2 supplementation to PAP therapy justifies current consensus guidelines for medical comorbidities to remain as exclusionary criteria for home management of SDB.11 Such a finding is responsive to calls for research to support appropriate patient selection for home-based management.1
The time spent below oxygen saturation of 90% has been associated with increased risk for all-cause mortality.14 Therefore, knowingly allowing patients to remain hypoxic under the auspices of a prospective research study with informed consent would be opposed to the concept of beneficence.15 Allowing persistent hypoxemia may place research participants under risk, however small, when participating in such a research study. Therefore, we chose to perform a retrospective chart review of laboratory-based polysomnography patients as opposed to a prospective study of home-sleep study patients in order to prevent possible and posited harm that is inherent to a prospective study design. We know that ventilatory response and the arousal stimulus to hypoxia are blunted in both nonrapid eye movement and rapid eye movement sleep.20,21 Sustained nocturnal hypoxia may predispose such susceptible patients to cardiac arrhythmias and hence increase morbidity and mortality.
According to the portable monitoring task force of the American Academy of Sleep Medicine, HSAT is not recommended for the diagnosis of OSA in patients with significant comorbid medical conditions, including, but not limited to, moderate to severe pulmonary disease, neuromuscular disease, or congestive heart failure and other sleep disorders.22 Although such guidelines exist, health insurance companies modify such criteria to impose restrictions on the use of laboratory-based polysomnography. For example, some insurance companies require BMI > 50 kg/m2 as a threshold above which laboratory-based polysomnography would be allowable. Based upon our data, BMI > 35 kg/m2 was associated with odds ratio of 3.6 (95% CI; 1.9, 7.0; p < 0.0001). We originally used a threshold of 35 kg/m2 because it is the accepted threshold for defining morbid obesity; however, we realize that there are some who have variably applied other higher thresholds such as 40 or 45 kg/m2. When compared with a threshold of 35 kg/m2, the application of higher thresholds weakened the association of such definitions of morbid obesity with the need for unanticipated oxygen supplementation (Table 2). Application of such higher BMI thresholds as a cost-saving measure to triage patients away from laboratory sleep studies could potentially decrease our ability to identify patients who may need unanticipated O2 supplementation following HSAT.
Additionally, currently, there is no age limit for HSAT and home-based initiation of autoPAP therapy. Our study suggests that older individuals may be more likely to require nocturnal O2 supplementation to PAP therapy was not anticipated prior to testing. Conceivably, such a finding may be due to the fact that many elderly patients may suffer from as yet undiagnosed COPD or other medical conditions that may increase the likelihood for unanticipated O2 supplementation.
Most studies that validated the use of HSAT excluded patients with comorbid medical problems.11 Two studies, however, included patients with comorbid medical conditions but in neither of these studies was autoPAP therapy initiated following the diagnosis of SDB.23,24 Home-based initiation of autoPAP therapy would have been unable to detect concurrent persistent hypoxia and the need for unanticipated O2 supplementation in addition to PAP therapy in patients with significant medical comorbidities.
There was no association between heart failure and unanticipated need for nocturnal O2 supplementation to PAP therapy in our study. Such results are in line with recent findings of utility of HSAT in two cohort studies of patients following myocardial infarction or patients with heart failure.25,26 There are no studies, however, that test the performance of autoPAP in the presence of significant medical co-morbidities including heart failure as outlined by the American Academy of Sleep Medicine.3,11 Most studies of autoPAP therapy excluded participants with significant comorbidities.18,27–29 Our prediction tool of positional change in oxygen saturation in combination with age and medical comorbidities could help clinicians identify patients who are most likely to need unanticipated O2 entrainment to PAP therapy and hence were not ideal candidates for home-based management of SDB.
Several physiological changes occur with normal aging and may have contributed to age older than 50 y being associated with unanticipated need for nocturnal O2 supplementation to PAP therapy. Various physiological changes in the elderly occur, such as increased chest wall compliance, reduction in lung elastic recoil, and reduction in respiratory muscle strength.30 Such changes may contribute to alveolar hypoventilation and an increase in closing volume of the lungs leading to nocturnal hypoxemia. Additionally, ventilatory response to hypercapnia and hypoxia are reduced in the elderly.31 Previously, investigators have demonstrated that in patients with obesity, standing posture improves oxygenation when compared to the supine position.32 Moreover, effect of body position on respiratory physiology of patients with COPD has been studied in great detail by others, suggesting that the supine position places the respiratory system at a disadvantage for performing gas exchange function that would require compensatory changes.33–36 Such a “stress test” of the respiratory system could identify individuals who are more likely to be hypoxemic.
In a recent study, Polese and colleagues validated HSAT in the elderly (age older than 65 y) and reported that HSAT could be extended to elderly patients with high probability of SDB, but they too excluded patients with significant comorbidities.37 Conceivably, the age-based effect observed in our study may have been due to as-yet undiagnosed medical comorbidities (Table 2). Moreover, in obese patients, Steier and colleagues found that supine posture resulted in elevated gastric pressure and end-expiratory esophageal pressures in obese individuals when compared to control patients, and that such mechanisms may contribute to nocturnal hypoventilation and hypoxemia.38 Others have reported that a restrictive defect noted on pulmonary function testing in morbidly obese individuals was associated with failure of continuous PAP therapy when compared to bilevel PAP therapy in treating obesity hypoventilation syndrome. Such findings suggest that such underlying mechanisms may play a role in the inadequate treatment of SDB in morbidly obese patients.39
Patients with COPD experience reduction in minute ventilation by nearly 25% and elevated upper airway resistance during sleep when compared to wakefulness.40 In addition to hypoventilation, ventilation perfusion mismatch, reduced functional residual capacity, and diminished responsiveness to hypoxia and CO2 retention can occur and contribute to nocturnal hypoxia.41,42 Such mechanisms may have played a role in the observed association between COPD and need for unanticipated nocturnal O2 supplementation to PAP therapy in our study (Table 2).
Limitations
There are several limitations to our study. First, this is a retrospective case-control study and such analyses are subject to bias that is inherent to such a retrospective analysis. However, we selected continuous patients in order to prevent such a bias. Second, the initiation of oxygen therapy on the night of the study is subject to the technician's measurement of apneahypopnea index on the night of the study to ensure that such patients' sleep-disordered breathing is adequately controlled. To account for such an operator error, we included the cases based on whether the interpreting sleep physician agreed with the sleep technician's decision and issued a prescription for oxygen upon review of the scored polysomnography. Third, the sample size of patients with heart failure was too small for us to understand the effects of types of heart failure on the need for unanticipated oxygen therapy.
CONCLUSIONS
Management pathways guided by simple prediction tools including advanced age, BMI, history of comorbid COPD, and postural desaturation of oxygen may help clinicians predict patients who may need unanticipated nocturnal O2 supplementation in addition to PAP therapy. Such findings provide scientific evidence to support the current consensus-derived practice guidelines that exclude such patients from undergoing HSAT and home-based autoPAP therapy.
DISCLOSURE STATEMENT
This information was presented as an abstract/poster presentation at the SLEEP 2015 meeting in Seattle in June 2015. Work for this study was conducted at University of Arizona, Tucson, AZ. This was not an industry supported study. This work was supported by the National Institutes of Health Grants (HL095799 to Dr. Parthasarathy); PCORI contract (IHS-1306-02505 and EAIN #3394-UOA to Dr. Parthasarathy). The statements in this manuscript are solely the responsibility of the author and do not necessarily represent the views of PCORI, its Board of Governors or Methodology Committee. The funding institutions did not have any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Dr. Parthasarathy reports grants from NIH/NHLBI, grants from Patient Centered Outcomes Research Institute, grants from US Department of Defense, grants from NIH (National Cancer Institute) NCI, grants from US Department of Army, grants from Johrei Institute, personal fees from American Academy of Sleep Medicine, personal fees from American College of Chest Physicians, nonfinancial support from National Center for Sleep Disorders Research of the NIH (NHLBI), personal fees from UpToDate Inc., personal fees from Philips-Respironics, Inc. and Vapotherm, Inc., grants from Younes Sleep Technologies, Ltd., grants from Niveus Medical Inc., grants from Philips-Respironics, Inc., outside the submitted work; In addition, Dr. Parthasarathy has a patent UA 14-018 U.S.S.N. 61/884,654; PTAS 502570970 (Home breathing device) pending. Dr. Grandner reports grants from NIH/NHLBI and NIH/NIEHS, personal fees from Nexalin Technologies, Bayer Inc., and FitBit. The above-mentioned conflicts including the patent are unrelated to the topic of this paper. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
SP had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, including and especially any adverse effects. SS, SP, AF, and SP contributed substantially to the study design, data analysis and interpretation, and the writing of the manuscript.
ABBREVIATIONS
- autoPAP
automatic positive airway pressure therapy
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CI
confidence interval
- HSAT
home sleep apnea testing
- OR
odds ratio
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- ROC
receiver operating characteristics
- SDB
sleep-disordered breathing
- SpO2
oxygen saturation
REFERENCES
- 1.Kuna ST, Badr MS, Kimoff RJ, et al. An official ATS/AASM/ACCP/ERS workshop report: research priorities in ambulatory management of adults with obstructive sleep apnea. Proc Am Thorac Soc. 2011;8(1):1–16. doi: 10.1513/pats.2009-042WS. [DOI] [PubMed] [Google Scholar]
- 2.Atwood CW., Jr How few signals are needed to diagnose sleep apnea? Sleep. 2014;37(12):1883–1884. doi: 10.5665/sleep.4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collop NA. Home sleep testing: it is not about the test. Chest. 2010;138(2):245–246. doi: 10.1378/chest.10-0712. [DOI] [PubMed] [Google Scholar]
- 4.Thomas RJ, Bianchi MT. Changing the direction of sleep medicine: business can boom, but it is not as usual. J Clin Sleep Med. 2013;9(9):977–979. doi: 10.5664/jcsm.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bianchi MT. Evidence that home apnea testing does not follow AASM practice guidelines--or Bayes' theorem. J Clin Sleep Med. 2015;11(2):189. doi: 10.5664/jcsm.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson ME. Coding and billing for home (out-of-center) sleep testing. Chest. 2013;143:539–543. doi: 10.1378/chest.12-0425. [DOI] [PubMed] [Google Scholar]
- 7.Cairns A, Poulos G, Bogan R. Who is getting tested for obstructive sleep apnea using a portable recording system? Test results from 193,221 patients. J Clin Sleep Med. 2014;10:1193–1198. doi: 10.5664/jcsm.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown LK. Point: should board certification in sleep be required to prescribe CPAP therapy on the basis of home sleep testing? Yes. Chest. 2013;144:1752–1754. doi: 10.1378/chest.13-1697. [DOI] [PubMed] [Google Scholar]
- 9.Punjabi NM, Aurora RN, Patil SP. Home sleep testing for obstructive sleep apnea: one night is enough! Chest. 2013;143:291–294. doi: 10.1378/chest.12-2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung F, Liao P, Yang Y, et al. Postoperative sleep-disordered breathing in patients without preoperative sleep apnea. Anesth Analg. 2015;120:1214–1224. doi: 10.1213/ANE.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 11.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–747. [PMC free article] [PubMed] [Google Scholar]
- 12.Parthasarathy S. CON: thoughtful steps informed by more comparative effectiveness research is needed in home testing. J Clin Sleep Med. 2013;9:9–12. doi: 10.5664/jcsm.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Shayeb M, Topfer LA, Stafinski T, et al. Diagnostic accuracy of level 3 portable sleep tests versus level 1 polysomnography for sleep-disordered breathing: a systematic review and meta-analysis. CMAJ. 2014;186:E25–E51. doi: 10.1503/cmaj.130952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Punjabi NM, Caffo BS, Goodwin JL, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Capron AM. (Almost) everything you ever wanted to know about informed consent. [Review of: Faden, RR and Beauchamp, TL. A history and theory of informed concsent. New York and Oxford: Oxford University Press, 1986] Med Humanit Rev. 1987;1:78–82. [PubMed] [Google Scholar]
- 16.Hudson KL, Guttmacher AE, Collins FS. In support of SUPPORT--a view from the NIH. N Engl J Med. 2013;368:2349–5231. doi: 10.1056/NEJMp1306986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4:157–171. [PMC free article] [PubMed] [Google Scholar]
- 18.Nussbaumer Y, Bloch KE, Genser T, et al. Equivalence of autoadjusted and constant continuous positive airway pressure in home treatment of sleep apnea. Chest. 2006;129:638–643. doi: 10.1378/chest.129.3.638. [DOI] [PubMed] [Google Scholar]
- 19.Antic NA, Buchan C, Esterman A, et al. A randomized controlled trial of nurse-led care for symptomatic moderate-severe obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:501–508. doi: 10.1164/rccm.200810-1558OC. [DOI] [PubMed] [Google Scholar]
- 20.Douglas NJ, White DP, Weil JV, et al. Hypoxic ventilatory response decreases during sleep in normal men. Am Rev Respir Dis. 1982;125:286–289. doi: 10.1164/arrd.1982.125.3.286. [DOI] [PubMed] [Google Scholar]
- 21.Berthon-Jones M, Sullivan CE. Ventilatory and arousal responses to hypoxia in sleeping humans. Am Rev Respir Dis. 1982;125:632–639. doi: 10.1164/arrd.1982.125.6.632. [DOI] [PubMed] [Google Scholar]
- 22.Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–737. [PMC free article] [PubMed] [Google Scholar]
- 23.Cirignotta F, Mondini S, Gerardi R, et al. Unreliability of automatic scoring of MESAM 4 in assessing patients with complicated obstructive sleep apnea syndrome. Chest. 2001;119:1387–1389. doi: 10.1378/chest.119.5.1387. [DOI] [PubMed] [Google Scholar]
- 24.Zou D, Grote L, Peker Y, et al. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006;29:367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 25.Ludka O, Stepanova R, Vyskocilova M, et al. Sleep apnea prevalence in acute myocardial infarction--the Sleep Apnea in Post-acute Myocardial Infarction Patients (SAPAMI) Study. Int J Cardiol. 2014;176:13–19. doi: 10.1016/j.ijcard.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abraham WT, Trupp RJ, Phillips B, et al. Effect of treatment with continuous positive airway pressure or oxygen on sleep-disordered breathing in patients with heart failure: results of the Sleep Events, Arrhythmias, and Respiratory Analysis in Chronic Heart Failure (SEARCH) study. Congest Heart Fail. 2008;14:197–201. doi: 10.1111/j.1751-7133.2008.07841.x. [DOI] [PubMed] [Google Scholar]
- 27.Kuna ST, Gurubhagavatula I, Maislin G, et al. Noninferiority of functional outcome in ambulatory management of obstructive sleep apnea. Am J Respir Crit Care Med. 2011;183:1238–1244. doi: 10.1164/rccm.201011-1770OC. [DOI] [PubMed] [Google Scholar]
- 28.Berry RB, Hill G, Thompson L, et al. Portable monitoring and autotitration versus polysomnography for the diagnosis and treatment of sleep apnea. Sleep. 2008;31:1423–1431. [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen CL, Auckley D, Benca R, et al. A multisite randomized trial of portable sleep studies and positive airway pressure autotitration versus laboratory-based polysomnography for the diagnosis and treatment of obstructive sleep apnea: the HomePAP study. Sleep. 2012;35:757–767. doi: 10.5665/sleep.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janssens JP, Pache JC, Nicod LP. Physiological changes in respiratory function associated with ageing. Eur Respir J. 1999;13:197–205. doi: 10.1034/j.1399-3003.1999.13a36.x. [DOI] [PubMed] [Google Scholar]
- 31.Peterson DD, Pack AI, Silage DA, et al. Effects of aging on ventilatory and occlusion pressure responses to hypoxia and hypercapnia. Am Rev Respir Dis. 1981;124:387–391. doi: 10.1164/arrd.1981.124.4.387. [DOI] [PubMed] [Google Scholar]
- 32.Hakala K, Mustajoki P, Aittomaki J, et al. Effect of weight loss and body position on pulmonary function and gas exchange abnormalities in morbid obesity. Int J Obes Relat Metab Disord. 1995;19:343–346. [PubMed] [Google Scholar]
- 33.Druz WS, Sharp JT. Electrical and mechanical activity of the diaphragm accompanying body position in severe chronic obstructive pulmonary disease. Am Rev Respir Dis. 1982;125:275–280. doi: 10.1164/arrd.1982.125.3.275. [DOI] [PubMed] [Google Scholar]
- 34.Sharp JT, Druz W, Danon J, et al. Respiratory muscle function and the use of respiratory muscle electromyography in the evaluation of respiratory regulation. Chest. 1976;70:150–154. doi: 10.1378/chest.70.1_supplement.150. [DOI] [PubMed] [Google Scholar]
- 35.Sharp JT, Druz WS, Kondragunta VR. Diaphragmatic responses to body position changes in obese patients with obstructive sleep apnea. Am Rev Respir Dis. 1986;133:32–37. doi: 10.1164/arrd.1986.133.1.32. [DOI] [PubMed] [Google Scholar]
- 36.Vellody VP, Nassery M, Druz WS, et al. Effects of body position change on thoracoabdominal motion. J Appl Physiol Respir Environ Exerc Physiol. 1978;45:581–589. doi: 10.1152/jappl.1978.45.4.581. [DOI] [PubMed] [Google Scholar]
- 37.Polese JF, Santos-Silva R, de Oliveira Ferrari PM, et al. Is portable monitoring for diagnosing obstructive sleep apnea syndrome suitable in elderly population? Sleep Breath. 2013;17:679–686. doi: 10.1007/s11325-012-0742-y. [DOI] [PubMed] [Google Scholar]
- 38.Steier J, Lunt A, Hart N, Polkey MI, Moxham J. Observational study of the effect of obesity on lung volumes. Thorax. 2014;69(8):752–759. doi: 10.1136/thoraxjnl-2014-205148. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee D, Yee BJ, Piper AJ, et al. Obesity hypoventilation syndrome: hypoxemia during continuous positive airway pressure. Chest. 2007;131(6):1678–1684. doi: 10.1378/chest.06-2447. [DOI] [PubMed] [Google Scholar]
- 40.O'Donoghue FJ, Catcheside PG, Eckert DJ, et al. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol. 2004;559(Pt 2):663–667. doi: 10.1113/jphysiol.2004.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Little SA, Elkholy MM, Chalmers GW, et al. Predictors of nocturnal oxygen desaturation in patients with COPD. Respir Med. 1999;93(3):202–207. doi: 10.1016/s0954-6111(99)90009-4. [DOI] [PubMed] [Google Scholar]
- 42.Sanders MH, Newman AB, Haggerty CL, et al. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]


