Abstract
Amyotrophic lateral sclerosis (ALS) is characterized by the degeneration of motor neurons resulting in a catastrophic loss of motor function. Current therapies are severely limited owing to a poor mechanistic understanding of the pathobiology. Mutations in a large number of genes have now been linked to ALS, including SOD1, TARDBP (TDP-43), FUS and C9orf72. Functional analyses of these genes and their pathogenic mutations have provided great insights into the underlying disease mechanisms. Defective axonal transport is hypothesized to be a key factor in the selective vulnerability of motor nerves due to their extraordinary length and evidence that ALS occurs as a distal axonopathy. Axonal transport is seen as an early pathogenic event that precedes cell loss and clinical symptoms and so represents an upstream mechanism for therapeutic targeting. Studies have begun to describe the impact of a few pathogenic mutations on axonal transport but a broad survey across a range of models and cargos is warranted. Here, we assessed the axonal transport of different cargos in multiple Drosophila models of ALS. We found that axonal transport defects are common across all models tested, although they often showed a differential effect between mitochondria and vesicle cargos. Motor deficits were also common across the models and generally worsened with age, though surprisingly there was not a clear correlation between the severity of axonal transport defects and motor ability. These results further support defects in axonal transport as a common factor in models of ALS that may contribute to the pathogenic process.
Introduction
Amyotrophic lateral sclerosis (ALS) is a typically adult onset progressive neurodegenerative disorder and the most common form of motor neuron disease. It is characterized by the loss of both the upper and lower motor neurons representing a catastrophic loss of motor function. The condition is fatal, usually due to respiratory failure, with an average life expectancy of 3–5 years from diagnosis (1). There is no cure and current therapies are severely limited owing to a poor mechanistic understanding of the pathobiology. Although the majority of ALS cases is sporadic, ∼10% are monogenic, familial forms. A large number of genes have now been linked to ALS, including SOD1, TARDBP (TDP-43), FUS and C9orf72. Functional analyses of these genes and pathogenic mutations have provided great insight into the underlying disease mechanisms (1,2).
TDP-43 is a multi-functional DNA/RNA-binding protein that shuttles between the nucleus and cytoplasm (3). In the nucleus, it plays many roles in transcription and RNA processing (4–7). In the cytoplasm, TDP-43 localizes to stress granules, P-bodies and RNA transport granules, and is involved in the regulation and spatial distribution of RNAs (8–12). FUS is also a DNA/RNA-binding protein that undergoes nuclear/cytoplasmic shuttling, with functions in RNA processing (13,14). The targets of TDP-43 and FUS RNA processing number in the thousands in animal models, although there appears to be only limited overlap (13,15). However, TDP-43 and FUS targets show some functional commonality suggesting defects in TDP-43 or FUS function could lead to common pathogenic outcomes (15,16).
Expansion of a hexanucleotide repeat GGGGCC in the first intron of C9orf72 is the most common genetic cause of ALS (17,18). Bidirectional transcription of these repeats forms nuclear RNA foci which sequester RNA-binding proteins (RBPs) (17,19–24). Moreover, these repeats undergo repeat associated non-ATG (RAN) translation, giving rise to a series of dipeptide repeat proteins (DPRs). These DPRs have a high propensity to aggregate and form inclusions in C9orf72 associated patient tissue (25–28). Recent research has suggested that pathogenicity is specifically associated with arginine containing DPRs (29,30).
These insights into the molecular causes of ALS, however, still do not provide a clear rationale for the selective cell-type vulnerability implicit in the disease. A defining feature of motor neurons is the extraordinary length of their axons. This characteristic, coupled with evidence indicating that ALS occurs as a distal axonopathy, has led to defective axonal transport being implicated as a key initiating contributor to the selective vulnerability of motor nerves. Emerging evidence has begun to highlight the impact of pathogenic mutations on axonal transport. For example, expression of mutant SOD1 in motor nerves causes axonal transport defects as an early pathology that precedes cell loss and clinical symptoms (31,32). Multiple types of cargo are affected including mitochondria (33), neurofilaments (32) and vesicles (34). Overexpression of the wild-type and several pathogenic mutant TDP-43 proteins leads to early onset mitochondrial transport dysfunction (35,36); however, this was not observed in another study (8). Thus, further work remains to be done to elucidate the relationship between axonal transport and degeneration in TDP-43 and other ALS models.
Here, we analyzed fast axonal transport in larval motor neurons of Drosophila models of TARDBP (TDP-43), FUS and C9orf72. We also analyzed the effect of loss-of-function mutants of the Drosophila orthologs of TDP-43 and FUS, TBPH and caz, respectively. The motor activities of larvae and adults in these models were assessed to correlate potential defects in axonal transport with locomotor deficits. We found that axonal transport defects are common across all models tested, although they often showed a differential effect between mitochondria and vesicle cargos. Motor deficits were also common across the models, though did not always show a clear correlation between the severity of axonal transport defects and motor ability.
Results
Gain and loss of TDP-43 function have differential effects on axonal transport
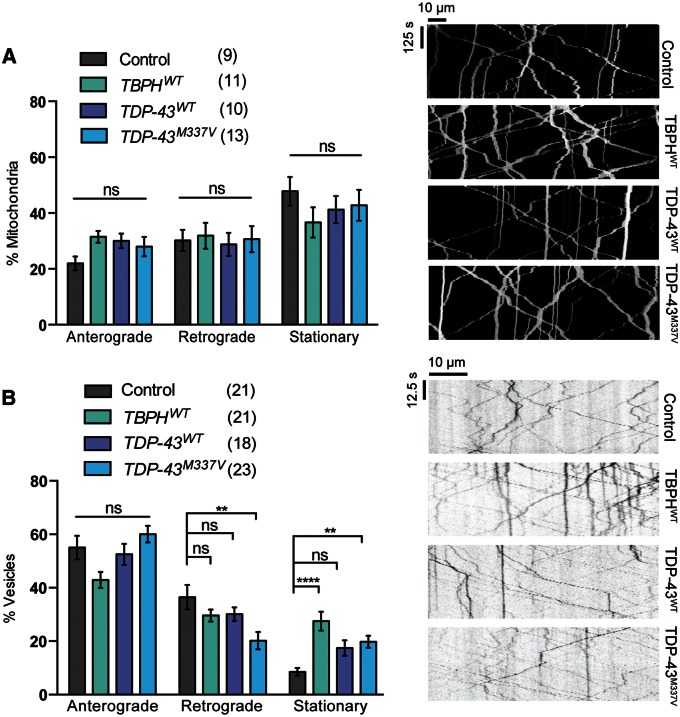
Mutations in TDP-43 cause ALS in an autosomal dominant manner and likely impact on neuronal function in a multitude of ways. We first analyzed whether ectopic expression of a pathogenic variant of TDP-43, M337V, caused any disruption of axonal transport in Drosophila motor nerves in comparison to TDP-43WT. To minimize the likelihood of artifacts from different expression levels or from disruption of genomic insertion, we made use of transgenes generated in the same integration site and expressing at equivalent levels (see Materials and Methods). Somewhat surprisingly no significant effect on mitochondrial transport was observed for any of the transgenes (Fig. 1A). However, when we analyzed a different transport cargo—vesicles loaded with GFP-fused neuropeptide Y (NPY.GFP)—although TDP-43WT did not cause any significant defects, TDP-43M337V expression caused a decrease in motility, with a concomitant significant increase in the stationary fraction (Fig. 1B). Overexpression of the fly homolog of TDP-43, named TBPH, also interfered with the transport of vesicles but not mitochondria (Fig. 1).
Figure 1.
Overexpression of TDP-43 affects the axonal transport of vesicles but not mitochondria. Live imaging analysis of (A) mitochondrial transport or (B) NPY containing vesicles in Drosophila motor axons overexpressing either Drosophila TBPH or human TDP-43 variants. Mitochondria are marked by UAS-mito.GFP and vesicles by UAS-NPY.GFP, driven by CCAP-GAL4. The number in brackets indicates the number of movies analyzed. Representative kymographs of the indicated genotypes are shown. Control genotype; (A) CCAP-GAL4/+; UAS-mito.GFP/UAS-lacZ, (B) CCAP-GAL4/+; UAS-NPY.GFP/UAS-lacZ. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test: **P < 0.01, ****P < 0.0001. Charts show mean ± SEM.
We next addressed whether TDP-43 normally plays a role in axonal transport by examining whether loss of the endogenous TBPH disrupted transport. We used a trans-heterozygous combination of the independently derived TBPH1 and the TBPHΔ23 deletion mutants to avoid potential genetic background effects. Loss of TBPH caused an overall increase in the stationary fraction of mitochondria (Fig. 2A). Although there was an observable decrease in both anterograde and retrograde transport, this only reached significance in the anterograde direction. In contrast, we found no impact of loss of TBPH on vesicle transport (Fig. 2B).
Figure 2.
Loss of TBPH inhibits axonal transport of mitochondria but not vesicles. Live imaging analysis of (A) mitochondrial transport or (B) NPY containing vesicles in Drosophila motor axons. Transheterozygous TBPH mutants were analyzed alone or in combination with transgenic expression of wild-type TBPH, or wild-type or a pathogenic variant of TDP-43. The number in brackets indicates the number of movies analyzed. Representative kymographs of the indicated genotypes are shown. Control genotype; (A) CCAP-GAL4/+; UAS-mito.GFP/UAS-lacZ, (B) CCAP-GAL4/+; UAS-NPY.GFP/+. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test: *P < 0.05, **P < 0.01. Charts show mean ± SEM.
Importantly, the disruption of mitochondrial transport could be completely rescued by re-expression of a TBPHWT transgene (Fig. 2A), verifying that this effect is a direct consequence of the genetic loss of TBPH. Interestingly, the loss of mitochondrial transport was also rescued by the ectopic expression of human TDP-43WT and by the ALS-linked variant TDP-43M337V. This cross-species rescue is consistent with previous reports and reflects their close homology, but also reveals that the pathogenic variant can broadly function as normal. Overall, these results indicate that dysregulated TDP-43 expression can affect axonal transport but cargoes are affected differently, and suggest that the pathogenic mutation may increase the susceptibility to disease.
Axonal transport disruption in TDP-43 models correlates with behavioral deficits
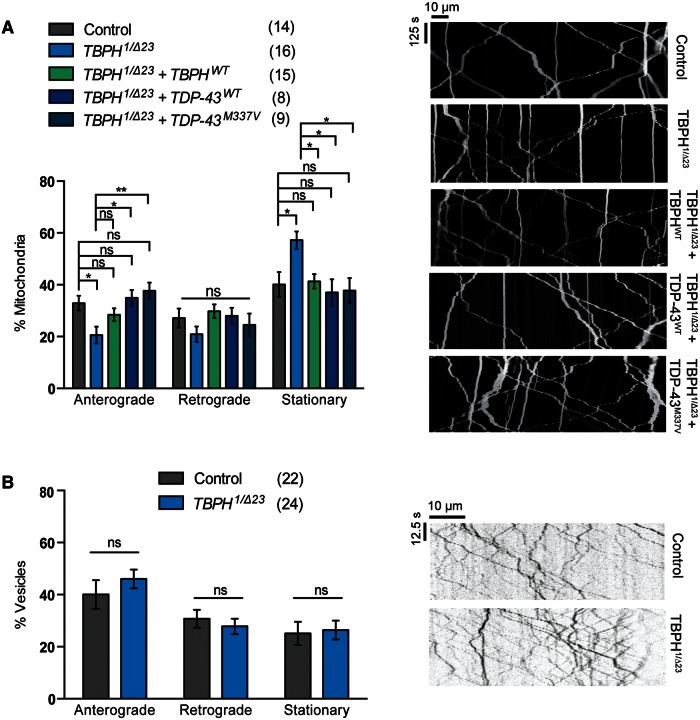
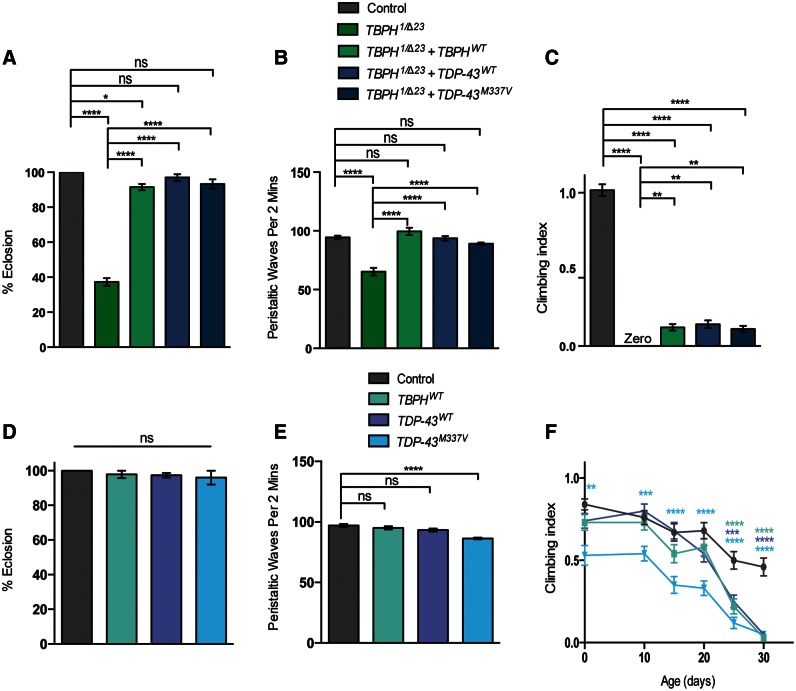
A prime motivation for this study was to correlate disruptions in axonal transport to decline in motor neuron function; the cardinal clinical feature of ALS. Thus, flies were examined for their neuromuscular function using a number of assays. One indicator of severe motor dysfunction is the inability of mutant flies to eclose from the pupal case after undergoing metamorphosis. For TBPH mutants, <40% of pupae produce viable adults (Fig. 3A), all of whom died within 5 days (37,38). As a more direct measure, we analyzed larval crawling and found that TBPH mutants exhibit a significant motor deficit (Fig. 3B). Furthermore, the few adult mutants that do eclose are uncoordinated and fail to register any climbing capacity (Fig. 3C). These phenotypes could all be rescued by the re-expression of either fly or human TDP-43 variants. For eclosion and larval crawling, the phenotypes were rescued back to control levels; however, the adult locomotion showed very limited rescue suggesting that cell types other than motor neurons substantially contribute to this severe phenotype.
Figure 3.
Gain and loss of TBPH/TDP-43 cause motor behavioral defects. TBPH mutants were analyzed alone or in combination with transgenic expression of wild-type TBPH, or wild-type or a pathogenic variant of TDP-43, were assayed for (A) eclosion, (B) larval crawling, or (C) adult climbing ability. Transgenes were expressed in motor neurons via the D42-GAL4 driver. Animals overexpressing TBPH or TDP-43 variants in a wild-type background were assayed for (D) eclosion, (E) larval crawling, or (F) adult climbing ability. N (animals) ≥ 100 (A, D), ≥20 (B, E), ≥50 (C, F). Control genotypes are TBPH1/+; D42-GAL4/+ (A–C) and D42-GAL4/UAS-lacZ (D–F). The mutant genotype carries heterozygous D42-GAL4 in the background. Data in (C) and (F) are normalized to control. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test, except (C) and (F) which were analyzed by Kruskal–Wallis non-parametric test with Dunn’s correction: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Comparisons in (C) are with control. Charts show mean ± SEM.
In contrast, the overexpression of the TBPH/TDP-43 has relatively modest effects on motor function. Overexpression of wild-type TBPH/TDP-43 does not perturb viability, larval crawling or climbing ability in young adult flies (Fig. 3D–F). However, the ectopic expression of TDP-43M337V caused a modest loss of larval locomotion but had a more pronounced effect on adult climbing. Interestingly, when analyzed with age, the climbing behavior in all three conditions progressively worsened, with the effect of wild-type TBPH/TDP-43 overexpression eventually mirroring that of the pathogenic mutant (Fig. 3F). Overall, these findings support a relationship between the observed transport defects and aberrant motor behavior.
Loss or gain of caz causes broad axonal transport disruption but gain of FUS specifically disrupts transport of vesicles
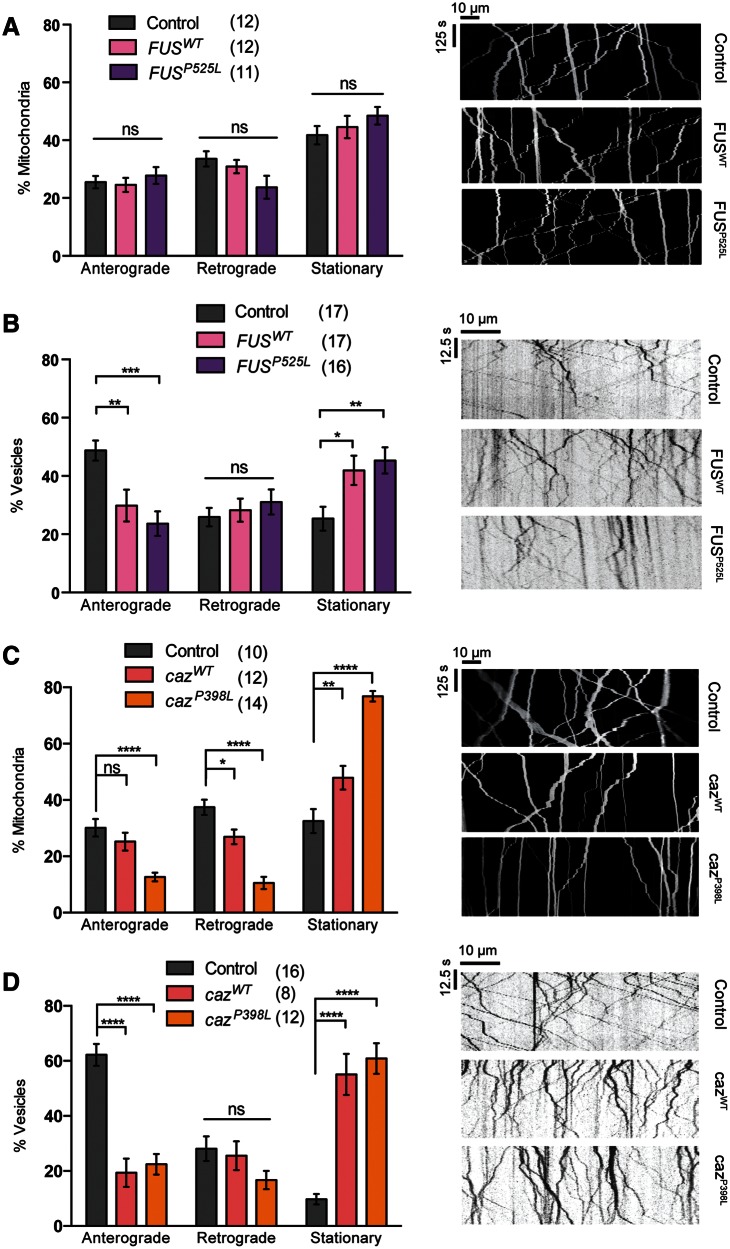
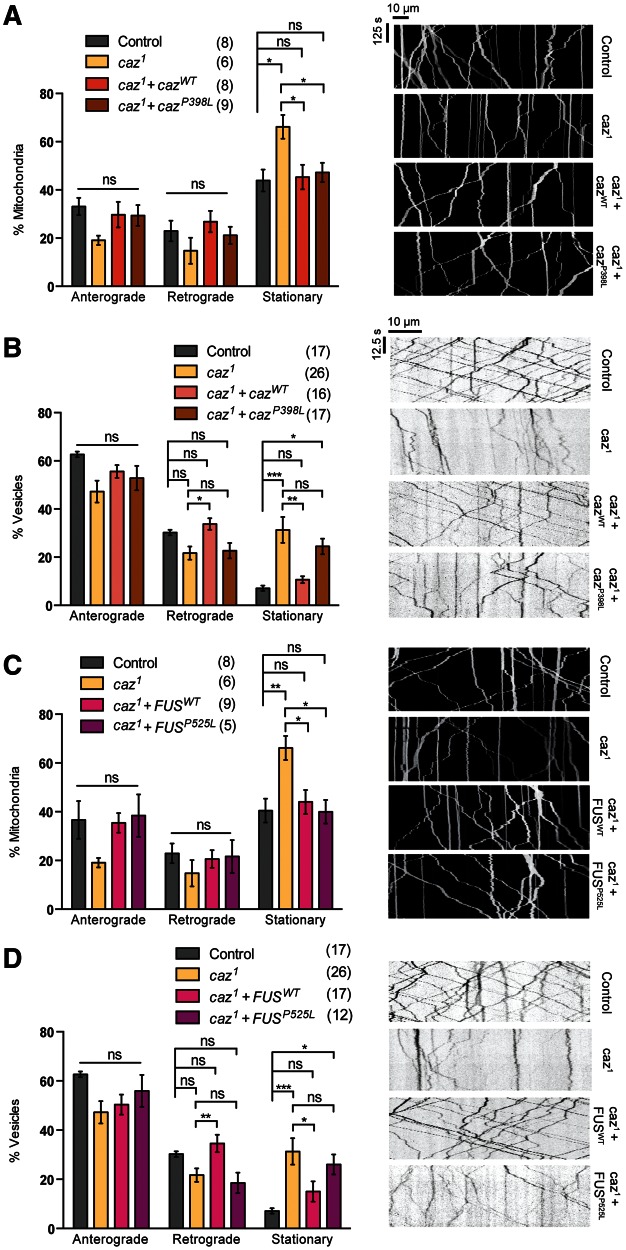
We next sought to broaden our analysis and determine whether axonal transport deficits were apparent in other models of ALS; thus, we analyzed Drosophila models of FUS and its homolog cabeza (caz). Ectopic expression of FUSWTor a pathogenic variant FUSP525L caused no disruption to mitochondrial transport (Fig. 4A). However, the expression of both FUS variants led to an increase in the stationary fraction of vesicles (Fig. 4B). Interestingly, this appeared to be due to a selective decrease in anterograde movement. In comparison, overexpression of cazWT and the pathogenic equivalent cazP398L inhibited the transport of both mitochondria and vesicles (Fig. 4C and D). Notably, although both caz variants had a similar effect on vesicle transport, the pathogenic variant had a substantially more marked effect on mitochondrial transport.
Figure 4.
Overexpression of FUS or caz differentially inhibits mitochondrial and vesicle transport. Live imaging analysis of (A, C) mitochondrial transport and (B, D) vesicle transport in Drosophila motor axons. Animals overexpressing wild-type or a pathogenic variant of FUS (A and B) or the Drosophila homolog caz (C and D) were analyzed. Representative kymographs of the indicated genotypes are displayed. The number in brackets indicates number of movies analyzed. Control genotype; (A, B) CCAP-GAL4/+; UAS-mito.GFP/UAS-lacZ, (C, D) CCAP-GAL4/+; UAS-NPY.GFP/UAS-lacZ. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Charts show mean ± SEM.
Analyzing the caz1 null mutant, we found a significant decrease in the transport of both mitochondria and vesicles (Fig. 5A and B). These phenotypes could be completely rescued by the re-expression of cazWT (Fig. 5A and B). It is worth noting that although vesicle transport was rescued by cazWT under standard conditions (i.e. raised at 25 °C), animals needed to be raised at 29 °C, increasing transgene expression levels, to see a complete rescue of the mitochondrial transport. Interestingly, the re-expression of the pathogenic variant, cazP398L, had differential rescuing effects. Although it was able to rescue the mitochondrial transport defect, it was unable to rescue the disruption in vesicle transport. Similarly, expression of FUSWT was able to rescue the caz1 mutant transport deficit of mitochondria and vesicles (Fig. 5C and D). We also saw a strikingly similar effect from the FUSP525L variant as observed for the equivalent cazP398L; the mitochondrial transport was rescued but vesicle transport was not (Fig. 5C and D).
Figure 5.
Loss of caz inhibits mitochondrial and vesicle transport. Live imaging analysis of (A, C) mitochondrial transport and (B, D) vesicle transport in Drosophila motor axons. caz1 mutants were analyzed alone or in combination with transgenic expression of wild-type or a pathogenic variant of caz (A and B) or FUS (C and D) were analyzed. Representative kymographs of the indicated genotypes are shown. The number in brackets indicates the number of movies analyzed. Control genotype; (A, B) CCAP-GAL4/+; UAS-mito.GFP/lacZ, (C, D) CCAP-GAL4/+; UAS-NPY.GFP/lacZ. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test: *P < 0.05, **P < 0.01, ***P < 0.001. Charts show mean ± SEM.
Axonal transport disruption in FUS/caz models correlates with behavioral deficits
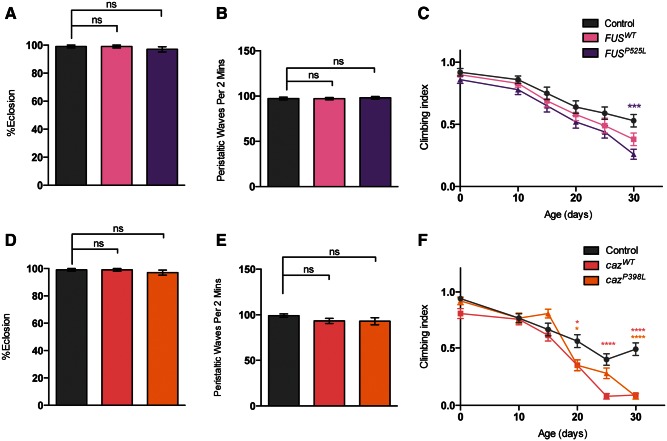
Analyzing the effects of FUS/caz mutations on motor ability, we found that overexpression of either FUS or caz variants had almost no effect on eclosion, larval crawling or young adult locomotion (Fig. 6A–F). However, climbing ability began to decline more rapidly in adults overexpressing the pathogenic FUSP525L (Fig. 6C). Overexpression of either form of caz significantly perturbed motor ability to a similar extent (Fig. 6F).
Figure 6.
Expression of FUS/caz variants has limited effect on motor behavior. Animals overexpressing wild-type or a pathogenic variant of FUS (A–C) or caz (D–F) in a wild-type background were assayed for (A and D) eclosion, (B and E) larval crawling, or (C and F) adult climbing ability, driven by D42-GAL4. N (animals) ≥ 100 (A, D), ≥20 (B, E), ≥50 (C, F). Control genotype is D42-GAL4/UAS-lacZ. Data in (C) and (F) are normalized to control. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test, except (C) and (F) which were analyzed by Kruskal–Wallis non-parametric test with Dunn’s correction: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Charts show mean ± SEM.
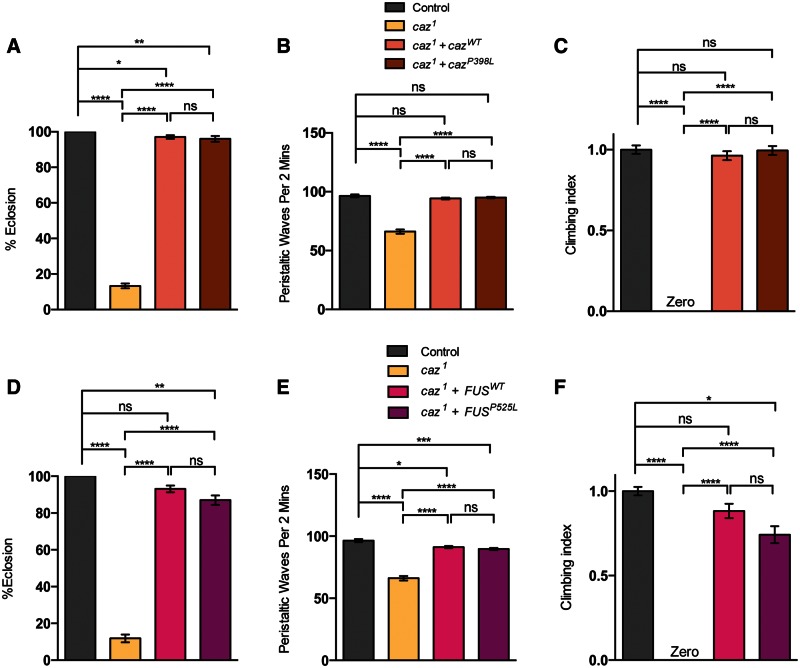
caz1mutants had a dramatic reduction in eclosion (Fig. 7A), as reported previously (39). Larval crawling was also significantly reduced, whereas the escaper adult mutants failed to register any climbing activity, reminiscent of the TBPH mutants (Fig. 7B and C). These phenotypes could be substantially rescued by the re-expression of either cazWT or FUSWT as previously reported (39). The pathogenic variant was also able to rescue these phenotypes to a level similar to the wild-type version (Fig. 7A–F).
Figure 7.
Loss of caz causes reduced motor ability. caz1 mutants were analyzed alone or in combination with transgenic expression of wild-type or a pathogenic variant of caz or FUS, driven by D42-GAL4. The indicated genotypes were assayed for (A and D) eclosion, (B and E) larval crawling, or (C and F) adult climbing ability. N (animals) ≥ 100 (A, D), ≥20 (B, E), ≥50 (C, F). Control genotype is D42-GAL4/UAS-lacZ. The mutant genotype carries heterozygous D42-GAL4 in the background. Data in (C) and (F) are normalized to control. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test, except (C) and (F) which were analyzed by Kruskal–Wallis non-parametric test with Dunn’s correction: *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Charts show mean ± SEM.
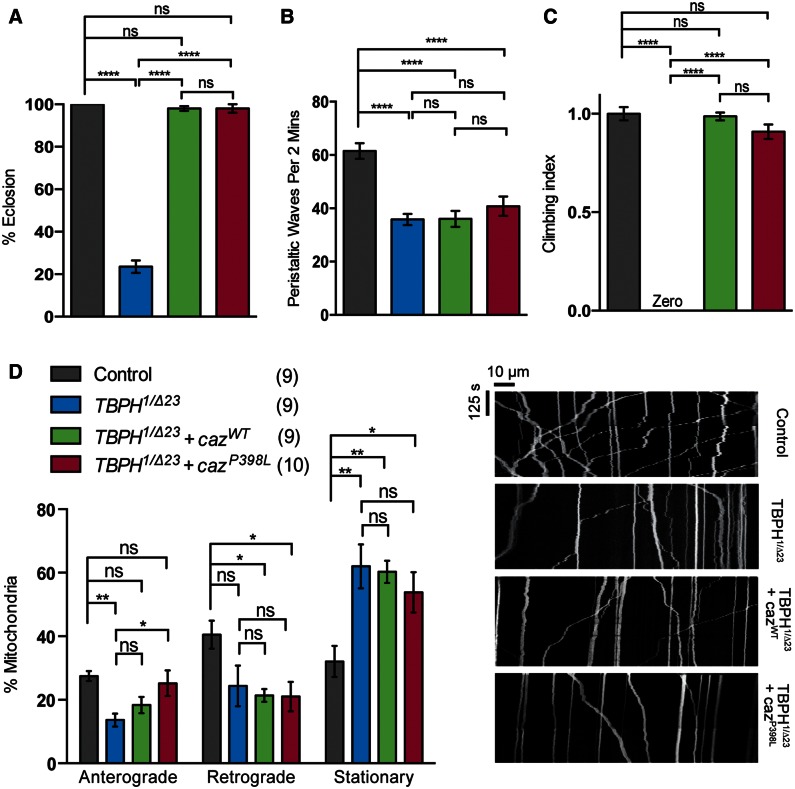
caz overexpression rescues TBPH motor function but not axonal transport
TDP-43 and FUS, and their fly orthologs, have been shown to genetically interact. The reduced viability and lifespan of TBPH mutants were rescued by neuronal overexpression of caz, whereas the locomotor deficit was partially rescued; however, overexpression of TBPH did not alter the caz1 phenotypes (39). In agreement with these findings, we also saw a complete rescue of eclosion and adult climbing ability (Fig. 8A and C).
Figure 8.
Overexpression of caz can rescue behavioral but not transport phenotypes in TBPH mutants. TBPH mutants were analyzed alone or in combination with transgenic expression of caz variants and assayed for (A) eclosion, (B) larval crawling and (C) adult climbing ability. (D) Live imaging analysis of mitochondrial transport in Drosophila motor axons. The number in brackets indicates the number of movies analyzed and representative kymographs of the indicated genotypes are shown. N (animals) ≥ 100 (A), ≥20 (B), ≥50 (C). Control genotypes; (A, B, C) D42-GAL4/UAS-lacZ, (D) CCAP-GAL4/+; UAS-mito.GFP/+. The mutant genotype carries heterozygous D42-GAL4 in the background. Data in (C) are normalized to control. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test, except (C) which was analyzed by Kruskal–Wallis non-parametric test with Dunn’s correction: *P < 0.05, **P < 0.01, ****P < 0.0001. Charts show mean ± SEM.
In light of this, we assayed whether the mitochondrial axonal transport defect (Fig. 5) was also rescued. Surprisingly, the transport defect in TBPH mutants was not rescued by overexpression of either cazWT or cazP398L (Fig. 8D). However, consistent with this we found that larval crawling was not rescued by caz expression (Fig. 8B). Therefore, it appears that although TBPH and caz act within a genetic pathway influencing viability and adult motor behaviors, this is not shared in the mechanisms that disrupt axonal transport.
C9orf72-linked hexanucleotide repeats severely impair transport but only mildly disrupts behavior
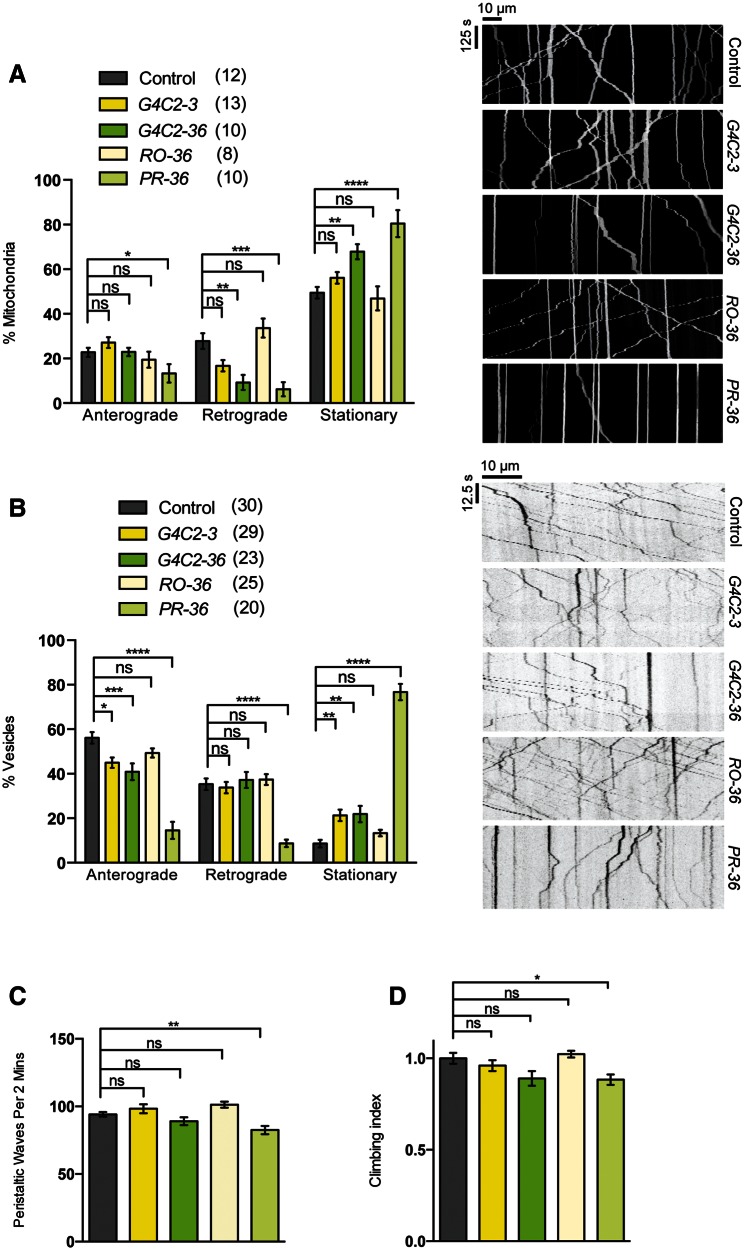
The third genetic model of ALS assessed in this study was the C9orf72-associated hexanucleotide (GGGGCC [G4C2]) repeats. Transgenic expression of a non-pathogenic repeat length (G4C2-3) had no effect on mitochondrial transport; however, expression of 36 repeats (G4C2-36), previously shown to cause neurotoxicity (30), caused a significant increase in the stationary fraction of mitochondria (Fig. 9A). To assess whether this was due to RNA toxicity or the production of arginine-containing dipeptide repeats, we analyzed animals expressing ‘RNA-only’ (RO-36) or proline–arginine encoding (PR-36) transcripts (30) and found that this effect was specifically attributable to the dipeptide repeats (Fig. 9A). Interestingly, vesicle transport was already slightly disrupted by G4C2-3, which was not substantially increased by G4C2-36 expression (Fig. 9B). Although the RO-36 repeats were again non-toxic, the expression of PR-36 caused a severe disruption of vesicle transport (Fig. 9B).
Figure 9.
C9orf72-related dipeptide repeats inhibit axonal transport and cause a mild motor deficit. Animals expressing control length (G4C2-3) or expanded (G4C2-36) hexanucleotide repeats, expanded RNA-only (RO-36) or proline-arginine dipeptide repeats (PR-36) were analyzed for axonal transport of (A) mitochondria, (B) vesicles, or motor function as (C) larval crawling or (D) adult climbing ability. The number in brackets indicates the number of movies analyzed and representative kymographs of the indicated genotypes are shown. N (animals) ≥ 20 (C), ≥50 (D). Control genotypes; (A) CCAP-GAL4/+;UAS-mito.GFP/+, (B) CCAP-GAL4/+; UAS-NPY.GFP/UAS-lacZ, (C, D) D42-GAL4/UAS-lacZ. Data in (D) are normalized to control. Statistical analysis was performed using one-way ANOVA with Sidak’s multiple comparison test, except (D) which was analyzed by Kruskal–Wallis non-parametric test with Dunn’s correction: *P< 0.05, **P< 0.01, ***P < 0.001, ****P < 0.0001. Charts show mean ± SEM.
Despite the axonal transport phenotypes, neuromuscular function appears to be only mildly affected. There were no obvious effects on viability (data not shown), and the locomotor capacity of larvae and young adults was modestly affected only by PR-36 (Fig. 9C and D). Therefore, it seems that these disruptions to axonal transport are reasonably well tolerated in young flies, although a progressive aged-related decline in neuromuscular function was not tested.
Discussion
In this study we sought to determine whether disruption of axonal transport is a common feature of ALS pathogenesis, which may underlie the disruption of neuromuscular function and loss of motor ability. The neuromuscular junction is highly energetically demanding and accordingly is dependent on correct distribution and function of mitochondria (40). Thus, disruption in mitochondrial transport has long been postulated to contribute to neuronal dysfunction, impacting on energy supply and calcium buffering capacity at the neuromuscular junction. Nonetheless, many other cellular components need to be transported to synapses to support proper functioning. Here, using previously characterized Drosophila models of ALS, we examined the transport of two different cargos and correlated any transport deficiencies with motor ability. Overall, we found that axonal transport deficits are a common feature in all the models analyzed here, but the severity and cargo selectivity differ substantially between models. Moreover, the severity of motor deficits does not always mirror the severity of axonal transport defects.
Gain and loss of TDP-43/TBPH exert differential effects on axonal transport. The overexpression of TDP-43 variants did not affect mitochondrial axonal transport but impaired vesicle transport, whereas loss of TBPH caused a decrease in the transport of mitochondria but vesicle transport was unaffected. The relatively modest deficit in vesicle transport in the overexpression models correlated with modest locomotor deficits, whereas the loss of mitochondrial transport in TBPH mutants correlated with severe motor impairment.
Both gain and loss of function of FUS/caz affected axonal transport, though the effects were generally more widespread. Although the overexpression of caz variants affected both mitochondria and vesicle transport, overexpression of FUS variants only perturbed vesicle transport. Loss of caz substantially affected both vesicle and mitochondrial transport. The effects on motor ability were similar to TDP-43/TBPH; although the caz mutants exhibit severe developmental and locomotor defects, the overexpression models have only modest effects on adult locomotion that become more pronounced with age.
Axonal transport was also disrupted in the C9orf72 models. The expanded pure G4C2-36 repeats caused a disruption of mitochondrial transport; however, the effect on the transport of vesicles is more complicated as the G4C2-3 repeats also slightly inhibit vesicles transport. Nevertheless, results from the RO-36 and PR-36 transgenes further support the view that toxicity of these expanded repeats is caused by DPR production and not ‘RNA toxicity’ as the RO-36 transgene appeared inert in all assays whereas the PR-36 transgene was highly toxic, substantially inhibiting mitochondrial and vesicle transport. Interestingly, expression of this transgene caused only modest motor deficits.
Thus, our data indicate that axonal transport deficits are a common feature in all the models analyzed here. Disruption of axonal transport has also been noted in several vertebrate models of ALS, most notably in models of SOD1 mutations and TDP-43 models. We chose to not investigate the impact of SOD1 mutations as they have been fairly well characterized in vertebrates, and have been found to affect neurofilaments (31,32), mitochondria (33) and vesicles (34). TDP-43 has been reported to modestly affect axonal transport of mitochondria (35), although, consistent with our observations, disruption of mitochondrial transport was not substantiated in another study (8). To our knowledge, our results are the first reporting an impact of FUS or G4C2-repeats on axonal transport. Hence, our findings confirm and extend the body of evidence indicating a general impact of axonal transport deficits in diverse models of ALS. It is also notable that in the vertebrate studies no clear pattern of disruption has emerged, further supporting a more generalized disruption of axonal transport.
Although this study has highlighted axonal transport deficits as a common feature in Drosophila models of ALS, axonal transport deficits are by no means specific to ALS because they have also been observed in other disease models. Axonal transport has been shown to be disrupted in models of Alzheimer’s disease (AD), polyglutamine (polyQ) diseases such as Huntington’s disease, peripheral neuropathies and Parkinson’s disease (PD). It is notable that the proteins in question in these disease models are all functionally quite different from each other and from the ALS-related disease factors studied here, indicating that diverse mechanisms can disrupt axonal transport. The models of AD implicate Tau-linked disruption of microtubules or dysregulation of kinases and phosphatases to impair transport (41). Huntingtin has been shown to interact with motor proteins via HAP-1; however, axonal transport disrupted is also disrupted by expanded polyQ in other disease contexts and thought to arise indirectly from blockages caused by aggregated polyQ (42). A wide array of mechanisms have been proposed for peripheral neuropathies but many center on cytoskeleton, molecular motors and disruption of mitochondrial distribution and function (43). Related to PD, an elegant mechanism has been elucidated by which PINK1 and Parkin specifically regulate the transport of mitochondria by regulating the turnover of the Miro–Milton complex that connects mitochondria to kinesin (44). More recently, mutations in LRRK2 have been shown interfere with axonal transport by influencing the acetylation state of microtubules, although the exact mechanisms are not clear (45). Thus, it is clear that disturbances to multiple and diverse cellular processes have the potential to disrupt axonal transport.
This study did not seek to determine the mechanism(s) of axonal transport defects but emerging evidence strongly implicates RNA dysregulation in TDP-43, FUS and G4C2-repeats mechanisms. TDP-43 and FUS function as part of ribonucleoprotein complexes in the regulation of RNA metabolism, and dysregulation leads to widespread effects on transcription and RNA splicing and transport. A growing consensus proposes that these RBPs sequester mRNAs in stress granules (46). Recent work has revealed that TDP-43 and related RBPs form stress granules via liquid–liquid phase separation (47). Moreover, pathogenic mutations enhance the propensity for RBPs to fibrilize within these granules, which is proposed to interfere with stress granule disassembly.
Both loss and gain of TDP-43 and FUS result in neurodegenerative phenotypes (48–51), indicating that correct levels of these proteins are critical. Our results support the concept that the stoichiometry of ribonucleoprotein complexes is a key factor, and if the complex composition is altered functioning is impaired. Moreover, changes to the stoichiometry of ribonucleoprotein complexes may result in differential effects on their various targets; this may reflect the differences observed in the cargos that are affected by different mutations.
Both TDP-43 and FUS have been shown to bind and regulate RNAs of several motor proteins including KIF13 and KLC1 (7), and KIF5C, KIF1B and KIF3A (13,52), respectively. KIF13 is a kinesin 3 motor involved in vesicle transport, whereas KIF5C, KIF1B and KIF3A are involved in the axonal transport of mitochondria and vesicles. KLC1 is a kinesin light chain, which interacts with several kinesin heavy chain proteins. Interestingly, TDP-43 has also been reported to bind the mRNA of TRAK1 (13), homologous to Drosophila Milton, an adaptor protein that along with Miro specifically mediates the binding of mitochondria to the motor Kinesin 1 (53). Dysregulation of TRAK1/milton may underlie the mitochondria-specific effect observed in TBPH mutants.
Although it is early days for understanding the mechanism(s) by which C9orf72 G4C2 repeat expansions cause disease, no clear picture is emerging that explains the selective neuronal vulnerability. The formation of RNA foci from the expanded G4C2 repeats again presents the possibility for wide-spread RNA dysregulation as the pathogenic cause, but this seems unlikely in light of the compelling observations from Drosophila that arginine-containing DPRs are highly neurotoxic whereas ‘RNA only’ repeats are not (30). Furthermore, recent reports have shown that inhibiting nuclear export of these repeats prevents much of the toxicity (54–56). Thus, the effects of cytoplasmic DPR toxicity may more indirectly affect sensitive cellular processes such as axonal transport. In summary, our findings support axonal transport defects as a shared phenomenon in multiple models of ALS, highlighting this as a potential common upstream target for therapeutic manipulation.
Materials and Methods
Drosophila genetics
Drosophila were raised under standard conditions at 25 °C on food consisting of agar, cornmeal, molasses and yeast unless otherwise stated. The UAS transgenes for TBPH, TDP-43, caz and FUS, which are all inserted in the attP2 landing site, as well as the caz1 mutant were a gift from Brian McCabe (Columbia University) (51). The TBPH1 line was a gift from Aaron Voigt (RWTH Aachen University) (57) and the TBPHΔ23 a gift from Fabian Feiguin (ICGEB, Trieste) (38). All C9orf72-related transgenic lines, which are all inserted in the attP40 landing site, were a gift from Adrian Isaacs and Linda Partridge (University College London) (30). UAS-NPY.GFP was a gift from Iain Robinson (Plymouth University) (58). UAS-lacZ, UAS-mito.GFP, D42-GAL4 and CCAP-GAL4 were obtained from the Bloomington Drosophila Stock Centre (Bloomington, IN).
Dissection and imaging of axonal transport
Analysis of axonal transport was performed live on third instar larvae as previously described (45,59). Briefly, larvae were pinned at each end dorsal side up to a Sylgard (Sigma 761028) slide and covered in dissection solution: 128 mm NaCl, 1 mm EGTA, 4 mm MgCl2, 2 mm KCl, 5 mm HEPES and 36 mm sucrose, adjusted to pH 7.2. Larvae were cut along the dorsal midline using micro-dissection scissors, the sides pinned back and the internal organs removed. Movies were taken using an Olympus FV1000 fluoview confocal microscope with a 60× water immersion lens (NA 0.90 Olympus LUMPFL). Images were captured at a rate of 1 frame per 5 s for 100 frames for mitochondria, or 1 frame per 0.5 s for 100 frames for vesicles. CCAP-GAL4 was used as it expresses in a very sparse population of cells which secrete the neuropeptide CCAP (Crustacean Cardio-Active Peptide). These neurons send out a single axon in the segmental nerve thus facilitating precise imaging of transport in an individual axon.
Analysis of axonal transport
Movies were processed and analyzed using the ImageJ software as previously described to produce kymographs (45). These were used to manually score cargo as anterograde, retrograde or stationary. Scoring was undertaken blind by two independent researchers (K.R.B. and V.K.G.) and only concordant scores were retained.
Eclosion
Pupae were placed in groups of 25 to a vial and the proportion of adult flies that emerged was assessed. Animals from at least three replicate crosses were analyzed.
Larval locomotion
Control and experimental crosses were established at 25 °C and transferred to fresh vials every 2 days. Vials containing wandering third instar larvae were coded by an independent researcher, placed at 23 °C and left to acclimatize for 2 h. Individual larvae were taken and rinsed in distilled water to remove any residual food, placed on a 2% agarose plate under a viewing microscope and left to acclimatize for 5 s. The number of peristaltic movements in 2 min was counted by direct observation. Animals from at least three replicate crosses were analyzed.
Adult climbing
Male flies between 0 and 3 days post-eclosion were placed at 23 °C to acclimatize for 1 h. The flies were then transferred to the climbing tubes at a maximum number of 25 per tube for a further 1 h. Flies were introduced into a negative geotaxis counter-current apparatus and given 10 s to climb at each position for total of five attempts. A climbing index score was generated based on the number of flies at each of the positions. The score for each genotype within an experiment was normalized to the control. Animals from at least three replicate crosses were analyzed. For the longitudinal study of the effects of age-related neurodegeneration on climbing ability, male flies were aged in cohorts and the climbing assay was performed at set time points as indicated. Between assays, the flies were transferred every 2 days into fresh vials with no more than 25 flies per vial.
Statistical analysis
Calculations were performed using GraphPad Prism 6.0. Adult climbing analysis is not normally distributed so the data were analyzed using Kruskal–Wallis non-parametric test with Dunn’s correction for multiple comparisons. All other quantifications passed the D’Agostino & Pearson omnibus normality test. For axonal transport, eclosion and larval crawling significance was determined by one-way analysis of variance (ANOVA) with the Sidak multiple comparison test (being more powerful than Bonferroni’s correction).
Acknowledgements
The authors thank the following for generously sharing reagents; Brian McCabe, Fabian Feiguin, Aaron Voigt, Adrian Isaacs, Linda Partridge and Iain Robinson. They also thank Kurt de Vos and Andrew Grierson (University of Sheffield) for technical advice and discussions, and the rest of the Whitworth laboratory for discussions.
Conflict of Interest statement. None declared.
Funding
ERC Starting Grant (no. 309742) and MRC core funding (MC-A070-5PSB0); Studentship awarded as part of the MRC Centre for Developmental and Biomedical Genetics (Grant G070091) at the University of Sheffield to K.R.B.; EMBO Long-Term Fellowship to V.L.H. The Wellcome Trust provided support for the Sheffield Light Microscopy Facility (GR077544AIA). Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Funding to pay the Open Access publication charges for this article was provided by the ERC Starting Grant and MRC core funding.
References
- 1.Robberecht W., Philips T. (2013) The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci., 14, 248–264. [DOI] [PubMed] [Google Scholar]
- 2.Peters O.M., Ghasemi M., Brown R.H., Jr. (2015) Emerging mechanisms of molecular pathology in ALS. J. Clin. Invest., 125, 1767–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayala Y.M., Zago P., D'Ambrogio A., Xu Y.F., Petrucelli L., Buratti E., Baralle F.E. (2008) Structural determinants of the cellular localization and shuttling of TDP-43. J. Cell. Sci., 121, 3778–3785. [DOI] [PubMed] [Google Scholar]
- 4.Polymenidou M., Lagier-Tourenne C., Hutt K.R., Huelga S.C., Moran J., Liang T.Y., Ling S.C., Sun E., Wancewicz E., Mazur C. et al. (2011) Long pre-mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat. Neurosci., 14, 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sephton C.F., Cenik C., Kucukural A., Dammer E.B., Cenik B., Han Y., Dewey C.M., Roth F.P., Herz J., Peng J. et al. (2011) Identification of neuronal RNA targets of TDP-43-containing ribonucleoprotein complexes. J. Biol. Chem., 286, 1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tollervey J.R., Curk T., Rogelj B., Briese M., Cereda M., Kayikci M., Konig J., Hortobagyi T., Nishimura A.L., Zupunski V. et al. (2011) Characterizing the RNA targets and position-dependent splicing regulation by TDP-43. Nat. Neurosci., 14, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao S., Sanelli T., Dib S., Sheps D., Findlater J., Bilbao J., Keith J., Zinman L., Rogaeva E., Robertson J. (2011) RNA targets of TDP-43 identified by UV-CLIP are deregulated in ALS. Mol. Cell. Neurosci., 47, 167–180. [DOI] [PubMed] [Google Scholar]
- 8.Alami N.H., Smith R.B., Carrasco M.A., Williams L.A., Winborn C.S., Han S.S., Kiskinis E., Winborn B., Freibaum B.D., Kanagaraj A. et al. (2014) Axonal transport of TDP-43 mRNA granules is impaired by ALS-causing mutations. Neuron, 81, 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colombrita C., Zennaro E., Fallini C., Weber M., Sommacal A., Buratti E., Silani V., Ratti A. (2009) TDP-43 is recruited to stress granules in conditions of oxidative insult. J. Neurochem., 111, 1051–1061. [DOI] [PubMed] [Google Scholar]
- 10.Fallini C., Bassell G.J., Rossoll W. (2012) The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Hum. Mol. Genet., 21, 3703–3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu-Yesucevitz L., Bilgutay A., Zhang Y.J., Vanderweyde T., Citro A., Mehta T., Zaarur N., McKee A., Bowser R., Sherman M. et al. (2010) Tar DNA binding protein-43 (TDP-43) associates with stress granules: analysis of cultured cells and pathological brain tissue. PLoS One, 5, e13250.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang I.F., Wu L.S., Chang H.Y., Shen C.K. (2008) TDP-43, the signature protein of FTLD-U, is a neuronal activity-responsive factor. J. Neurochem., 105, 797–806. [DOI] [PubMed] [Google Scholar]
- 13.Colombrita C., Onesto E., Megiorni F., Pizzuti A., Baralle F.E., Buratti E., Silani V., Ratti A. (2012) TDP-43 and FUS RNA-binding proteins bind distinct sets of cytoplasmic messenger RNAs and differently regulate their post-transcriptional fate in motoneuron-like cells. J. Biol. Chem., 287, 15635–15647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zinszner H., Sok J., Immanuel D., Yin Y., Ron D. (1997) TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling. J. Cell. Sci., 110 (Pt 15), 1741–1750. [DOI] [PubMed] [Google Scholar]
- 15.Honda D., Ishigaki S., Iguchi Y., Fujioka Y., Udagawa T., Masuda A., Ohno K., Katsuno M., Sobue G. (2013) The ALS/FTLD-related RNA-binding proteins TDP-43 and FUS have common downstream RNA targets in cortical neurons. FEBS Open Bio, 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lagier-Tourenne C., Polymenidou M., Hutt K.R., Vu A.Q., Baughn M., Huelga S.C., Clutario K.M., Ling S.C., Liang T.Y., Mazur C. et al. (2012) Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci., 15, 1488–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeJesus-Hernandez M., Mackenzie I.R., Boeve B.F., Boxer A.L., Baker M., Rutherford N.J., Nicholson A.M., Finch N.A., Flynn H, Adamson J. et al. (2011) Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron, 72, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Renton A.E., Majounie E., Waite A., Simon-Sanchez J., Rollinson S., Gibbs J.R., Schymick J.C., Laaksovirta H., van Swieten J.C., Myllykangas L. et al. (2011) A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron, 72, 257–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper-Knock J., Walsh M.J., Higginbottom A., Robin Highley J., Dickman M.J., Edbauer D., Ince P.G., Wharton S.B., Wilson S.A., Kirby J. et al. (2014) Sequestration of multiple RNA recognition motif-containing proteins by C9orf72 repeat expansions. Brain, 137, 2040–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly C.J., Zhang P.W., Pham J.T., Haeusler A.R., Mistry N.A., Vidensky S., Daley E.L., Poth E.M., Hoover B., Fines D.M. et al. (2013) RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron, 80, 415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee Y.B., Chen H.J., Peres J.N., Gomez-Deza J., Attig J., Stalekar M., Troakes C., Nishimura A.L., Scotter E.L., Vance C. et al. (2013) Hexanucleotide repeats in ALS/FTD form length-dependent RNA foci, sequester RNA binding proteins, and are neurotoxic. Cell Rep., 5, 1178–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori K., Lammich S., Mackenzie I.R., Forne I., Zilow S., Kretzschmar H., Edbauer D., Janssens J., Kleinberger G., Cruts M. et al. (2013) hnRNP A3 binds to GGGGCC repeats and is a constituent of p62-positive/TDP43-negative inclusions in the hippocampus of patients with C9orf72 mutations. Acta Neuropathol., 125, 413–423. [DOI] [PubMed] [Google Scholar]
- 23.Sareen D., O'Rourke J.G., Meera P., Muhammad A.K., Grant S., Simpkinson M., Bell S., Carmona S., Ornelas L., Sahabian A. et al. (2013) Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci. Transl. Med., 5, 208ra149.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Z., Poidevin M., Li X., Li Y., Shu L., Nelson D.L., Li H., Hales C.M., Gearing M, Wingo T.S. et al. (2013) Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc. Natl. Acad. Sci. U. S. A., 110, 7778–7783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackenzie I.R., Arzberger T., Kremmer E., Troost D., Lorenzl S., Mori K., Weng S.M., Haass C., Kretzschmar H.A., Edbauer D. et al. (2013) Dipeptide repeat protein pathology in C9ORF72 mutation cases: clinico-pathological correlations. Acta Neuropathol., 126, 859–879. [DOI] [PubMed] [Google Scholar]
- 26.Mori K., Arzberger T., Grasser F.A., Gijselinck I., May S., Rentzsch K., Weng S.M., Schludi M.H., van der Zee J., Cruts M. et al. (2013) Bidirectional transcripts of the expanded C9orf72 hexanucleotide repeat are translated into aggregating dipeptide repeat proteins. Acta Neuropathol., 126, 881–893. [DOI] [PubMed] [Google Scholar]
- 27.Mori K., Weng S.M., Arzberger T., May S., Rentzsch K., Kremmer E., Schmid B., Kretzschmar H.A., Cruts M, Van Broeckhoven C. et al. (2013) The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science, 339, 1335–1338. [DOI] [PubMed] [Google Scholar]
- 28.Zu T., Liu Y., Banez-Coronel M., Reid T., Pletnikova O., Lewis J., Miller T.M., Harms M.B., Falchook A.E., Subramony S.H. et al. (2013) RAN proteins and RNA foci from antisense transcripts in C9ORF72 ALS and frontotemporal dementia. Proc. Natl. Acad. Sci. U. S. A., 110, E4968–E4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon I., Xiang S., Kato M., Wu L., Theodoropoulos P., Wang T., Kim J., Yun J., Xie Y., McKnight S.L. (2014) Poly-dipeptides encoded by the C9orf72 repeats bind nucleoli, impede RNA biogenesis, and kill cells. Science, 345, 1139–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizielinska S., Gronke S., Niccoli T., Ridler C.E., Clayton E.L., Devoy A., Moens T., Norona F.E., Woollacott I.O., Pietrzyk J. et al. (2014) C9orf72 repeat expansions cause neurodegeneration in Drosophila through arginine-rich proteins. Science, 345, 1192–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williamson T.L., Cleveland D.W. (1999) Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci., 2, 50–56. [DOI] [PubMed] [Google Scholar]
- 32.Zhang B., Tu P., Abtahian F., Trojanowski J.Q., Lee V.M. (1997) Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell. Biol., 139, 1307–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Vos K.J., Chapman A.L., Tennant M.E., Manser C., Tudor E.L., Lau K.F., Brownlees J., Ackerley S., Shaw P.J., McLoughlin D.M. et al. (2007) Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum. Mol. Genet., 16, 2720–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilsland L.G., Sahai E., Kelly G., Golding M., Greensmith L., Schiavo G. (2010) Deficits in axonal transport precede ALS symptoms in vivo. Proc. Natl. Acad. Sci. U. S. A., 107, 20523–20528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magrane J., Cortez C., Gan W.B., Manfredi G. (2014) Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum. Mol. Genet., 23, 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Li L., Lin W.L., Dickson D.W., Petrucelli L., Zhang T., Wang X. (2013) The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum. Mol. Genet., 22, 4706–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaper D.C., Adachi Y., Sutcliffe B., Humphrey D.M., Elliott C.J., Stepto A., Ludlow Z.N., Vanden Broeck L., Callaerts P., Dermaut B, et al. (2013) Loss and gain of Drosophila TDP-43 impair synaptic efficacy and motor control leading to age-related neurodegeneration by loss-of-function phenotypes. Hum. Mol. Genet., 22, 1539–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feiguin F., Godena V.K., Romano G., D'Ambrogio A., Klima R., Baralle F.E. (2009) Depletion of TDP-43 affects Drosophila motoneurons terminal synapsis and locomotive behavior. FEBS Lett., 583, 1586–1592. [DOI] [PubMed] [Google Scholar]
- 39.Wang J.W., Brent J.R., Tomlinson A., Shneider N.A., McCabe B.D. (2011) The ALS-associated proteins FUS and TDP-43 function together to affect Drosophila locomotion and life span. J. Clin. Invest., 121, 4118–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MacAskill A.F., Kittler J.T. (2010) Control of mitochondrial transport and localization in neurons. Trends Cell. Biol., 20, 102–112. [DOI] [PubMed] [Google Scholar]
- 41.Kanaan N.M., Pigino G.F., Brady S.T., Lazarov O., Binder L.I., Morfini G.A. (2013) Axonal degeneration in Alzheimer’s disease: when signaling abnormalities meet the axonal transport system. Exp. Neurol., 246, 44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunawardena S., Goldstein L.S. (2005) Polyglutamine diseases and transport problems: deadly traffic jams on neuronal highways. Arch. Neurol., 62, 46–51. [DOI] [PubMed] [Google Scholar]
- 43.Pareyson D., Saveri P., Piscosquito G. (2014) Charcot-Marie-Tooth Disease and Related Hereditary Neuropathies: from gene function to associated phenotypes. Curr. Mol. Med., 14, 1009–1033. [DOI] [PubMed] [Google Scholar]
- 44.Wang X., Winter D., Ashrafi G., Schlehe J., Wong Y.L., Selkoe D., Rice S., Steen J., LaVoie M.J., Schwarz T.L. (2011) PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell, 147, 893–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Godena V.K., Brookes-Hocking N., Moller A., Shaw G., Oswald M., Sancho R.M., Miller C.C., Whitworth A.J., De Vos K.J. (2014) Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat. Commun., 5, 5245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bowden H., Dormann D. (2016) Altered mRNP granule dynamics in FTLD pathogenesis. J. Neurochem, 138 (Suppl. 1), 112–133. [DOI] [PubMed] [Google Scholar]
- 47.Molliex A., Temirov J., Lee J., Coughlin M., Kanagaraj A.P., Kim H.J., Mittag T., Taylor J.P. (2015) Phase separation by low complexity domains promotes stress granule assembly and drives pathological fibrillization. Cell, 163, 123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou H., Huang C., Chen H., Wang D., Landel C.P., Xia P.Y., Bowser R., Liu Y.J., Xia X.G. (2010) Transgenic rat model of neurodegeneration caused by mutation in the TDP gene. PLoS Genet., 6, e1000887.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kraemer B.C., Schuck T., Wheeler J.M., Robinson L.C., Trojanowski J.Q., Lee V.M., Schellenberg G.D. (2010) Loss of murine TDP-43 disrupts motor function and plays an essential role in embryogenesis. Acta Neuropathol., 119, 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hicks G.G., Singh N., Nashabi A., Mai S., Bozek G., Klewes L., Arapovic D., White E.K., Koury M.J., Oltz E.M. et al. (2000) Fus deficiency in mice results in defective B-lymphocyte development and activation, high levels of chromosomal instability and perinatal death. Nat. Genet., 24, 175–179. [DOI] [PubMed] [Google Scholar]
- 51.Huang C., Zhou H., Tong J., Chen H., Liu Y.J., Wang D., Wei X., Xia X.G. (2011) FUS transgenic rats develop the phenotypes of amyotrophic lateral sclerosis and frontotemporal lobar degeneration. PLoS Genet., 7, e1002011.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hoell J.I., Larsson E., Runge S., Nusbaum J.D., Duggimpudi S., Farazi T.A., Hafner M., Borkhardt A., Sander C., Tuschl T. (2011) RNA targets of wild-type and mutant FET family proteins. Nat. Struct. Mol. Biol., 18, 1428–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glater E.E., Megeath L.J., Stowers R.S., Schwarz T.L. (2006) Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J. Cell. Biol., 173, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freibaum B.D., Lu Y., Lopez-Gonzalez R., Kim N.C., Almeida S., Lee K.H., Badders N., Valentine M., Miller B.L., Wong P.C. et al. (2015) GGGGCC repeat expansion in C9orf72 compromises nucleocytoplasmic transport. Nature, 525, 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang K., Donnelly C.J., Haeusler A.R., Grima J.C., Machamer J.B., Steinwald P., Daley E.L., Miller S.J., Cunningham K.M, Vidensky S. et al. (2015) The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature, 525, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jovicic A., Mertens J., Boeynaems S., Bogaert E., Chai N., Yamada S.B., Paul J.W., 3rd, Sun S., Herdy J.R., Bieri G. et al. (2015) Modifiers of C9orf72 dipeptide repeat toxicity connect nucleocytoplasmic transport defects to FTD/ALS. Nat. Neurosci., 18, 1226–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiesel F.C., Voigt A., Weber S.S., Van den Haute C., Waldenmaier A., Gorner K., Walter M., Anderson M.L., Kern J.V, Rasse T.M, et al. (2010) Knockdown of transactive response DNA-binding protein (TDP-43) downregulates histone deacetylase 6. Embo J., 29, 209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mudher A., Shepherd D., Newman T.A., Mildren P., Jukes J.P., Squire A., Mears A., Drummond J.A., Berg S, MacKay D. et al. (2004) GSK-3beta inhibition reverses axonal transport defects and behavioural phenotypes in Drosophila. Mol. Psychiatry, 9, 522–530. [DOI] [PubMed] [Google Scholar]
- 59.Wang X., Schwarz T.L. (2009) Imaging axonal transport of mitochondria. Methods Enzymol., 457, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]