Abstract
Bestrophin1 (BEST1) is expressed in human retinal pigment epithelium (RPE) and mutations in the BEST1 gene commonly cause retinal dysfunction and macular degeneration. BEST1 is presumed to assemble into a calcium-activated chloride channel and be involved in chloride transport but there is no direct evidence in live human RPE cells to support this idea. To test whether BEST1 functions as a chloride channel in living tissue, BEST1-mutant RPE (R218H, L234P, A243T) were generated from patient-derived induced pluripotent stem cells and compared with wild-type RPE in a retinal environment, using a biosensor that visualizes calcium-induced chloride ion flux in the cell. Calcium stimulation elicited chloride ion export in normal RPE but not in RPE derived from three patients with BEST1 mutations. These data, along with three-dimensional modeling, provide evidence that BEST1 assembles into a key calcium-sensing chloride channel in human RPE.
Introduction
Channelopathies are diseases due to mutations in channels that transport ions across cell membranes (1). To develop targeted therapies to specific channelopathies, it is crucial to understand precisely how disease mutations affect ion conductance. Vitelliform macular degeneration (VMD, OMIM#153700) is a blinding, retinal channelopathy caused by mutations in BESTROPHIN1 (BEST1, MIM#607854) (2,3). The gene product BESTROPHIN1 (BEST1) is thought to comprise a transmembrane anion channel expressed at the basolateral membrane of human retinal pigment epithelium (RPE) (4). It is also speculated that BEST1 is the Ca2+-activated chloride channel (CaCC) responsible for the electrooculogram (EOG) light peak (5,6). Still the question remains whether BEST1 functions as a chloride (Cl−) channel directly or indirectly regulates Cl− conductance via another channel (7–10).
In human VMD caused by BEST mutations, H2O and lipofuscin accumulate under the retina (11) and the EOG light peak is deficient (12). But Best1 knockout mice show normal RPE CaCC activity and EOG responses (13–16), so controversy remains regarding the true function of BEST1 in human RPE. Also, some studies claim that BEST1 regulates Cl− uptake into the endoplasmic reticulum (ER) (6,15,17,18), while others suggest BEST1 regulates Cl− efflux at the basal lateral plasma membrane (19). This issue is also unresolved, since previous studies were limited by the use of heterologous cell lines and indirect methods of Cl− ion detection (20,21). Many previous studies of chloride current through bestrophin channels employed whole-cell, patch-clamp analysis of exogenously overexpressing HEK293 cells (21,22).
To circumvent the limits of heterologous cells and inconsistency of model organisms (23–27), we used patient-specific RPE (iRPE) and a novel physiological approach to visualize Cl− ions in RPE directly with a biosensor. With this method, BEST1 can be directly studied in real-time, in a native RPE environment with endogenous BEST1 expression, providing strong evidence that BEST1 is a key calcium-sensing chloride channel in human RPE, and essential for RPE viability.
Results
RPE derived from BEST1 mutant patients
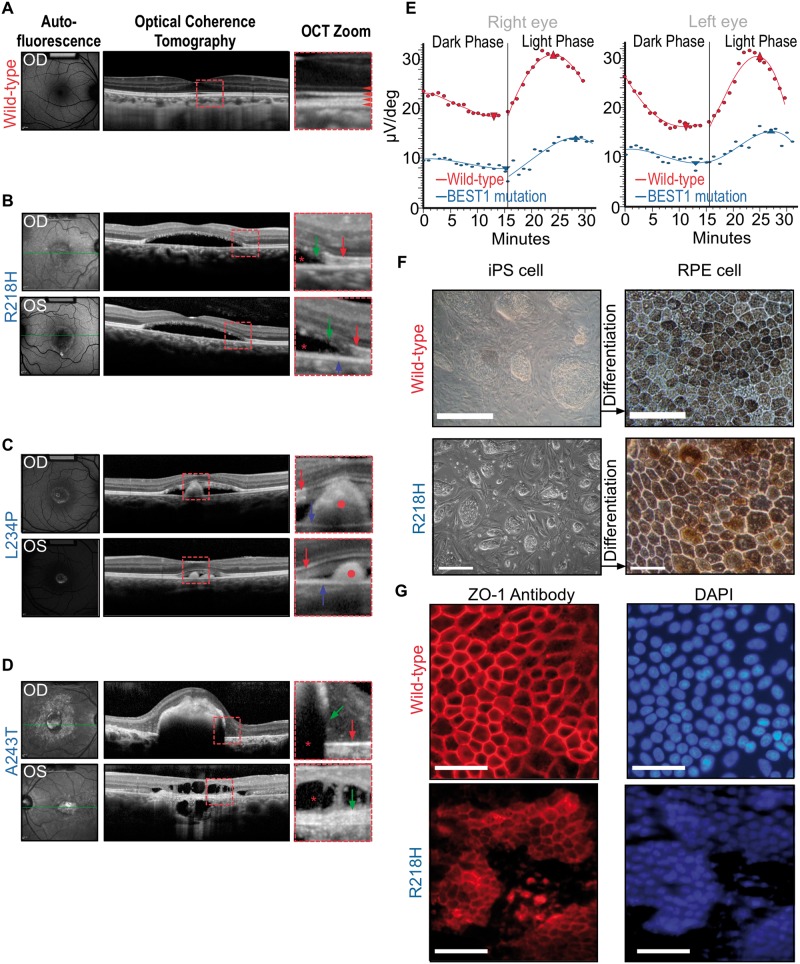
Patients with BEST1 mutations display characteristic retinal morphology and electrographic abnormalities suggesting defects in RPE fluid transport. We took advantage of this set of symptoms to uncover BEST1 mutations. An R218H mutation was detected in a 52-year-old with probable lipofuscin deposition (vitelliform lesion), a thin photoreceptor layer and diseased RPE with extensive subretinal serous fluid (Fig. 1B). A second A243T mutation was in a 61-year-old male who had retinal atrophy and disintegration of the photoreceptor mitochondrial band (Fig. 1C), indicating phagocytosis by the RPE had disrupted the outer segment. Mutations in R218 and A243 are known to cause BEST1-VMD (19). The third mutation was a previously undescribed substitution, L234P, in a 7-year-old boy with vitelliform lesions in both eyes, subretinal serous fluid and retinal outer segment debris (Fig. 1D). Each subject showed evidence of defective RPE fluid transport, a mutation in BEST1 and electrophysiological changes consistent with BEST1 VMD (Fig. 1E). We derived induced pluripotent stem cells from these patients to generate functional RPE carrying mutant BEST1 (Fig. 1F and G); these cells allow direct measurement of the effect of VMD BEST1 mutations on chloride ion transport across the RPE cell membrane.
Figure 1.
Human BEST1 mutations cause defective fluid transport in RPE and accumulation of serous fluid (*) in the subretinal space with a defective EOG light peak. (A) Autofluorescence and optical coherence tomography (OCT) images from a normal eye. Red square shows zoomed in area (right panel). Red arrowheads point to the four distinct reflective bands of the outer retinal layers in a normal eye under OCT scan. From top to bottom: external limiting membrane—boundary between photoreceptor nuclei and inner segments; EZ (ellipsoid zone)—formed by mitochondria in the outer portion of photoreceptor inner segments; interdigitation zone—where photoreceptor outer segments (POSs) interface with the RPE; RPE (retinal pigmented epithelium). (B) Phenotypic manifestation in a 52-yo VMD patient with R218H mutation. Right (OD) and left (OS) eyes showed vitelliform lesions. The central photoreceptor layer is thin compared with that of a healthy control with a loss of integrity of the retinal bands, and serous fluid has developed subretinally in OCT imaging. Green arrows point to POS debris due to diseased RPE. Red arrows point to EZ. Blue arrow points to RPE layer. Red star marks the subretinal fluid accumulation. (C) Phenotypic manifestation in a 7-yo VMD patient with L234P mutation. Right and left eyes showed vitelliform lesions. Subretinal fluid and outer segment debris are visible in the SD-OCT images. Red circles mark POS debris due to diseased RPE. Red arrows point to EZ. Blue arrows point to RPE.(D) Phenotypic manifestation in a 61-yo VMD patient with A243T mutation; Photoreceptor bands have disintegrated at various points. Red star indicates subretinal fluid accumulation. Green arrows point to POS debris due to diseased RPE. Red arrow points to EZ (upper panel). (E) A reduction in EOG light rise is seen in BEST1 VMD. Light-rise of the EOG is generated by Cl− mediated depolarization of the basal membrane of the RPE. The graphs show mean EOG responses to light of a normal (red line) and a VMD patient with R218H mutation (blue line). The normal increase in electrical response to light began after ∼15 min and reached a peak after 25 min. In the mutant case, this light rise response is both attenuated and slower. (F) Characteristic light microscopy images of cultured iPSCs from a WT control (top panels) and from the R218H patient in A before differentiation (left), and the RPE cells derived from these iPS after 12 weeks of differentiation (right). Scale bar: 100 μm. (G) Characteristic RPE cultures from a WT control (top panels) and the R218H patient (bottom panels) immunolabeled for the epithelial specific tight junction marker ZO-1 (left) with a corresponding DAPI (nuclear) stain (right). Scale bar: 100 μm.
Structural modeling of human BEST1 mutations
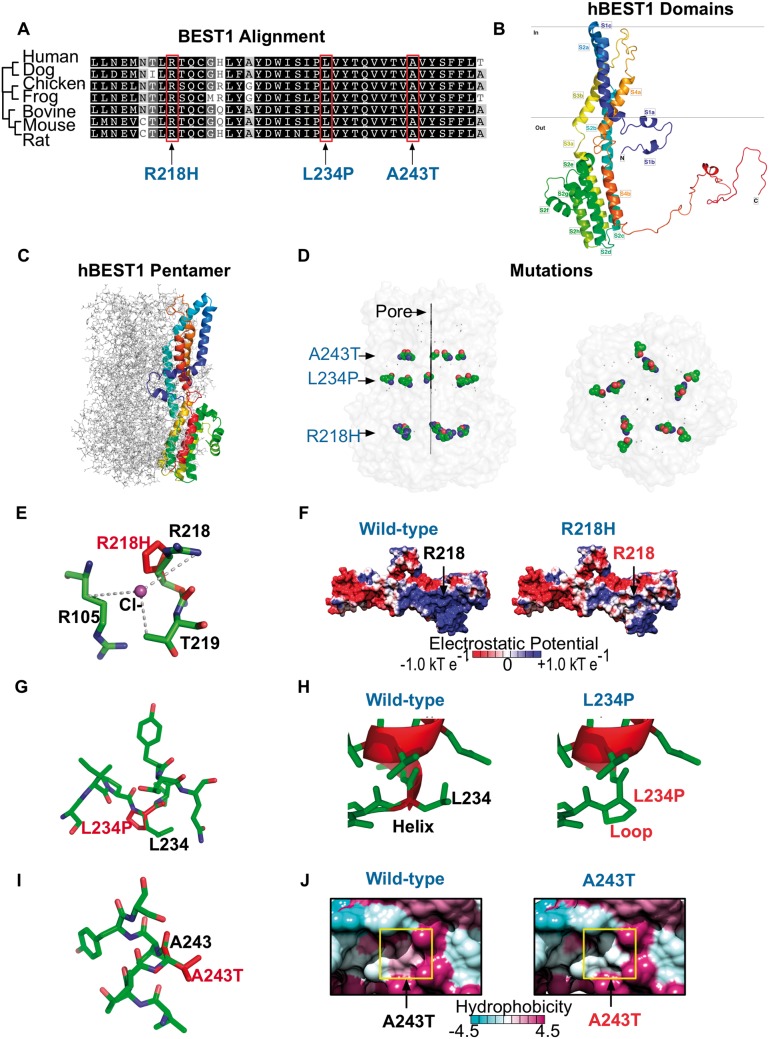
BEST1 is well conserved among species, especially in the N-terminal region, where many BEST1 missense mutations occur (28,29). Our patients’ mutations were in two adjacent alpha-helices, S3a and S3b, at residues conserved across seven species (Fig. 2A). To assess conservation of these residues, a PSI-BLAST identified 493 homologous sequences and ConSurf analysis was performed on the 150 sequences with the lowest E values. This analysis showed a minimum of 35% conservation between 150 bestrophin sequences. The residues mutated in our three patients had percent identity values of 97% (R218), 99% (A243) and 100% (L234) across 150 bestrophin sequences. The high degree of conservation highlights the probable importance of these amino acids for BEST1 function.
Figure 2.
Modeling of VMD mutations on human bestrophin-1 structure. (A) Species sequence alignments of BEST1 shows that the patient VMD mutations fell on conserved residues. (B) Homology model of hBEST1 generated using cBEST1 (PDB: 4RDQ) as a template. (C) Superimposition of the hBEST1 monomer on the channel pentamer. (D) Location of the patient VMD mutations in relationship to the channel pore. (E) Visualization of the putative Cl−-binding site in hBEST1 formed by the interface of R105, R218 and T219. The R218H mutation disrupts this binding site. (F) Electrostatic potential surfaces of the WT hBEST1 monomer and R218H hBEST1 reveal a change in positive to neutral potential in the residues surrounding the mutation. (G) Visualization of the location of L234P mutation in hBEST1. (H) Substitution of L234 with a proline would disrupt the secondary structure of the channel pore. Red represents helical residues while green represents residues that form disordered loops.(I) Visualization of the location of A243T mutation in hBEST1. (J) Hydrophobicity surface representations of the WT hBEST1 monomer and A243T mutant reveal a substitution of a hydrophobic residue to an uncharged, polar amino acid in a helix that is partially embedded in the membrane. The color scheme for the surface hydrophobicity is based on the Kyte and Doolittle scale.
High-resolution crystal structures exist for Klebsiella pneumoniae BEST1 (22) and chicken BEST1 (30) but the crystal structure of human BEST1 (hBEST1) remains undetermined. To predict how our patients’ mutations compromise the function of the hBEST1 channel, we generated three-dimensional models from previously solved crystal structures (Fig. 2B and C). Our hBEST1 model oriented the mutated residues along the channel pore interior, at the interface between adjacent hBEST1 subunits (Fig. 2D). Thus, the patient mutations were predicted to disrupt the stability of pentamer formation and affect channel function.
We modeled how individual mutations might affect hBEST1 function. R218H falls on a putative Cl− binding site in the bestrophin channel inner cavity (30). This binding site forms at the interface of three residues (R105, R218 and T219) on two adjacent helical subunits (Helix S2b and S2c; Fig. 2E). The arginine to histidine substitution appeared to shift the distance between the Cl−-binding residue and the anion and may decrease the local concentration of ions at the neck of the channel pore, thereby disrupting anion selectivity. Analysis of the hBEST1 electrostatic potential showed the residues surrounding the mutation change from a positive to a neutral charge, suggesting they bind less tightly to negatively charged ions, i.e. Cl− (Fig. 2F). Also, R218 appeared to be involved in a hydrogen-bonding network on the interface of two subunits, further highlighting that this mutation destabilizes the pentamer. The L234P mutation was in a buried α-helix that would likely be disrupted by a proline substitution, causing structural defects at the channel interface (Fig. 2G–H). The A243T mutation falls on helix S4a, which sits in a hydrophobic pocket that is partially embedded in the membrane. A substitution from a hydrophobic residue to a polar uncharged residue (Fig. 2J) could disrupt the association between adjacent subunits and potentially between the channel and the membrane. This three-dimensional modeling suggested the disease mutations reduce Cl− conductance, either by directly altering conductance or affecting the stability and formation of the hBEST1 pentamer.
Cl− current imaging of patient-derived RPE
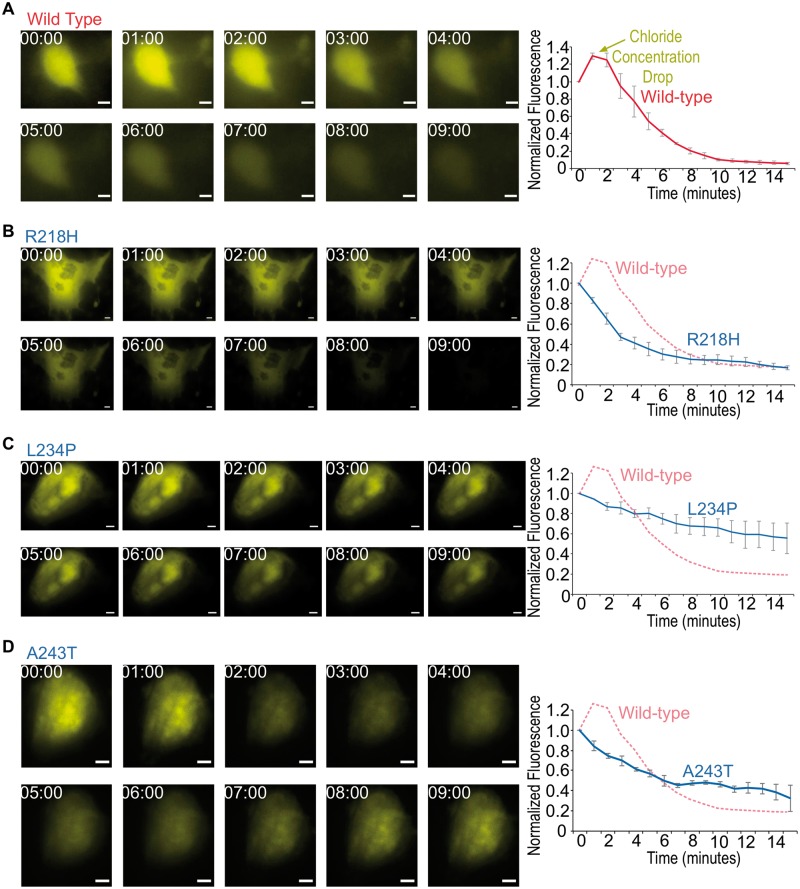
To test if BEST1 directly controls Cl− conductance in patient RPE cells, we performed live-cell imaging with a fluorescent anion biosensor (31). This biosensor is sensitive to Cl−, so that when intracellular [Cl−] increases, its YFP intensity drops, and vice versa (31). Cl− currents were measured in control and BEST1 mutant patient-derived RPE cells. Since BEST1 is predicted to be calcium-activated, the Ca2+ ionophore A23187 (32) was used to induce Ca2+ release from the ER and activate BEST1. Within 3 min of stimulation, wild-type (WT) RPE cells expressing the biosensor exhibited a decreased concentration of intracellular Cl−, followed by recovery within 15 min (Fig. 3A, Supplementary Material, Movie S1). In contrast, RPE cells derived from patients with BEST1 mutations (Fig. 1F) did not respond to this stimulation (Fig. 3B–D, Supplementary Material, Movies S2–S4), suggesting BEST1 mediates an efflux of Cl− in human RPE.
Figure 3.
Defective Cl− export in patient-specific iRPE revealed by biosensor imaging following calcium release. Representative still images of WT (A), R218H (B), L234P (C) and A243T (D) patient-derived RPE cells expressing Cl− biosensor and stimulated with 5 μM A23187 (left panels). Images were acquired at the given time in minutes after stimulation. Quantification of mean whole-cell biosensor emission intensity, normalized to t = 0 time point (right graphs). Data are represented as mean ± Standard error of the mean. Scale bar = 5 μm.
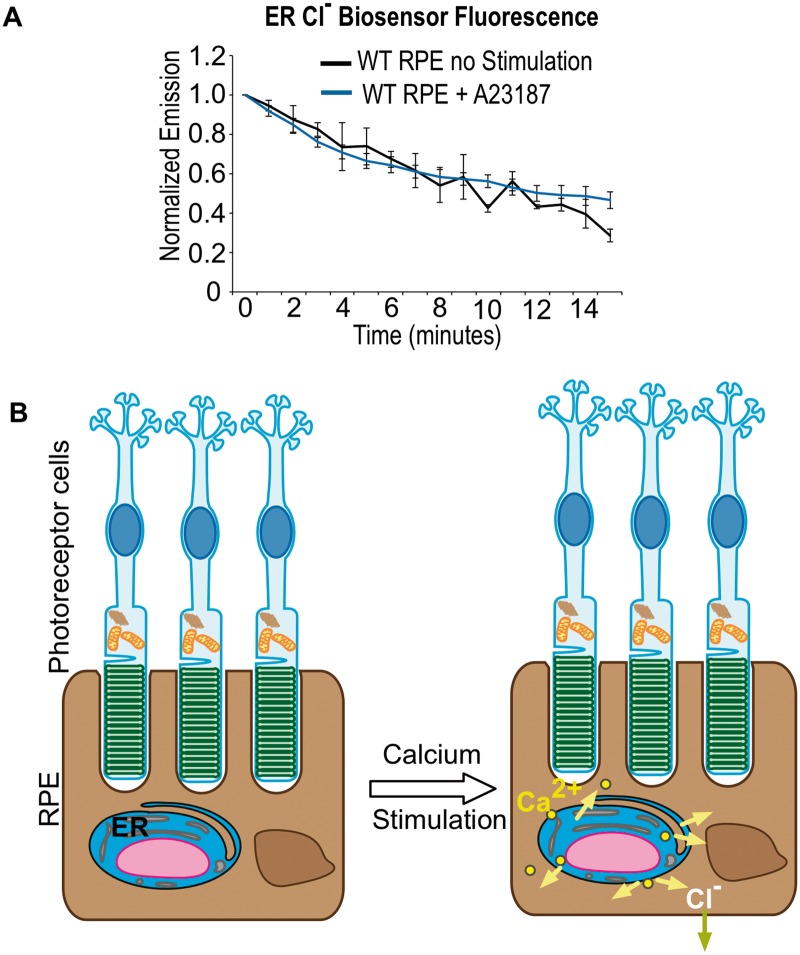
BEST1 has been hypothesized to function as an intracellular channel that conducts Cl− as the counter-ion that facilitates Ca2+ reentry into the ER (33,34). Based on the biosensor readout in WT RPE, intracellular Ca2+ release caused intracellular [Cl−] to drop. To test whether Cl− ions were moving out of the basolateral membrane or into the ER, the Cl− biosensor was targeted to the ER with an ER-localization signal sequence and KDEL (Lys-Asp-Glu-Leu) motif. Under the same stimulation and imaging conditions in WT RPE cells, we detected no significant change in [Cl−] in the ER (Fig. 4A). This negative result supports a model in which BEST1 does not function at the ER but instead conducts Cl− across the cell membrane (Fig. 4B).
Figure 4.
BEST1 Cl− current does not pass through ER. (A) Biosensor imaging of ER Cl− currents. Quantification of mean biosensor emission from WT RPE cells. Cl− biosensor was targeted to the ER and cells were imaged with and without A23187 stimulation. No significant change in Cl− ions was found in the ER. (B) Model of bestrophin-mediated Cl− current in RPE cells. Light induced activation of MerTK results in the generation of inositol-1,4,5-trisphosphate which triggers Ca2+ release from ER stores (pink arrows) and Cl− ion outflux (blue arrow).
Discussion
To develop effective therapies for the BEST1 VMD channelopathy, it is crucial to determine the mechanism linking genetic mutations to eye disease. Our novel biosensor imaging and computer modeling provide strong evidence that BEST1 is a Ca2+-activated Cl− channel, and VMD patient-derived BEST1-mutant RPE are defective in conducting Cl−. The phenotype only occurs in human RPE and not in mice likely because of cell and species-specific expression. Recently, it was shown that Best1 is expressed in mouse testes (not in mouse RPE), and that it’s exclusively localized in the cell membrane of human RPE (25).
Our structural models place the three VMD mutations at the interface between hBEST1 subunits. The residues at this interface are highly conserved across bestrophin sequences, likely because bonding interactions stabilize the multimeric structures. In contrast to mutations that disrupt channel assembly at the interface, the R218H mutation appears likely to disrupt anion selectivity along the pore, affecting chloride conductance directly. Other channelopathy studies have similarly highlighted how structural modeling can reveal disease mechanisms. For example, class II mutants of Cystic Fibrosis Transmembrane Conductance Regulator (e.g. ΔF508) likely form an unstable channel because they cannot fold correctly, contributing to development of cystic fibrosis (35). Similarly, predicted misfolding of the Cav2.1 subunit of P/Q-type Ca2 + channels likely causes episodic ataxia type-2 (EA2) (36).
Several types of BEST1 disease mutations prevent it from localizing normally to the basal cell membrane (25,37). The A243T mutant appears to be defective in Cl− conductance (38) because of mislocalization (37). This mutant may be analogous to a Y227N mutant, believed not to translocate efficiently to the basal membrane (39). In contrast, a nearby R218H mutation has no effect on membrane localization yet still causes the mutant phenotype (40–42). Structural modeling placed residue 218 within the channel pore, suggesting this mutation disrupts a Cl− binding site and renders BEST1 channels defective in conductance. Another novel mutation we described, L234P changes a highly conserved residue (Fig. 2F) near other disease mutations (Y227N, T237R, Q238R) that mislocalize to the cytoplasm (25,37). Chloride flux is likely blocked in BEST1-mutant RPE because of BEST1 channel mislocalization, anion transport or binding defects, or a combination of both (43). Detailed studies of BEST1 localization (25,37) also confirm this cell biological basis of dysfunction.
All three patient-specific RPE are defective in Cl− ion efflux in our biosensor assay and VMD mutations directly affect BEST1 in regulating Cl− exchange in the cell membrane. Since dark-adapted photoreceptors metabolize each molecule of glucose into six molecules of H2O and CO2, H2O accumulation is prevented by Cl− outflux in the basolateral membrane, which drives osmotic transport of H2O across RPE via aquaporins (34). In VMD, Cl− outflux is defective, preventing the Cl− driven transport of water and weakening adhesion of the RPE to the neuronal retina (44). Thus, therapies for BEST1 channelopathy should restore Cl− conductance in the RPE, to prevent subretinal H2O accumulation.
Materials and Methods
Live imaging
The collection of data used in this study was approved by the Institutional Review Board for Human Subjects Research at Columbia University Medical Center, was compliant with the Health Insurance Portability and Accountability Act and adhered to the tenets of the Declaration of Helsinki. Written informed consent was received from participants. Clinical examination and genetic testing was performed as previously described (45,46). Stereoscopic autoflourescent images and spectral domain optical coherence tomography were obtained using Spectralis Heidelberg (Heidelberg, Germany)
Homology modeling of human bestrophin-1
Homology models for hBEST1 were generated using MODELLER 9.14, as described previously (47,48). Briefly, a BLAST search for hBEST1 against the Protein Database (PDB) returned the structure of chicken BEST1 (PDB ID 4RDQ) as the top hit (Supplementary Material, Table S1). Ten models were generated using each template and superimposed well with a root mean square deviation that was <0.257 over 307 Cα atoms (Supplementary Material, Figure S1A. and B and Table S2). PyMOL and UCSF Chimera (49) were used to generate all structure figures (http://www.pymol.org/). Modeling of the channel pore surface was performed in MOLE 2.0 (50). Electrostatic potentials were calculated in APBS (51). Sequence alignments were performed using Geneious R8 (52).
Generation of patient iPSC
CytoTune-iPS Sendai Reprogramming Kit (53) (Life Technologies, Carlsbad, CA), was used to generate iPS with no genomic integration viral remnants. The iPSCs underwent four quality-control tests: karyotyping, genome sequencing (54), testing for the absence of Yamanaka transgenes and a pluripotency assay.
Differentiation of patient iPSCs into RPE
Human iPSCs were differentiated into RPE as we had done before (53). Phenotype was confirmed by assaying RPE-specific features: i) pigment expression and hexagonal cell shape (morphology), ii) expression of apical-basal polarity markers, iii) expression of RPE-specific markers (qPCR and immunoassays) and iv) phagocytic activity (data not shown). Primary antibodies are anti-RPE65 (rabbit, Santa Cruz; mouse, GeneTex), rabbit anti-bestrophin (Abcam) and rabbit anti-ZO-1 (Zymed). Secondary antibodies are Alexa Fluor 555 goat anti-rabbit IgG (Invitrogen).
Biosensor imaging
iRPE cells were seeded on matrigel (BD Biosciences) coated TransWell membranes (Corning). The Cl− biosensor (pcDNA3.1 EYFP H148Q/I152L) was purchased from Addgene and transfected into RPE cells with Lipofectamine 3000 (Invitrogen). Three days after transfection, individual biosensor-expressing RPE were imaged on a Lieca DM5000B upright fluorescent microscope with SPOT Imaging Solutions software. Cells were stimulated with 5 μM Ca2+ ionophore A23187 (IC50 ∼ 0.5–1 μM, Sigma, St Louis) (55) in PBS at 37°C and images were taken every minute for 15 min. All images were acquired at 40X magnification with a GFP filter set and 500 ms exposure. Raw images were processed using ImageJ software.
Supplementary Material
Supplementary Material is available at HMG online.
Acknowledgements
The authors thank Diana Colgan, Melinda Smits and Lokesh Gakhar for technical assistance.
Conflict of Interest statement. None declared.
Funding
The Barbara & Donald Jonas Laboratory of Regenerative Medicine and Bernard & Shirlee Brown Glaucoma Laboratory are supported by the National Institute of Health (5P30EY019007, R01EY018213, R01EY024698, R21AG050437); National Cancer Institute Core (5P30CA013696); the Research to Prevent Blindness (RPB) Physician-Scientist Award; unrestricted funds from RPB, New York, NY, USA. S.H.T. is a member of the RD-CURE Consortium and is supported by the Tistou and Charlotte Kerstan Foundation; the Schneeweiss Stem Cell Fund; New York State (C029572); the Foundation Fighting Blindness New York Regional Research Center Grant (C-NY05-0705-0312); the Joel Hoffman Fund; the Professor Gertrude Rothschild Stem Cell Foundation and the Gebroe Family Foundation. V.B.M. is supported by the National Institutes of Health (K08EY020530, R01EY016822); Doris Duke Charitable Foundation Grant #2013103 and RPB, New York, NY. G.V. is supported by the National Institute of Health (T32GM007337).
References
- 1.Berend K., van Hulsteijn L.H., Gans R.O. (2012) Chloride: the queen of electrolytes? Eur. J. Intern. Med., 23, 203–211. [DOI] [PubMed] [Google Scholar]
- 2.Marquardt A., Stohr H., Passmore L.A., Kramer F., Rivera A., Weber B.H. (1998) Mutations in a novel gene, VMD2, encoding a protein of unknown properties cause juvenile-onset vitelliform macular dystrophy (Best's disease). Hum. Mol. Genet., 7, 1517–1525. [DOI] [PubMed] [Google Scholar]
- 3.Petrukhin K., Koisti M.J., Bakall B., Li W., Xie G., Marknell T., Sandgren O., Forsman K., Holmgren G., Andreasson S. et al. (1998) Identification of the gene responsible for Best macular dystrophy. Nat. Genet., 19, 241–247. [DOI] [PubMed] [Google Scholar]
- 4.Marmorstein A.D., Marmorstein L.Y., Rayborn M., Wang X., Hollyfield J.G., Petrukhin K. (2000) Bestrophin, the product of the Best vitelliform macular dystrophy gene (VMD2), localizes to the basolateral plasma membrane of the retinal pigment epithelium. Proc. Natl Acad. Sci. U. S. A., 97, 12758–12763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fujii S., Gallemore R.P., Hughes B.A., Steinberg R.H. (1992) Direct evidence for a basolateral membrane Cl-conductance in toad retinal pigment epithelium. Am. J. Physiol., 262, C374–C383. [DOI] [PubMed] [Google Scholar]
- 6.Barro Soria R., Spitzner M., Schreiber R., Kunzelmann K. (2009) Bestrophin-1 enables Ca2+-activated Cl-conductance in epithelia. J. Biol. Chem., 284, 29405–29412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess R., Millar I.D., Leroy B.P., Urquhart J.E., Fearon I.M., De Baere E., Brown P.D., Robson A.G., Wright G.A., Kestelyn P. et al. (2008) Biallelic mutation of BEST1 causes a distinct retinopathy in humans. Am. J. Hum. Genet., 82, 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davidson A.E., Millar I.D., Urquhart J.E., Burgess-Mullan R., Shweikh Y., Parry N., O'Sullivan J., Maher G.J., McKibbin M., Downes S.M. et al. (2009) Missense mutations in a retinal pigment epithelium protein, bestrophin-1, cause retinitis pigmentosa. Am. J. Hum. Genet., 85, 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao Q., Hartzell H.C., Yu K. (2010) Bestrophins and retinopathies. Pflugers Arch., 460, 559–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kunzelmann K., Kongsuphol P., Chootip K., Toledo C., Martins J.R., Almaca J., Tian Y., Witzgall R., Ousingsawat J., Schreiber R. (2011) Role of the Ca2+ -activated Cl- channels bestrophin and anoctamin in epithelial cells. Biol. Chem., 392, 125–134. [DOI] [PubMed] [Google Scholar]
- 11.Weingeist T.A., Kobrin J.L., Watzke R.C. (1982) Histopathology of Best's macular dystrophy. Arch. Ophthalmol., 100, 1108–1114. [DOI] [PubMed] [Google Scholar]
- 12.Cross H.E., Bard L. (1974) Electro-oculography in Best's macular dystrophy. Am. J. Ophthalmol., 77, 46–50. [DOI] [PubMed] [Google Scholar]
- 13.Marmorstein L.Y., Wu J., McLaughlin P., Yocom J., Karl M.O., Neussert R., Wimmers S., Stanton J.B., Gregg R.G., Strauss O. et al. (2006) The light peak of the electroretinogram is dependent on voltage-gated calcium channels and antagonized by bestrophin (best-1). J. Gen. Physiol., 127, 577–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marmorstein A.D., Kinnick T.R. (2007) Focus on molecules: bestrophin (best-1). Exp. Eye Res., 85, 423–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barro-Soria R., Schreiber R., Kunzelmann K. (2008) Bestrophin 1 and 2 are components of the Ca(2+) activated Cl(-) conductance in mouse airways. Biochim. Biophys. Acta, 1783, 1993–2000. [DOI] [PubMed] [Google Scholar]
- 16.Marmorstein A.D., Cross H.E., Peachey N.S. (2009) Functional roles of bestrophins in ocular epithelia. Prog. Retin. Eye Res., 28, 206–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barro-Soria R., Aldehni F., Almaca J., Witzgall R., Schreiber R., Kunzelmann K. (2010) ER-localized bestrophin 1 activates Ca2+-dependent ion channels TMEM16A and SK4 possibly by acting as a counterion channel. Pflugers Arch., 459, 485–497. [DOI] [PubMed] [Google Scholar]
- 18.Neussert R., Muller C., Milenkovic V.M., Strauss O. (2010) The presence of bestrophin-1 modulates the Ca2+ recruitment from Ca2+ stores in the ER. Pflugers Arch., 460, 163–175. [DOI] [PubMed] [Google Scholar]
- 19.Hartzell H.C., Qu Z., Yu K., Xiao Q., Chien L.T. (2008) Molecular physiology of bestrophins: multifunctional membrane proteins linked to best disease and other retinopathies. Physiol. Rev., 88, 639–672. [DOI] [PubMed] [Google Scholar]
- 20.Strauss O., Muller C., Reichhart N., Tamm E.R., Gomez N.M. (2014) The role of bestrophin-1 in intracellular Ca(2+) signaling. Adv. Exp. Med. Biol., 801, 113–119. [DOI] [PubMed] [Google Scholar]
- 21.Marmorstein A.D., Kinnick T.R., Stanton J.B., Johnson A.A., Lynch R.M., Marmorstein L.Y. (2015) Bestrophin-1 influences transepithelial electrical properties and Ca2+ signaling in human retinal pigment epithelium. Mol. Vis., 21, 347–359. [PMC free article] [PubMed] [Google Scholar]
- 22.Yang T., Liu Q., Kloss B., Bruni R., Kalathur R.C., Guo Y., Kloppmann E., Rost B., Colecraft H.M., Hendrickson W.A. (2014) Structure and selectivity in bestrophin ion channels. Science, 346, 355–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh R., Shen W., Kuai D., Martin J.M., Guo X., Smith M.A., Perez E.T., Phillips M.J., Simonett J.M., Wallace K.A. et al. (2013) iPS cell modeling of Best disease: insights into the pathophysiology of an inherited macular degeneration. Hum. Mol. Genet., 22, 593–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright L.S., Phillips M.J., Pinilla I., Hei D., Gamm D.M. (2014) Induced pluripotent stem cells as custom therapeutics for retinal repair: progress and rationale. Exp. Eye Res., 123, 161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milenkovic A., Brandl C., Milenkovic V.M., Jendryke T., Sirianant L., Wanitchakool P., Zimmermann S., Reiff C.M., Horling F., Schrewe H. et al. (2015) Bestrophin 1 is indispensable for volume regulation in human retinal pigment epithelium cells. Proc. Natl Acad. Sci. U. S. A., 112, E2630–E2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y., Chan L., Nguyen H.V., Tsang S.H. (2016) Personalized medicine: cell and gene therapy based on patient-specific iPSC-derived retinal pigment epithelium cells. Adv. Exp. Med. Biol., 854, 549–555. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen H.V., Li Y., Tsang S.H. (2015) Patient-specific iPSC-derived RPE for modeling of retinal diseases. J. Clin. Med., 4, 567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stohr H., Marquardt A., Nanda I., Schmid M., Weber B.H. (2002) Three novel human VMD2-like genes are members of the evolutionary highly conserved RFP-TM family. Eur. J. Hum. Genet., 10, 281–284. [DOI] [PubMed] [Google Scholar]
- 29.Boon C.J., Klevering B.J., Leroy B.P., Hoyng C.B., Keunen J.E., den Hollander A.I. (2009) The spectrum of ocular phenotypes caused by mutations in the BEST1 gene. Prog. Retin. Eye Res., 28, 187–205. [DOI] [PubMed] [Google Scholar]
- 30.Kane Dickson V., Pedi L., Long S.B. (2014) Structure and insights into the function of a Ca(2+)-activated Cl(-) channel. Nature, 516, 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galietta L.J., Haggie P.M., Verkman A.S. (2001) Green fluorescent protein-based halide indicators with improved chloride and iodide affinities. FEBS Lett., 499, 220–224. [DOI] [PubMed] [Google Scholar]
- 32.Babcock D.F., First N.L., Lardy H.A. (1976) Action of ionophore A23187 at the cellular level. Separation of effects at the plasma and mitochondrial membranes. J. Biol. Chem., 251, 3881–3886. [PubMed] [Google Scholar]
- 33.Constable P.A. (2014) A perspective on the mechanism of the light-rise of the electrooculogram. Invest. Ophthalmol. Vis. Sci., 55, 2669–2673. [DOI] [PubMed] [Google Scholar]
- 34.Reichhart N., Strauß O. (2014) Ion channels and transporters of the retinal pigment epithelium. Exp. Eye Res., 126, 27–37. [DOI] [PubMed] [Google Scholar]
- 35.Okiyoneda T., Lukacs G.L. (2012) Fixing cystic fibrosis by correcting CFTR domain assembly. J. Cell Biol., 199, 199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mezghrani A., Monteil A., Watschinger K., Sinnegger-Brauns M.J., Barrere C., Bourinet E., Nargeot J., Striessnig J., Lory P. (2008) A destructive interaction mechanism accounts for dominant-negative effects of misfolded mutants of voltage-gated calcium channels. J. Neurosci., 28, 4501–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milenkovic V.M., Rohrl E., Weber B.H., Strauss O. (2011) Disease-associated missense mutations in bestrophin-1 affect cellular trafficking and anion conductance. J. Cell Sci., 124, 2988–2996. [DOI] [PubMed] [Google Scholar]
- 38.Yu K., Cui Y., Hartzell H.C. (2006) The bestrophin mutation A243V, linked to adult-onset vitelliform macular dystrophy, impairs its chloride channel function. Invest. Ophthalmol. Vis. Sci., 47, 4956–4961. [DOI] [PubMed] [Google Scholar]
- 39.Mullins R.F., Oh K.T., Heffron E., Hageman G.S., Stone E.M. (2005) Late development of vitelliform lesions and flecks in a patient with best disease: clinicopathologic correlation. Arch. Ophthalmol., 123, 1588–1594. [DOI] [PubMed] [Google Scholar]
- 40.Caldwell G.M., Kakuk L.E., Griesinger I.B., Simpson S.A., Nowak N.J., Small K.W., Maumenee I.H., Rosenfeld P.J., Sieving P.A., Shows T.B. et al. (1999) Bestrophin gene mutations in patients with Best vitelliform macular dystrophy. Genomics, 58, 98–101. [DOI] [PubMed] [Google Scholar]
- 41.Marchant D., Gogat K., Boutboul S., Pequignot M., Sternberg C., Dureau P., Roche O., Uteza Y., Hache J.C., Puech B. et al. (2001) Identification of novel VMD2 gene mutations in patients with best vitelliform macular dystrophy. Hum. Mutat., 17, 235. [DOI] [PubMed] [Google Scholar]
- 42.Atchaneeyasakul L.O., Jinda W., Sakolsatayadorn N., Trinavarat A., Ruangvoravate N., Thanasombatskul N., Thongnoppakhun W., Limwongse C. (2008) Mutation analysis of the VMD2 gene in thai families with best macular dystrophy. Ophthalmic Genet., 29, 139–144. [DOI] [PubMed] [Google Scholar]
- 43.Yang T., Justus S., Li Y., Tsang S.H. (2015) BEST1: the best target for gene and cell therapies. Mol. Ther., 23, 1805–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frambach D.A., Roy C.E., Valentine J.L., Weiter J.J. (1989) Precocious retinal adhesion is affected by furosemide and ouabain. Curr. Eye Res., 8, 553–556. [DOI] [PubMed] [Google Scholar]
- 45.Bassuk A.G., Sujirakul T., Tsang S.H., Mahajan V.B. (2014) A novel RPGR mutation masquerading as Stargardt disease. Br. J. Ophthalmol., 98, 709–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncker T., Greenberg J.P., Ramachandran R., Hood D.C., Smith R.T., Hirose T., Woods R.L., Tsang S.H., Delori F.C., Sparrow J.R. (2014) Quantitative fundus autofluorescence and optical coherence tomography in best vitelliform macular dystrophy. Invest. Ophthalmol. Vis. Sci., 55, 1471–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eswar N., Webb B., Marti-Renom M.A., Madhusudhan M.S., Eramian D., Shen M.Y., Pieper U., Sali A. (2007) Comparative protein structure modeling using MODELLER. Curr. Protoc. Protein Sci., 50, 2.9: 2.9.1-2.9.31. [DOI] [PubMed] [Google Scholar]
- 48.Bassuk A.G., Yeh S., Wu S., Martin D.F., Tsang S.H., Gakhar L., Mahajan V.B. (2015) Structural modeling of a novel CAPN5 mutation that causes uveitis and neovascular retinal detachment. PLoS One, 10, e0122352.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. (2004) UCSF chimera–a visualization system for exploratory research and analysis. J. Comput. Chem., 25, 1605–1612. [DOI] [PubMed] [Google Scholar]
- 50.Sehnal D., Svobodova Varekova R., Berka K., Pravda L., Navratilova V., Banas P., Ionescu C.M., Otyepka M., Koca J. (2013) MOLE 2.0: advanced approach for analysis of biomacromolecular channels. J. Cheminform., 5, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baker N.A., Sept D., Joseph S., Holst M.J., McCammon J.A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl Acad. Sci. U. S. A., 98, 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C. et al. (2012) Geneious basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics, 28, 1647–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Tsai Y.T., Hsu C.W., Erol D., Yang J., Wu W.H., Davis R.J., Egli D., Tsang S.H. (2012) Long-term safety and efficacy of human-induced pluripotent stem cell (iPS) grafts in a preclinical model of retinitis pigmentosa. Mol. Med., 18, 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bock C., Kiskinis E., Verstappen G., Gu H., Boulting G., Smith Z.D., Ziller M., Croft G.F., Amoroso M.W., Oakley D.H. et al. (2011) Reference maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell, 144, 439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hall M.O., Abrams T.A., Mittag T.W. (1991) ROS ingestion by RPE cells is turned off by increased protein kinase C activity and by increased calcium. Exp. Eye Res., 52, 591–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.