Abstract
Among the most diagnostic manifestations of Cushing’s syndrome (CS) are those involving the skin; they include violaceous striae, facial acne, hirsutism, acanthosis nigricans (AN), fungal infections, hyperpigmentation (Hp) and easy bruisability. Fortunately, most resolve within a year or two after cure of CS, although light-colored striae can persist for years depending on the age of the patients. AN, Hp, and bruisability usually resolve within months after cure in almost all ages. Facial plethora (along with acne and other facial skin changes) is a typical sign of CS that is due to increased perfusion. It resolves immediately after curative therapy of CS. Typically, the severity of the manifestations does not correlate with the biochemical indices of the disease, pointing to age, gender, genetic and skin-type differences that determine the cutaneous manifestations of CS.
Keywords: Cushing’s syndrome, glucocorticoids, skin, acne, plethora, hyperpigmentation, bruise, striae, hirsutism, acanthosis
Skin findings in Cushing’s syndrome
There are only a few studies focusing on the skin manifestations of Cushing’s syndrome (CS) caused by endogenous glucocorticoids (1–4) (Figure 1). Skin striae due to hypercortisolism are often wide and purple, which contrasts with the narrow and pale or pink striae of rapid weight gain (5). Facial acne and hirsutism are attributed to increased adrenal androgen and/or cortisol secretion (1,2). Women and prepubertal children with CS typically have fine downy facial lanugo hair and, in addition to acne and hirsutism, may also have temporal scalp hair regression (1,2,5). Acanthosis nigricans is a velvety, hyperpigmented thickened plaque occurring most often at the nape of the neck and the axilla, which is often associated with insulin resistance and CS (6). Superficial fungal infections are common in CS and are caused by cortisol-induced immune suppression (2, 7) and glucose intolerance (1). In addition, in CS, the skin often shows signs of lacerations that have healed poorly and may be hyperpigmented (1).
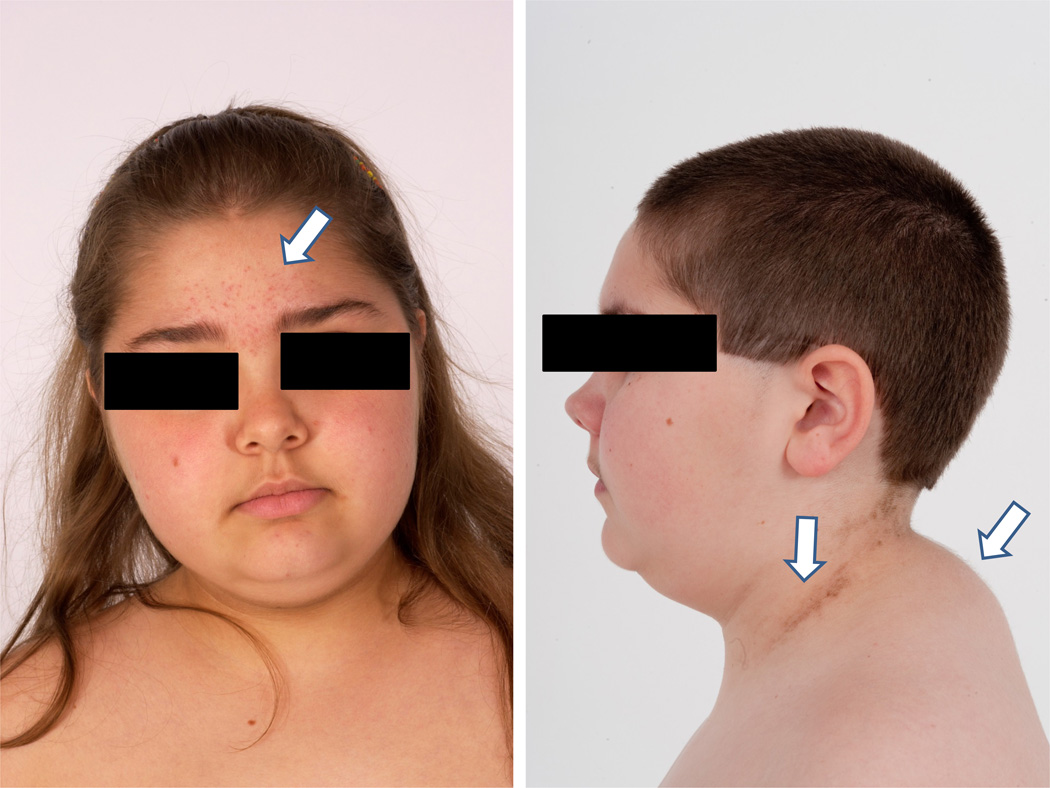
Figure 1.
Two pediatric patients with endogenous CS. A. Typical acne on the forehead (arrow) and elsewhere on the plethoric face of this girl with CS; B. A boy with CS had impressive acanthosis nigricans (arrow) on the lateral neck, and a web (arrow) on the back.
What happens to cutaneous manifestations of CS after cure?
Like the other manifestations of CS, cutaneous symptoms decrease dramatically within the first few months to a year postoperatively. In pediatric patients, none of the skin manifestations of CS was present after the first 12 months following the surgery, with the exception of lightly pigmented striae (1), which were present for up to 18 months postoperatively. The response of the skin to the cure of hypercortisolism is comparable, albeit somewhat faster, to the recovery of other affected systems, such as the hypothalamic-pituitary-adrenal (HPA), thyroid and somatotropic axes (8–12). The HPA axis is suppressed during the first post-operative year in most patients with CS, although the pituitary corticotroph cells demonstrate normal responses to stimuli (12). The defective pituitary-thyroid axis returns to normalcy usually within the first 3 postoperative months, but defective thyrotropin secretion can be seen in some patients for up to 12 months (13). Patients with CS have marked suppression of their growth hormone (GH) secretion during their illness, which persists for at least the first 12 months of the convalescence period following the surgery, and often leads to a compromised final adult height (9, 11). Almost all symptoms and signs of CS recover back to normal (or near normal) also within the first 12 months post-operatively (12–18). Finally, the significant decrease in bone turnover that is seen in children with CS (19, 20), which is comparable to that observed in other conditions associated with chronic endogenous hypercortisolism (21) recovers at least when checked in childhood and young adulthood (20).
Pathophysiology of skin findings in CS
The persistence of striae for several months following CS remission underlines the significant effects of glucocorticoids on epidermal structure and physiology. The glucocorticoid receptor (GR) is highly expressed in the basal cells but it is barely detectable in the other layers of the epidermis (22). The localization of the GR in basal corneocytes, as well as the negative effects of glucocorticoids on keratinocyte growth factor and type-I and-III collagen gene expression (23–26) suggest that endogenous cortisol in CS suppresses not only wound healing but also normal skin growth and turnover. In one study, these processes were significantly inhibited after a 3-day topical betamethasone application, and did not recover even after a 2-week corticoid-free period (26). Interestingly, there was no difference in the degree of collagen synthesis suppression between the young (mean age 23 years) and the old (64 years) subjects of the same study (26). It has been suggested that local retinoic acid may prevent corticosteroid-induced atrophy (27), but this has not been tested in the striae of patients with CS. Repeated trauma and sun exposure may delay the healing of glucocorticoid-induced striae (28); thus, a usual recommendation to our patients during the recovery period is avoidance of these factors.
Although endogenous hypercortisolism is obviously responsible for most of the skin manifestations of CS, we found that the severity of these symptoms did not correlate with glucocorticoid levels preoperatively (1). This suggests that genetic differences in skin sensitivity or other factors, such as the temporal pattern of glucocorticoid secretion may have been responsible for the observed variability. In general, inter-individual differences in clinical symptomatology are more common in endogenous than exogenous hypercortisolism (29). It has been observed, for example, that, although posterior subcapsular cataracts in chronic iatrogenic CS appear to be dose- and duration-dependent, endogenous hypercortisolism of equal severity only infrequently lead to cataract formation (30).
Facial plethora in CS
Facial plethora in CS is another sign that does not appear to correlate with the levels of glucocorticoids (31,32). In a recent study, we showed that plethora, an ancient clinical sign, is due to increase perfusion of the face under the influence of excess glucocorticoids (31). Along with acne, hirsutism, and the occasional skin infection, plethora is characteristic of the face of a patient with CS and appears to be among the first clinical signs to resolve after curative therapy (Figure 2).
Figure 2.
Facial plethora (circled) in pediatric and adult patients with CS (A, C, E, G, I, K) and its resolution following cure (B, D, F, H, J, L and M; the last 2 pictures are from the same patient immediately [L] and 1 year after surgery [M])). See also reference 31.
Concluding remarks
In CS, the skin is affected at multiple sites; most lesions heal within the first year after surgical cure of the disease, with the exception of striae which may take longer. Hirsutism also may persist. Plethora is a characteristic sign of CS that resolves first after cure. These effects do not correlate with the levels of circulating glucocorticoids during active disease pointing to idiosyncratic (likely to be genetic) response of the skin to excess glucocorticoids.
Acknowledgments
This review was supported by the research project Z01-HD008920 (Principal Investigator: Dr. Constantine A Stratakis) of the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), National Institutes of Health (NIH), Bethesda, MD, USA.
Footnotes
Compliance with ethical standards
Conflict of interest
The author declares that he has no conflict of interest.
REFERENCES
- 1.Stratakis CA, Mastorakos G, Mitsiades NS, Mitsiades CS, Chrousos GP. Skin manifestations of Cushing disease in children and adolescents before and after the resolution of hypercortisolemia. Pediatr Dermatol. 1998;15(4):253–258. doi: 10.1046/j.1525-1470.1998.1998015253.x. [DOI] [PubMed] [Google Scholar]
- 2.Lionakis MS, Kontoyiannis DP. Glucocorticoids and invasive fungal infections. Lancet. 2003 Nov 29;362(9398):1828–1838. doi: 10.1016/S0140-6736(03)14904-5. [DOI] [PubMed] [Google Scholar]
- 3.Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006 Jun;15(6):406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 4.Guillot B. Glucocorticoid-induced cutaneous adverse events. Rev Med Interne. 2013 May;34(5):310–314. doi: 10.1016/j.revmed.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Thiboutot DM. Dermatological manifestations of endocrine disorders. J Clin Endocrinol Metab. 1995;80(10):3082–3087. doi: 10.1210/jcem.80.10.7559901. [DOI] [PubMed] [Google Scholar]
- 6.Matsuoka LY, Wortsman J, Goldman J. Acanthosis nigricans. Clin Dermatol. 1993 Jan-Mar;11(1):21–25. doi: 10.1016/0738-081x(93)90076-o. [DOI] [PubMed] [Google Scholar]
- 7.Feingold KR, Elias PM. Endocrine-skin interactions: cutaneous manifestations of pituitary disease, thyroid disease, calcium disorders and diabetes. J. Am. Acad. Dermatol. 1987;17:921–940. doi: 10.1016/s0190-9622(87)70282-5. [DOI] [PubMed] [Google Scholar]
- 8.Gomez MT, Magiakou MA, Mastorakos G, Chrousos GP. The pituitary corticotroph is not the rate limiting step in the postoperative recovery of the hypothalamic-pituitary-adrenal axis in patients with Cushing syndrome. J Clin Endocrinol Metab. 1993;77(1):173–177. doi: 10.1210/jcem.77.1.8392083. [DOI] [PubMed] [Google Scholar]
- 9.Magiakou MA, Mastorakos GM, Gomez T, Rose SR, Chrousos GP. Suppressed spontaneous and stimulated growth hormone secretion in patients with Cushing’s disease. J Clin Endocrinol Metab. 1994;78(1):131–137. doi: 10.1210/jcem.78.1.7507118. [DOI] [PubMed] [Google Scholar]
- 10.Stratakis CA. Cushing syndrome in pediatrics. Endocrinol Metab Clin North Am. 2012 Dec;41(4):793–803. doi: 10.1016/j.ecl.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gourgari E, Lodish M, Keil M, Wesley R, Hill S, Xekouki P, Lyssikatos C, Belyavskaya E, De La Luz SM, Stratakis CA. Post-operative growth is different in various forms of pediatric Cushing's syndrome. Endocr Relat Cancer. 2014;21(6):L27–L31. doi: 10.1530/ERC-14-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lodish M, Dunn SV, Sinaii N, Keil MF, Stratakis CA. Recovery of the hypothalamic-pituitary-adrenal axis in children and adolescents after surgical cure of Cushing's disease. J Clin Endocrinol Metab. 2012 May;97(5):1483–1491. doi: 10.1210/jc.2011-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stratakis CA, Magiakou MA, Mastorakos G, Passaro M, Oldfield EH, Cutler GB, Chrousos GP. Thyroid function in children with Cushing disease before and after surgical cure. J Pediatr. 1997 Dec;131(6):905–909. doi: 10.1016/s0022-3476(97)70041-6. [DOI] [PubMed] [Google Scholar]
- 14.Lacroix A, Feelders RA, Stratakis CA, Nieman LK. Cushing's syndrome. Lancet. 2015 Aug 29;386(9996):913–927. doi: 10.1016/S0140-6736(14)61375-1. [DOI] [PubMed] [Google Scholar]
- 15.Lodish MB, Gourgari E, Sinaii N, Hill S, Libuit L, Mastroyannis S, Keil M, Batista DL, Stratakis CA. Skeletal maturation in children with Cushing syndrome is not consistently delayed: the role of corticotropin, obesity, and steroid hormones, and the effect of surgical cure. J Pediatr. 2014 Apr;164(4):801–806. doi: 10.1016/j.jpeds.2013.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keil MF, Graf J, Gokarn N, Stratakis CA. Anthropometric measures and fasting insulin levels in children before and after cure of Cushing syndrome. Clin Nutr. 2012 Jun;31(3):359–363. doi: 10.1016/j.clnu.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Briassoulis G, Damjanovic S, Xekouki P, Lefebvre H, Stratakis CA. The glucocorticoid receptor and its expression in the anterior pituitary and the adrenal cortex: a source of variation in hypothalamic-pituitary-adrenal axis function; implications for pituitary and adrenal tumors. Endocr Pract. 2011 Nov-Dec;17(6):941–948. doi: 10.4158/EP11061.RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista DL, Oldfield EH, Keil MF, Stratakis CA. Postoperative testing to predict recurrent Cushing disease in children. J Clin Endocrinol Metab. 2009 Aug;94(8):2757–2765. doi: 10.1210/jc.2009-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lodish MB, Hsiao HP, Serbis A, Sinaii N, Rothenbuhler A, Keil MF, Boikos SA, Reynolds JC, Stratakis CA. Effects of Cushing disease on bone mineral density in a pediatric population. J Pediatr. 2010 Jun;156(6):1001–1005. doi: 10.1016/j.jpeds.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keil MF, Merke DP, Gandhi R, Wiggs EA, Obunse K, Stratakis CA. Quality of life in children and adolescents 1-year after cure of Cushing syndrome: a prospective study. Clin Endocrinol (Oxf) 2009 Sep;71(3):326–333. doi: 10.1111/j.1365-2265.2008.03515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Michelson D, Stratakis CA, Hill L, Reynolds J, et al. Bone mineral density in women with depression. N Engl J Med. 1996;335:1176–1181. doi: 10.1056/NEJM199610173351602. [DOI] [PubMed] [Google Scholar]
- 22.Arem AJ, Kirscher CW. Analysis of striae. Plast Reconstr Surg. 1980;65:22–29. doi: 10.1097/00006534-198001000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Serres M, Viac J, Schmitt DB. Glucocorticoid receptor localization in human epidermal cells. Arch Dermatol Res. 1996;288:140–146. doi: 10.1007/BF02505823. [DOI] [PubMed] [Google Scholar]
- 24.Chedid M, Hoyle JR, Csaky KG, Rubin JS. Glucocorticoids inhibit keratinocyte growth factor production in primary dermal fibroblasts. Endocrinology. 1996;137:2232–2237. doi: 10.1210/endo.137.6.8641170. [DOI] [PubMed] [Google Scholar]
- 25.Meisler N, Shull S, Xie R, Long GL, Asher M, Connoly JP, Cutroneo KR. Glucocorticoids coordinately regulate type I collagen pro-alpha 1 promoter activity thorugh both the glucocorticoid and TGF-b response elements: a novel mechanism of glucococrtocid regulation of eukaryotic genes. J Cell Biochem. 1995;59:376–388. doi: 10.1002/jcb.240590309. [DOI] [PubMed] [Google Scholar]
- 26.Brauchle M, Fassler R, Werner S. Suppression of keratinocyte growth factor expression by glucocorticoids in vitro and during wound healing. J Invest Dermatol. 1995;105:579–584. doi: 10.1111/1523-1747.ep12323521. [DOI] [PubMed] [Google Scholar]
- 27.Haapasaari KM, Risteli J, Oikarinen A. Recovery of human skin collagen synthesis after short-term topical corticosteroid treatment and comparison between young and old subjects. J Dermatol. 1996;135:65–69. [PubMed] [Google Scholar]
- 28.McMichael AJ, Griffiths CE, Talwar HS, Finkel LJ, Rafal ES, Hamilton TA, Voorhees JJ. Concurrent application of tretinoin (retinoic acid) partially protects against corticosteroid-induced epidermal atrophy. Br J Dermatol. 1996;135:60–64. [PubMed] [Google Scholar]
- 29.Nesbitt LT., Jr Minimizing complications from systemic glucocorticoid use. Dermatol. Clin. 1995;13:925–939. [PubMed] [Google Scholar]
- 30.Bouzas EA, Mastorakos G, Friedman TC, Scott MH, Chrousos GP, Kaiser-Kupfer MI. Posterior subcapsular cataract in endogenous Cushing syndrome: An uncommon manifestation. Invest Ophthalmol Vis Sci. 1993;34:3497–3500. [PubMed] [Google Scholar]
- 31.Afshari A, Ardeshirpour Y, Lodish MB, Gourgari E, Sinaii N, Keil M, Belyavskaya E, Lyssikatos C, Chowdhry FA, Chernomordik V, Anderson AA, Mazzuchi TA, Gandjbakhche A, Stratakis CA. Facial Plethora: Modern Technology for Quantifying an Ancient Clinical Sign and Its Use in Cushing Syndrome. J Clin Endocrinol Metab. 2015 Oct;100(10):3928–3933. doi: 10.1210/jc.2015-2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hannah-Shmouni F, Stratakis CA, Koch CA. Flushing in (neuro)endocrinology. Rev Endocr Metab Disord. 2016 doi: 10.1007/s11154-016-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]