Abstract
The role of aggregate formation in the pathophysiology of Huntington’s disease (HD) remains uncertain. However, the temporal appearance of aggregates tends to correlate with the onset of symptoms and the numbers of neuropil aggregates correlate with the progression of clinical disease. Using highly sensitive immunohistochemical methods we have detected the appearance of diffuse aggregates during embryonic development in the R6/2 and YAC128 mouse models of HD. These are initially seen in developing axonal tracts and appear to spread throughout the cerebrum in the early neonate.
Keywords: Huntington’s disease, mouse models, aggregation, development
Huntington’s disease is a devastating autosomal dominant neurodegenerative disease caused by the expansion of a CAG trinucleotide repeat, coding for the amino acid glutamine, in the first exon of the huntingtin gene (HTT) which encodes the huntingtin protein (HTT) [1]. Expansions of 40 or more CAG repeats invariably cause disease with the age of onset correlating with the size of the repeat expansion and a median age of onset in the mid-40s [2]. Intermediate expansions of 36 to 39 repeats show reduced penetrance. There are currently no disease-modifying treatments and FDA-approved medications provide only symptomatic relief.
The pathology of HD has been defined by the pronounced degeneration of the striatum and cortex, associated with the appearance of intranuclear inclusions and neuropil aggregates comprised of amyloid-like fragments derived from the N-terminal region of mutant huntingtin (mHTT) and containing the expanded polyglutamine segment [3]. Although a correlation exists between the density of neuropil aggregates in HD and the severity of clinical pre-mortem symptoms [4], the exact role of these aggregates in the pathophysiology of the disease remains unclear. This uncertainty arises in part from the study of animal models of HD where a lack of correlation between the numbers of inclusions and the symptoms of disease can be striking. For example, the YAC128 mouse develops large numbers of nuclear inclusions in addition to large numbers of neuropil aggregates, while the similarly symptomatic BACHD97 transgenic mice have considerably smaller numbers of neuropil aggregates and no intranuclear inclusions, Furthermore, behavioral abnormalities have been found to precede the appearance of conventional inclusions in a transgenic rat model of HD [5]. Nevertheless, the presence of inclusions in all animal models of HD, appearing around the onset of symptoms of disease provides compelling evidence for a central role of aggregation and aggregate formation. In several diseases, collectively termed protein aggregation disorders, it has been proposed that neurotoxicity may be mediated by oligomeric forms and that mature inclusions may actually be protective; although evidence in some experimental models of HD supports this concept[6, 7], this does not exclude the possibility that some subset of aggregates or intermediate in the aggregation process may play a pivotal role in the disease. Moreover, numerous studies have reproduced many of the pathological sequelae of HD by the exogenous addition of aggregates of mutant huntingtin or of synthetic polyglutamine aggregates leading to the concept that these disorders may spread through the brain in a prion-like manner [8, 9].
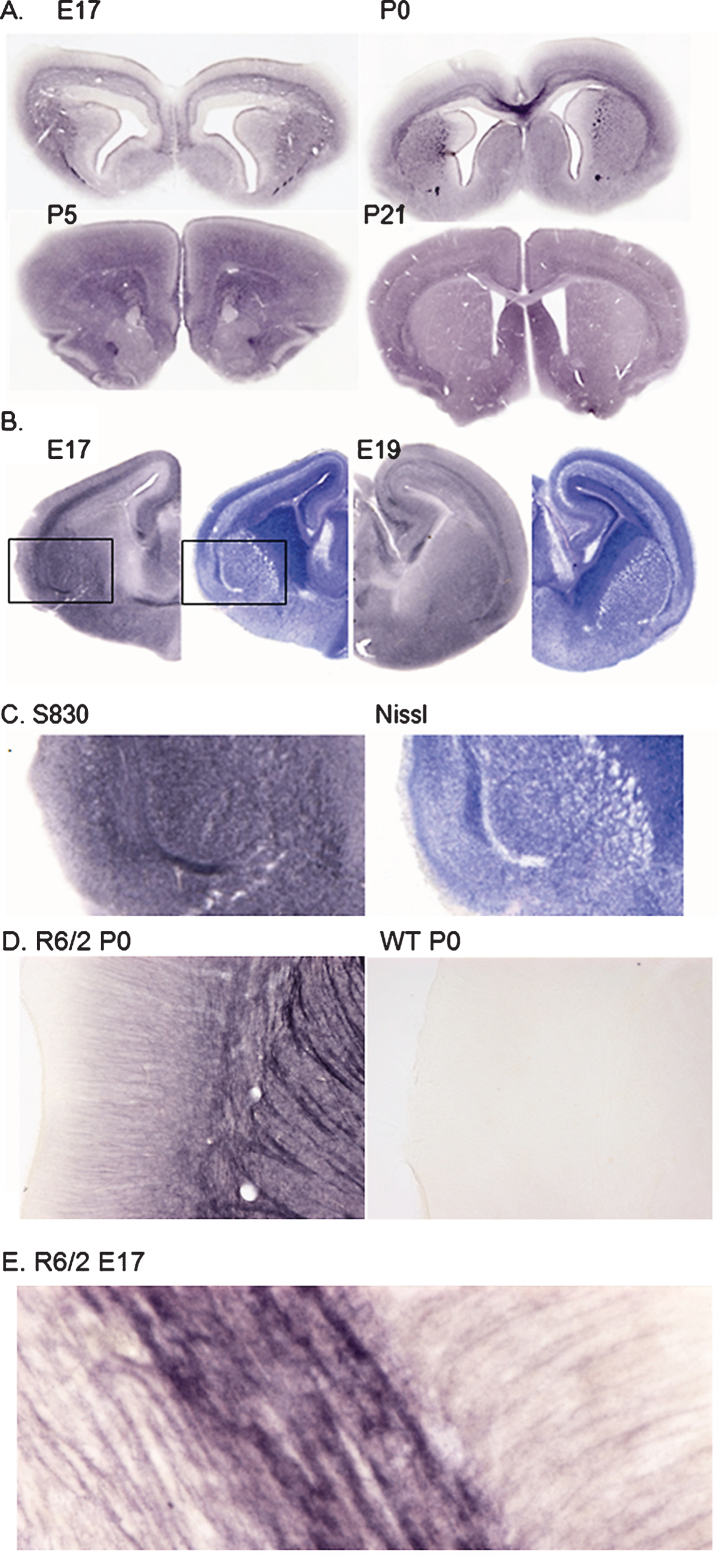
During an evaluation of the earliest stages of inclusion pathology in the R6/2 mouse, a high density of staining with the aggregate-specific antibody, S830 [10], was seen throughout the cerebral neuropil particularly in younger (2-3 weeks) animals. This polyclonal sheep antibody was raised against a recombinant GST-exon 1 HTT fusion protein with 53Q and reacts in a highly selective and sensitive manner with mHTT aggregates and in fixed tissue is unreactive with mHTT or native huntingtin. The use of a xenogeneic antibody permits the use of highly sensitive indirect immunohistochemical methods which cannot be used for mouse monoclonal antibodies such as mEM48 and the MW series due to the invariable presence of background levels of mouse IgG in mouse brain sections. To determine the age of onset of this reactivity we examined brains from E17 embryos and P0 and P5 pups. As shown in Fig. 1A, dense diffuse staining of axon bundles was seen at E17; at P0 mixed diffuse staining of immature white matter and brain parenchyma was seen. At P5 diffuse staining was limited to the neuropil and by P21 the density of neuropil staining was markedly reduced coincident with the onset of formation of neuropil aggregates and the intranuclear accumulation of mHTT aggregates (Fig. 1A). A strikingly similar diffuse staining of developing white matter was seen with embryonic YAC128 mice (Fig. 1B), despite the relatively protracted prodromal period prior to neurodegenerative pathology in this model [11, 12].
Fig.1.
A. S830 staining of R6/2 mouse brain at E17, P0, P5 and P21 showing staining initially restricted to developing white matter tracts and appearing extracellular. B. S830 (left) and Nissl (right) staining of YAC128 at E17 and E19 showing preponderance of S830 staining associated with developing white matter tracts and fiber bundles. C. Enlargement of areas shown in B: S830 (left) and Nissl (right) reveal inverse relationship between S830 and Nissl staining, particularly in the developing external capsule and fiber bundles in the developing striatum. D. Enlargement of a similar region in P0 R6/2 mouse brain showing diffuse staining of corticostriatal fibers with weak staining of cortical dendrites (left); staining of the same region in WT P0 brain (right) under identical conditions reveals the total absence of immunoreactivity with S830. E. High power view of external capsule of E17 R6/2 mouse brain stained with S830 showing diffuse nature of staining and complete absence of discrete mHTT aggregates; field size: 200×80 μ.
In order to determine whether there was a correlation between the level of persistent diffuse S830 reactivity and symptoms of disease we took advantage of two models in which aggregate formation had been reported to occur in the absence of significant changes in motor function or behavior. The asymptomatic ‘short-stop’ mouse expresses a truncated expanded HTT fragment encoded by exons 1 and 2 that had been reported to lead to a greater density of mHTT inclusions than seen in the YAC128 [13]. We performed a quantitative analysis of the density of the S830 reactivity between YAC128 and short-stop sections from mice aged 6 months. We found that there was a significant elevation in intercellular S830 immunoreactivity in the YAC128 as compared to the short-stop (arbitrary intensity units: 35.9±9.7 and 22.2±5.6 respectively; t-test: p = 0.032). The rescue of the BACHD phenotype by the expression of a human transgene encoding αB-crystallin under the GFAP promoter was recently described, in which the total number of S830 positive neuropil aggregates was reported to be unaffected by the presence of the transgene, although the number of large aggregates was significantly reduced [14]. When sections from mice aged 12 months were analyzed for the density of extracellular reactivity in cortex and striatum we found that the double transgenics displayed a lower level (arbitrary intensity units): 70.2±2.6 vs. 82.3±14.2 (t-test: p = 0.06) than in the BACHD mice.
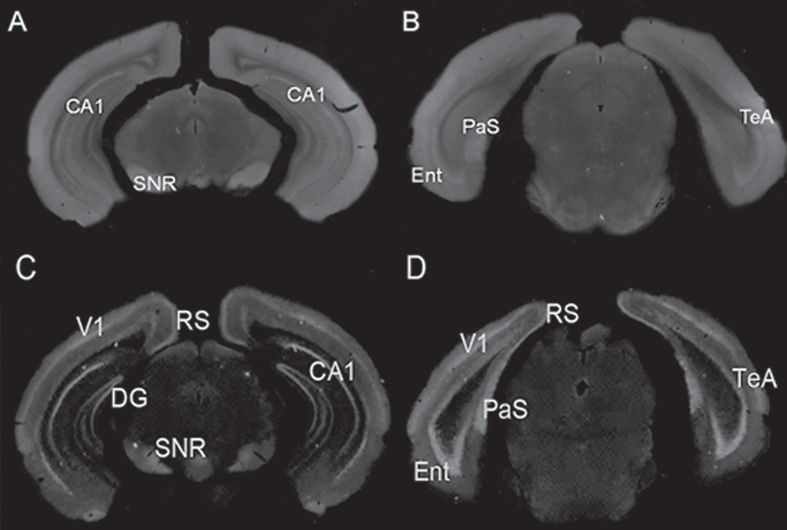
A further example of diffuse aggregate staining was seen in zQ175 heterozygous knock-in mice (Fig. 2). At 7 weeks (the earliest age studied) these mice exhibited diffuse S830 staining in several regions, predominantly in those that first developed mHTT inclusions at later ages. This staining was essentially absent from zQ175 homozygous mice which by this age already expressed widespread neuronal intranuclear accumulations of mHTT and numerous small neuropil aggregates.
Fig.2.
S830 staining of 7 week-old heterozygous (A, B) and homozygous (C, D) zQ175 mouse brain. In heterozygous Q175 mice, A and B, minimal neuronal nuclear cellular staining was limited to the CA1 region, the parasubiculum (PaS) and deep layer V neurons in entorhinal cortex (Ent, TeA), while diffuse staining (reduced or absent at later ages) was present in these regions and in the substantia nigra (SNR). At 7 weeks of age homozygous zQ175 mouse brain, C and D, showed exclusively neuronal nuclear staining, in CA1 region of dentate gyrus (DG), in parasubiculum (PaS), and in cortex, with primary visual (V1), somatosensory (S1), temporal association (TeA), retrosplenial (RS) and entorhinal (Ent) shown here.
We conclude that a novel form of mHTT aggregate is generated in the brains of HD models during embryonic development and that this persists for a variable period of time in the post-natal mouse in a diffuse form, distributed widely throughout the cerebrum. These ‘proto-aggregates’ are the earliest form of mHTT aggregate yet detected by immunohistochemical methods. The similarity in embryonic distribution between the R6/2 and YAC128 is taken to suggest that either the intact mHTT in the YAC128 mouse is efficiently processed to an N-terminal fragment or that an N-terminal mHTT fragment is generated by incomplete splicing [15] or by RAN translation [16]. Additionally, given accumulating evidence that HD includes neurodevelopmental abnormalities [17–19], these observations support a role for mHTT aggregation in the earliest stages of the disease. Finally, recent studies have shown that certain features of Huntington’s disease in the adult BACHD mouse model were retained when the expression of mutant protein was switched off at weaning [20], confirming an important role for expression of the mutant protein during development. Confirmation of a role for proto-aggregates in the pathophysiology of disease will add significantly to our understanding of HD and potentially provide novel targets for pharmacological intervention and disease prevention.
CONFLICT OF INTEREST
The authors have no conflict of interest to report.
ACKNOWLEDGMENTS
Funding was provided by the Hereditary Diseases Foundation and CHDI (APO, GPB), NIH/NIEHS RO1 ES016931 (ABB), and NIH/NIEHS T32 ESES007028 (ABB and TJB). YAC128 and short stop mouse brains were provided by Michael Hayden at the University of Vancouver and BACHD and αB-crystallin double transgenic mouse brains were provided by Steven Finkbeiner at the University of California at San Francisco.
REFERENCES
- [1]. MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, et al. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group Cell. 1993;72(6):971–83. [DOI] [PubMed] [Google Scholar]
- [2]. Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR. International Huntington’s Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin Genet. 2004;65(4):267–77. [DOI] [PubMed] [Google Scholar]
- [3]. Lunkes A, Lindenberg KS, Ben-Haïem L, Weber C, Devys D, Landwehrmeyer GB, et al. Proteases acting on mutant huntingtin generate cleaved products that differentially build up cytoplasmic and nuclear inclusions. Mol Cell. 2002;(2):259–69. [DOI] [PubMed] [Google Scholar]
- [4]. Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, Rye D, et al. Nuclear and neuropil aggregates inHuntington’s disease: Relationship to neuropathology. J Neurosci. 1999;19(7):2522–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Nguyen HP, Kobbe P, Rahne H, Wörpel T, Jäger B, Stephan M, et al. Behavioral abnormalities precede neuropathological markers in rats transgenic for Huntington’s disease. Hum Mol Genet. 2006;15(21):3177–94. [DOI] [PubMed] [Google Scholar]
- [6]. Arrasate M, Mitra S, Schweitzer ES, Segal MR, Finkbeiner S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature. 2004;431(7010):805–10. [DOI] [PubMed] [Google Scholar]
- [7]. Miller J, Arrasate M, Brooks E, Libeu CP, Legleiter J, Hatters D, et al. Identifying polyglutamine protein species in situ that best predict neurodegeneration. Nat Chem Biol. 2011;7(12):925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Tan Z, Dai W, van Erp TG, Overman J, Demuro A, Digman MA, et al. Huntington’s disease cerebrospinal fluid seeds aggregation of mutant huntingtin. Mol Psychiatry. 2015;(11):1286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Pearce MM, Spartz EJ, Hong W, Luo L, Kopito RR. Prion-like transmission of neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun. 2015;6:6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Sathasivam K, Woodman B, Mahal A, Bertaux F, Wanker EE, Shima DT, et al. Centrosome disorganization in fibroblast cultures derived from R6/2 Huntington’s disease (HD) transgenic mice and HD patients. Hum Mol Genet. 2001;10(21):2425–35. [DOI] [PubMed] [Google Scholar]
- [11]. Van Raamsdonk JM, Murphy Z, Slow EJ, Leavitt BR, Hayden MR. Selective degeneration and nuclear localization of mutant huntingtin in the YAC128 mouse model of Huntington disease. Hum Mol Genet. 2005;14(24):3823–35. [DOI] [PubMed] [Google Scholar]
- [12]. Brooks SP, Jones L, Dunnett SB. Comparative analysis of pathology and behavioural phenotypes in mouse models of Huntington’s disease. Brain Res Bull. 2012;88(2-3):81–93. [DOI] [PubMed] [Google Scholar]
- [13]. Slow EJ, Graham RK, Osmand AP, Devon RS, Lu G, et al. Absence of behavioral abnormalities and neurodegeneration in vivo despite widespread neuronal huntingtin inclusions. Proc Natl Acad Sci U S A. 2005;102(32):11402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Oliveira AO, Osmand A, Outeiro TF, Muchowski PJ, Finkbeiner S. αB-Crystallin overexpression in astrocytes modulates the phenotype of the BACHD mouse model of Huntington’s disease. Hum Mol Genet. 2016;25(9):1677–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Sathasivam K, Neueder A, Gipson TA, Landles C, Benjamin AC, Bondulich MK, et al. Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease. Proc Natl Acad Sci U S A. 2013;110(6):2366–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Bañez-Coronel M, Ayhan F, Tarabochia AD, Zu T, Perez BA, Tusi SK, et al. RAN translation in Huntington disease. Neuron. 2015;88(4):667–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Nopoulos PC, Aylward EH, Ross CA, Mills JA, Langbehn DR, Johnson HJ, et al. Smaller intracranial volume in prodromal Huntington’s disease: Evidence for abnormal neurodevelopment. Brain. 2011;134(Pt 1):137–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Humbert S. Is Huntington disease a developmental disorder? EMBO Rep 2010;11(12):899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Kerschbamer E, Biagioli M. Huntington’s disease as neurodevelopmental disorder: Altered chromatin regulation, coding, and non-coding RNA transcription. Front Neurosci. 2015;9, 509 http://doi.org/10.3389/fnins.2015.00509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Molero AE, Arteaga-Bracho EE, Chen CH, Gulinello M, Winchester ML, Pichamoorthy N, et al. Selective expression of mutant huntingtin during development recapitulates characteristic features of Huntington’s disease. Proc Natl Acad Sci U S A. 2016;113(20):5736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]