ABSTRACT
Antibiotic intervention is an effective treatment strategy for many bacterial infections and liberates bacterial antigens and stimulatory products that can induce an inflammatory response. Despite the opportunity for bacterial killing to enhance the development of adaptive immunity, patients treated successfully with antibiotics can suffer from reinfection. Studies in mouse models of Salmonella and Chlamydia infection also demonstrate that early antibiotic intervention reduces host protective immunity to subsequent infection. This heightened susceptibility to reinfection correlates with poor development of Th1 and antibody responses in antibiotic-treated mice but can be overcome by delayed antibiotic intervention, thus suggesting a requirement for sustained T cell stimulation for protection. Although the contribution of memory T cell subsets is imperfectly understood in both of these infection models, a protective role for noncirculating memory cells is suggested by recent studies. Together, these data propose a model where antibiotic treatment specifically interrupts tissue-resident memory T cell formation. Greater understanding of the mechanistic basis of this phenomenon might suggest therapeutic interventions to restore a protective memory response in antibiotic-treated patients, thus reducing the incidence of reinfection.
INTRODUCTION
Since the discovery of penicillin in 1928, antibiotics have been widely used to treat bacterial infections, and as a result, bacteria have rapidly developed antibiotic resistance (1, 2). The development of multidrug-resistant (MDR) bacteria is now a critical issue in modern medicine, with the concern that serious bacterial infections will reemerge in the 21st century in the absence of effective treatment options (3–6). Despite this important issue, antibiotics remain an effective treatment option for many common infectious diseases.
An adaptive immune response to infection is initiated by recognition of foreign protein antigens in the presence of local inflammation (7). The contextual inflammatory cues come from innate immune cells that encounter bacterial products, and these signals profoundly affect the subsequent adaptive immune response (8). This initial activation stage occurs within local lymph nodes and causes low-frequency naive T cells and B cells to produce an army of effector cells to eradicate a complex pathogen (9, 10). Effective antibiotic therapy will kill a large number of bacteria, thus liberating antigen for lymphocyte recognition and releasing bacterial products that can amplify local inflammatory responses. Thus, antibiotics have a direct effect on bacterial growth but also have the potential to enhance an ongoing pathogen-specific adaptive immune response. However, many studies have shown that antibiotic administration can paradoxically weaken immune memory, leaving a recovered host fully susceptible to reinfection with the same pathogen (11–13). The mechanistic basis for this detrimental effect of antibiotics on immune memory and protection is incompletely understood. A more detailed understanding of this phenomenon might allow the development of targeted strategies to encourage immune memory development and support long-lasting protection from reinfection. In this review, we will discuss this issue in the context of recent findings from mouse models of Salmonella and Chlamydia infection, since both models show a detrimental effect of antibiotics upon the development of immune memory.
HUMAN SALMONELLA AND CHLAMYDIA INFECTIONS
Salmonella bacteria cause a variety of clinical diseases, depending on the bacterial serovar and the underlying susceptibility of the infected host (14, 15). In many low-income countries with limited infrastructure, Salmonella enterica serovars Typhi and Paratyphi are transferred via the fecal-oral route and can cause enteric fever (16). While enteric fever can be successfully treated using antibiotics, the prevalence of multidrug-resistant strains is increasingly an impediment to treatment in areas where it is endemic (13). The administration of ciprofloxacin (a fluoroquine derivative) for 7 to 14 days is often sufficient to ensure the recovery of infected patients, but this depends upon the local prevalence of MDR strains (13, 17). Interestingly, even when treatment is successful, a cohort of patients suffer relapsing disease or can be reinfected with different Salmonella Typhi or Paratyphi strains at a later date. Thus, the successful resolution of primary infection with antibiotics does not guarantee the acquisition of protective immunity to reinfection.
Salmonella are not the only intracellular bacteria for which a lack of secondary protection is observed following antibiotic treatment. Chlamydia trachomatis is an obligate intracellular bacterium that causes ocular and sexually transmitted infections worldwide (18). In the United States, Chlamydia causes over 1.4 million sexually transmitted infections annually, and the health care costs associated with these infections amount to $500 million every year (19, 20). Immunity to Chlamydia infection in asymptomatic women develops slowly, and 50% of women continue to shed bacteria for a year (21). Since persistent or recurrent infection is a major risk factor for pelvic inflammatory disease (22, 23), Chlamydia control programs were introduced to reduce the burden of disease. These “seek and treat” programs have not reduced the incidence of Chlamydia infection but have reduced the incidence of associated pathology (24–27). However, reinfection is often observed following successful antibiotic treatment (24, 28), indicating that protective memory responses fail to develop in antibiotic-treated patients. Indeed, it has been argued that antibiotic treatment is counterproductive to the generation of Chlamydia immunity, an idea that is often referred to as the “arrested immunity” hypothesis (12). Recent clinical data support this hypothesis, since women who spontaneously resolve Chlamydia infection have a lower incidence of reinfection than antibiotic-treated women (29). Furthermore, gamma interferon (IFN-γ)-producing Chlamydia-specific Th1 cells develop slowly and do not persist in the circulation of women after effective antibiotic treatment (30). Thus, the high reinfection rates found in large population studies could actually be a consequence of early intervention programs that seek to screen and treat Chlamydia-infected women (24, 31). Together, these studies suggest findings that parallel those from Salmonella-infected patients and indicate that protective immunity does not develop effectively following antibiotic treatment of Chlamydia.
PROTECTIVE IMMUNITY TO INTRACELLULAR BACTERIA
Since antibiotic treatment appears to have a negative impact on host protective immunity to secondary bacterial infection, it is vitally important to determine the mechanism of this phenomenon. The basic cellular immune responses to Salmonella and Chlamydia infection have been elucidated in mouse models and share common features (32–34). As expected for intracellular bacteria, CD4 Th1 cells that express T-bet and produce IFN-γ are critical for bacterial clearance. Thus, mice lacking major histocompatibility complex (MHC) class II-restricted T cells, T-bet, or IFN-γ succumb to primary infection with attenuated S. enterica serovar Typhimurium, an infection that resolves naturally in wild-type mice (35, 36). In contrast, mice lacking MHC class I-restricted CD8 T cells or B cells display only minor deficiencies in clearing primary Salmonella infection (37–39). Similarly, mice lacking MHC class II-restricted CD4 T cells or IFN-γ have difficulty resolving primary Chlamydia infection (40, 41), and yet, CD8 T cells or B cells are not essential (40, 42, 43). Together, these data point to a major role for CD4 Th1 cells in primary clearance of both Salmonella and Chlamydia infections. However, despite the fact that Salmonella and Chlamydia replicate intracellularly, antibody responses can play an important additive role during secondary infection (38, 39, 44, 45). Thus, memory CD4 T cells and circulating antibody can both be involved in effective clearance of bacteria during secondary infection (32, 33).
Persisting memory T cells contain at least three distinct subsets, each displaying different functional capabilities and tissue-homing potential (46, 47). Central memory T cells (TCM) recirculate between the blood and lymph fluid and have low immediate effector potential, similar to naive T cells. In contrast, effector memory T cells (TEM) display high immediate effector potential and can recirculate between blood and nonlymphoid tissues, anatomical locations where they are likely to encounter secondary bacterial infection. Finally, a population of resident memory T cells (TRM) remains within nonlymphoid tissues and has high immediate effector potential. This heterogeneity in T cell memory is important, since some infections show a greater reliance on tissue-resident versus circulating memory cells for pathogen clearance (48). While the protective contribution of distinct T cell memory subsets has not been fully explored in Salmonella infection models, recent data demonstrate that TRM CD4 T cells are critically important for immunity to Chlamydia infection (49). Thus, deficiency in secondary protective immunity to Salmonella and Chlamydia infection seems likely to involve CD4 T cell memory and may reflect an alteration in generating a specific protective subset.
ANTIBIOTIC CLEARANCE OF SALMONELLA IN THE MOUSE MODEL
Two different mouse models are commonly used to investigate the immune response to Salmonella infection (50). The first model involves infecting genetically resistant mice with virulent Salmonella Typhimurium, thus allowing detailed study of innate and adaptive immune responses during the natural resolution of Salmonella infection (51). The alternative approach is to infect genetically susceptible mice with attenuated Salmonella strains, again allowing basic analysis of immune responses to primary bacterial infection (10). The obvious caveat to this second model is that the bacteria used are not fully virulent; however, the basic mechanism of primary clearance appears similar in both models. As noted above, protective immunity to secondary infection requires the cooperation of CD4 T cells and Salmonella-specific antibody responses. Importantly, in the genetically resistant model, protective immunity to reinfection can be transferred by antibody alone (52, 53), making this model less useful for examining CD4 T cell memory. Since humans require MHC class II-restricted T cell responses for efficient resolution of Salmonella infection (54), the susceptible mouse model is often used when studying the protective role of CD4 T cells against secondary infection.
Since many human typhoid infections are resolved by antibiotic treatment, our laboratory previously developed a mouse model in which susceptible mice were challenged with highly virulent Salmonella bacteria and antibiotics used to resolve primary infection. This proved more difficult than expected, and a full 5 weeks of enrofloxacin administration was required for C57BL/6 mice to completely resolve a primary infection with Salmonella Typhimurium (55). Live imaging experiments demonstrated that while viable bacteria were cleared from the spleen, liver, and bone marrow within 72 h of antibiotic treatment, a small population of bacteria persisted in mesenteric lymph node (MLN) phagocytes for several weeks after treatment (56). Thus, the removal of antibiotics before this population was eradicated allowed the outgrowth of bacteria and resumption of clinical disease. Indeed, relapse of primary infection was previously observed after treatment of murine infection with ampicillin and is also common to human salmonellosis (16, 57). Importantly, this relapsing disease does not require the development of antibiotic resistance, and these late-outgrowth bacteria remain susceptible to antibiotics (55). Similarly, in the resistant mouse model, recovery from primary infection is associated with continued bacterial shedding, indicating a chronic infection (58). Although persisting infection in both models is often localized to the MLNs (56, 58), surgical removal of the MLNs actually increased relapsing disease in antibiotic-treated mice (56), suggesting that the true site of bacterial persistence is more likely to be upstream from MLN afferent lymph drainage. Salmonella persistence has been correlated with the ability of some bacteria to enter a dormant state in which they are largely refractory to the effect of antibiotics (59, 60). However, recent studies have shown that outgrowing bacteria actually derive from bacteria with an intermediate growth phenotype that remain partially sensitive to antibiotic treatment (61). Thus, Salmonella bacteria display the ability to persist in the face of antibiotic treatment and in mice genetically predisposed to resolve primary infection. Given the wide availability of bacterial antigens for recognition by the host innate and adaptive immune system in these models, it is perplexing that relapsing infection can still occur many weeks after primary infection. The inability of the host immune system to effectively clear persistent or relapsing Salmonella infection might be due to effective immune evasion strategies employed by the bacteria (62–64).
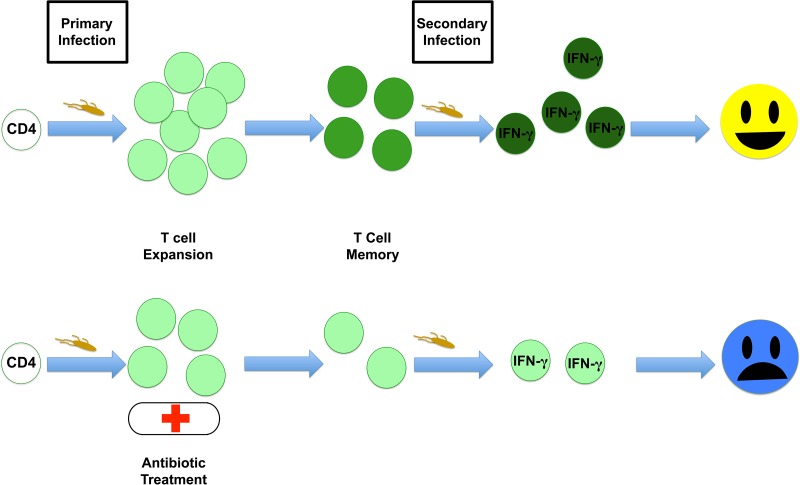
Susceptible mice treated for at least 5 weeks with enrofloxacin were able to fully resolve primary Salmonella infection, and these mice did not suffer from relapsing disease (55). This model therefore allowed a comparison of secondary protective immunity following antibiotic resolution of infection and naturally resolved primary infection with attenuated bacteria. While mice administered attenuated bacteria generated robust protective immunity to secondary infection, antibiotic-treated mice remained susceptible to Salmonella infection, although they survived longer than naive mice (55). These data point to an underlying deficiency in the acquisition of protective memory following antibiotic treatment that is similar to the susceptibility to reinfection observed in human disease (16). The weak protective immunity that was evident in antibiotic-treated mice required MHC class II-restricted T cells, B cells, and IFN-γ (55). When adaptive immune responses were examined in antibiotic-treated mice, it was noted that antibody responses were slightly depressed and the frequency of IFN-γ-producing cells was significantly reduced (55, 56). In recent experiments using MHC class II tetramers to track endogenous CD4 T cell responses to Salmonella, our laboratory has found that antibiotic treatment reduces the overall size of the Salmonella-specific CD4 memory pool (J. M. Benoun and S. J. McSorley, unpublished data). Thus, appropriate protective mechanisms appear to be engaged in antibiotic-treated mice, but the overall response is reduced, preventing a robust response to secondary infection (Fig. 1).
FIG 1 .
Antibiotic treatment reduces the development of T cell memory. Primary bacterial infection initiates the expansion of T cells that aid in clearance of the bacteria, leaving a pool of memory T cells behind. Upon secondary infection, these memory cells have acquired effector potential and can eliminate bacteria rapidly. Antibiotic treatment of primary infection truncates T cell expansion and limits memory cell development, preventing an effective response to secondary infection.
Previous studies have shown that CD4 clonal expansion and memory formation require sustained antigen presentation over several days (65, 66). Although antibiotic treatment liberates antigens from dead bacteria, it seems likely that this period of antigen presentation could be short, thus adversely impacting CD4 memory. Indeed, when antigen presentation is prolonged by delaying antibiotic intervention, this allows robust protective responses to emerge (56). This increased protection also coincides with a gradual increase in Salmonella-specific Th1 responses (56), suggesting that a prolonged period of antigen stimulation allows the recovery of memory development. Indeed, full recovery of Th1 responses required 14 days of exposure to live bacteria prior to antibiotic intervention (56). Thus, a major factor driving the detrimental impact of antibiotics upon protective memory responses is rapid elimination of bacterial antigens before memory CD4 T cells fully develop. Since CD8 T cells are thought to be less dependent on sustained antigen presentation (65–68), infections that rely on CD8 T cells for pathogen clearance might show less of a detrimental effect of antibiotics upon memory development. However, although initial CD8 responses occur normally with only a short period of antigen presentation, CD8 memory responses are still dependent to some extent upon prolonged antigen stimulation (69). In addition to sustained antigen stimulation, CD4 T cells also require costimulatory signals and local cytokines to develop appropriate functional responses (7). The availability of these costimulatory signals and cytokines is also likely to diminish as antibiotics rapidly resolve a primary bacterial infection. It will therefore be important to define precisely which of these signals is lacking in antibiotic-treated mice, since it may be possible to deliver these signals therapeutically during the period of antibiotic treatment.
ANTIBIOTIC CLEARANCE OF CHLAMYDIA IN THE MOUSE MODEL
Investigators studying immunity to Chlamydia make use of two complementary mouse models (32, 70). In the first model, investigators use Chlamydia trachomatis to infect in-bred mouse strains, which has the obvious advantage of using the human pathogen directly in these model studies. However, Chlamydia trachomatis is not a natural pathogen of mice, and many laboratories therefore choose to examine immunity to Chlamydia muridarum, which causes an ascending reproductive tract infection following vaginal inoculation. Chlamydia bacteria have the ability to fuse and form noninfectious aberrant bodies under antibiotic pressure, and this form of the pathogen is known to be refractory to antibiotic killing (71, 72). There is also evidence that Chlamydia can persist in the intestine during an immune response or antibiotic treatment that can effectively clear bacteria from the reproductive tract (73, 74). Thus, Chlamydia and Salmonella each have the ability to persist in the face of antibiotic treatment, and for both these organisms, this may involve low-level chronic infection of intestinal tissues.
Secondary protective immunity has been examined following antibiotic clearance of Chlamydia infection in the mouse model. As with the Salmonella mouse model, successful treatment of Chlamydia infection in mice reduced protective immunity to subsequent secondary challenge compared to the protective immunity in mice that resolved primary infection naturally (75). Similar to Salmonella infection, the mechanistic basis of this arrested immunity is largely unknown but can be mitigated by delaying the start of antibiotic treatment (75), presumably because this allows more time for CD4 T cell memory to develop. Interestingly, administering a suboptimal dose of antibiotic creates a self-limiting subclinical infection, and these mice develop stronger adaptive responses and robust protective immunity as a result (76). The detrimental effect of antibiotic intervention in the Chlamydia model also correlates with lower Chlamydia-specific antibody responses and a reduced ability of splenocytes to produce IFN-γ in response to Chlamydia antigens (75). Thus, similar to the Salmonella model, rapid bacterial clearance using antibiotics is associated with the development of a dysfunctional memory response.
While CD4 T cells are essential for immunity to genital C. muridarum infection, the specific contribution of each TCM, TEM, and TRM subset has yet to be firmly established. Importantly, noncirculating tissue-resident memory (TRM) T cells have been identified as an essential component of protection at mucosal surfaces (77). Cells of this population typically have rapid effector capability, adopt tissue-specific differentiation patterns, and can initiate rapid innate immune responses to secondary infection (77–79). However, given the tissue-restricted localization of TRM T cells and the difficulty quantifying this population by flow cytometry, a comparative analysis of these subsets in the Chlamydia model is not trivial to complete (80). Recent studies have shown that repeated use of antibiotics to control a primary Chlamydia infection induces effector memory CD4 T cells in local draining lymph nodes (81), but the relationship between this population and effective protective immunity has not been established. As noted above, data from parabiosis experiments have shown that nonrecirculating memory cells are critical for protective immunity using a prototype Chlamydia vaccine (49). Thus, noncirculating TRM T cells would seem to be an integral component of protective immunity to secondary infection with Chlamydia (34). Therefore, it seems likely that antibiotic treatment during Chlamydia infection will adversely affect the development of Chlamydia-specific TRM within the reproductive tract, but this issue has yet to be examined experimentally.
CONCLUSION
A number of experiments from Salmonella and Chlamydia infection models suggest that early antibiotic intervention impedes the development of effective protective memory, which is largely mediated by Th1 CD4 cells. The duration of antigen presentation and inflammatory stimulation are known to be key variables in the generation of CD4 T cell memory, and both of these variables are likely to be adversely affected by antibiotic administration. It is not yet clear whether this deficiency in CD4 T cell responses could be overcome by administering additional antigen or adjuvants during the period of antibiotic administration. Using the Salmonella infection model, we administered purified bacterial flagellin to mice being treated with antibiotic and detected the recovery of robust protective immunity to secondary infection (55). This experiment suggests that it may be possible to support the development of an effective memory lymphocyte population during antibiotic administration and that this can ultimately be of considerable benefit to the host. However, given the role of bacterial flagellin as a CD4 T cell target antigen and an inducer of inflammatory responses (82, 83), it still remains unclear whether providing antigen or adjuvant is the most effective strategy to recover protective memory responses. Future experiments will focus on whether the immune responses that are boosted in this context come from circulating or noncirculating memory cells. Greater understanding of the mechanisms of impaired adaptive immune responses after antibiotic treatment may allow simple and effective therapeutic strategies that could be easily translated to protect against repeated bacterial infections.
ACKNOWLEDGMENT
This work was supported by grants from the National Institutes of Health to S.J.M. (grants number AI056172, AI103422, and AI117303).
Footnotes
Citation Benoun JM, Labuda JC, McSorley SJ. 2016. Collateral damage: detrimental effect of antibiotics on the development of protective immune memory. mBio 7(6):e01520-16. doi:10.1128/mBio.01520-16.
REFERENCES
- 1.Aminov RI. 2010. A brief history of the antibiotic era: lessons learned and challenges for the future. Front Microbiol 1:134. doi: 10.3389/fmicb.2010.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abraham EP, Chain E. 1988. An enzyme from bacteria able to destroy penicillin. 1940. Rev Infect Dis 10:677–678. [PubMed] [Google Scholar]
- 3.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 4.Ikram R, Psutka R, Carter A, Priest P. 2015. An outbreak of multi-drug resistant Escherichia coli urinary tract infection in an elderly population: a case-control study of risk factors. BMC Infect Dis 15:224. doi: 10.1186/s12879-015-0974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schrag SJ, McGee L, Whitney CG, Beall B, Craig AS, Choate ME, Jorgensen JH, Facklam RR, Klugman KP, Active Bacterial Core Surveillance Team . 2004. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob Agents Chemother 48:3016–3023. doi: 10.1128/AAC.48.8.3016-3023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nathan C. 2004. Antibiotics at the crossroads. Nature 431:899–902. doi: 10.1038/431899a. [DOI] [PubMed] [Google Scholar]
- 7.Jenkins MK, Khoruts A, Ingulli E, Mueller DL, McSorley SJ, Reinhardt RL, Itano A, Pape KA. 2001. In vivo activation of antigen-specific CD4 T cells. Annu Rev Immunol 19:23–45. doi: 10.1146/annurev.immunol.19.1.23. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki A, Medzhitov R. 2015. Control of adaptive immunity by the innate immune system. Nat Immunol 16:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jenkins MK, Moon JJ. 2012. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J Immunol 188:4135–4140. doi: 10.4049/jimmunol.1102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McSorley SJ. 2014. Immunity to intestinal pathogens: lessons learned from Salmonella. Immunol Rev 260:168–182. doi: 10.1111/imr.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verver S, Warren RM, Beyers N, Richardson M, van der Spuy GD, Borgdorff MW, Enarson DA, Behr MA, van Helden PD. 2005. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med 171:1430–1435. doi: 10.1164/rccm.200409-1200OC. [DOI] [PubMed] [Google Scholar]
- 12.Brunham RC, Rekart ML. 2008. The arrested immunity hypothesis and the epidemiology of chlamydia control. Sex Transm Dis 35:53–54. doi: 10.1097/OLQ.0b013e31815e41a3. [DOI] [PubMed] [Google Scholar]
- 13.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos RL, Zhang S, Tsolis RM, Kingsley RA, Adams LG, Bäumler AJ. 2001. Animal models of salmonella infections: enteritis versus typhoid fever. Microbes Infect 3:1335–1344. doi: 10.1016/S1286-4579(01)01495-2. [DOI] [PubMed] [Google Scholar]
- 15.Keestra-Gounder AM, Tsolis RM, Bäumler AJ. 2015. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 13:206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 16.Parry CM, Hien TT, Dougan G, White NJ, Farrar JJ. 2002. Typhoid fever. N Engl J Med 347:1770–1782. doi: 10.1056/NEJMra020201. [DOI] [PubMed] [Google Scholar]
- 17.Kariuki S, Gordon MA, Feasey N, Parry CM. 2015. Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 33(Suppl 3):C21–C29. doi: 10.1016/j.vaccine.2015.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. 2015. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting. PLoS One 10:e0143304. doi: 10.1371/journal.pone.0143304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention 2015. Sexually transmitted disease surveillance 2014. CDC, Atlanta, GA: http://www.cdc.gov/std/stats14/toc.htm. [Google Scholar]
- 20.Owusu-Edusei K Jr, Doshi SR, Apt BS, Gift TL. 2010. The direct cost of chlamydial infections: estimates for the employer-sponsored privately insured population in the United States, 2003-2007. Sex Transm Dis 37:519–521. doi: 10.1097/OLQ.0b013e3181d73e4c. [DOI] [PubMed] [Google Scholar]
- 21.Molano M, Meijer CJ, Weiderpass E, Arslan A, Posso H, Franceschi S, Ronderos M, Muñoz N, van den Brule AJ. 2005. The natural course of Chlamydia trachomatis infection in asymptomatic Colombian women: a 5-year follow-up study. J Infect Dis 191:907–916. doi: 10.1086/428287. [DOI] [PubMed] [Google Scholar]
- 22.Hillis SD, Owens LM, Marchbanks PA, Amsterdam LF, Mac Kenzie WR. 1997. Recurrent chlamydial infections increase the risks of hospitalization for ectopic pregnancy and pelvic inflammatory disease. Am J Obstet Gynecol 176:103–107. doi: 10.1016/S0002-9378(97)80020-8. [DOI] [PubMed] [Google Scholar]
- 23.Brunham RC, Gottlieb SL, Paavonen J. 2015. Pelvic inflammatory disease. N Engl J Med 372:2039–2048. doi: 10.1056/NEJMra1411426. [DOI] [PubMed] [Google Scholar]
- 24.Brunham RC, Pourbohloul B, Mak S, White R, Rekart ML. 2005. The unexpected impact of a Chlamydia trachomatis infection control program on susceptibility to reinfection. J Infect Dis 192:1836–1844. doi: 10.1086/497341. [DOI] [PubMed] [Google Scholar]
- 25.Gottlieb SL, Martin DH, Xu F, Byrne GI, Brunham RC. 2010. Summary: the natural history and immunobiology of Chlamydia trachomatis genital infection and implications for chlamydia control. J Infect Dis 201(Suppl 2):S190–S204. doi: 10.1086/652401. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention 2013. Sexually transmitted disease surveillance 2012. CDC, Atlanta, GA; http://www.cdc.gov/std/stats12/toc.htm. [Google Scholar]
- 27.Gottlieb SL, Xu F, Brunham RC. 2013. Screening and treating Chlamydia trachomatis genital infection to prevent pelvic inflammatory disease: interpretation of findings from randomized controlled trials. Sex Transm Dis 40:97–102. doi: 10.1097/OLQ.0b013e31827bd637. [DOI] [PubMed] [Google Scholar]
- 28.Hosenfeld CB, Workowski KA, Berman S, Zaidi A, Dyson J, Mosure D, Bolan G, Bauer HM. 2009. Repeat infection with chlamydia and gonorrhea among females: a systematic review of the literature. Sex Transm Dis 36:478–489. doi: 10.1097/OLQ.0b013e3181a2a933. [DOI] [PubMed] [Google Scholar]
- 29.Geisler WM, Lensing SY, Press CG, Hook EW. 2013. Spontaneous resolution of genital Chlamydia trachomatis infection in women and protection from reinfection. J Infect Dis 207:1850–1856. doi: 10.1093/infdis/jit094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicetti Miguel RD, Reighard SD, Chavez JM, Rabe LK, Maryak SA, Wiesenfeld HC, Cherpes TL. 2012. Transient detection of chlamydial-specific Th1 memory cells in the peripheral circulation of women with history of Chlamydia trachomatis genital tract infection. Am J Reprod Immunol 68:499–506. doi: 10.1111/aji.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Généeux M, Leclerc P, Bédard L, Allard R. 2010. Upsurge of chlamydial reinfection in a large Canadian city: an indication of suboptimal chlamydia screening practices? Can J Public Health 101:420–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farris CM, Morrison RP. 2011. Vaccination against chlamydia genital infection utilizing the murine C. muridarum model. Infect Immun 79:986–996. doi: 10.1128/IAI.00881-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffin AJ, McSorley SJ. 2011. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol 4:371–382. doi: 10.1038/mi.2011.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson RM, Brunham RC. 2016. Tissue-resident T cells as the central paradigm of chlamydia immunity. Infect Immun 84:868–873. doi: 10.1128/IAI.01378-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess J, Ladel C, Miko D, Kaufmann SH. 1996. Salmonella typhimurium aroA- infection in gene-targeted immunodeficient mice: major role of CD4+ TCR-alpha beta cells and IFN-gamma in bacterial clearance independent of intracellular location. J Immunol 156:3321–3326. [PubMed] [Google Scholar]
- 36.Ravindran R, Foley J, Stoklasek T, Glimcher LH, McSorley SJ. 2005. Expression of T-bet by CD4 T cells is essential for resistance to Salmonella infection. J Immunol 175:4603–4610. doi: 10.4049/jimmunol.175.7.4603. [DOI] [PubMed] [Google Scholar]
- 37.Lee SJ, Dunmire S, McSorley SJ. 2012. MHC class-I-restricted CD8 T cells play a protective role during primary Salmonella infection. Immunol Lett 148:138–143. doi: 10.1016/j.imlet.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McSorley SJ, Jenkins MK. 2000. Antibody is required for protection against virulent but not attenuated Salmonella enterica serovar Typhimurium. Infect Immun 68:3344–3348. doi: 10.1128/IAI.68.6.3344-3348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mastroeni P, Simmons C, Fowler R, Hormaeche CE, Dougan G. 2000. Igh-6(−/-) (B-cell-deficient) mice fail to mount solid acquired resistance to oral challenge with virulent Salmonella enterica serovar Typhimurium and show impaired Th1 T-cell responses to Salmonella antigens. Infect Immun 68:46–53. doi: 10.1128/IAI.68.1.46-53.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison RP, Feilzer K, Tumas DB. 1995. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect Immun 63:4661–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry LL, Feilzer K, Caldwell HD. 1997. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J Immunol 158:3344–3352. [PubMed] [Google Scholar]
- 42.Su H, Feilzer K, Caldwell HD, Morrison RP. 1997. Chlamydia trachomatis genital tract infection of antibody-deficient gene knockout mice. Infect Immun 65:1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li LX, McSorley SJ. 2013. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog 9:e1003707. doi: 10.1371/journal.ppat.1003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nanton MR, Way SS, Shlomchik MJ, McSorley SJ. 2012. Cutting edge: B cells are essential for protective immunity against Salmonella independent of antibody secretion. J Immunol 189:5503–5507. doi: 10.4049/jimmunol.1201413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morrison SG, Morrison RP. 2005. A predominant role for antibody in acquired immunity to chlamydial genital tract reinfection. J Immunol 175:7536–7542. doi: 10.4049/jimmunol.175.11.7536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jameson SC, Masopust D. 2009. Diversity in T cell memory: an embarrassment of riches. Immunity 31:859–871. doi: 10.1016/j.immuni.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carbone FR. 2015. Tissue-resident memory T cells and fixed immune surveillance in nonlymphoid organs. J Immunol 195:17–22. doi: 10.4049/jimmunol.1500515. [DOI] [PubMed] [Google Scholar]
- 48.Turner DL, Farber DL. 2014. Mucosal resident memory CD4 T cells in protection and immunopathology. Front Immunol 5:331. doi: 10.3389/fimmu.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stary G, Olive A, Radovic-Moreno AF, Gondek D, Alvarez D, Basto PA, Perro M, Vrbanac VD, Tager AM, Shi J, Yethon JA, Farokhzad OC, Langer R, Starnbach MN, von Andrian UH. 2015. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science 348:aaa8205. doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moon JJ, McSorley SJ. 2009. Tracking the dynamics of salmonella specific T cell responses. Curr Top Microbiol Immunol 334:179–198. doi: 10.1007/978-3-540-93864-4_8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Broz P, Monack DM. 2011. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev 243:174–190. doi: 10.1111/j.1600-065X.2011.01041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eisenstein TK, Killar LM, Sultzer BM. 1984. Immunity to infection with Salmonella typhimurium: mouse-strain differences in vaccine- and serum-mediated protection. J Infect Dis 150:425–435. doi: 10.1093/infdis/150.3.425. [DOI] [PubMed] [Google Scholar]
- 53.Johanns TM, Law CY, Kalekar LA, O’Donnell H, Ertelt JM, Rowe JH, Way SS. 2011. Early eradication of persistent Salmonella infection primes antibody-mediated protective immunity to recurrent infection. Microbes Infect 13:322–330. doi: 10.1016/j.micinf.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunstan SJ, Hue NT, Han B, Li Z, Tram TT, Sim KS, Parry CM, Chinh NT, Vinh H, Lan NP, Thieu NT, Vinh PV, Koirala S, Dongol S, Arjyal A, Karkey A, Shilpakar O, Dolecek C, Foo JN, Phuong le T, Lanh MN, Do T, Aung T, Hon DN, Teo YY, Hibberd ML, Anders KL, Okada Y, Raychaudhuri S, Simmons CP, Baker S, de Bakker PI, Basnyat B, Hien TT, Farrar JJ, Khor CC. 2014. Variation at HLA-DRB1 is associated with resistance to enteric fever. Nat Genet 46:1333–1336. doi: 10.1038/ng.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Griffin A, Baraho-Hassan D, McSorley SJ. 2009. Successful treatment of bacterial infection hinders development of acquired immunity. J Immunol 183:1263–1270. doi: 10.4049/jimmunol.0900772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Griffin AJ, McSorley SJ. 2011. Generation of Salmonella-specific Th1 cells requires sustained antigen stimulation. Vaccine 29:2697–2704. doi: 10.1016/j.vaccine.2011.01.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maskell DJ, Hormaeche CE. 1985. Relapse following cessation of antibiotic therapy for mouse typhoid in resistant and susceptible mice infected with salmonellae of differing virulence. J Infect Dis 152:1044–1049. doi: 10.1093/infdis/152.5.1044. [DOI] [PubMed] [Google Scholar]
- 58.Monack DM, Bouley DM, Falkow S. 2004. Salmonella typhimurium persists within macrophages in the mesenteric lymph nodes of chronically infected Nramp1+/+ mice and can be reactivated by IFNgamma neutralization. J Exp Med 199:231–241. doi: 10.1084/jem.20031319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, Holden DW. 2010. Dynamics of intracellular bacterial replication at the single cell level. Proc Natl Acad Sci U S A 107:3746–3751. doi: 10.1073/pnas.1000041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helaine S, Cheverton AM, Watson KG, Faure LM, Matthews SA, Holden DW. 2014. Internalization of Salmonella by macrophages induces formation of nonreplicating persisters. Science 343:204–208. doi: 10.1126/science.1244705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Claudi B, Spröte P, Chirkova A, Personnic N, Zankl J, Schürmann N, Schmidt A, Bumann D. 2014. Phenotypic variation of Salmonella in host tissues delays eradication by antimicrobial chemotherapy. Cell 158:722–733. doi: 10.1016/j.cell.2014.06.045. [DOI] [PubMed] [Google Scholar]
- 62.Bueno SM, González PA, Schwebach JR, Kalergis AM. 2007. T cell immunity evasion by virulent Salmonella enterica. Immunol Lett 111:14–20. doi: 10.1016/j.imlet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan A, Nanton M, Griffin A, McSorley SJ. 2009. Culling of activated CD4 T cells during typhoid is driven by Salmonella virulence genes. J Immunol 182:7838–7845. doi: 10.4049/jimmunol.0900382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bedoui S, Kupz A, Wijburg OL, Walduck AK, Rescigno M, Strugnell RA. 2010. Different bacterial pathogens, different strategies, yet the aim is the same: evasion of intestinal dendritic cell recognition. J Immunol 184:2237–2242. doi: 10.4049/jimmunol.0902871. [DOI] [PubMed] [Google Scholar]
- 65.Obst R, van Santen HM, Mathis D, Benoist C. 2005. Antigen persistence is required throughout the expansion phase of a CD4(+) T cell response. J Exp Med 201:1555–1565. doi: 10.1084/jem.20042521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rabenstein H, Behrendt AC, Ellwart JW, Naumann R, Horsch M, Beckers J, Obst R.. 2014. Differential kinetics of antigen dependency of CD4+ and CD8+ T cells. J Immunol 192:3507–3517. doi: 10.4049/jimmunol.1302725. [DOI] [PubMed] [Google Scholar]
- 67.Kaech SM, Ahmed R. 2001. Memory CD8 T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol 2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wong P, Pamer EG. 2001. Cutting edge: antigen-independent CD8 T cell proliferation. J Immunol 166:5864–5868. doi: 10.4049/jimmunol.166.10.5864. [DOI] [PubMed] [Google Scholar]
- 69.Williams MA, Bevan MJ. 2004. Shortening the infectious period does not alter expansion of CD8 T cells but diminishes their capacity to differentiate into memory cells. J Immunol 173:6694–6702. doi: 10.4049/jimmunol.173.11.6694. [DOI] [PubMed] [Google Scholar]
- 70.Roan NR, Starnbach MN. 2008. Immune-mediated control of chlamydia infection. Cell Microbiol 10:9–19. doi: 10.1111/j.1462-5822.2007.01069.x. [DOI] [PubMed] [Google Scholar]
- 71.Schoborg RV. 2011. Chlamydia persistence—a tool to dissect chlamydia-host interactions. Microbes Infect 13:649–662. doi: 10.1016/j.micinf.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Phillips Campbell R, Kintner J, Whittimore J, Schoborg RV. 2012. Chlamydia muridarum enters a viable but non-infectious state in amoxicillin-treated BALB/c mice. Microbes Infect 14:1177–1185. doi: 10.1016/j.micinf.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rank RG, Yeruva L. 2014. Hidden in plain sight: chlamydial gastrointestinal infection and its relevance to persistence in human genital infection. Infect Immun 82:1362–1371. doi: 10.1128/IAI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang Q, Huang Y, Gong S, Yang Z, Sun X, Schenken R, Zhong G. 2015. In vivo and ex vivo imaging reveals a long-lasting chlamydial infection in the mouse gastrointestinal tract following genital tract inoculation. Infect Immun 83:3568–3577. doi: 10.1128/IAI.00673-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su H, Morrison R, Messer R, Whitmire W, Hughes S, Caldwell HD. 1999. The effect of doxycycline treatment on the development of protective immunity in a murine model of chlamydial genital infection. J Infect Dis 180:1252–1258. doi: 10.1086/315046. [DOI] [PubMed] [Google Scholar]
- 76.Su H, Messer R, Whitmire W, Hughes S, Caldwell HD. 2000. Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains. Infect Immun 68:192–196. doi: 10.1128/IAI.68.1.192-196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Masopust D, Picker LJ. 2012. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schenkel JM, Fraser KA, Vezys V, Masopust D. 2013. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol 14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schenkel JM, Fraser KA, Beura LK, Pauken KE, Vezys V, Masopust D. 2014. T cell memory. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science 346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, Masopust D. 2015. Quantifying memory CD8 T cells reveals regionalization of immunosurveillance. Cell 161:737–749. doi: 10.1016/j.cell.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Riley MM, Zurenski MA, Frazer LC, O’Connell CM, Andrews CW, Mintus M, Darville T. 2012. The recall response induced by genital challenge with Chlamydia muridarum protects the oviduct from pathology but not from reinfection. Infect Immun 80:2194–2203. doi: 10.1128/IAI.00169-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McSorley SJ, Cookson BT, Jenkins MK. 2000. Characterization of CD.4+ T cell responses during natural infection with Salmonella typhimurium. J Immunol 164:986–993. doi: 10.4049/jimmunol.164.2.986. [DOI] [PubMed] [Google Scholar]
- 83.McSorley SJ, Ehst BD, Yu Y, Gewirtz AT. 2002. Bacterial flagellin is an effective adjuvant for CD4 T cells in vivo. J Immunol 169:3914–3919. doi: 10.4049/jimmunol.169.7.3914. [DOI] [PubMed] [Google Scholar]