Abstract
Although dramatic postnatal changes in maternal behavior have long been noted, we are only now beginning to understand the neurobiological mechanisms that support this transition. The present paper synthesizes growing insights from both animal and human research to provide an overview of the plasticity of the mother’s brain, with a particular emphasis on the oxytocin system. We examine plasticity observed within the oxytocin system and discuss how these changes mediate an array of other adaptations observed within the maternal brain. We outline factors that affect the oxytocin-mediated plasticity of the maternal brain and review evidence linking disruptions in oxytocin functions to challenges in maternal adaptation. We conclude by suggesting a strategy for intervention with mothers who may be at risk for maladjustment during this transition to motherhood, while highlighting areas where further research is needed.
Mothers across all mammalian species undergo a host of changes in their neurobiology and behavior as they embark on motherhood. An organism previously devoted to her own needs and survival now becomes primarily concerned about the care and nurturance of her offspring. Although this phenomenon has long been observed, we are only now starting to understand the complex processes behind such a dramatic transition. Recent advances in neuroscience have begun to unveil hormonal adaptations that occur during the course of pregnancy, childbirth, and post-partum caregiving. These changes have been associated with the structural and functional plasticity of the mother’s brain as it undergoes this critical transition (Kim, Strathearn, & Swain, 2016).
Of all the hormones that are involved at the onset of motherhood, oxytocin (OT) has received extensive attention, both from the scientific community and the general public. The present paper aims to synthesize the growing body of research on the role of OT in regulating plasticity in the mother’s brain during pregnancy and the postpartum period. We focus on the OT system for several reasons. The OT system is a key system supporting neurobiobehavioral adaptation during pregnancy, childbirth, and postpartum caregiving in a number of species. It has received close scrutiny over the past few decades for its role in the onset and maintenance of maternal behavior (Meyer-Lindenberg, Domes, Kirsch, & Heinrichs, 2011). Less than optimal adjustment to motherhood has increasingly been linked to OT-related dysfunctions (Kim, 2015; Strathearn, 2011). This, in turn, has fueled optimism that clinical interventions targeting the OT system may offer promise to mothers at risk for maladaptation.
In what follows, we first provide an overview of the OT system and discuss the role of OT in maternal behavior. Second, we review alterations that take place within the OT system leading to, and following the commencement of, motherhood and examine OT-mediated neural plasticity in the maternal brain. Third, we outline factors known to modulate functions of the OT system and address how these variations may shape maternal adaptation during pregnancy and the postpartum period. Finally, we conclude by synthesizing the literature with regard to the use of OT as a therapeutic agent for mothers for whom clinical interventions may be beneficial.
Overview of the Oxytocin System
The neuropeptide hormone OT is synthesized in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus and released into the peripheral circulation from the posterior pituitary gland (Gimpl & Fahrenholz, 2001). The peptide’s peripheral actions include its well-known functions in reproduction, namely uterine contraction during labor and milk ejection during lactation. OT is also involved in processes that contribute to cardiovascular and hydroelectrolytic homeostasis, such as regulation of blood pressure, heart rate, body fluid volume, and sodium excretion (Costa et al., 2005). Within the central nervous system, OT neurons project between brain regions known to be critical for maternal behavior, including the medial preoptic area (MPOA), bed nucleus of the stria terminalis (BNST), ventral tegmental area (VTA), ventral striatum (VS), and amygdala (AMY). OT’s central actions, particularly its role in the regulation of attachment and bond formation, have recently received considerable attention (Feldman, 2012; Meyer-Lindenberg et al., 2011). In the present paper, we address one of the central actions of OT, namely its regulation of maternal behavior.
Of all social bonds, the bond between a mother and her offspring has the strongest and most well-documented ties with OT. Studies using animal models have widely documented OT’s role in the onset and maintenance of maternal behavior. Virgin rats are normally aversive toward rat pups but display a wide range of maternal behavior following intraventricular injection of OT (Pedersen, Ascher, Monroe, & Prange, 1982). Similarly, injection of OT into the cerebrospinal fluid of sheep induces maternal-like behavior toward unfamiliar lambs, even in nonparent ewes (Kendrick, Keverne, & Baldwin, 1987). Conversely, disruption of central OT activity inhibits the expression of maternal behavior. OT antagonist administration into the VTA blocks maternal behavior in rat mothers (Pedersen, Caldwell, Walker, Ayers, & Mason, 1994). OT knockout mice (Ragnauth et al., 2005) and transgenic mice with reduced OT neurons in the PVN (Li et al., 1999) display impaired maternal behavior. The density of OT receptors in the AMY and BNST has been associated with the quality of maternal care observed in rats (Champagne, Diorio, Sharma, & Meaney, 2001).
Oxytocin-Related Plasticity During Pregnancy and the Postpartum Period
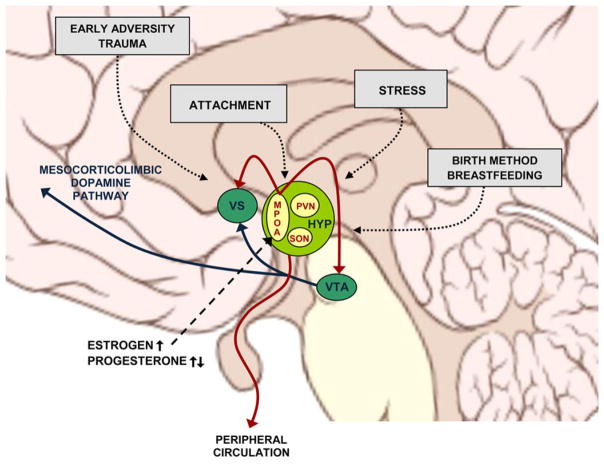
Considering the paramount role that OT plays in regulating maternal behavior, it is no surprise that the OT system undergoes a fundamental structural and functional reorganization during pregnancy and the postpartum period (Hillerer, Reber, Neumann, & Slattery, 2011). Here we review changes that take place at (a) the molecular and cellular level and (b) the neuroendocrine level (Figure 4.1).
Figure 4.1. Oxytocin-related maternal brain plasticity.
Structural and functional plasticity is observed in the OT neurons of the PVN, SON, and MPOA of the HYP during late pregnancy, childbirth, and postpartum. These changes coordinate an array of other adaptations critical for the maternal brain, including those within the mesocorticolimbic dopamine circuitry. MPOA = medial preoptic area; PVN = paraventricular nucleus; SON = supraoptic nucleus; HYP = hypothalamus; VS = ventral striatum; VTA = ventral tegmental area. Brain schematic by P. J. Lynch (2006; CC BY 2.5).
Molecular and Cellular Level
In animal models, morphological and physiological plasticity has long been observed in the PVN and SON of the hypothalamus during late pregnancy, parturition, and lactation (Hatton, 1990). Cell bodies and dendrites of OT neurons become closely juxtaposed around this time, reducing interneuronal membrane distances. New synapses are formed at a substantially increased rate, allowing a single synaptic bouton to make contact with multiple postsynaptic cell bodies or dendrites (Theodosis, Piet, Poulain, & Oliet, 2004). Alterations are also found in the length and geometry of the dendritic trees of OT neurons, which allow synaptic signals to travel more efficiently to cell bodies (Stern & Armstrong, 1998). These structural adjustments functionally move neurons in the direction of increased excitability, and are observed starting in late pregnancy and throughout the postpartum lactation period (Hatton, 1997). Around the time of parturition, OT receptor and OT mRNA expressions are observed to increase notably in the PVN of the hypothalamus (Zingg et al., 1995).
Striking plasticity has also been observed in MPOA neurons, which show a notable increase in the size of cell bodies in late pregnancy, when increased activity on the part of the MPOA becomes critical in preparation for imminent parturition (Keyser-Marcus et al., 2001). The cell body volumes return to their prepregnancy baseline as the postpartum period progresses and the mother’s pup-directed behaviors become increasingly contingent on external cues from pups.
Neuroendocrine Level
Shifts that occur within the OT system support and orchestrate the mother’s adaptations during pregnancy and the postpartum period. Until late pregnancy, responsiveness of OT neurons remains inhibited by metabolites of progesterone (Russell, Leng, & Douglas, 2003). Estrogen and progesterone levels rise steadily during pregnancy and OT storage accumulates accordingly in the pituitary. When close to term, a precipitous drop is observed in progesterone levels, signaling the fall of a mechanism that inhibits OT release (Brunton, Russell, & Hirst, 2014). The MPOA of the hypothalamus, a region with abundant OT receptors, is responsible for monitoring a cascade of neuroendocrine alterations throughout pregnancy. Upon sensing the drop in progesterone shortly preceding parturition, the maternal brain increases OT receptor expression and OT production rises subsequently (Rilling & Young, 2014). Parturition is characterized by a large amount of OT secretion, in parallel with increased basal activity in oxytocinergic cells (Russell, Leng, & Douglas, 2003). The MPOA continues to serve as a key neural substrate supporting maternal adaptation postparturition and is a region that stimulates the onset of maternal behavior via interaction with the mesolimbic dopamine (DA) circuitry, including the VTA and VS (Rilling & Young, 2014; Stolzenberg & Numan, 2011). In response to cues of the offspring in postpartum, the MPOA induces DA release into the mesocorticolimbic DA pathway, enhancing the perceived appetitive value of the offspring, ultimately facilitating the mother’s offspring-directed behavior.
Elegantly designed animal models continue to tease apart the involvement of OT in maternal brain plasticity around parturition. Human studies have been constrained by methodological limitations and have largely relied on peripheral assessments of OT—plasma, salivary, and urinary—as proxies for OT functions in the brain. Available data suggest that peripheral OT levels increase over the course of pregnancy (Feldman, 2012). This rise in OT primes the mother to provide optimal care for the infant (Gordon, Zagoory-Sharon, Leckman, & Feldman, 2010), whereas postpartum interactions with the infant further stimulate OT release in mothers (Kim, Fonagy, Koos, Dorsett, & Strathearn, 2014; Strathearn, Fonagy, Amico, & Montague, 2009). As the mother–infant relationship matures, the interaction between the mother’s OT and DA systems is strengthened, infusing the mother’s infant-directed interactions with a unique sense of reward (Strathearn, 2011).
Accumulating evidence from both animal and human research has reported high intraindividual stability and high interindividual variability in the mother’s OT functions (Gordon et al., 2010; Levine, Zagoory-Sharon, Feldman, & Weller, 2007). This has led to the understanding that OT-related functions may constitute a traitlike characteristic that undergirds the mother’s adaptation to motherhood (Strathearn, Iyengar, Fonagy, & Kim, 2012). It is to this topic that we turn next.
Factors Influencing Oxytocin-Related Maternal Brain Plasticity
Given the centrality of OT functions in adaptation to motherhood, considerable attention has been paid to factors that regulate the mother’s OT-related functions in pregnancy and postpartum. Next, we review two distal and two proximal factors that have received scrutiny to date.
Early-Life Adversity or Trauma
Early-life adversity or trauma is often considered one of the most important factors implicated in the link between the mother’s OT functions and her adaptation to motherhood. Female rodents exposed to early-life stress show dampened OT functions in the PVN, VS, or BNST in adulthood compared to those without a history of adversity (Bales, Boone, Epperson, Hoffman, & Carter, 2011; Veenema, Bredewold, & Neumann, 2007). Similarly, a history of childhood trauma or stress has consistently been associated with low levels of cerebrospinal fluid and plasma OT measured in adult women (Bertsch, Schmidinger, Neumann, & Herpertz, 2013; Heim et al., 2009). Among different types of trauma, the strongest effects appear for childhood emotional abuse and neglect (Bertsch et al., 2013; Heim et al., 2009), underscoring their potential links to disrupted attachment. Several studies have reported a dose-dependent inverse relationship between peripheral OT concentrations and the number of trauma exposures (Heim et al., 2009; Opacka-Juffry & Mohiyeddini, 2012). The timing of trauma is also important because similar experiences in childhood, but not in adolescence or adulthood, predict OT concentrations in adulthood (Opacka-Juffry & Mohiyeddini, 2012).
Attachment
A pattern of insecure attachment has also shown consistent links to OT functioning in adulthood. Peripheral OT levels are lower in mothers who have insecure forms of attachment (Samuel et al., 2015; Strathearn et al., 2009). The reduced densities of OT receptors in brain regions critical for attachment formation are believed to underlie both the mother’s insecure attachment and her low OT synthesis. Rodent studies have provided a fine-grained examination of the mechanisms by which early maternal care shapes long-term development of the OT system. Compromised caregiving that rats receive early in life is directly associated with the reduced OT receptor densities observed in their brains (e.g., MPOA, BNST, and PVN) and with their low OT activity profiles subsequently assessed in adulthood (Champagne et al., 2001; Champagne et al., 2006). Importantly, cross-fostering studies suggest that these effects are not associated with genetic variation, but rather may be more a consequence of disrupted early maternal caregiving, mediated by the changes in epigenetic regulation via altered DNA methylation (Champagne et al., 2006). Unfortunately, human research on the respective contributions of genetics, epigenetics, and environment is currently limited by methodological challenges.
Perinatal Stress
The perinatal period, from late pregnancy to the lactation period in particular, is considered a time in which a multitude of neurobiobehavioral alterations work in concert to produce attenuated stress reactivity in the mother (Heinrichs et al., 2001). One such alteration involves the upregulated OT system examined earlier, which exerts inhibitory effects on the stress-induced responsivity of the hypothalamic–pituitary–adrenal (HPA) axis. However, when the amount and intensity of stress surpass what the mother can effectively manage, her equilibrium becomes disrupted and her susceptibility to experiencing challenges in adaptation increases. Chronic perinatal stress may prevent an adaptive rise in OT mRNA expression in the hypothalamic PVN around the time of parturition (Hillerer et al., 2011). Chronic gestational stress may also reduce OT receptor bindings in the MPOA, BNST, and AMYG and elicit suboptimal behavioral adaptations in rodent mothers, who, in prior pregnancies, demonstrated high OT functions and positive behavioral adaptations in the absence of excess stress (Champagne & Meaney, 2006). Similarly, in humans, the body of evidence demonstrating the role of OT in buffering against the deleterious effects of stress on maternal adaptation continues to grow (Zelkowitz et al., 2014). Negative links have also been established between levels of perinatal stress and OT functions in pregnancy and postpartum (Samuel et al., 2015).
Childbirth and Feeding Methods
A series of events around parturition can also modify OT functions in the mother. Vaginal delivery increases central and peripheral levels of OT in rats and sheep (Kendrick, Keverne, Hinton, & Goode, 1991; Neumann, Russell, & Landgraf, 1993). Similar results have been found in women undergoing labor, whereas Cesarean delivery in the absence of labor may lead to a diminished OT response during childbirth (Takagi et al., 1985) and dampened maternal neural responses to their own infant’s cries in the early postpartum period, particularly in brain regions critical for empathy, motivation, and reward, including OT-innervated regions (Swain et al., 2008). Although not extensively studied, some have speculated that various pharmacological manipulations that are common during childbirth may also affect the mother’s OT functions. These groups suggest that the intrapartum use of synthetic OT (Pitocin; used to induce labor), OT receptor antagonists (Atosiban; used to suppress labor), or exogenous opioids (morphine; used as analgesic) may dampen endogenous OT functions, at least for the short-term, and may lead to postpartum alterations in OT functions (Olza-Fernandez, Marin Gabriel, Gil-Sanchez, Garcia-Segura, & Arevalo, 2014).
Breastfeeding has often been considered a pinnacle of attachment experience between the mother and infant, and has been reported to induce OT release in both rodent and human mothers (Olza-Fernandez et al., 2014). In early postpartum women, breastfeeding enhances neural responses to own-infant cries; interestingly, this occurs in brain regions that largely overlap with those that are more active following vaginal versus Cesarean delivery (Kim et al., 2011; Kim et al., 2016). Similar results have been found in lactating postpartum rodents. Suckling is observed to induce OT release, which, in turn, serves to enhance neuronal activity in reward- and emotion-related brain regions critically involved in postpartum maternal care (Febo, Numan, & Ferris, 2005; Ferris et al., 2005). From a population perspective, a large longitudinal study demonstrated that breastfeeding duration was inversely associated with state reported maternally perpetrated neglect, suggesting that the OT-related effects of breastfeeding may be protective in the long-term (Strathearn, Mamun, Najman, & O’Callaghan, 2009).
Variations in Oxytocin Function and Adaptation to Motherhood
There exists a solid body of research documenting that variations in maternal OT functions systematically predict individual differences in adaptation to motherhood. First, the mother’s OT functions are directly associated with her ability to provide optimal caregiving. In rodents, OT activity profiles and OT receptor densities measured in brain regions critical for bond formation robustly predict the quality of caregiving displayed by mothers (Champagne et al., 2001). Comparable results have been found in humans. Mothers who display less than optimal bonds with their infants exhibit a compromised rise in OT levels over the course of pregnancy (Levine et al., 2007), reduced baseline OT levels during the postpartum period (Gordon et al., 2010), and blunted OT release during interaction with their infants (Feldman, Gordon, Schneiderman, Weisman, & Zagoory-Sharon, 2010).
Second, disrupted OT functions are also linked to maternal psychopathology, particularly during the perinatal period. Symptoms of post-partum depression and anxiety correlate negatively with peripheral OT functions in pregnancy and the postpartum period (Eapen et al., 2014; Skrundz, Bolten, Nast, Hellhammer, & Meinlschmidt, 2011). Reports from animal models support stress-attenuating and antidepressive properties of OT, with intraventricular and intraperitoneal injections of OT leading to attenuated depressive-like behaviors in mice, an effect similar to that shown by standard antidepressants (Kim, Soeken, et al., 2014). This line of work has contributed to a rapidly increasing number of studies utilizing intranasally administered OT to determine whether pharmacological alterations of OT enhance socio-emotional functions while ameliorating a range of dysfunctions in mothers facing challenges in their adaptation (Bartz, Zaki, Bolger, & Ochsner, 2011; Macdonald & Macdonald, 2010). If an increase in OT levels promotes behavioral adaptation and facilitates OT-mediated adaptation of the maternal brain that is critical during pregnancy and postpartum, can such an intervention offer promise to at-risk mothers?
Pharmacological Effects of Oxytocin: A Novel Treatment for Adaptation to Motherhood?
Despite the initial optimism that surrounded the pharmacological potential of OT, results reported to date have been mixed. Early enthusiasm was supported by reports that administration of OT indeed enhanced socio-emotional adaptation, largely in normative, healthy individuals (Macdonald & Macdonald, 2010). However, the initial excitement has become gradually tempered by subsequent reports documenting that OT administration fails to yield significant effects in some individuals, most often in individuals with a history of disrupted attachment. There have been particularly puzzling reports of OT exacerbating existing dysfunctions, which have been termed “paradoxical effects” and are primarily documented in individuals with suboptimal attachment experiences (Bartz, Simeon, et al., 2011; Kim & Strathearn, under review). In attempts to reconcile these seemingly divergent findings, the prevailing view of OT as a prosocial neuropeptide is currently undergoing scrutiny and revision. Rather than universally enhancing socio-emotional functions, it is now proposed that OT’s primary mechanism of action may lie in increasing the salience of social cues (Shamay-Tsoory, 2010), mobilizing approach behaviors (Feeser et al., 2014; Kemp & Guastella, 2010), or activating affiliative motives in interaction with the mesolimbic DA motivational circuitry (Tops, 2010; Walker & McGlone, 2013). Although the field has not yet reached a consensus in this regard, it is important to note that the outcome of any one of the aforementioned three effects would vary as a function of the quality of early attachment of the given individual. When pharmacologically increased OT highlights the salience of social cues, activates affiliative motives, and/or increases approach behaviors, individuals with secure attachment histories are more likely to display an increase in their prosocial behavior. However, similar interventions may intensify negative emotions, activate memories of suboptimal attachment experiences, and thereby exacerbate existing dysfunctions in individuals who have had repeated social experiences wherein their safety and security were undermined (Bartz, Simeon, et al., 2011).
These evolving perspectives on the mechanisms of OT actions are particularly imperative in considering the pharmacological potential of OT. As was previously discussed, the mother’s history of attachment appears to be a critical factor modifying the functions of her OT system (Kim, 2015). Although increasing OT levels pharmacologically appears to be a promising intervention for mothers with disrupted attachment and suboptimal OT functions who may be at risk for maladjustment, such intervention may ironically have the effect of magnifying preexisting expectations about affiliative experiences and associated emotions, inducing paradoxical effects in those who need the intervention the most. What appears to be paramount for these mothers, prior to considering the pharmacological use of OT, is an examination and modification of their underlying expectations about relationships that were shaped by their suboptimal early experiences (Kim & Strathearn, under review). As this examination and modification unfold, whether via psychotherapy or through other means, the pharmacological use of OT may prove to be helpful in amplifying the salience of new relational expectations and associated emotions. OT may also be used to augment affiliative motives or mobilize approach behaviors when such interventions may be beneficial rather than destabilizing.
Several theoretical and clinical gaps remain to be bridged before intranasal OT can be used as a therapeutic agent or adjunct for mothers. More data are needed to clarify the nature of OT dysregulation in at-risk mothers and to flesh out more precise mechanisms by which these dysregulations modify the OT-mediated plasticity of the maternal brain. Future studies also need to account for variables known to affect levels of OT in mothers, including age, medication, contraceptive intake, and menstrual cycle phase. Much of extant data on OT administration have relied on studies of men to minimize the confounding effects of these variables. Levels of naturally produced OT undergo more frequent and wide fluctuation in women, particularly in women who are in the process of becoming mothers. The patterns of OT functions induced by OT administration will inevitably be complicated by these fluctuations, and the resulting effects need to be teased apart carefully. The effects of repeated OT administration on naturally occurring OT release in mothers should also be studied in greater detail, along with its interactions with an array of neuroendocrine adaptations that take place during pregnancy and the postpartum period.
Acknowledgments
This work was supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development [R01HD065819 and R03HD080998] and the National Institute on Drug Abuse [R01DA026437]. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes or the National Institutes of Health.
Contributor Information
Sohye Kim, Assistant Professor, Departments of Obstetrics and Gynecology, Psychiatry, and Pediatrics, Baylor College of Medicine; Clinical Psychologist, The Women’s Place, Center for Reproductive Psychiatry, Pavilion for Women, Texas Children’s Hospital.
Lane Strathearn, Professor, Department of Pediatrics, University of Iowa Carver College of Medicine; Director, Division of Developmental and Behavioral Pediatrics; Physician Director, Center for Disabilities and Development, University of Iowa Children’s Hospital.
References
- Bales KL, Boone E, Epperson P, Hoffman G, Carter CS. Are behavioral effects of early experience mediated by oxytocin? Front Psychiatry. 2011;2:24. doi: 10.3389/fpsyt.2011.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, Kim S, Crystal S, Braun A, … Hollander E. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience. 2011;6(5):556–563. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: Context and person matter. Trends in Cognitive Science. 2011;15(7):301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Schmidinger I, Neumann ID, Herpertz SC. Reduced plasma oxytocin levels in female patients with borderline personality disorder. Hormones and Behavior. 2013;63(3):424–429. doi: 10.1016/j.yhbeh.2012.11.013. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA, Hirst JJ. Allopregnanolone in the brain: Protecting pregnancy and birth outcomes. Progress in Neurobiology. 2014;113:106–136. doi: 10.1016/j.pneurobio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proceedings of the National Academy of Sciences, USA. 2001;98(22):12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ. Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biological Psychiatry. 2006;59(12):1227–1235. doi: 10.1016/j.biopsych.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Weaver IC, Diorio J, Dymov S, Szyf M, Meaney MJ. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147(6):2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Costa ESRH, Pereira-Junior PP, Oliveira PF, Olivares EL, Werneck-de-Castro JP, Mello DB, … Campos-de-Carvalho AC. Cardiac effects of oxytocin: Is there a role for this peptide in cardiovascular homeostasis? Regulatory Peptides. 2005;132(1–3):107–112. doi: 10.1016/j.regpep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N, Silove DM. Separation anxiety, attachment and inter-personal representations: Disentangling the role of oxytocin in the perinatal period. PLoS One. 2014;9(9):e107745. doi: 10.1371/journal.pone.0107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience. 2005;25(50):11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeser M, Fan Y, Weigand A, Hahn A, Gartner M, Aust S, … Grimm S. The beneficial effect of oxytocin on avoidance-related facial emotion recognition depends on early life stress experience. Psychopharmacology (Berlin) 2014;231(24):4735–4744. doi: 10.1007/s00213-014-3631-1. [DOI] [PubMed] [Google Scholar]
- Feldman R. Oxytocin and social affiliation in humans. Hormones and Behavior. 2012;61(3):380–391. doi: 10.1016/j.yhbeh.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent-infant contact. Psychoneuroendocrinology. 2010;35(8):1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. Journal of Neuroscience. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: Structure, function, and regulation. Physiology Review. 2001;81(2):629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biological Psychiatry. 2010;68(4):377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI. Emerging concepts of structure-function dynamics in adult brain: The hypothalamo-neurohypophysial system. Progress in Neurobioogyl. 1990;34(6):437–504. doi: 10.1016/0301-0082(90)90017-b. [DOI] [PubMed] [Google Scholar]
- Hatton GI. Function-related plasticity in hypothalamus. Annual Review of Neuroscience. 1997;20:375–397. doi: 10.1146/annurev.neuro.20.1.375. [DOI] [PubMed] [Google Scholar]
- Heim C, Young LJ, Newport DJ, Mletzko T, Miller AH, Nemeroff CB. Lower CSF oxytocin concentrations in women with a history of childhood abuse. Molecular Psychiatry. 2009;14(10):954–958. doi: 10.1038/mp.2008.112. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum C, Ehlert U, Hellhammer DH. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. Journal of Clinical Endocrinology and Metabolism. 2001;86(10):4798–4804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Reber SO, Neumann ID, Slattery DA. Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: Implications for postpartum anxiety and mood disorders. Endocrinology. 2011;152(10):3930–3940. doi: 10.1210/en.2011-1091. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. Oxytocin: Prosocial behavior, social salience, or approach-related behavior? Biological Psychiatry. 2010;67(6):e33–34. doi: 10.1016/j.biopsych.2009.11.019. author reply e35. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Baldwin BA. Intracerebroventricular oxytocin stimulates maternal behaviour in the sheep. Neuroendocrinology. 1987;46(1):56–61. doi: 10.1159/000124796. [DOI] [PubMed] [Google Scholar]
- Kendrick KM, Keverne EB, Hinton MR, Goode JA. Cerebrospinal fluid and plasma concentrations of oxytocin and vasopressin during parturition and vaginocervical stimulation in the sheep. Brain Research Bulletin. 1991;26(5):803–807. doi: 10.1016/0361-9230(91)90178-m. [DOI] [PubMed] [Google Scholar]
- Keyser-Marcus L, Stafisso-Sandoz G, Gerecke K, Jasnow A, Nightingale L, Lambert KG, … Kinsley CH. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Research Bulletin. 2001;55(6):737–745. doi: 10.1016/s0361-9230(01)00554-8. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry. 2011;52(8):907–915. doi: 10.1111/j.1469-7610.2011.02406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE. The maternal brain and its plasticity in humans. Hormones and Behavior. 2016;77:113–123. doi: 10.1016/j.yhbeh.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. The mind in the making: Developmental and neurobiological origins of mentalizing. Personality Disorders: Theory, Research, and Treatment. 2015;6(4):356–365. doi: 10.1037/per0000102. [DOI] [PubMed] [Google Scholar]
- Kim S, Fonagy P, Koos O, Dorsett K, Strathearn L. Maternal oxytocin response predicts mother-to-infant gaze. Brain Research. 2014;1580:133–142. doi: 10.1016/j.brainres.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L. Oxytocin and postpartum depression: Delivering on what’s known and what’s not. Brain Research. 2014;1580:219–232. doi: 10.1016/j.brainres.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Strathearn L. Trauma, mothering, and intergenerational transmission: A synthesis of behavioral and oxytocin research. Psychoanalytic Study of the Child under review. [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: Individual patterns and maternal-fetal attachment. Peptides. 2007;28(6):1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Li L, Keverne EB, Aparicio SA, Ishino F, Barton SC, Surani MA. Regulation of maternal behavior and offspring growth by paternally expressed Peg3. Science. 1999;284(5412):330–333. doi: 10.1126/science.284.5412.330. [DOI] [PubMed] [Google Scholar]
- Macdonald K, Macdonald TM. The peptide that binds: A systematic review of oxytocin and its prosocial effects in humans. Harvard Review of Psychiatry. 2010;18(1):1–21. doi: 10.3109/10673220903523615. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. National Review of Neuroscience. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Neumann I, Russell JA, Landgraf R. Oxytocin and vasopressin release within the supraoptic and paraventricular nuclei of pregnant, parturient and lactating rats: A microdialysis study. Neuroscience. 1993;53(1):65–75. doi: 10.1016/0306-4522(93)90285-n. [DOI] [PubMed] [Google Scholar]
- Olza-Fernandez I, Marin Gabriel MA, Gil-Sanchez A, Garcia-Segura LM, Arevalo MA. Neuroendocrinology of childbirth and mother-child attachment: The basis of an etiopathogenic model of perinatal neurobiological disorders. Frontiers in Neuroendocrinology. 2014;35(4):459–472. doi: 10.1016/j.yfrne.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Opacka-Juffry J, Mohiyeddini C. Experience of stress in childhood negatively correlates with plasma oxytocin concentration in adult men. Stress. 2012;15(1):1–10. doi: 10.3109/10253890.2011.560309. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216(4546):648–650. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behavioral Neuroscience. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Ragnauth AK, Devidze N, Moy V, Finley K, Goodwillie A, Kow LM, … Pfaff DW. Female oxytocin gene-knockout mice, in a semi-natural environment, display exaggerated aggressive behavior. Genes and Brain Behavior. 2005;4(4):229–239. doi: 10.1111/j.1601-183X.2005.00118.x. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345(6198):771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA, Leng G, Douglas AJ. The magnocellular oxytocin system, the fount of maternity: Adaptations in pregnancy. Front in Neuroendocrinology. 2003;24(1):27–61. doi: 10.1016/s0091-3022(02)00104-8. [DOI] [PubMed] [Google Scholar]
- Samuel S, Hayton B, Gold I, Feeley N, Carter CS, Zelkowitz P. Attachment security and recent stressful life events predict oxytocin levels: A pilot study of pregnant women with high levels of cumulative psychosocial adversity. Attachment and Human Development. 2015;17(3):272–287. doi: 10.1080/14616734.2015.1029951. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG. One hormonal system for love and envy: A reply to Tops. Biological Psychiatry. 2010;67(1):e7. doi: 10.1016/j.biopsych.2009.08.032. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36(9):1886–1893. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JE, Armstrong WE. Reorganization of the dendritic trees of oxytocin and vasopressin neurons of the rat supraoptic nucleus during lactation. Journal of Neuroscience. 1998;18(3):841–853. doi: 10.1523/JNEUROSCI.18-03-00841.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neuroscience and Biobehavioral Reviews. 2011;35(3):826–847. doi: 10.1016/j.neubiorev.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Strathearn L. Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology. 2011;23(11):1054–1065. doi: 10.1111/j.1365-2826.2011.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34(13):2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Iyengar U, Fonagy P, Kim S. Maternal oxytocin response during mother-infant interaction: Associations with adult temperament. Hormones and Behavior. 2012;61(3):429–435. doi: 10.1016/j.yhbeh.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Abdullah M, Najman JM, O’Callaghan M. Does breast-feeding protect against substantiated child abuse and neglect? A 15-year cohort study. Pediatrics. 2009;123(2):483–493. doi: 10.1542/peds.2007-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry. 2008;49(10):1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi T, Tanizawa O, Otsuki Y, Sugita N, Haruta M, Yamaji K. Oxytocin in the cerebrospinal fluid and plasma of pregnant and nonpregnant subjects. Hormones and Metabolism Research. 1985;17(6):308–310. doi: 10.1055/s-2007-1013526. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Piet R, Poulain DA, Oliet SH. Neuronal, glial and synaptic remodeling in the adult hypothalamus: Functional consequences and role of cell surface and extracellular matrix adhesion molecules. Neurochemistry International. 2004;45(4):491–501. doi: 10.1016/j.neuint.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Tops M. Oxytocin: Envy or engagement in others? Biological Psychiatry. 2010;67(1):e5–6. doi: 10.1016/j.biopsych.2009.08.032. author reply e7. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, Neumann ID. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32(5):437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Walker SC, McGlone FP. The social brain: Neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides. 2013;47(6):379–393. doi: 10.1016/j.npep.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, … Levin P. Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Hormones and Behavior. 2014;66(2):351–360. doi: 10.1016/j.yhbeh.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Zingg HH, Rozen F, Breton C, Larcher A, Neculcea J, Chu K, … Arslan A. Gonadal steroid regulation of oxytocin and oxytocin receptor gene expression. Advances in Experimental Medicine and Biology. 1995;395:395–404. [PubMed] [Google Scholar]