Summary
Liver X receptors (LXRs) are regulators of cholesterol metabolism that also modulate immune responses. Inactivation of LXR α and β in mice leads to autoimmunity; however, how the regulation of cholesterol metabolism contributes to autoimmunity is unclear. Here we found that cholesterol loading of CD11c+ cells triggered the development of autoimmunity, whereas preventing excess lipid accumulation by promoting cholesterol efflux was therapeutic. LXRβ-deficient mice crossed to the hyperlipidemic ApoE-deficient background or challenged with a high-cholesterol diet developed autoantibodies. Cholesterol accumulation in lymphoid organs promoted T cell priming and stimulated the production of the B cell growth factors Baff and April. Conversely, B cell expansion and the development of autoantibodies in ApoE−/−Lxrβ−/− mice was reversed by ApoA-I expression. These findings implicate cholesterol imbalance as a contributor to immune dysfunction and suggest that stimulating HDL-dependent reverse cholesterol transport could be beneficial in the setting of autoimmune disease.
Graphical Abstract

Introduction
The liver X receptors α and β (LXRα and LXRβ) are members of nuclear receptor superfamily that regulate cholesterol, fatty acid and phospholipid metabolism (Calkin and Tontonoz, 2012; Hong and Tontonoz, 2014; Rong et al., 2013). In peripheral cell types, including macrophages, excess cholesterol accumulation leads to LXR activation, which in turn promotes cholesterol efflux to lipid-poor apolipoprotein A-I (ApoA-I) and high-density lipoprotein (HDL) acceptors through induction of the sterol transporters Abca1 and Abcg1. As a result, excess cholesterol from the periphery is shuttled by HDL to the liver for excretion through the reverse cholesterol transport pathway. LXRs also control the expression of genes involved in bile production and cholesterol excretion in liver, and regulate cholesterol and fatty acid absorption in intestine (Peet et al., 1998; Repa et al., 2002; Repa et al., 2000; Wang et al., 2016). Collectively, the systemic regulation of genes involved in cholesterol metabolism by LXRs is crucial for the maintenance of sterol homeostasis. Dysregulation of the LXR pathway leads to systemic and cellular cholesterol overload and the development of atherosclerosis (Bradley et al., 2007; Hong et al., 2012a; Tangirala et al., 2002).
In addition to their importance in lipid metabolism, LXRs are also known to regulate immune cell function (Kidani and Bensinger, 2012; Spann and Glass, 2013). One of the best-characterized roles of LXRs in immune cells is to inhibit inflammatory gene expression triggered by toll-like receptors (TLRs) (Castrillo et al., 2003; Ghisletti et al., 2007; Glass and Saijo, 2010; Joseph et al., 2004; Joseph et al., 2003). We recently demonstrated that this anti-inflammatory effect is attributable at least in part to primary effects of LXRs on cellular lipid metabolism. We showed that transcriptional induction of Abca1 by LXRs promotes cholesterol efflux and alters plasma membrane cholesterol distribution, resulting in the attenuation of MAPKs and NFκB signaling downstream of TLRs (Ito et al., 2015). These findings suggest that the dual role of LXRs in lipid homeostasis and immune cell function have common mechanistic underpinnings.
Mice lacking LXRs exhibit age-dependent systemic autoimmune disease, and pharmacological activation of LXRs with a synthetic agonist attenuates disease progression in a mouse model of lupus-like autoimmunity (A-Gonzalez et al., 2009). One mechanism underlying the development of autoimmunity in the setting of LXR deficiency is a defect in the phagocytic clearance of apoptotic cells (A-Gonzalez et al., 2009). Activation of LXRs by phagocytosed lipids activates a positive feedback loop to promote efficient apoptotic cell clearance through the induction of the plasma membrane efferocytosis receptor Mertk. LXRs have also been shown to modulate lymphocyte proliferation by linking cellular cholesterol availability to cell division (Bensinger et al., 2008). Although these prior findings suggest the crosstalk between cholesterol metabolism and immune functions are likely to be relevant to the development of autoimmune disease-related pathologies, the question of whether altered cellular cholesterol levels per se contributes the pathogenesis of autoimmunity has not been addressed.
We found that hypercholesterolemia and the consequent accumulation of excess cholesterol in immune cells played a causal role in the development of autoimmune disease in mice. We further showed that cholesterol accumulation in antigen-presenting cells stimulated the production of B-cell proliferation factors and promoted T cell priming through antigen presentation, thereby driving the expansion of autoreactive B cells. Finally, we showed that promoting reverse cholesterol transport by overexpressing the HDL constituent ApoA-I confered protection from the development of autoimmune disease. These data outline a critical role for LXR signaling in coupling immune cell cholesterol homeostasis with systemic immune responses, and suggest that promoting reverse cholesterol transport could have therapeutic utility in autoimmune disease.
Results
Hypercholesterolemia in LXRβ-deficient mice provokes the development of lupus-like disease
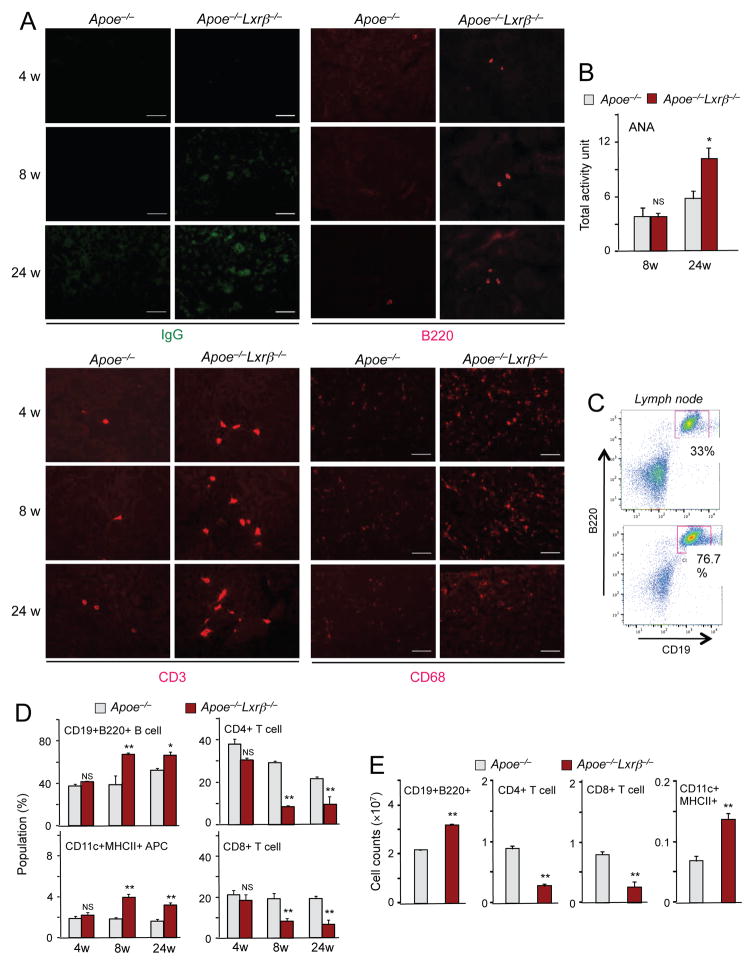
We previously reported that Lxrαβ−/− mice, but not Lxrα−/− or Lxrβ−/− mice, exhibit age-dependent systemic autoimmune disease (A-Gonzalez et al., 2009). Given that LXRs are major regulators of cholesterol homeostasis, these findings suggest that excess cellular and/or systemic cholesterol accumulation could be a driver of autoimmunity. We therefore speculated that challenging mice deficient in a single LXR subtype with excess cholesterol might provoke the development of autoimmune disease. To explore this possibility, we generated Lxrβ−/− mice on the ApoE-null background (Hong et al., 2012a). Loss of ApoE markedly raises plasma cholesterol levels in mice due to the critical importance of ApoE as a ligand for the LDL receptor (Zhang et al., 1992). Whereas mice lacking LXRβ (A-Gonzalez et al., 2009) or ApoE alone did not show evidence of autoimmunity, Apoe−/−Lxrβ−/− mice exhibited the age-dependent deposition of IgG-containing immune complexes and infiltration of B cells (B220+), T cells (CD3+) and macrophages (CD68+) in kidneys (Figure 1A). Accordingly, elevated levels of total immunoglobulins and antibodies against nuclear proteins (ANA, antinuclear antibodies) were detected in the plasma of Apoe−/−Lxrβ−/− mice (Figures 1B and S1A).
Figure 1. Deletion of Lxrβ on an ApoE-null background is sufficient to provoke autoimmune disease.
(A) Kidney sections from indicated ages of Apoe−/− and Apoe−/−Lxrβ−/− mice immunostained for IgG, B220, CD3 and CD68. (B) Plasma samples from 8-week-old and 24-week-old Apoe−/− and Apoe−/−Lxrβ−/− mice were analyzed for anti-nuclear antibody (ANA) titers by ELISA. (C) Flow cytometric analysis of CD19+B220+ B cells from lymph node of 8-week-old Apoe−/− and Apoe−/− Lxrβ−/− mice. (D) Percentages of indicated cell populations in lymph node of Apoe−/− and Apoe−/−Lxrβ−/− mice analyzed by flow cytometry. (E) Cell counts of the indicated cell populations in lymph node of ApoE−/− and Apoe−/−Lxrβ−/− mice analyzed by flow cytometry. N=4–6 per group. Statistical analysis was performed with Student’s t test. *p < 0.05, **p < 0.01, NS, not significant. Error bars represent means +/− SEM. See also Figure S1.
Apoe−/−Lxrβ−/− mice also exhibited lymphadenopathy and splenomegaly compared to control Apoe−/− mice (data not shown), and expanded populations of CD19+ B220+ B cells and CD11c+MHC class II+ cells (which can include dendritic cells and other antigen-presenting cells) in lymph node and spleen (Figures 1C, 1D, S1B and S1C). Cell counts revealed increased B cells, increased CD11c+MHC class II+ cells and decreased T cells in Apoe−/−Lxrβ−/− compared to Apoe−/− mice (Fig. 1E and S1D). We analyzed the abundance of CD11c+ B cells, as these cells have been causally associated with autoimmune disease (Rubstov et al., 2011; Naradikian et al., 2016). These age/autoimmune-associated B cells have been reported to be most abundant in spleen. We observed no difference in relative or absolute abundance of this B cell population between Apoe−/− and Apoe−/−Lxrβ−/− mice in spleen or lymph node (Fig. S1E, F).
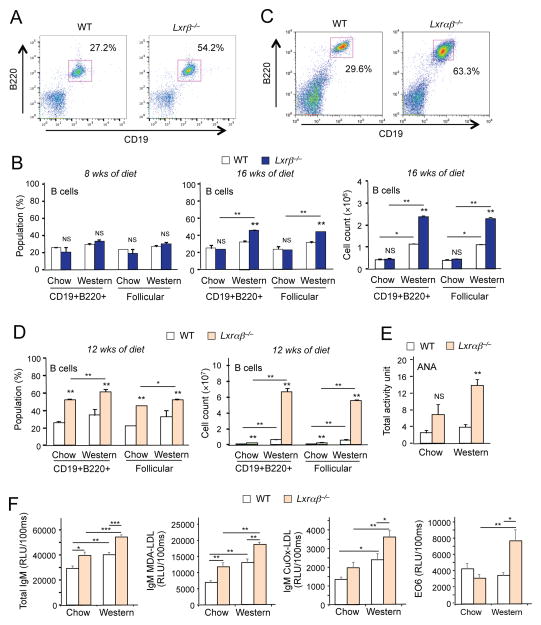
As an alternative strategy to perturb systemic cholesterol balance, we challenged Lxrβ −/− mice with Western diet (0.12% cholesterol) for 8 or 16 weeks. Although diet alone was not sufficient to induce immunoglobulin deposition in kidneys in Lxrβ−/− mice (data not shown), there was an increase in the frequency of B cells in lymph nodes compared to wild-type mice when fed Western diet for 16 weeks, and cell counts confirmed an absolute increase in B cell number (Figures 2A and 2B). We also observed an increase in the absolute number of CD11c+ B cells in lymph node this model (Fig. S2A); however, this increase paralleled the increase in total B cell number (c.f. Fig. 2B) and there was no increase in the relative abundance of these cells compared to other cell types in lymph node or spleen (Fig. S2B). These results suggest that chronic dietary cholesterol excess in the setting of LXRβ deficiency also leads to immune dysregulation and provokes B cell expansion.
Figure 2. Feeding a high cholesterol diet promotes autoimmunity.
(A) Flow cytometric analysis of CD19+B220+ B cells from lymph node of wild-type and Lxrβ−/− mice fed Western diet for 16 weeks. (B) Percentages and cell counts of the indicated cell populations in lymph node of wild-type and Lxrβ−/− mice fed chow or Western diet for 8 weeks or 16 weeks analyzed by flow cytometry. (C) Flow cytometric analysis of CD19+B220+ B cells from lymph node of wild-type and Lxrαβ−/− mice fed Western diet for 12 weeks. (D) Percentages and cell counts of the indicated cell populations in lymph node of wild-type and Lxrαβ−/− mice fed chow or Western diet for 12 weeks analyzed by flow cytometry. (E) Plasma samples from wild-type and Lxrαβ−/− mice fed chow and Western diet for 12 weeks analyzed for the titer of ANA by ELISA. (F) Plasma samples from wild-type and Lxrαβ−/− mice fed chow or Western diet for 12 weeks analyzed for the titers of total IgM, IgM against Cu-OxLDL, MDA-LDL and EO6 by ELISA. N=4–6 per group. Statistical analysis was performed with two-way ANOVA. *p < 0.05, **p < 0.01, NS, not significant. Error bars represent means +/− SEM. See also Figure S2.
To further perturb cholesterol homeostasis in the Western-diet fed model, we employed mice lacking both LXRα and LXRβ, which have an even more severe defect in cellular cholesterol efflux (Hong et al., 2012a; Tangirala et al., 2002). Lxrαβ−/− mice exhibited B cell expansion compared to wild-type controls even on chow diet, as indicated by increased percentage and absolute number of B cells (Figures 2C and 2D). Feeding Lxrαβ−/− mice Western diet greatly accelerated the production of circulating ANA (Figure 2E). We also assessed titers of total IgM as well as IgM-specific autoantibodies against oxidized phospholipids (EO6), copper-oxidized low-density lipoprotein (Cu-OxLDL) and malondialdehyde-modified low-density lipoprotein (MDA-LDL). With the exception of E06, titres of all of these antibodies were higher in Lxrαβ−/− mice than wild-type mice fed standard chow, and were further increased in response to Western diet feeding (Figure 2F). Although EO6 titres were not different between genotypes on chow diet, they were markedly induced in Western diet-fed Lxrαβ −/− mice. Collectively, the data of Figures 1 and 2 show that the development of autoimmune disease in mice is progressively exacerbated by both increasing systemic cholesterol delivery and by decreasing LXR pathway activity.
Cholesterol accumulation in lymphoid organs promotes the production of Baff and April
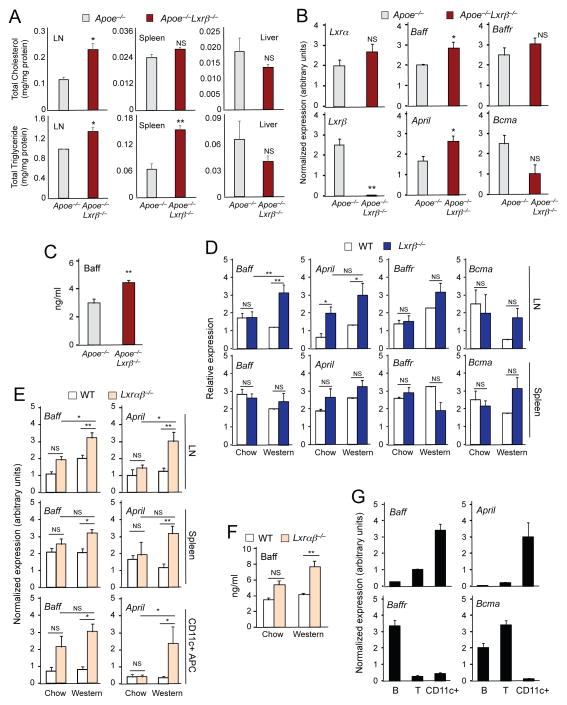
To further investigate whether the hypercholesterolemia associated with the ApoE−/− background or Western-diet feeding was a contributor to immune cell changes in LXR-deficient mice, we analyzed tissue lipid content in lymph node and spleen. Levels of cholesterol and triglyceride in the lymph nodes, and of triglyceride in the spleen were higher in Apoe−/−Lxrβ−/− mice compared to Apoe−/− controls, whereas there was no difference in lipid content in the liver between genotypes (Figure 3A).
Figure 3. Cholesterol accumulation in lymphoid organs promotes the production of Baff and April.
(A) Lipid was extracted from lymph node, spleen and liver of 8-week-old Apoe−/− and Apoe−/−Lxrβ−/− mice. Total masses of cholesterol and triglyceride were determined by colorimetric methods. N=3. (B) Gene expression in lymph node of 8-week-old Apoe−/− and Apoe−/−Lxrβ−/− mice analyzed by real-time PCR. (C) Plasma Baff concentration in 8-week-old ApoE−/− and Apoe−/−Lxrβ−/− mice determined by ELISA. (D) Gene expression in lymph node (upper) and spleen (bottom) of wild-type and Lxrβ−/− mice fed chow or Western diet for 16 weeks analyzed by real-time PCR. (E) Gene expression in lymph node (upper), spleen (middle) and CD11c+ antigen-presenting cells (APC) (bottom) of wild-type and Lxrαβ−/− mice fed chow or Western diet for 12 weeks analyzed by real-time PCR. (F) Plasma Baff concentration in wild-type and Lxrαβ−/− mice fed chow or Western diet for 12 weeks determined by ELISA. (G) Pan B cells, pan T cells and APC were isolated from spleen of wild-type mice. Gene expression was analyzed by real-time PCR. N=4–6 per group. Statistical analysis was performed with Student’s t test (A–C) and two-way ANOVA (D–F). *p < 0.05, **p < 0.01, NS, not significant. Error bars represent means +/− SEM.
We next asked whether the excess cholesterol accumulation in Apoe−/−Lxrβ −/− mice was associated with altered production of immune mediators. Baff and April are tumor necrosis family (TNF) ligand family members that are known to support the survival and differentiation of B cells and to play an important role in the development of autoimmune diseases (Mackay and Schneider, 2009). The mRNA expression levels of Baff and April in lymph nodes and the protein concentration of Baff in plasma were higher in Apoe−/−Lxrβ−/− mice compared to Apoe−/− controls (Figures 3B and 3C). By contrast, expression of the receptors for these mediators, Baffr and Bcma, was not different between the genotypes (Figure 3B). The expression of Baff and April was similar between wild-type and Lxrβ −/− mice fed a chow diet; however, Western diet resulted in significantly increased Baff and April in LXRβ-deficient lymph node (Figure 3D). No difference was seen in levels of mRNAs encoding the receptors Baff-R and Bcma in lymph node or in spleen between wild-type and Lxrβ−/− mice. Baff and April expression was also induced in lymph node, spleen and isolated CD11c+ APCs from Lxrαβ−/− mice in response to Western diet feeding (Figure 3E). Protein levels of Baff in plasma were correspondingly elevated in Western diet-fed Lxrαβ−/− mice (Figure 3F).
To determine the source of Baff and April production, we examined gene expression in isolated B cells, T cells and APCs. Expression levels of Baff and April were substantially higher in CD11c+ APCs compared to T cells or B cells, strongly suggesting that APCs were the primary source of these mediators in our model. By contrast, Baffr expression was restricted to B cells, and Bcma was restricted to B and T cells (Figures 3G and S3B). Together, these data suggest that cellular lipid accumulation, in this case due to the combination of hypercholesterolemia and impairment of LXR-dependent cholesterol efflux, induces the expression of Baff and April, and this is associated with the development of autoimmune-like pathology.
Loss of LXRβ expression in hematopoietic cells promotes the development of autoimmune disease
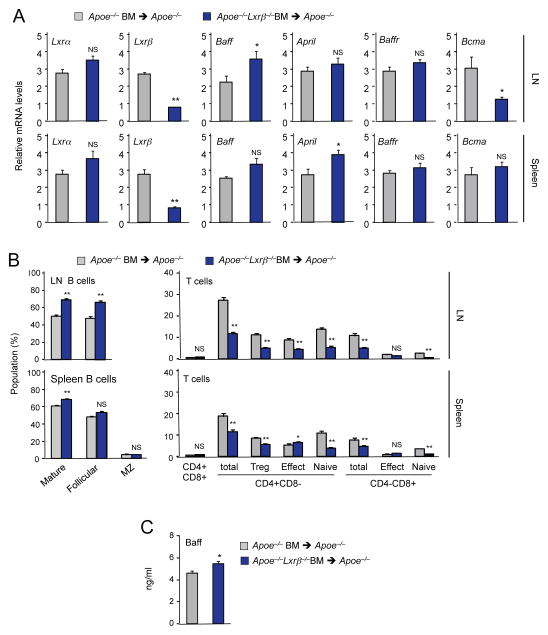
To elucidate if the development of autoimmune disease in Apoe−/−Lxrβ−/− mice was caused by a hematopoietic cell-intrinsic defect, we performed bone marrow transplants. Bone marrow cells from 8 week-old Apoe−/− or Apoe−/−Lxrβ−/− mice were transplanted into 8 week-old Apoe−/− recipient mice. Recipients were then maintained on standard chow diet for 12 weeks. Lxrβ gene expression was greatly reduced in lymph node and spleen from recipients of Apoe−/−Lxrβ −/− bone marrow compared to recipients of Apoe−/− bone marrow, whereas Lxrα expression was not different, confirming the efficacy of the transplant (Figure 4A). The frequency of B cells was higher, and the frequency of T cells was correspondingly lower, in lymph nodes and spleens of Apoe−/− mice transplanted with Apoe−/−Lxrβ−/− bone marrow cells, compared to those transplanted with Apoe−/− cells (Figure 4B). Expression of Baff transcripts in lymph node and of April transcripts as in spleen assessed by realtime PCR was also higher in ApoE−/− mice transplanted with Apoe−/−Lxrβ −/− bone marrow cells (Figure 4A). Although the difference in April expression in lymph node and Baff expression in spleen did not reach statistical significance, these levels trended higher in ApoE−/− mice transplanted with Apoe−/−Lxrβ−/− bone marrow cells. Plasma Baff levels were also higher in Apoe−/− mice transplanted with Apoe−/−Lxrβ−/− bone marrow cells (Figure 4C). These results indicate that lack of LXRβ expression in hematopoietic cells leads to B-cell hyperplasia in the setting of ApoE deficiency.
Figure 4. Loss of LXRβ expression in hematopoietic cells promotes the development of autoimmune disease.
Bone marrow cells from Apoe−/− and Apoe−/−Lxrβ−/− mice were transplanted into Apoe−/− mice. Mice were analyzed 12 weeks after the transplantation. (A) Gene expression in from lymph node and spleen of Apoe−/− mice transplanted with bone marrow cells from Apoe−/− or Apoe−/− Lxrβ−/− mice analyzed by real-time PCR. (B) Percentages of indicated cell populations in lymph node and spleen of Apoe−/− mice transplanted with bone marrow cells from Apoe−/− or Apoe−/−Lxrβ−/− mice analyzed by flow cytometry. (C) Plasma Baff concentration in Apoe−/− mice transplanted with bone marrow cells from Apoe−/− or Apoe−/−Lxrβ−/− mice determined by ELISA. N=6 per group. Statistical analysis was performed with Student’s t test. *p < 0.05, **p < 0.01, NS, not significant. Error bars represent means +/− SEM.
Susceptibility to autoimmune disease in LXRβ-deficient mice is not due to lymphocyte-intrinsic effects
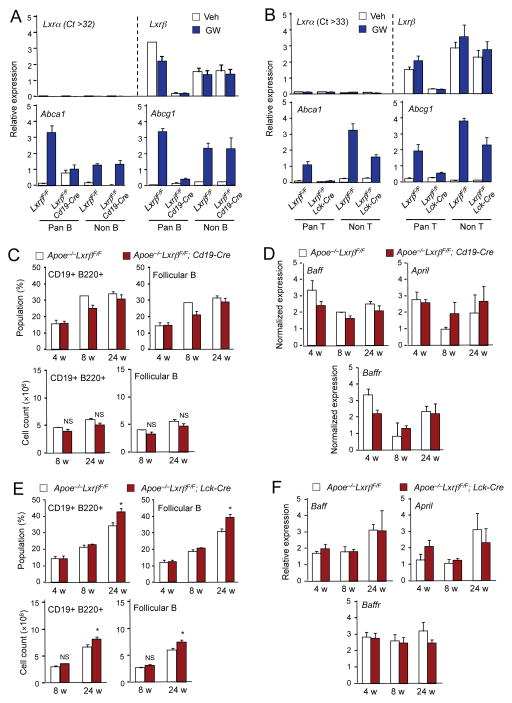
To further clarify the cell types driving the development of autoimmune disease in the absence of LXRβ signaling, Lxrβfl/fl mice (Figure S3A) were crossed with either Cd19-Cre transgenic mice to create B cell-specific LXRβ-deficient (Lxrβfl/fl; Cd19-Cre) mice, or with Lck-Cre transgenic mice to create T cell-specific LXRβ-deficient (Lxrβfl/fl; Lck-Cre) mice. Expression of Lxrβ was markedly reduced in pan-B cells from Lxrβfl/fl; Cd19-Cre mice, but was preserved in all other cells of hematopoietic origin (CD4+, CD8a+, CD11b+, CD43+, CD49b+, CD90.2+, Ly-6C/G+, and TER119+) (Figures 5A and S3B). Lxrα was barely detectable in B cells and its expression was not affected by Lxrβ deletion. Moreover, expression of the LXR target genes Abca1 and Abcg1 was induced by LXR agonist GW3965 in wild-type B cells but not in B cells from Lxrβfl/fl; Cd19-Cre mice (Figure 5A). Similarly, expression of Lxrβ was lost and the induction of Abca1 and Abcg1 in response to LXR agonist was abolished in pan-T cells from Lxrβfl/fl; Lck-Cre mice (Figures 5B and S3B).
Figure 5. Loss of LXRβ in lymphocyte does not affect the development of autoimmune disease.
(A) Pan-B cells and non-B cells were isolated from spleen of LxrβF/F (F/F) and LxrβF/F; CD19-Cre (B-KO) mice. Cells were treated with GW3965 (1μM) overnight. Gene expression was analyzed by real-time PCR. (B) Pan-T cells and non-T cells were isolated from spleen of LxrβF/F (F/F) and LxrβF/F; LCK-Cre (T-KO) mice. Cells were treated with GW 3965 (1μM) overnight. Gene expression was analyzed by real-time PCR. (C) Percentages and cell counts of the indicated cell populations in lymph node of Apoe−/−LxrβF/F and ApoE−/−LxrβF/F; CD19-Cre mice analyzed by flow cytometry. (D) Gene expression in from lymph node of Apoe−/−LxrβF/Fand Apoe−/−LxrβF/F; CD19-Cre mice analyzed by real-time PCR. (E) Percentages and cell counts of the indicated cell populations in lymph node of Apoe−/−LxrβF/Fand Apoe−/−LxrβF/F; Lck-Cre mice analyzed by flow cytometry. (F) Gene expression in lymph node from Apoe−/−LxrβF/Fand Apoe−/−LxrβF/F; Lck-Cre mice analyzed by real-time PCR. N=4–5 per group. Statistical analysis was performed with Student’s t test. *p < 0.05, NS, not significant. Error bars represent means +/− SEM. See also Figures S3 and S4.
We then crossed Lxrβfl/fl; Cd19-Cre mice, Lxrβfl/fl; Lck-Cre mice, and their respective controls with Apoe−/− mice to determine whether LXRβ expression specifically in B cells or T cells was critical for the development of autoimmune disease. Surprisingly, the frequency and absolute number of B cells, as well as the expression of Baff and April was not affected by LXRβ deletion in B cells (Figures 5C, 5D and S4A, S4B). Although there was a slight increase in the B cell population in the setting of T-cell specific LXRβ deletion at 24 weeks of age (Figure 5E), this effect was weaker and occurred much later than the expansion observed in global Apoe−/−Lxrβ −/− mice. Moreover, there was no difference in Baff and April gene expression in Apoe−/− mice lacking LXRβ in T cells (Figures 5F and S4C, S4D). Collectively, these results suggest that the action of LXRβ in B cells and T cells is not the primary determinant of susceptibility to autoimmune disease in our model.
Altered antigen presentation drives autoimmune disease in LXR-deficient mice
Changes in the function of antigen presenting cells have been linked to autoimmunity (Ganguly et al., 2013). To examine if lipid accumulation was associated with changes in the differentiation or activation of dendritic cells in vitro, bone marrow cells from Lxrαβ−/− and control mice were differentiated into dendritic cells with GM-CSF for 8 days. The CD11c+MHC class IIhi population was slightly enriched in Lxrαβ−/− cells after differentiation, but this difference did not reach significance (Figure S5A). TLR4 stimulation by LPS for 24 hours after differentiation induced comparable dendritic maturation in both groups, as revealed by a shift of the population from CD11c+MHC class IImid to CD11c+MHC class IIhi and the induction of CD86 expression (Figure S5A,B). Thus, the difference in dendritic cell population in lymph node and spleen of Lxrαβ−/− mice in response to western diet did not correlate with altered differentiation or maturation of bone marrow cells into dendritic cells in vitro.
We hypothesized that cellular lipid accumulation in the setting of hyperlipidemia and LXR deficiency might alter APC function and predispose to autoimmunity. To test this idea, we fed control and Lxrαβ−/− mice Western diet for 12 weeks and analyzed CD11c+MHC class II+ number and function. Lxrαβ−/− mice had more CD11c+MHC class II+ cells in both lymph node and spleen (Figure S6A). Moreover, CD11c+MHC class II+ cells in lymph node and spleen from Lxrαβ−/− mice showed marked lipid accumulation compared to those from wild-type controls fed similar diet (Figure 6A). The majority of the cholesterol in these lipid-loaded cells was esterified and there was no difference in the percentage of free versus esterified cholesterol between genotypes (Figure S6B). There was no difference in the frequency of CD11c+MHC class II+ cells or intracellular lipid content between the genotypes on chow diet (Figures S5C, S6D).
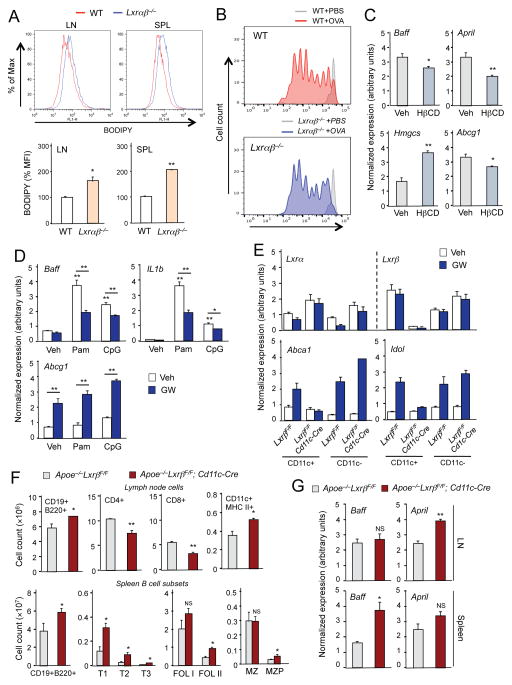
Figure 6. Altered antigen-presenting cell function in LXR-deficient mice.
(A) Lipid content in CD11c+ APCs was analyzed by staining cells from lymph node and spleen of wild-type and Lxrαβ−/− mice fed Western diet for 12 weeks with BODIPY. MFI in CD11c+ APC population was determined by flow cytometry. (B) CFSE dilution of adoptively-transferred OT-1 T cells from lymph node and spleen of wild-type and Lxrαβ−/− mice fed Western diet for 16 weeks. On day 3 after challenge with ovalbumin (200 μg/mouse), CFSE-labeled OT-1 T cells were assessed by flow cytometry gating on CD8+TCR Va2+. (C) Bone marrow-derived dendritic cells were incubated with hydroxypropyl-β-cyclodextrin (HβCD, 10 mM) for 1 hr. Gene expression was analyzed by real-time PCR. (D) Bone marrow–derived dendritic cells were pretreated with GW3965 (1 μM) overnight, followed by stimulation with Pam3CSK4 (100 ng/ml) or CpG (1 μM) for 24 hr. Gene expression was analyzed by real-time PCR. (E) CD11c+ and CD11c− cells were isolated from spleen of Apoe−/−LxrβF/F and Apoe−/−LxrβF/F; Cd11c-Cre mice. Cells were treated with GW3965 (1μM) overnight. Gene expression was analyzed by real-time PCR. (F) Cell counts of the indicated cell populations in lymph node of ApoE−/−LxrβF/F and −/−LxrβF/F; Cd11c-Cre mice at 8 weeks of age analyzed by flow cytometry. (G) Gene expression in lymph node and spleen of Apoe−/−LxrβF/F and Apoe−/−LxrβF/F; CD11c-Cre mice analyzed by real-time PCR. N=4–6 per group. Statistical analysis was performed with Student’s t test (A, C, F, G) and two-way ANOVA (D). *p < 0.05, **p < 0.01. Error bars represent means +/− SEM. See also Figures S5 and S6.
To examine the ability to present antigen and to stimulate T cell proliferation in vivo, we adoptively transferred CFSE-labeled CD8+ T cells from OT-1 transgenic mice into Lxrαβ−/− or wild-type mice fed Western diet for 16 weeks. One day later, the mice were challenged with ovalbumin and 3 additional days later, we assessed the dilution of CFSE in donor CD8+ TCR Vα2 T cells from the recipient mice (Figure S5C). Although T cells proliferated in response to OVA stimulation in both genotypes, this response was more robust in Lxrαβ−/− mice compared to wild-type mice. The population of cells failing to respond to antigen (G0 peak at right) was much higher in wild-type mice than in Lxrαβ−/− mice. These findings demonstrate that Western diet-fed Lxrαβ−/− mice have enhanced ability to present antigen and to prime T cells (Figure 6B).
We next evaluated the effect of diet and cholesterol content on Baff and April expression in CD11c+ APCs. Bone marrow cells from WT mice were differentiated to dendritic cells and treated with hydroxyl beta cyclodextrin (HβCD) to forcibly extract cholesterol from cellular membranes. As expected, we found increased expression of the SREBP2 target gene Hmgcs and increased expression of the LXR target Abcg1 in response to HβCD, confirming that cellular cholesterol content was reduced (Fig 6C). Furthermore, loss of cellular cholesterol reduced expression of both Baff and April. Thus, expression of Baff and April is responsive to cellular sterol status. In line with these findings, Western diet feeding of Lxrαβ−/− mice provoked expression of Baff and April in CD11c+MHC class II+ cells in vivo (Fig. 3E, bottom). We also found that treatment of bone marrow-derived dendritic cells with the synthetic LXR agonist GW3965 blunted the induction of Baff expression in response to TLR activation, consistent with the idea that LXR activity limits Baff expression in the setting of inflammatory stimuli (Fig. 6D).
To directly assess the role of CD11c+ antigen-presenting cells in the development of autoimmune-related pathology in LXR-deficient mice, we generated mice lacking LXRβ in CD11c+ cells by crossing Apoe−/− Lxrβfl/fl mice with Cd11c-Cre transgenics. Interestingly, although both LXRα and LXRβ are highly expressed in activated macrophages in vitro, we found that resident CD11c+ cells isolated from mouse spleen expressed higher levels of LXRβ compared to LXRα (Fig. 6E). Consistent with this observation, splenic APCs isolated from Apoe−/−Lxrβfl/fl; Cd11c-Cre mice failed to induce expression of Abca1 or Idol in response to GW3965 treatment (Fig. 6E). Moreover, these mice developed all of the autoimmune-related phenotypes observed in the global Apoe−/−Lxrβ−/− mice, including B-cell hyperplasia and elevated Baff and April expression (Fig. 6F,G). These data strongly suggest that one or more population of CD11c+cells are a major driver of systemic immune dysfunction in the setting of hypercholesterolemia.
ApoA-I expression ameliorates autoimmune disease in ApoE−/−Lxrβ−/− mice
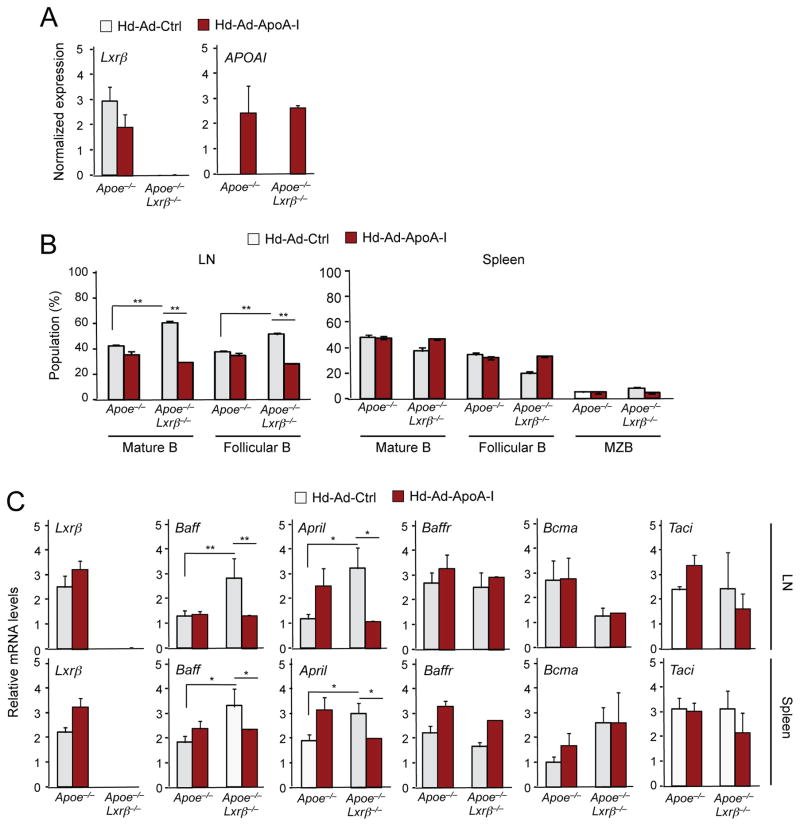
To test directly the causal relationship between cellular lipid accumulation and the development of autoimmune disease, we asked whether promoting cholesterol efflux through systemic administration of ApoA-I, the primary protein constituent of HDL, would ameliorate the immune dysfunction provoked by ApoE and LXRβ deficiency. Prior studies have reported that plasma ApoA-I levels were increased and maintained for 12 weeks after one-time transduction with helper-dependent adenovirus expressing ApoA-I (Belalcazar et al., 2003; Wilhelm et al., 2009). We injected Apoe−/−Lxrβ−/− mice and control Apoe−/− mice with helper-dependent adenovirus expressing human ApoA-I (Hd-Ad-ApoA-I) or control virus (Hd-Ad-Ctrl) for 12 weeks starting at 6 weeks of age. Consistent with prior reports, we detected human APOAI gene expression in liver from Hd-Ad-ApoA-I-treated animals 12 weeks after injection (Figure 7A). Lxrβ expression was absent in liver from Apoe−/−Lxrβ−/− mice as expected (Figure 7A). Remarkably, the expansion of the B cell population observed in ApoE−/−Lxrβ−/− mice was completely reversed by ApoA-I treatment (Figure 7B). ApoA-I treatment did not affect the B cell population in ApoE−/−mice (Figure 7B), indicating that the effects of ApoA-I were specific to the setting of LXRβ deficiency. In line with the effect on B cell numbers, the increased expression of Baff and April expression in lymph node and spleen ApoE−/− Lxrβ−/− mice compared to Apoe−/− controls was also suppressed by ApoA-I treatment (Figure 7C). A similar effect was observed on plasma Baff protein concentrations (Figure 7D). Furthermore, the elevated plasma levels of ANA in Apoe−/−Lxrβ−/− mice were also abrogated by ApoA-I (Figure 7D). We observed no difference in the number of CD11c+ B cells in lymph node or spleen between genotypes in this experiment, either in the presence or absence of ApoA-I (Fig. S7). These observations suggest that peripheral cholesterol accumulation per se, rather than some other consequence of ApoE and LXRβ deficiency, is the primary driver of autoimmune disease in Apoe−/− Lxrβ−/− mice.
Figure 7. ApoA-I expression ameliorates autoimmune disease in mice deficient in ApoE and LXRβ.
6 week-old Apoe−/− and Apoe−/−Lxrβ−/− mice were injected with Hd-Ad-control or Hd-Ad-ApoA-I. The mice were analyzed on 12 weeks after the injection. (A) Gene expression in liver of Apoe−/− and Apoe−/−Lxrβ−/− mice injected Hd-Ad-control or Hd-Ad-ApoA-I. (B) Percentages of indicated cell populations in lymph node and spleen of Apoe−/− and Apoe−/− Lxrβ−/− mice injected Hd-Ad-control or Ad-ApoA-I were analyzed by flow cytometry. (C) Gene expression in lymph node and spleen of Apoe−/− and Apoe−/−Lxrβ−/− mice injected with Hd-Ad-control or Ad-ApoA-I was analyzed by real-time PCR. (D) Plasma samples from Apoe−/− and Apoe−/−Lxrβ−/− mice injected with Hd-Ad-control or Ad-ApoA-I were analyzed for the presence of Baff (upper) and ANA (bottom) by ELISA. N=4–6 per group. Statistical analysis was performed with two-way ANOVA. *p < 0.05, **p < 0.01. Error bars represent means +/− SEM. See also Figure S7.
Discussion
Loss of both LXRα and LXRβ causes age-dependent lupus-like disease in mice (A-Gonzalez et al., 2009). However, the role of cholesterol metabolism per se in the development of autoimmunity is unclear. In this study we demonstrated that, in combination with lipid overload, modest impairment of cholesterol efflux through deletion of the LXRβ alone was sufficient to induce the development of overt autoimmune disease. We further showed that CD11c+ cells, which include several cell type specialized to present antigen) are the principle drivers of autoimmune-related pathology and that cholesterol loading of these cells in vivo promotes the expression of Baff and April, critical factors in B cell survival and expansion. The emergence of these phenotypes could be prevented by accelerating reverse cholesterol transport through sustained expression of ApoA-I. These observations reveal roles for LXR signaling in APCs and outline pathways for pathophysiological crosstalk between cholesterol metabolism and immune responses.
B cells play fundamental roles in the development of autoimmune diseases. B cell-depletion strategies have shown promise as therapy for various autoimmune disorders. Baff (also known as BLys or TNFSF13B) and April (also known as TNFSF13A) are TNF-superfamily ligands that promote B-cell proliferation and survival (Mackay and Schneider, 2009). Baff-deficient mice have considerable reductions in mature B cell populations and total serum immunoglobulins (Schiemann et al., 2001). Conversely, Baff-transgenic mice show increases in mature B cell numbers and serum immunoglobulins, and develop systemic lupus-like disease (Khare et al., 2000; Mackay et al., 1999). In this work, we found that B cell hyperplasia in hyperlipidemic LXRβ-deficient mice was associated with increased production of Baff and April in lymphoid organs and that CD11c+MHC class II+ cells were the primary source of these factors. However, a causal role for these factors is not established by the present study, and further work will be needed to directly test their contribution to the phenotypes of LXR-deficient mice. It would be of interest to know if blocking the induction of Baff and April would affect the development of autoimmunity in Apoe−/−Lxrβ−/− mice.
We recently reported that regulation of cholesterol content in lipid raft by LXRs affects NFκB-dependent inflammatory gene expression by controlling the recruitment of MyD88 and TRAF6 (Ito et al., 2015). An array of NFκB-dependent genes are upregulated in LXR-deficient cells due to loss of ABCA1-dependent cholesterol efflux. We found that accumulation of cholesterol in lipid rafts enhances the recruitment of MyD88 and TRAF6 and promotes downstream TLR signaling. Given that TLRs are highly expressed in antigen-presenting cells and both Baff and April are known NFκB-dependent genes (Fu et al., 2006; Tong et al., 2016), increased accumulation of cholesterol in lipid rafts in antigen-presenting cells is likely to be the underlying mechanism of Baff and April induction in LXR-deficient mice. In support of this hypothesis, activation of LXR with synthetic agonist blunted the induction of Baff in response to TLR activation.
We also established that cholesterol loading of antigen-presenting cells in vivo is a stimulus for Baff and April production. We found that accumulation of intracellular cholesterol in response to feeding a high-cholesterol diet enhanced the expression of Baff and April in APCs. Conversely, reducing intracellular cholesterol by treatment with HβCD in vitro, or promoting ApoA-I-dependent cholesterol efflux in vivo, reduced the levels of Baff and April. Finally, loss of LXRβ expression specifically in CD11c+ cells in vivo was sufficient to promote Baff and April expression and B-cell expansion on an ApoE-deficient background.
Our data also implicate dysfunctional antigen presentation and T-cell priming in LXR-null mice as another mechanism underlying their autoimmune-related symptomology. Analysis of T cell proliferation showed that antigen presentation was enhanced in the setting of hypercholesterolemia and cellular cholesterol accumulation. This finding is consistent with previous reports that pharmacologic LXR activation inhibits T cell priming (Geyeregger et al., 2007; Torocsik et al., 2010). HDL and ApoA-I treatment has also been reported to disrupt lipid rafts and attenuate MHC class II localization to rafts in antigen presenting cells (Wang et al., 2012). It is also possible that altered APC numbers contribute to disease development in LXR-deficient mice. LXR-deficient mice had expanded CD11c+MHC class II+ populations and this was exacerbated by Western-diet feeding. Mice lacking the LXR-target genes Abca1 and Abcg1, which promote cholesterol efflux, have a dramatic expansion of hematopoietic stem cells in their bone marrow (Westerterp et al., 2012; Yvan-Charvet et al., 2010). Effects of reduced Abca1 and Abcg1 expression on hematopoiesis may also contribute to the CD11c+MHC class II+ expansion in LXR-deficient mice.
Lymphocyte proliferation is enhanced by cellular cholesterol accumulation and LXRβ is an intrinsic regulator of lymphocyte proliferation (Bensinger et al., 2008). However, in contrast to CD11c-selective knockouts, deletion of LXRβ in B cells or T cells in combination with ApoE deficiency did not have a dominant effect on autoimmune responses. T cell-specific LXRβ-deficient mice on an ApoE-null background did show an expansion in the B cell population as the mice age, suggesting that a T cell-intrinsic effect of LXRβ may also contribute to B cell expansion.
Recent reports have shown that CD11c+ age/autoimmune-associated B cells appear in spleen during the development of autoimmune disease (Rubstov et al., 2011; Naradikian et al., 2016). Although splenic CD11c+ B cells were not consistently altered in the various cholesterol-overloaded mouse models used here, we did note an expansion of this population specifically in lymph node in the setting of western diet feeding. The fact that deletion of LXR in all B cells did not recapitulate the phenotype of global LXR deficiency argues against autoimmune B cells being the primary driver of disease in our models. However, since our CD11c-Cre driver deletes in this B cell population as well as in CD11c+MHC class II+ cells, we cannot exclude the possibility that combined loss of LXRβ in both APCs and CD11c+ B cells may contribute to the development of cholesterol-induced autoimmunity.
A major physiological function of HDL is to accept excess cholesterol effluxed from cells in peripheral tissues and subsequently transport it to the liver for excretion. This reverse cholesterol transport pathway is critical for the maintenance of systemic cholesterol homeostasis. Prior studies have shown that LXRs are the major transcriptional regulators of a battery of genes involved in reverse cholesterol transport. Inactivation of LXRs in mice impairs the efflux of cholesterol from macrophages and other cells, and causes whole body cholesterol overload (Bradley et al., 2007; Hong et al., 2012a; Tangirala et al., 2002). ApoA-I is the principal protein component of HDL. Previous work has shown that reverse cholesterol transport can be enhanced therapeutically through exogenous administration of ApoA-I (Smith, 2010). Overexpression of ApoA-I is even sufficient to counter cellular cholesterol accumulation in the setting of combined deficiency in the key cholesterol efflux transporters Abca1 and Abcg1 (Yvan-Charvet et al., 2010), or in combined deficiency in LDL receptor and ApoA1 (Wilhelm et al., 2009). The observation that the development of autoantibodies were prevented by sustained ApoA-I treatment in our studies directly implicates cellular lipid loading per se in the development of B cell hyperplasia and autoimmunity. While LXR and ApoE deficiency may have other physiological consequences besides promoting cellular lipid accumulation, one would not expect such effects to be countered by the cholesterol acceptor protein ApoA-I.
Finally, it is interesting to note that several reports have documented pro-inflammatory HDL in patients with autoimmune disease, and this HDL is less effective at promoting cholesterol efflux (Charles-Schoeman et al., 2012; McMahon et al., 2006; Ronda et al., 2014). Dysfunctional HDL may be a consequence of systemic inflammation (Chait et al., 2005) and this vicious cycle could promote lipid accumulation in CD11c+ cells and worsen autoimmune disease. Cholesterol lowering statin drug have been reported to have immunomodulatory properties and to attenuate autoimmune disease in animals and in some clinical trials (Fessler, 2015; Greenwood et al., 2006). Additional studies are needed to better define the links between altered reverse cholesterol transport, immune cell lipid accumulation, and the development of autoimmune disease. Given the ability of ApoA-I administration to ameliorate disease development in our mouse models, further exploration of the potential therapeutic utility of promoting cellular cholesterol efflux in the setting of human autoimmunity is warranted.
Experimental Procedures
Animal Studies
Lxrα−/−, Lxrβ−/−, and Lxrαβ−/− mice (C57Bl/6, greater than 10 generations backcrossed) were provided by David Mangelsdorf and bred with C57Bl/6 Apoe−/− mice from the Jackson Laboratory (Zhang et al., 1992). Lxrβfl/fl mice were generated by the Mouse Clinical Institute – Institut Clinique de la Souris (ICS) and generously provided by Johan Auwerx (Figure S3A). They were bred with CD19-Cre, LCK-Cre or CD11c-Cre mice from Jackson Laboratory. For bone marrow transplantation studies, recipient Apoe−/− mice (8 weeks of age) were lethally irradiated with 900 rads and transplanted with 3 × 106 bone marrow cells from 8-week-old donors (Apoe−/− or Apoe−/−Lxrβ−/−) via tail vein injection as previously described (Tangirala et al., 2002). Mice were maintained on standard chow diet for 12 weeks after bone marrow transplantation. For perturbation of systemic cholesterol, mice were fed either standard chow or Western diet (21% fat, 0.21% cholesterol: D12079B; Research Diets, Inc.) for indicated time. For long-term overexpression of ApoA1, 6 week-old Apoe−/− and Apoe−/−LXRβ−/− mice were injected via tail vein with HD-Ad-ApoA1 (4.5×1012 particles/kg) or equivalent amount of control HD-Ad (Belalcazar et al., 2003). OT-I transgenic mice were obtained from Jackson Laboratory. All mice were maintained on a 12-hour light/dark cycle and had access to food and water ad libitum. Animal studies were performed in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the University of California, Los Angeles Animal Research Committee guidelines.
Cell Preparation
Spleen and lymph node were digested with 1mg/ml Collagenase D (Roche Diagnostics) and 40 U/ml DNase I (SIGMA) in HBSS at 37 °C for 45 min. Antigen-presenting cells were isolated using CD11c UltraPure microbeads and MidiMACS separator (Miltenyi Biotec). Pan B cells, Pan T and CD8+ T cells were isolated with EasySep mouse B cell isolation kit, mouse T cell isolation kit and mouse CD8+ T cell isolation kit, respectively (Stemcell Technologies) and cultured in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, 100 mg/ml streptomycin, 2 mM L-glutamine and 50 mM 2-ME. Dendritic cells were differentiated from bone marrow cells. Bone marrow cells were obtained from femurs and tibias and 2×106 cells were cultured RPMI 1640 supplemented with 10% FBS, 10% GM-CSF conditioned medium, 100 U/mL penicillin, 100 mg/ml streptomycin, 2 mM L-glutamine and 50 mM 2-ME in 10 cm petri dish for 7 days. For complete maturation, non-adherent cells were re-plated into new petri dish with 2 μg/ml LPS for 24 hours. For cellular cholesterol modification, bone marrow-derived dendritic cells were placed in DMEM containing 0.5% FBS, 5 μM simvastatin plus 100 μM mevalonic acid for 4 hr then incubated with hydroxypropyl-β-cyclodextrin (Trappsol) (10 mM) to deplete cholesterol.
Reagents and Flow Cytometry
Synthetic LXR ligand GW3965 was provided by T. Wilson (GlaxoSmithKline). Single cell suspension from peripheral skin-draining inguinal, brachial and axillary lymph nodes and spleens were incubated with anti-mouse CD16/32 antibody (BioLegend) to prevent nonspecific binding of antibodies and stained with fluorescence-conjugated antibodies in 0.1% BSA, 5mM EDTA in PBS. Following conjugated antibodies were used for the staining: B220 (RA3-6B2), CD3 (145-2C11), CD8 (53-6.7, BD), 7AAD (BD Biosciences), CD19 (6D5), IgM (RMM-1), CD21 (7E9), CD4 (GK1.5), TCR Vα2 (B20.1), IgD (11-26c.2a), (BioLegend), CD23 (B3B4), CD93 (AA4.1), MHC class II (M5) (eBioscience), CD11c (N418), CD86 (GL-1) (Tonbo Biosciences) and DAPI (Molecular Probes). After washing, cells were analyzed on FACSCalibur, FACSVerse or LSRII (BD bioscience) with FlowJo software (Treestar) as previously described (Allman and Pillai, 2008; Muppidi et al., 2011).
Histology
Tissues were collected and embedded in OCT compound (Tissue-Tek) and frozen in liquid nitrogen. 4 μm frozen sections were air-dried, fixed with 4% paraformaldehyde, blocked with 6% BSA and 2% preimmune serum in PBS, and stained with fluorescence-conjugated antibodies. Sections were mounted in the presence of ProLong Gold Antifade Reagent with DAPI (Invitrogen).
Gene Expression
Total RNA was isolated from cells and tissues with Trizol (Invitrogen), following the manufacturer’s instruction. 500ng of total RNA was used for cDNA synthesis and quantified by real-time PCR using SYBR Green (Diagenode) and ABI 7900 or QuantStudio 6 Flex. Gene expression levels were normalized to 36B4. Primer sequences are listed in Table 1.
Measurement of Antibody Titers and Baff
Total immunoglobulin titers were determined by flowcytometric assay using mouse Immunoglobulin Isoryping Panel 6 plex kit (eBioscience). Total IgM and specific antibody titers were determined by chemiluminescent enzyme immunoassays as previously described (Baldan et al., 2014). In brief, 96-well plate were coated with IgM (Sigma-Aldrich), copper oxidized low-density lipoprotein (Cu-OxLDL) and malondialdehyde-modified low-density lipoprotein (MDA-LDL) at 5 mg/ml in PBS overnight at 4°C. Plates were blocked with 1% BSA in TBS, and serially diluted antiserum from individual mice was added. Binding of the IgM antibodies was detected with alkaline phosphatase (AP) conjugated IgM (Sigma-Aldrich). Bound plasma IgM levels were assessed using Lumi-Phos 530 (Lumigen) and Dynex luminometer (Dynex Technologies). For the measurement of T15 clonotypic (EO6) antibodies, binding of T15 clonotypic IgM antibodies to wells coated with monoclonal anti-T15 isotypic antibody AB1-2 (ATCC) was detected by using biotinylated AB1-2 followed by AP-labeled NeutrAdvin. Plasma Baff levels were measured by ELISA kit (R&D System) following manufacturer’s protocol.
Tissue and cell lipid analysis
For tissue lipid measurement, lipids were extracted from tissues using the Folch method. Briefly, chloroform extracts were dried under nitrogen and re-solubilized in water. Cholesterol content was determined using a commercially available enzymatic kit (Sigma-Aldrich). For cellular lipid measurement, cells were stained with BODIPY (Molecular Probes) and analyzed by flow cytometry. Cellular free-/esterified-cholesterol was determined with Amplex Red cholesterol assay kit (Molecular Probes).
Antigen-presenting Cell Functional Assay
For analysis of antigen uptake, isolated CD11c+ antigen-presenting cells were incubated with 5 μg/ml OVA-Alexa Fluor 647 (Molecular Probes) at 37 °C for 15 min and analyzed MFI by flow cytometry. In vivo analysis of antigen presentation was done as described previously (Quah et al., 2007). Single cell suspensions of OT-I T cells were labeled with CFSE (carboxyfluorescein diacetate succinimidyl ester, Molecular Probes) according to manufacturer’s protocol. CFSE-labeled OT-I T cells were adoptively transferred into wildtype or Lxrαβ−/− mice through retro-orbital sinus, and then the mice were challenged with ovalbumin (200 μg/mouse) or PBS by intraperitoneal injection. Mice were sacrificed 3 days later and lymph node and spleen were harvested. Single cells were stained for the surface expression of CD8, TCR Vα2 and CD11c, incubated with DAPI, and analyzed by flow cytometry (Figure S6).
Statistical Analysis
Statistics were performed using two-way ANOVA with post hoc tests or Student’s t test to compare to the control group. P < 0.05 was considered statistically significant. Data was presented as means ± SEM.
Supplementary Material
Acknowledgments
We are grateful to Johan Auwerx and the Institut Clinique de la Souris for the LXRβ-floxed mice. We thank members of the Tontonoz laboratory for helpful discussion and technical advice. This work was supported by NIH grants HL030568, DK063491, and DK063491 to P.T., AI093768 to S.J.B., and Ministerio de Economia y Competitividad (MINECO) grant SAF2014-56819-R to A.C.
Footnotes
Author Contributions
A.I. Designed and performed experiments and wrote the manuscript, C.H. designed and performed experiments, K.O. designed experiments and synthesized essential reagents, J.V.S. performed experiments, C.D. performed experiments, J.L.W. designed experiments, M.D. performed experiments, A.C. designed and performed experiments, S.J.B. designed experiments, L.C. designed experiments and synthesized essential reagents, and P.T. designed experiments, supervised the study and wrote the manuscript.
Disclosures
JLW has patents and patent applications for the use of oxidation specific antibodies, which are held by the University of California, San Diego. None of the other authors has a financial interest related to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, Deniz J, Ramirez C, Diaz M, Gallardo G, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allman D, Pillai S. Peripheral B cell subsets. Curr Opin Immunol. 2008;20:149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldan A, Gonen A, Choung C, Que X, Marquart TJ, Hernandez I, Bjorkhem I, Ford DA, Witztum JL, Tarling EJ. ABCG1 is required for pulmonary B-1 B cell and natural antibody homeostasis. J Immunol. 2014;193:5637–5648. doi: 10.4049/jimmunol.1400606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, Beaudet A, Chan L. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, Shih R, Parks JS, Edwards PA, Jamieson BD, Tontonoz P. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MN, Hong C, Chen M, Joseph SB, Wilpitz DC, Wang X, Lusis AJ, Collins A, Hseuh WA, Collins JL, et al. Ligand activation of LXR beta reverses atherosclerosis and cellular cholesterol overload in mice lacking LXR alpha and apoE. J Clin Invest. 2007;117:2337–2346. doi: 10.1172/JCI31909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, Tontonoz P. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- Chait A, Han CY, Oram JF, Heinecke JW. Thematic review series: The immune system and atherogenesis. Lipoprotein-associated inflammatory proteins: markers or mediators of cardiovascular disease? J Lipid Res. 2005;46:389–403. doi: 10.1194/jlr.R400017-JLR200. [DOI] [PubMed] [Google Scholar]
- Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, Taylor M, McMahon M, Paulus HE, Reddy ST. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–1162. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fessler MB. Regulation of Adaptive Immunity in Health and Disease by Cholesterol Metabolism. Curr Allergy Asthma Rep. 2015;15:48. doi: 10.1007/s11882-015-0548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Lin-Lee YC, Pham LV, Tamayo A, Yoshimura L, Ford RJ. Constitutive NF-kappaB and NFAT activation leads to stimulation of the BLyS survival pathway in aggressive B-cell lymphomas. Blood. 2006;107:4540–4548. doi: 10.1182/blood-2005-10-4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly D, Haak S, Sisirak V, Reizis B. The role of dendritic cells in autoimmunity. Nat Rev Immunol. 2013;13:566–577. doi: 10.1038/nri3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Saemann MD, Zlabinger GJ, Maurer D, Stulnig TM. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- Ghisletti S, Huang W, Ogawa S, Pascual G, Lin ME, Willson TM, Rosenfeld MG, Glass CK. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol Cell. 2007;25:57–70. doi: 10.1016/j.molcel.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat Rev Immunol. 2010;10:365–376. doi: 10.1038/nri2748. [DOI] [PubMed] [Google Scholar]
- Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Bradley MN, Rong X, Wang X, Wagner A, Grijalva V, Castellani LW, Salazar J, Realegeno S, Boyadjian R, et al. LXRalpha is uniquely required for maximal reverse cholesterol transport and atheroprotection in ApoE-deficient mice. J Lipid Res. 2012a;53:1126–1133. doi: 10.1194/jlr.M022061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Kidani Y, NAG, Phung T, Ito A, Rong X, Ericson K, Mikkola H, Beaven SW, Miller LS, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest. 2012b;122:337–347. doi: 10.1172/JCI58393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–444. doi: 10.1038/nrd4280. [DOI] [PubMed] [Google Scholar]
- Ito A, Hong C, Rong X, Zhu X, Tarling EJ, Hedde PN, Gratton E, Parks J, Tontonoz P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife. 2015;4:e08009. doi: 10.7554/eLife.08009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O’Connell RM, Cheng G, Saez E, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309. doi: 10.1016/j.cell.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–219. doi: 10.1038/nm820. [DOI] [PubMed] [Google Scholar]
- Khare SD, Sarosi I, Xia XZ, McCabe S, Miner K, Solovyev I, Hawkins N, Kelley M, Chang D, Van G, et al. Severe B cell hyperplasia and autoimmune disease in TALL-1 transgenic mice. Proc Natl Acad Sci U S A. 2000;97:3370–3375. doi: 10.1073/pnas.050580697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidani Y, Bensinger SJ. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunol Rev. 2012;249:72–83. doi: 10.1111/j.1600-065X.2012.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon M, Grossman J, FitzGerald J, Dahlin-Lee E, Wallace DJ, Thong BY, Badsha H, Kalunian K, Charles C, Navab M, et al. Proinflammatory high-density lipoprotein as a biomarker for atherosclerosis in patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Rheum. 2006;54:2541–2549. doi: 10.1002/art.21976. [DOI] [PubMed] [Google Scholar]
- Muppidi JR, Arnon TI, Bronevetsky Y, Veerapen N, Tanaka M, Besra GS, Cyster JG. Cannabinoid receptor 2 positions and retains marginal zone B cells within the splenic marginal zone. J Exp Med. 2011;208:1941–1948. doi: 10.1084/jem.20111083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naradikian MS, Hao Y, Cancro MP. Age-associated B cells: key mediators of both protective and autoreactive humoral responses. Immunol Rev. 2016;269:118–29. doi: 10.1111/imr.12380. [DOI] [PubMed] [Google Scholar]
- Peet DJ, Turley SD, Ma W, Janowski BA, Lobaccaro JM, Hammer RE, Mangelsdorf DJ. Cholesterol and bile acid metabolism are impaired in mice lacking the nuclear oxysterol receptor LXR alpha. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Berge KE, Pomajzl C, Richardson JA, Hobbs H, Mangelsdorf DJ. Regulation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 by the liver X receptors alpha and beta. J Biol Chem. 2002;277:18793–18800. doi: 10.1074/jbc.M109927200. [DOI] [PubMed] [Google Scholar]
- Repa JJ, Turley SD, Lobaccaro JA, Medina J, Li L, Lustig K, Shan B, Heyman RA, Dietschy JM, Mangelsdorf DJ. Regulation of absorption and ABC1-mediated efflux of cholesterol by RXR heterodimers. Science. 2000;289:1524–1529. doi: 10.1126/science.289.5484.1524. [DOI] [PubMed] [Google Scholar]
- Ronda N, Favari E, Borghi MO, Ingegnoli F, Gerosa M, Chighizola C, Zimetti F, Adorni MP, Bernini F, Meroni PL. Impaired serum cholesterol efflux capacity in rheumatoid arthritis and systemic lupus erythematosus. Ann Rheum Dis. 2014;73:609–615. doi: 10.1136/annrheumdis-2012-202914. [DOI] [PubMed] [Google Scholar]
- Rong X, Albert CJ, Hong C, Duerr MA, Chamberlain BT, Tarling EJ, Ito A, Gao J, Wang B, Edwards PA, et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubtsov AV, Rubtsova K, Fischer A, Meehan RT, Gillis JZ, Kappler JW, Marrack P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c+ B-cell population is important for the development of autoimmunity. Blood. 2011;118:1305–15. doi: 10.1182/blood-2011-01-331462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- Smith JD. Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2010;11:989–996. [PMC free article] [PubMed] [Google Scholar]
- Spann NJ, Glass CK. Sterols and oxysterols in immune cell function. Nat Immunol. 2013;14:893–900. doi: 10.1038/ni.2681. [DOI] [PubMed] [Google Scholar]
- Tangirala RK, Bischoff ED, Joseph SB, Wagner BL, Walczak R, Laffitte BA, Daige CL, Thomas D, Heyman RA, Mangelsdorf DJ, et al. Identification of macrophage liver X receptors as inhibitors of atherosclerosis. Proc Natl Acad Sci U S A. 2002;99:11896–11901. doi: 10.1073/pnas.182199799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AJ, Liu X, Thomas BJ, Lissner MM, Baker MR, Senagolage MD, Allred AL, Barish GD, Smale ST. A Stringent Systems Approach Uncovers Gene-Specific Mechanisms Regulating Inflammation. Cell. 2016;165:165–179. doi: 10.1016/j.cell.2016.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torocsik D, Barath M, Benko S, Szeles L, Dezso B, Poliska S, Hegyi Z, Homolya L, Szatmari I, Lanyi A, Nagy L. Activation of liver X receptor sensitizes human dendritic cells to inflammatory stimuli. J Immunol. 2010;184:5456–5465. doi: 10.4049/jimmunol.0902399. [DOI] [PubMed] [Google Scholar]
- Wang B, Rong X, Duerr MA, Hermanson DJ, Hedde PN, Wong JS, de Aguiar Vallim TQ, Cravatt BF, Gratton E, Ford DA, Tontonoz P. Intestinal phospholipid remodeling is required for dietary-lipid uptake and survival on a high-fat diet. Cell Metab. 2016;23:492–504. doi: 10.1016/j.cmet.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Yuan SG, Peng DQ, Zhao SP. HDL and ApoA-I inhibit antigen presentation-mediated T cell activation by disrupting lipid rafts in antigen presenting cells. Atherosclerosis. 2012;225:105–114. doi: 10.1016/j.atherosclerosis.2012.07.029. [DOI] [PubMed] [Google Scholar]
- Westerterp M, Gourion-Arsiquaud S, Murphy AJ, Shih A, Cremers S, Levine RL, Tall AR, Yvan-Charvet L. Regulation of hematopoietic stem and progenitor cell mobilization by cholesterol efflux pathways. Cell Stem Cell. 2012;11:195–206. doi: 10.1016/j.stem.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm AJ, Zabalawi M, Grayson JM, Weant AE, Major AS, Owen J, Bharadwaj M, Walzem R, Chan L, Oka K, et al. Apolipoprotein A-I and its role in lymphocyte cholesterol homeostasis and autoimmunity. Arterioscler Thromb Vasc Biol. 2009;29:843–849. doi: 10.1161/ATVBAHA.108.183442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yvan-Charvet L, Pagler T, Gautier EL, Avagyan S, Siry RL, Han S, Welch CL, Wang N, Randolph GJ, Snoeck HW, Tall AR. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328:1689–1693. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.