Abstract
Over the last thirty years, the world has seen HIV circulate the globe, affecting 33 million people to date and killing 2 million people a year. The disease has affected developed and developing countries alike, and in the U.S., remains one of the top ten leading causes of death. Many regions of the world are highly impacted by this disease, including sub-Saharan Africa, South and South-East Asia, and Eastern Europe. Fortunately, multilateral, global efforts, along with successful developments in diagnostic tools and anti-retroviral drugs (ARVs) have successfully curbed the spread of HIV over the last ten years. In spite of this fact, access to HIV treatment and preventive healthcare is varying and limited in developing countries. A lack of healthcare infrastructure, financial support, and healthcare workers are some logistical factors that are responsible. HIV stigmatization, discrimination, and inadequate education pose additional social challenges that are hindering countries from advancing in HIV prevention. This review focuses on current technological tools that are used for HIV diagnosis and ongoing research that is aimed at addressing the conditions in low-resource settings. Recent developments in microfluidic applications and mobile health technologies are promising approaches to building a compact, portable, and robust device that can provide information-rich, real-time diagnoses. We also discuss the role that governments, healthcare workers, and even researchers can play in order to increase the acceptance of newly introduced devices and treatments in rural communities.
TOC image
II. Introduction
Human Immunodeficiency Virus (HIV) is a pandemic that currently threatens over 33.4 million lives worldwide [4]. Despite the widespread attention it has received over the last few decades, the illness still lacks a cure and in some cases even lacks appropriate diagnostic technologies. It is widely held that HIV had its origins in Africa, and was the result of an interspecies crossover event from other primates. Although the specifics of this event remain circumstantial at best, transmission of the disease amongst humans has been found to occur through the mixing of bodily fluids. This can result from sexual activity, blood contact via open wounds or syringes, or maternal perfusion of the virus to a fetus during pregnancy [6]. Once infected, the virus quickly attacks the host immune system, leaving the individual susceptible to contracting opportunistic infections that can otherwise be as harmless as the common cold.
Pathologically, the virus causes this primarily through its attack on CD4+ T-helper (Th) lymphocytes [6, 7], and more specifically, HIV-specific CD4+ cells [8]. Th lymphocytes come in two chief varieties: type-1 and type-2. Type 1 lymphocytes are responsible for protecting the host from viral infections. They do so by activating a cytotoxic T lymphocyte (CTL)-mediated response against viral particles. However, this response is thwarted by the destruction of the type-1 cells. Type-2 lymphocytes are responsible for protecting against infections that are neutralized by host antibodies. By destroying type-2 cells, HIV leaves the individual susceptible to all other infections that the body would otherwise be capable of responding to.
Following two to four weeks of incubation and active viral replication, CD4+ cell counts fall precipitously. HIV kills Th lymphocytes through two main mechanisms. The first is through cell lysis due to viral reproduction. The second and more common mechanism is through induced cell apoptosis. The incorporation of viral DNA into that of the host cell causes the production of transcription factors that upregulate killer signals and downregulate anti-apoptotic signals [10]. Countering the infection, the sharp drop in CD4+ cells activates an acute CD8+ host response that identifies and attacks infected cells. Antibodies eventually take over this role through seroconversion [6]. This immune response quickly decreases the viral load and stabilizes CD4+ cell counts. This process typically takes a month, and is characterized symptomatically by fever, sore throat, rash, and throat and esophageal sores [6]. Due to the vague nature of these symptoms, this acute HIV infection is often misdiagnosed as a simple flu or cold. Following the acute infection phase, the virus remains in a latent state for anywhere from two weeks to twenty years. During this time, the viral load steadily increases and CD4+ cell counts steadily decrease.
Once Th lymphocyte counts reach a critical level, the individual is unable to mount any cell-mediated immune response—a state more commonly described as Acquired Immunodeficiency Syndrome (AIDS). Clinically, this occurs at a CD4+ count below 200/μl of blood, which marks the threshold for antiretroviral drug therapy (ART) [10, 11]. This complete failure of the host immune system leaves the individual susceptible to any infection, and can make even the most harmless of infections opportunistic and life threatening. Augmenting this state further is the ability of HIV to induce apoptosis in non-infected immune cells such as macrophages and dendritic cells [6, 7, 10]. Thus, in addition to its ability to directly threaten Th lymphocytes, the virus can moreover utilizes a systemic communication mechanism to advance its destructive capabilities.
In 2008 alone, HIV transmission led to 2.7 million new cases and resulted in approximately 2.0 million AIDS-related deaths worldwide [4]. HIV/AIDS remains one of the top ten causes of death globally (Table 1). The timeline in Figure 1 shows the growth of the HIV pandemic over the past two decades: the prevalence of HIV has increased three-fold, and currently represents over a 20% increase over numbers reported in 2000 [4]; however, the advent of successful HIV diagnostics, drug therapies, and preventative public policies has helped curb the rate of infection and HIV-related deaths since its peak in 1996.
Table 1.
Leading causes of death in the world as of 2004. Data provided by the WHO.
| Rank | Cause | Deaths in Millions |
|---|---|---|
| 1 | Coronary heart disease | 7.20 |
| 2 | Stroke and other cerebrovascular diseases | 5.71 |
| 3 | Lower respiratory infections | 4.18 |
| 4 | Chronic obstructive pulmonary disease | 3.02 |
| 5 | Diarrheal diseases | 2.16 |
| 6 | HIV/AIDS | 2.04 |
| 7 | Tuberculosis | 1.46 |
| 8 | Trachea, bronchus, lung cancers | 1.32 |
| 9 | Road traffic accidents | 1.27 |
| 10 | Prematurity and low birth weight | 1.18 |
| Low income countries, 2004 | ||
| Rank | Cause | Deaths in Millions |
| 1 | Lower respiratory infections | 2.94 |
| 2 | Coronary heart disease | 2.47 |
| 3 | Diarrheal diseases | 1.81 |
| 4 | HIV/AIDS | 1.51 |
| 5 | Stroke and other cerebrovascular diseases | 1.48 |
| 6 | Chronic obstructive pulmonary disease | 0.94 |
| 7 | Tuberculosis | 0.91 |
| 8 | Neonatal infections | 0.90 |
| 9 | Malaria | 0.86 |
| 10 | Prematurity and low birth weight | 0.84 |
Figure 1.
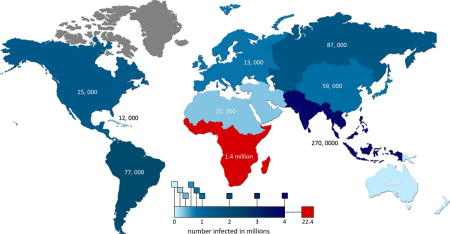
Map depicting the number of people infected globally by region (in color) along with the number of HIV/AIDS related deaths in 2008 superimposed.
In spite of this recent trend, the disease continues to spread rampantly throughout the world, particularly increasing in prevalence throughout Eastern Europe, Central and Southeast Asia, and sub-Saharan Africa. Africa itself accounts for approximately 67% of global infections, with Southern Africa housing 9 countries with some of the highest incidences of HIV in the world: 26% of adults in Swaziland, 24% in Botswana, 23.2% in Lesotho, and 5.7 million in South Africa—the largest population within any one country, globally [4]. The global HIV trend is summarized in Figure 1.
Reversing the epidemic is one of the goals of many developing countries, and it is the intent of the UN Millennium Project to achieve this by 2015. To this end, many countries have taken sociological steps to prevent the spread of HIV through appropriate sex education or abstinence advocacy programs; however, universal access to HIV diagnostics and treatments remains of indignant concern. In Kenya, it is estimated that 83% of the population remains undiagnosed [4]. Such a disgraceful statistic is not due to a lack of diagnostic technologies, but rather due to a lack of necessary technicians, patient motivation, and access in rural villages.
For adult diagnosis, common tests include CD4+ cell enumeration and HIV antibody tests [12, 13]; however, the problem with effective diagnosis of the adult population is largely organizational. Cost-effective diagnostic technologies are available, but the appropriate infrastructure and education to support large-scale HIV diagnosis programs is missing [2]. Even still, more robust technologies that allow in-field testing remain to be developed and would prove to be infinitely helpful for successful diagnoses.
For early infant diagnosis, reliable, cost-effective, and robust technologies simply do not exist. Available testing strategies for adults in developing regions are virtually impossible to use for diagnosing infants under the age of 18 months [14]. The only strategies available are extremely limited and time-consuming, delaying the speed with which infants may begin treatment. Tragically, those that live beyond 18 months with the disease are simply too advanced in their cases for effective treatment.
For those lucky enough to receive HIV treatment and care, CD4+ cell count and viral load assays are currently crucial tools that indicate the progression of HIV and the efficacy of highly active antiretroviral therapy (HAART) [15, 16]. Continual monitoring, four times a year, is necessary for the rest of the patient’s life [17]. These methods are time-intensive, require training, and only available to large clinics that have access to the necessary equipment [15]. Patients from rural areas who have little or no access to these monitoring technologies are at a disadvantage for their HIV treatments.
Several antiretroviral drugs (ARVs) are available to slow down the progression of HIV/AIDS and improve the prognosis of the individual, from an average of 11 years to 20 years [18–20]. HIV drug therapy usually consists of multiple ARVs that constitute HAART treatment. These drugs act in a variety of ways to prevent HIV replication and spreading. While effective, the drugs often cause nausea, vomiting, and other negative side effects. Irregularly taking these drugs or inappropriate dosages can cause drug resistance and drug toxicity [21]. Additional burdens on a person with HIV include lifestyle changes, financial burden, decreased productivity, and hindered social life. Successful HAART requires a lifetime commitment.
In this review, we focus on HIV diagnostic and monitoring technologies that address the unique circumstances of low-resource settings. We begin with a summary of current technologies in use and address the associated challenges and technological needs. Next, we discuss novel methods under ongoing development that address these needs. In particular, we focus on the use of microfluidics to both decrease the costs and increase the portability and robustness of the devices. We then focus on mobile health technology and how it can alleviate logistical challenges such as the lack of health workers and limited access to healthcare. We end with a discussion of the social and political challenges that significantly hamper the fight against preventative HIV, as well as various avenues that are available to counter them.
III. Current Diagnostic Tools
Table 2 summarizes the technologies currently in use for HIV diagnosis and viral load monitoring. In particular, the WHO has published a number of technologies that overcome difficulties in resource-poor settings [22–27]. These tests fall into two main categories: enzyme-linked immunosorbent assays (ELISAs) and Simple/Rapid assays (S/R). Here we discuss these conventional HIV technologies, their related drawbacks, as well as newer technologies that have been developed to overcome those challenges within a cost-effective and logistically compatible platform.
Table 2.
Summary of current types of techniques used for HIV diagnosis or monitoring. Values are approximate and based on literature searches.
| Available Diagnostics for HIV | Method | Current uses | Requires extensive technical training? | Requires expensive, bulky equipment? | High through-put? | Average Cost | Operation Time | Suitable for low-resource settings? |
|---|---|---|---|---|---|---|---|---|
| Antigen/Antibody ELISAs | Detects antibodies to HIV or HIV antigens | Confirmatory diagnosis for HIV in adults | ✓ | ✓ | ✓ | $0.60–1.50/test | ~2 hours | × |
| Viral Nucleic Acid Amplification (i.e. RT-PCR or PCR) | Detects and quantifies viral RNA or DNA | Confirmatory diagnosis for HIV Monitoring of HIV/AIDS disease state and efficacy of ART |
✓ | ✓ | ✓ | $12/test | ~ hours | × |
| Flow Cytometry | Quantifies CD4+ cells | Monitoring of HIV/AIDS disease state and efficacy of ART | ✓ | ✓ | × | $50/test | ~ 1 hour | × |
| Lateral Flow Diagnostics (Immuno-chromatographic) | Detects antibodies to HIV or HIV antigens | Screening tool for HIV in adults only | × | × | × | $1–3/test | seconds to minutes | ✓ |
| Western Blots | Detects antibodies to HIV | Confirmatory diagnosis for HIV in adults | ✓ | ✓ | × | $20–30/test | ~ hours | × |
Flow Cytometry
Among the numerous technologies used to diagnose HIV/AIDS, CD4+ cell enumeration through flow cytometry is among the oldest. Flow cytometry analyzes physical and chemical characteristics of particles in a fluid flow and has been a useful tool in clinical diagnostics and biological analysis for decades [29–33]. A basic flow cytometer involves a narrow fluid flow of single file particles interrogated by a laser. This either excites fluorescent dyes or is used to measure light scattering dynamics. A simple schematic of this arrangement is illustrated in Figure 3a.
Figure 3.
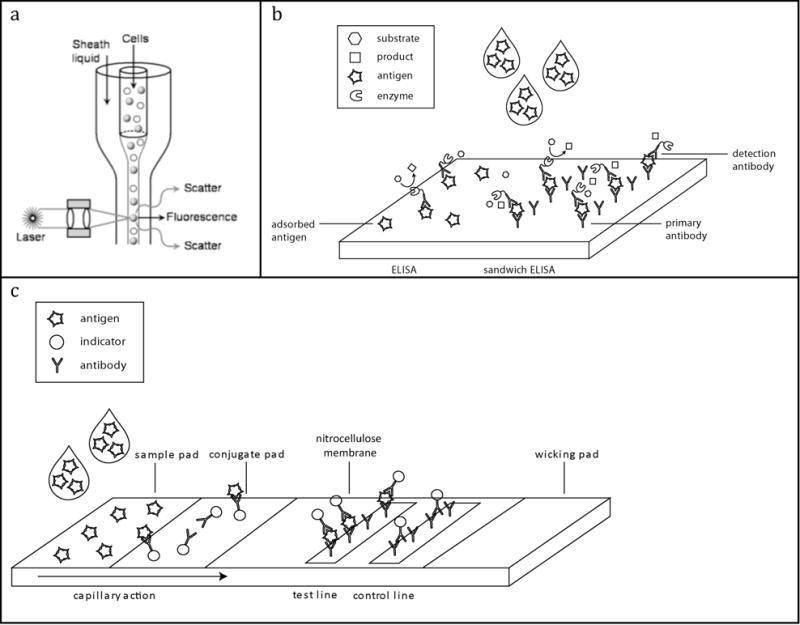
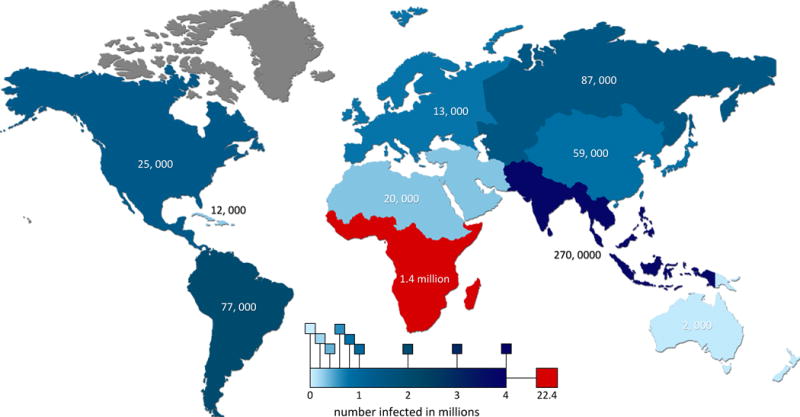
(a) Cells are injected into the core of a sheath flow and confined to a narrow single-file stream by hydrodynamic focusing. As fluorescent labeled cells flow past a focused laser beam, they generate light scattering and fluorescence emission measured by optics and electronics. Reprinted with the permission of Reference 28 and IOP Publishing Ltd. (b) Schematic of regular and sandwich ELISA assays. Antigen in regular ELISA nonspecifically binds to substrate while those in sandwich ELISA are immobilized by primary antibodies. (c) Schematic of lateral flow assay. Sample is applied to the sample pad and travels up toward the wicking pad via capillary action. Antigens present in the sample are bound by indicator-conjugated antibodies in the conjugate pad. Test and control lines bind to antigen-indicator antibody conjugates as they flow past.
Clinically, CD4+ counts have been found to be a much more reliable indicator for the onset of opportunistic infections in comparison to viral load. While the viral load within an individual may be very high, it is only when CD4+ counts are depleted that the onset of AIDS begins and the individual is at risk for infection [26]. In general, flow cytometers can provide absolute CD4+ cell counts or CD4+ leukocyte percentages. Although CD4+ percentage is more reliable for HIV diagnosis, absolute CD4+ counts are more precise due to more uniform standards for enumerating these cells [26].
In the face of this challenge, flow cytometry still offers a high-throughput, multiparamatric technology for HIV diagnostics. Moreover, it has also proven its usefulness in monitoring pediatric HIV [16]; however, it requires the use of bulky and expensive bench-top laboratory equipment that fall short of the logistical challenges evident in the developing world [13]. It is a technology that simply is not portable or accessible enough to serve as an efficient and reliable diagnostic tool.
ELISA Assays
ELISAs remain part of the gold standard for HIV diagnosis [34], boasting 100% sensitivity. ELISA tests are based on the activity of antibody-antigen interactions; if the test sample contains the target antigens, antigen-specific antibodies will bind and detect the target molecules. Enzymes linked to these antibody probes typically produce light used to quantify the amount of antigen present. Different enzymes can be used to produce other measureable signals. In contrast to normal ELISAs that rely on nonspecific binding of the antigen to the test plate, sandwich ELISAs use primary antibodies to achieve this. This significantly increases the sensitivity of the test by immobilizing all antigens and ensuring that none are competitively excluded by other proteins and washed away before detection. Figure 3b highlights the two methods.
ELISA tests remain a great option for use in large health centers that screen a high number of sera per day. They allow high throughput screening and good sensitivity [26]; however, ELISA tests require the use of large and expensive equipment that must be well maintained and cannot be used in the field. For this reason, many other S/R assays have been developed.
Simple and Rapid Assays
S/R assays provide the ability to perform diagnoses in resource-limited settings such as rural health clinics or within voluntary counseling and testing (VCT) services. This is due to their portable and robust nature. Moreover, S/R tests typically take fewer than fifteen minutes to perform, greatly minimizing the risk of sample mix-ups and greatly increasing the speed with which a diagnosis can be given. They are also cheap and easy-to-use among health workers that have little to no training [25].
Lateral Flow Tests
Lateral flow tests are among the most common of S/R assays [25, 27], and like other chromatography techniques, rely on capillary action. Lateral flow tests use strips with an absorbent layer coated with detection antibodies. A small drop of fluid sample causes antibody-antigen pairs to diffuse to a detection zone, providing a diagnosis. This zone typically contains a test line and a control line [27]. If both the test and control lines become visible, then the target antigen is present in the sample. If only the control line becomes visible, then no antigen is present. A diagram is presented in Figure 3c.
Sensitivity remains an issue for lateral flow assays such that additional tests are recommended to make an adequate diagnosis [27]. Despite this, lateral flow tests have become a cheap, simple, and useful HIV diagnostic tool since their initial use for home pregnancy tests. More recent advances in lateral flow assays have enabled the use of whole blood, eliminating the need to extract plasma from blood samples [29, 31, 32].
Saliva and Urine Tests
In addition to sera that is primarily used for lateral flow tests, a number of other S/R technologies have become available that make diagnoses based on saliva or urine samples [24]. These technologies still depend on either the ELISA or lateral flow platforms, but provide diagnoses using more easily obtainable fluid samples. This is of particular concern when social norms come in the way of extracting blood. Moreover, oral and urine samples eliminate the use of needles, reducing the risk of infections that are introduced through testing [24].
These technologies, however, still carry their respective disadvantages from ELISAs and lateral flow tests. Lateral flow tests already are not sensitive enough to make a complete and reliable diagnosis, and are thus only used for screenings. Although ELISAs can be much more sensitive, these tests are also subject to secondary confirmatory testing using another ELISA assay or a Western Blot. Given the reduced concentration of HIV antibodies in both urine and saliva when compared to serum, the sensitivity of these fluid-based tests is a chief concern.
Western Blot
Western Blot (WB) tests are regarded as the most reliable and sensitive form of confirmatory testing [34]. In a WB assay, a sample containing antigen is separated using gel electrophoresis with SDS-PAGE. Once separated, the antigens in the gel are transferred to a nitrocellulose membrane that is exposed to antibodies specific to a particular antigen. Colorimetric chemistry is then used to produce a color response if antigen-antibody pairs are formed. A positive color response indicates the presence of the target antigen within the original sample.
Although the WB assay is a highly sensitive platform, the technique again requires complex and expensive equipment. The use of this equipment also requires considerable technical training. These conditions represent significant challenges to resource-poor settings such as those seen in the developing world. Furthermore, WB tests can often produce inconclusive results. For these reasons, WB has more recently begun to be replaced by secondary ELISA assays that provide reliable confirmatory diagnoses [26]; however, the hesitation with which this transition is being made underscores the serious need for more sensitive and reliable in-field testing procedures that provide results similar to the WB assay in a more portable, reliable, and economical platform.
Nucleic Acid Amplification
PCR, RT-PCR, and NASBA (nucleic acid sequence based amplification) techniques are among the most used for monitoring HIV viral load [15]. RT-PCR targets, amplifies, and quantifies specific viral genes through the activity of reverse transcriptase on viral RNA. The final amount of amplified viral product is then quantified using one of various detection mechanisms. This information is used to determine the appropriate dose of ARV drugs, effectiveness of ART, and the current HIV/AIDS disease state of the patient. PCR is also used to detect HIV in infants younger than 18 months. Newer research suggests that early infant diagnosis can be done using NASBA kits as well [14, 35].
Although this method is very reliable for both diagnosing HIV and determining viral load, it again uses intricate and expensive instrumentation that is scarcely available in the developing world. In Zambia, only a single facility in the whole country is equipped with an RT-PCR instrument. Thus, samples that are taken in the field must be transported to this facility for testing, and the results transported back. The round-trip ferrying can take on the order of months, complicating efforts to effectively diagnose and treat HIV infected persons. A more effective tool would again be something that is capable of supplying similar results using a portable, reliable, and economical platform.
Early Infant Diagnostics for HIV
Early infant diagnostics and treatments for HIV still lag far behind those for adults. Box 1 describes the urgent need for improvements in this field. The usual serological tests for HIV cannot be used in the first 18 months of a child’s life because maternal antibodies remain in fetal blood. These antibodies unequivocally generate false positives. Accordingly, the use of more appropriate diagnostic tools is vital for infants, particularly because up to half of HIV-infected infants under the age of 18 months will die [2].
Box 1. Pediatric HIV.
Pediatric HIV in sub-Saharan Africa accounts for a staggering 90% of all global, child-HIV cases [2]. These children often have life expectancies shorter than their grandparents, and have very poor survival rates. By their second birthday, 35–59% of untreated children will die [3]. Many of these children are orphans who also lost their parents to AIDS. Without a primary caregiver, these children are forced to financially support themselves for nutrition and shelter. They pass on opportunities for school, and sometimes even ART programs designed for them [3].
Many children with HIV contracted the disease through mother-to-child transmission (MTCT). This may occur through pregnancy, delivery, or even breastfeeding. Unsurprisingly, the rate of MTCT in developing countries is significantly higher than in developed countries. This is due to the large number of HIV-positive women, high efficiency of MTCT, and lack of resources to reach, diagnose, and treat these women.
Fortunately, there are ways to decrease the impact of HIV on children. Studies have shown that early intervention with prophylactic and ARV drugs has shown to be effective at reducing MTCT [3, 5]. Longer ART regimens started during pregnancy are much more effective than those started after birth [5]. MTCT can also be prevented by reducing unwanted pregnancies [3]. Pediatric HIV treatments include variations in formula feeding or exclusive breastfeeding [9]. Ultimately, diagnosis of infants during their early stages of life enables them to benefit most from life-saving drug treatments.
In spite of the need, access to timely diagnostics for pediatric HIV is limited in developing countries. In these areas, PCR machines are few and far between rural communities. Not only does this make transport of the sample to the testing facility long (it can take up to four weeks for a result to be delivered back to a mother), but environmental factors such as heat make it necessary to refrigerate the sample during transport.
Viral DNA detection by PCR is the most prevalent technique to diagnose HIV in infants under this age in developing countries. One recent review evaluated the use of commercial oral HIV tests for infants, but found them to have poor sensitivity [36]. Additional recent studies showed that commercial HIV RNA assay kits (those that are used to monitor viral load) are comparable and more-easily accessible diagnostic tools for infants [14, 35].
More recently, the use of dried blood spots (DBS) in lieu of plasma samples have been shown to be a successful collecting method that simplifies diagnostics for infants [14, 35, 37]. Blood samples from the infant are taken, absorbed onto a filter paper, and then set out to dry. The filter paper can be directly transported by local mail services or regular ground transport to the testing facility for HIV RNA or DNA PCR analysis. According to Lofgren et al, DBS samples remain stable up to 10 weeks with desiccants and eliminate the need for refrigeration. Cross-contamination can be limited through proper training and storage techniques. Studies in remote villages across various countries, including Thailand, the Netherlands, and Tanzania, have shown that this method is a promising alternative to plasma samples for PCR analysis [14, 35, 38]. Further studies are needed before the WHO can validate the use of DBS for diagnosing HIV with PCR [14].
An alternative to PCR methods is the use of HIV RNA assays. One example is the Nuclisens EasyQ assay used in developing countries to monitor viral load. Studies across different countries have evaluated the NucliSens assay with DBS samples for viral load monitoring as a comparable alternative to using plasma and PCR [14, 35, 39]. In regards to diagnosing infants, Lilian et al recently showed that the Nuclisens assay can diagnose HIV with high specificity using DBS. Similar results were also found in Thailand and Tanzania the previous year. The main limitation to these quantitative assays is the lower specificity due to the fact that low levels of RNA in serum may not always be detected. This could be improved by changing the threshold of detection. On the whole, these results are very promising for pediatric diagnosis since HIV RNA assays are more readily accessible than PCR machines.
It is important to note that DBS testing does not solve the lagging transport time and lengthy waits for testing during influxes of samples. The average turnaround time in Tanzania is 23 days for an HIV PCR analysis, but could take up to 39 days [35]. Also, not all rural communities have access to reliable health or mail services. For now, mobile health technologies can quicken the turnaround time for HIV diagnosis in remote areas, while more long term solutions such as microfluidic PCR systems that provide on-site HIV diagnosis can continue to be developed.
Current Needs in HIV Diagnostics
Developments in HIV diagnostic tools and ARVs for HAART have been largely successful. The last 10 years have seen a reduced global rate of HIV infection and combined global efforts have fought to make HIV diagnostics and drugs affordable and available to developing countries (Figure 2). The primary technological challenges now for HIV prevention are (i) development of pediatric HIV diagnostics, (ii) introduction of rural HIV monitoring via CD4+ counts or viral load, and (iii) improvements to real-time HIV diagnoses. Microfluidic technologies provide new opportunities to address these challenges.
Figure 2.
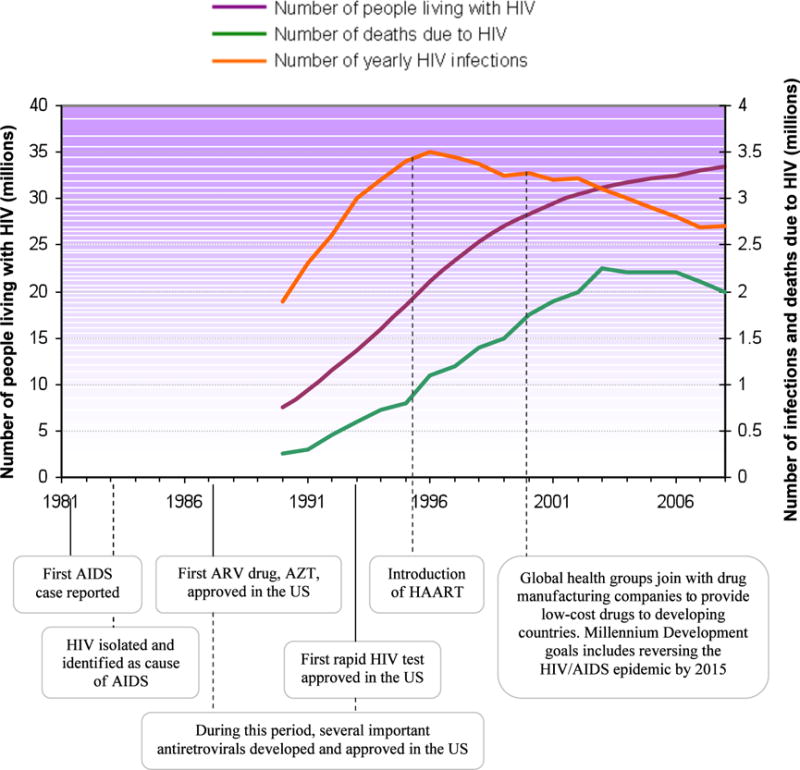
Global time-series of HIV infection and significant milestones in the fight against HIV. While the number of people living with HIV continues to increase yearly, the number of yearly infections and deaths due to HIV has decreased. Below the chart, we highlight significant achievements in relation to the number of people affected by HIV around the world. Sources: WHO, AVERT, and Kaiser Family Foundation.
IV. Progress in HIV Diagnostics using Microfluidics
Recent work with microfluidics has shown progress in miniaturizing conventional HIV diagnostic and monitoring methods such as ELISAs and CD4+ counters. In general, these tiny chips recreate the same immunoassays described above, but with micron-scale chambers and channels that are designed to maximize the efficiency of the reactions. Conceptually, a sample volume is loaded into a chamber and then pumped through microchannels in the chip. The microchannels are specially designed to isolate the target molecules such that they can be visualized or quantified. Varying the dimensions and shapes of these channels, combined with the appropriate mediums, manipulates the flow characteristics (such as shear stress contact time between the two phases) to maximize the capture/release of the target molecules.
Appropriately designed microfluidic devices provide several advantages over conventional diagnostics. They consume fewer reagents, have faster reaction times, and are potentially more affordable. Also, the tiny nature of microfluidic chips makes them candidates for use as compact, portable, and robust diagnostic tools. Many microfluidic devices are constantly being developed to perform a variety of bioassays, including PCR, ELISAs, and analyte quantification [11, 40, 41]. Below, we discuss the various considerations that come into play when designing these chips, and in particular, those that are vital for producing cheap, portable, and robust solutions for HIV diagnostics and quantification.
Material choice
Material choice is among the most important of design considerations. Although glassware is oftentimes a cheap investment, glass is not a cost-effective medium when dealing with dispensable chips. Thermoplastics and polymer-based materials like polydimethylsiloxane (PDMS), on the other hand, have proven to be extremely cheap. Consequently, they have been the material of choice for biomedical applications due not only to their low manufacturing cost, but also due to their biocompatibility and disposability [42, 43].
Light sources
Light sources are also a crucial component to diagnostics device that rely on optical techniques for quantification. Solid-state lasers are often the light source of choice for these applications. Although gas lasers typically offer better coherence of emitted light, solid-state lasers provide the ability to create light over a much wider range of wavelengths using only a fraction of the energy. This is particularly helpful in resource-poor regions where access to power is limited [44]. Furthermore, the greater range of wavelengths that can be produced with solid-state lasers also offers the potential to perform multiparametric studies that interrogate samples with multiple wavelengths.
In addition to using monochromatic lasers, studies have shown that commercially available fluorescent adaptors [45] can be used in conjunction with light microscopes to detect different diseases [46, 47]. These portable adaptors easily attach to light microscopes and can be powered by batteries or outlets. In addition, the adaptor tested in one study successfully diagnosed tuberculosis from sputum smear samples [45]. The adaptor, which is essentially an LED light source, focuses light of a particular wavelength on the sample. This light excites the fluorescent molecules on the sample, which in turn emit light at a new wavelength that the observer can visualize with a microscope.
Compartmentalizing optics
In order to utilize lasers in a microfluidic platform, optical components must also be miniaturized to work with the small length scales found on microfluidic chips. Consequently, the incorporation of optical features within such devices has also seen much work [11, 41]. Tung et al designed a device that incorporated optical fiber waveguides into grooves that were microfabricated into the device [48]. Optical fiber has the advantage of directing light through specific paths, and thus allows focusing of light within microfluidic devices. In their study, the optical guides focused light from a solid-state laser to a specific fluid flow interrogation point. The study also demonstrated the incorporation of multiple waveguides that converge at a single point for the purposes of multicolor analysis of the fluid flow. This provides the ability to construct a multiparametric platform to interrogate the sample.
In a separate study, Lee et al demonstrated the incorporation of optical waveguides without the need to embed external optical fibers into etched grooves [49]. Instead, channels for the waveguide were etched out and filled with an organic spin-on-glass (SOG) liquid. The extra fluid is then sucked out, leaving a thin layer of SOG within the waveguide channel. The SOG is then cured, creating a complete seal of the waveguide structure to the microfabricated device. In contrast to embedding optical fiber, this technique has the added advantage of eliminating any extra space between the waveguide structure and the microfabricated channel. This enhances the difference in the refractive indices, thereby enhancing the efficiency of the waveguide itself.
More recently, Cho et al have demonstrated the use of amorphous Teflon (Teflon AF) instead of SOG [50]. This Teflon AF is spin-coated onto the interior of the microchannels. In addition to forming a low refractive index cladding around the channel, the low surface energy of Teflon also prevents aggregation of components to the channel walls, thereby avoiding microchannel clogging by the fluid sample. Light is shone through the channels using optical fiber that is simply inserted into one end of the channel.
In addition to new techniques for introducing optical waveguides into microfluidic platforms, further research has also pursued the possibility of using other optical phenomenon in these devices. In particular, Ozcan et al conducted a proof-of-concept study that enumerated cells using a CCD chip that recorded the shadows of cells [51]. This method was further improved upon through the use of holographic imaging. In short, spatially coherent light scatters off the objects to be imaged. This scattered light interferes with reflected light, which is then recorded as a holographic image by the CCD. The novelty of this technique was in the use of a CCD chip to directly record data without the use of a lens system. This lensless system was further developed in a number of other studies. For future point-of-care devices, Stabayeva et al used this method to image CD4+ and CD8+ T cells on an antibody microarray [52]. The intensity of their secreted antibodies was also recorded. Tseng et al have further produced a prototype of a compact, lensless microscope on a mobile phone camera, bringing advancements to lensless technology one step closer to field use [53]. Additional refinements to this technique involved the use of multiple wavelengths of light to identify and quantify cells in a heterogeneous cell solution [52, 54–56].
Eliminating external sample preparation
Beyond advances in the incorporation of optical components into microfluidic chips, many devices also incorporate additional chambers and channels that can perform sample preparation on the device itself [41, 57]. Examples include cell lysis, reagent mixing, and isolation of certain biomolecules. This reduces the sample preparation time by the technician and improves the sensitivity of the assay. In whole blood, several other cell and protein components can interfere with a bioassay, skewing the actual results. For example, at low levels of CD4+ cells (200/μl), the device developed by Cheng et al had more inaccurate results due to monocyte contamination in the CD4+ capture chamber[12]. They obtained better purity of CD4+ T cells once they introduced a CD14+ (monocyte) depletion chamber prior to the CD4+ capture chamber (Figure 4). In a different study, Herr et al created a set of microfluidic chambers that could pre-treat saliva samples and then detect the presence of a model biomarker using electrophoresis [58]. However, whether these devices can become reusable and affordable remains a prudent question.
Figure 4.
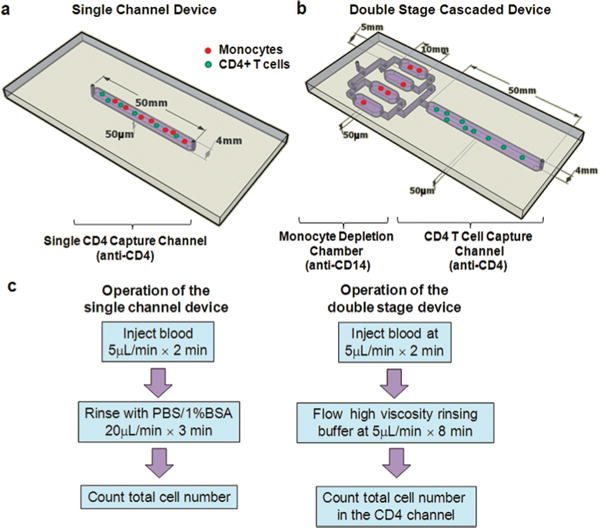
Microfluidic devices used in the study and the operation procedures. (a), (b) Schematics showing the geometry of the single channel (a) and two stage (b) devices used for cell counting in this paper. The CD4+ T cell capture channels of both devices were functionalized with an anti-CD4 antibody for target cell isolation. The two stage device also contains 4 parallel chambers upstream to the CD4 channel for monocyte depletion. (c) The operation procedures of the two counting devices. For the double-stage devices, two considerations were taken into account for the rinsing buffers: one is to quickly displace monocyte depleted blood to the CD4 capture channel and the second is to avoid shearing off specifically captured cells. These two considerations were studied in detail in this work. Reproduced from Reference 49 by permission of The Royal Society of Chemistry.
Eliminating external power sources
In developing countries, the supply of electricity can be sporadic and often is not widespread [44, 59]. Biomedical devices that can function under these conditions, whether by the use of battery sources or alternative energy would be advantageous. In particular, current microfluidic immunoassays rely on powered pumps and bulky, fluorescent detectors.
Martinez et al presented a novel concept to eliminate the need of an external power source, which most current bio-microdevices depend on. The scientists used a paper chromatography microfluidics platform where a blood sample wicks onto a hydrophillic paper sheet, carrying the sample through imprinted, hydrophobic channels with reactive chemistry to detect analytes [60]. A visual result can be easily seen and quantified by an analyst. The advantages of using paper are its low cost, availability, and ability to store well and be easily engineered. Furthermore, they demonstrated its potential for off-site, real-time analysis by photographing the colorimetric result with a mobile-phone camera and sending it to an off-site specialist.
Another alternative method is the use of solar power to charge batteries or devices [44]. In particular, innovative combinations of solar-powered phone batteries can power a biomedical device and could serve as a reliable, alternative power source. Mobile phone batteries are cheaper than conventional ones, less likely to be stolen, and ubiquitous throughout developing countries. As users often travel to charging stations in order to charge their phones, using these batteries would truly take advantage of the local resources.
Proof-of-concept studies have also been done to harness non-conventional energy sources in order to power biomedical devices. These include solar-powered pulse oximeters using mobile phone batteries, MEMS devices that consist of microarrays of thermocouples that turn body heat into electricity [61, 62], and devices that generate power from mechanical movement (such as walking or breathing) [63].
Diagnostic Devices Under Development
Microcytometers for viral monitoring
Several portable CD4+ T cell counters for HIV diagnosis and monitoring are being developed [11, 40, 64]. One such device developed by the Palo Alto Research Center is a microfluidic version of a flow-cytometer to quantify CD4+ T cells [65]. Zhu et al developed a miniature cytometry platform that enumerates lymphocytes using a printed antibody microarray, but uses RBC-depleted blood [66]. On the other hand, Cheng et al (2006) developed a simple, non-labeling glass slide that captures CD4+ cells from whole blood [67]. A small sample of blood is pumped through a single microchannel that contains anti-CD4+ antigens. No molecular labeling is required and only a light microscope is needed to visually count the isolated CD4+ cells. This makes it ideal for areas where light microscopes are more accessible than fluorescence detectors. With advances in tele-microscopy, this device could be combined into a compact HIV monitor in rural communities.
Miniaturized PCR for viral monitoring and infant diagnosis
Several versions of microfluidic PCR systems have also been developed in recent years [41, 68, 69]. The size of these systems makes them potentially more affordable and portable for use in resource-limited settings. Impressive new improvements are integrated PCR chips that include a means of quantitative detection. For example, Lee et al created a polymer-based MEMS device that performed both on-chip RT-PCR (incubation with RT, mixing with PCR mixture, and thermocycling) and chemiluminescent detection of the amplicon, all within 1 hour [68]. Their platform was portable, capable of detecting HIV, and required some external manipulation. Temperature was regulated by a control-system of infrared light sources.
Other microfluidic PCR platforms that use isothermal amplification methods may be even better suited for low-resource settings [41]. Loop-mediated and NASBA methods do not require thermocycling or the use of RT. Fang et al created a PDMS microfluidic chip that performed loop-mediated amplification of psuedorabies virus in 1 hour (Figure 5) [70]. A visible by-product that formed during amplification was a qualitative indication that amplification was successful. Furthermore, a fiber optic sensor could measure the absorbance and give a quantitative result, which could be used for viral load monitoring. While they demonstrated that the device isolated psuedorabies viral DNA, this method could easily be extended to HIV DNA.
Figure 5.
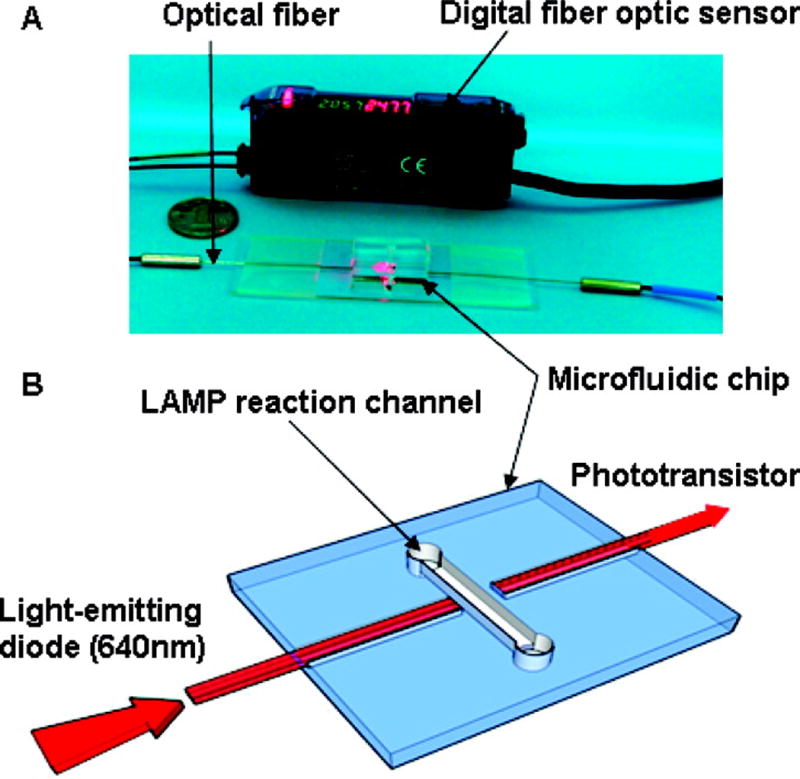
Photograph (A) and schematic illustration (B) of the quantitative analysis unit. Reprinted with permission from Reference 66. Copyright 2010 American Chemical Society.
Multiplex diagnostics using quantum dots
Quantum dots are a unique alternative to the conventional fluorescent molecules used to quantify viral load or CD4+ cells. Quantum dots (QDs) are a special class of semiconductors that are on the order of nanometers in scale. They can be used as inorganic fluorophores, with emission spectra that depend on the size of the quantum dot itself. Larger dots emit longer wavelengths (low energy) and smaller dots, of the same material, emit shorter wavelengths (high energy). Recently, the use of quantum dots has been demonstrated in live cell imaging and tracking [71]. As fluorophores, quantum dots are more stable, brighter, and can improve detection limits with higher signal-to-noise ratio. They may also be conjugated to a variety of biomolecules.
The true value of QDs for disease diagnostics lies in its potential to improve assay sensitivity and multiplex disease analysis [41]. They can also help reduce the reaction time and reagent consumption. However, QDs themselves are very costly to produce [41]. Most studies that have been performed using QDs for disease detection are proof-of-concept experiments that highlight their potential for diagnostics [57, 72–74].
Liu et al demonstrated a modified sandwich ELISA assay using antibody-coated QDs as the means of detection [73]. Briefly, antibody-coated microbeads bind the target virus antigens. A secondary antibody binds to the target virus, and then a complementary antibody-coated QD binds. Fluorescence from the QD could be visualized, recording the target binding events. Compared to conventional ELISAs, this method improved the detection limit of marine viruses, and could be extended for other small-scale, infectious disease immunoassays such as those for HIV [72].
For multiplex analysis, a single test could diagnose both HIV and tuberculosis, a secondary infection by which many AIDS patients die from [17]. These multiplex diagnoses may be useful for a doctor prescribing medication for an HIV patient. Klostranec et al demonstrated the detection of three viruses (HIV, HCB, and HBV) from a single, spiked blood sample using three different QDs attached to respective viral antibodies (Figure 6). Even though each QD was different, they were able to use a single activation wavelength. This is because QDs of different emission spectra can be excited by just a narrow bandwidth of light.
Figure 6.
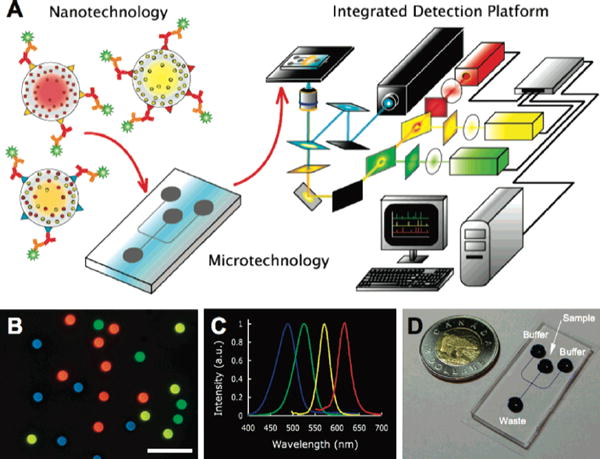
Diagnostic scheme. (A) Diagram illustrating the integration of Qdots, solution-based sandwich assay, microfluidics and fluorescence detection with custom software for high throughput, multiplexed blood-borne pathogen detection (see Supporting Information for details). (B) Fluorescence image of a collection of different color emitting, 5.0 μm diameter polystyrene QdotBs suitable for proteomic or genomic assays (Olympus 40× objective, 0.75 NA, scale bar) 20 μm). (C) Normalized Qdot emission profiles corresponding to the Qdots used for the barcodes in (B), all excited using 365 nm light. (D) Sample microfluidic chip, fabricated in polydimethylsiloxane with wells labeled. Channel dimensions are 100 μm wide by 15 μm high. Blue dye was used to visualize the channel intersection for electrokinetic focusing. Reprinted with permission from Reference 70. Copyright 2010 American Chemical Society.
Room for improvement
In spite of the exciting number of developments in microfluidic diagnostics, very few have been successfully commercialized [41]. Most microfluidic applications are still in their developing stages. Common drawbacks to the current developments include: (i) excessive sample prep or post-analysis by a technician, (ii) sub-par sensitivity and specificity of the diagnostic, as compared to current gold standards, (iii) the need for external power sources or pumps, (iv) expensive material choice, and (v) bulky auxiliary components that are not portable. As discussed, many of these problems have begun to be tackled, but more research is still required to translate those studies into effective, in-field diagnostic technologies.
From Bench to Bedside
In addition to the time required to successfully develop translatable diagnostic technologies, the time lag evident between the development of these technologies in laboratories and their successful and sustainable implementation in the field is a process that is often confusing and underexamined. Moreover, it is a factor that is commonly neglected when estimating the amount of time necessary to go from problem to solution. A manufactured solution is useless until it has been successfully implemented and becomes widely used within its target community. Figure 7 highlights the steps involved in this transition process from laboratory to field as adapted from Mabey et al (2004). Between each of these steps, it may take years as the diagnostic platform undergoes varying levels of scrutiny by several key players before even being presented to the target community.
Figure 7.
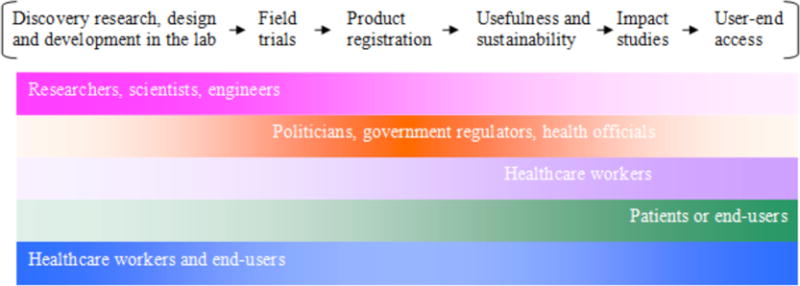
Timeline for the development of diagnostics.
V. Logistical Challenges
In spite of the success of HIV drugs and diagnostics over the last ten years, sub-Saharan Africa still grapples with the tremendous task of distributing these assets. In this region alone, HIV prevalence is over 5–10 times greater than the global average [2]. Moreover, as of 2008, less than half the people that need ART actually receive it [2]. It is clear that while there is progress in the HIV epidemic campaign, the progress is uneven throughout the world. The logistical challenges involved include individual financial burdens, the major lack of human resources for adequate healthcare in rural populations, and time-consuming delivery methods for healthcare to remote villages. Mobile health technology holds promising abilities to overcome these challenges and provide remote monitoring and real-time analysis and diagnosis.
Realizing Universal Access to HIV Treatments and Care
Over the last 10 years, a combination of global public policies has helped make anti-retroviral drugs cheaper and more available around the world, especially in low-income populations [2]. In 2000, the UN Millennium Goals included increasing universal access to antiretroviral drugs for people with HIV [75]. Health organizations including UNAIDS and WHO teamed up with pharmaceutical companies to provide cheaper drugs to developing countries. Since then, other drug companies have also produced discounted and generic drugs to these regions. The U.S. has also implemented programs to address HIV/AIDS in the developing world through the Global Health Initiative and PEPFAR (President’s Emergency Plan For AIDS Relief) [76]. Indeed, since 2004, the global number of deaths due to HIV/AIDS has decreased and the global prevalence of HIV has stabilized at ~1% [2].
Individual Financial Burdens
In spite of the increase in funding for HIV/AIDS programs around the world, further technological and policy efforts that can lower the cost of HIV diagnostics and drugs could alleviate the financial burden for all families. Between the richest and poorest homes in India, the financial cost of treatment and care for someone living with HIV ranges from 20% to 86% of the family’s total annual income [2].
In Africa, the majority of children with HIV are orphans and lack financial and nutritional support from an older adult. Even with free ART programs sponsored for children, without caretakers, many children drop out in order to provide for and support themselves [3]. Thus, it is evident that a combination of public policies and global efforts aimed toward children with HIV that provide low-cost ARV drugs will continue to be essential in reducing the HIV epidemic in the coming years.
Remote Monitoring
Access to healthcare is difficult for those that live in remote areas, especially in developing countries. Resource-limited settings often lack clinical facilities, causing health professionals to force natives to travel long distances to find health treatments. Healthcare workers play critical roles in delivering healthcare by traveling to rural areas, administering treatments, and referring patients that otherwise would not have traveled to nearby clinics. Unfortunately, a standing shortage of workers presents a major challenge for delivering health treatments, including those for HIV drugs [12, 75]. In some cases, blood samples must still be taken to a larger city with appropriate testing equipment. In Zambia, diagnosis of fetal blood samples for HIV can take more than two weeks. Workers that take samples in the field must transport them to a testing facility, test them via PCR, and send the results back to the patient. More often, thorough testing can take on the order of months. For pediatric HIV diagnosis in rural areas, this is frankly unacceptable, as ARV treatment is more effective the earlier it is taken.
For this reason, HIV diagnostic and monitoring tools that can be high throughput, easy to use, and decrease transit times would be extremely beneficial; it would reduce the need for more trained professionals and allow each worker to reach more rural populations. Current mobile health companies, such as ClickDiagnostics, have developed applications for remote monitoring and real-time diagnosis with the aid of a cell phone.
Mobile Health Technologies
Mobile health technologies are an extremely promising approach to bridge the gap between health professionals and those with limited access to healthcare. These mobile health applications can connect doctors, health care workers, patients, and even public health officials. Mobile technology companies targeted for environmental and health information have grown with the recent boom in mobile-phone users around the world. Africa alone gained 60 million cell phone users in 2007 and even remote villages in sub-Saharan Africa have communal cell phones [60]. Box 2 outlines the areas of impact for mobile health technology.
BOX 2. Impact areas of mobile health applications [1].
Education
Data Collection
Remote Monitoring
Communication and Training
Disease and Outbreak Tracking
Diagnostic and Treatment Support
Box 3 shows how various organizations, academic institutions, and entrepreneurship competitions have largely supported the mobile health movement over the last few years. While most applications are still in their development or trial phase, the field is quickly gaining momentum [1]. Further collaboration between these groups would help realize the potential impact that mobile health technologies can make.
BOX 3. Current organizations and groups that have supported Mobile Health Technology.
mHealth Alliance is a partnership between the Rockefeller, United Nations, and Vodafone Foundations that began in 2009. The Alliance sponsors “mHealth”, or mobile health, initiatives for healthcare and disaster relief across the world. In 2010, they began awarding the “mHealth Alliance Award” to the most promising mobile health technology presented at the Vodafone Wireless Innovation Competition. The winner receives $50,000 and networking support.
GOOGLE SMS in Uganda allows users to text short questions or search options and receive information on health tips, local clinics and their services, agricultural tips, and weather forecasts.
DataDyne is a non-profit organization that develops easy-to-use mobile health applications for data collection and mobile information programs in developing countries. They are a UN- and WHO- sponsored mobile health organization
Click Diagnostics develops mobile health applications for remote monitoring and diagnosis in Sub-Saharan Africa, namely Kenya, Ghana, Botswana, and Bangladesh. They were a winner of the MIT $100K Entrepreneurship Competition
Sana created a web-based mobile application for remote monitoring and diagnosis in Southeast Asia and Mexico. They received the mHealth Alliance Award in 2010 and were originally a spinoff from the Next Billion Network Program, a collection of mobile health technology projects, at MIT.
VodafoneWireless Innovation Project is a yearly competition that began in 2009, seeking innovative applications of wireless technology that would address global issues such as health, access to communication, economic development, and education. Among the winning projects were those related to telemedicine and telemicroscopy. Winners receive up to $300,000 in support of continuing their project.
Aspects of Mobile Health Technologies
Data Collection
By far, most pilot mobile health applications are for data collection. These applications, such as EpiSurveyor by DataDyne, allow the user to create their own survey questions on an external computer, which they can download onto a cell phone and take out into the field as a “digital clipboard.” In cases where healthcare workers do not have adequate expertise to advise patients, a growing number of applications also allow for trained professionals to remotely monitor and diagnose patients in rural communities. ClickDiagnostics have developed a mobile-phone platform for healthcare workers to record patients’ health information, take mobile-phone photos, and electronically send them to a health professional for diagnosis. Their application has had successes in Kenya, Ghana, Botwana, and Bangladesh.
SMS Reminders
SMS, or short message service, provides a cheap, text-based means of communication. According to Curioso et al, the average adherence to ART for HIV patients was 77% in resource-limited settings, and even lower in some resource-rich settings. Reminders by SMS may be a novel method for improving patient adherence to ART, reducing the risks of drug resistance and secondary infections [77]. SMS are not only quick and far-reaching, but the anonymity of receiving SMS messages for HIV reminders would draw less attention to individuals to whom HIV stigma is still an issue.
Integrating Diagnostic Tools – Telemedicine
The future of mobile health technology lies in the idea of telemedicine, where not only are health professionals connected with low-resource regions, but associated diagnostic tools are also encased in a compact, portable, and robust form. By far, microscopes have been the most successful diagnostic technology that has been integrated with mobile phones. Fletcher et al attached a microscope lens to a mobile phone camera and was able to generate pictures of sufficient quality for diagnosis; some were even able to producing high quality images (Vodafone competition). Similar constructs by groups in Italy and India demonstrated the ability to diagnose the presence of malaria through photos of Plasmodium falciparum in red blood cells [78, 79]. Martinez et al also used a PDMS lens as a cheap, alternative lens for less powerful camera phones [52]. Using only an LED and cell phone with a built-in camera, Tseng et al created an impressive, lensless microscope and imaged particles and cells ranging from 3 μm to 10 μm in size (Figures 8, 9) [53, 80].
Figure 8.
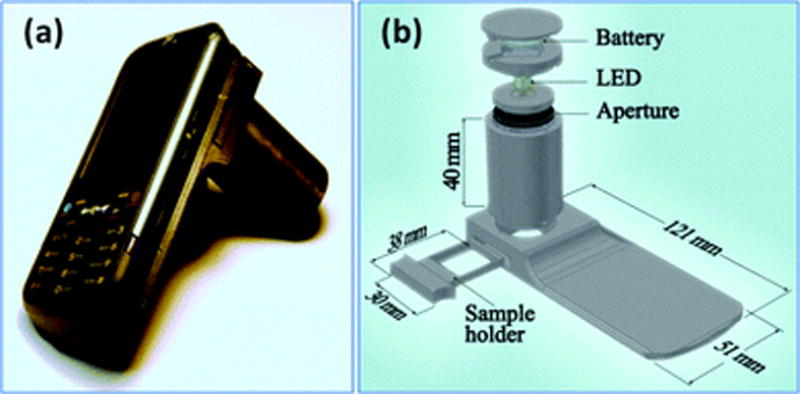
(a) A lensfree cellphone microscope which operates based on incoherent in-line holography is shown. The additional hardware installed on the cellphone weighs 38 grams (<1.4 ounces) and is composed of an inexpensive light emitting diode (at 587 nm) with an aperture of 100 m in front of the source. This cellphone microscope does not utilize any lenses or other bulky optical components and operates with a unit fringe magnification to claim the entire active area of the sensor as its imaging field of view. The samples to be imaged are loaded from the side through a mechanical sample holder. (b) Schematic diagram of the microscope attachment shown in (a) is illustrated. Reproduced from Reference 49 by permission of The Royal Society of Chemistry.
Figure 9.
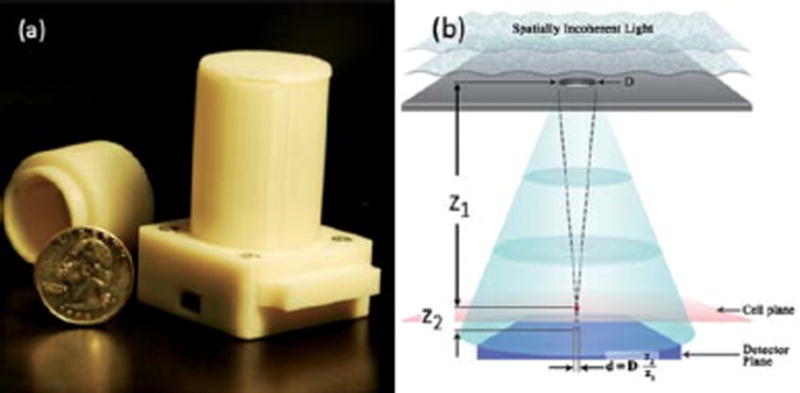
(a) A lensfree holographic on-chip microscope that weighs 46 grams is shown. It utilizes an LED source (at 591 nm) with an aperture of 50 to 100 μm in front of the source. The LED and the sensor are powered through USB connection from the side. This lensless holographic microscope operates with a unit fringe magnification to claim the entire active area of the sensor as its imaging field of view (24 mm2). For different designs refer to Fig. S1. (b) Schematics of the incoherent lensfree holographic microscope shown in (a). Drawing not to scale. Typical values: z1 2–5 cm, z2 < 1–2 mm, D 50–100 μm. Reproduced from Reference 77 by permission of The Royal Society of Chemistry.
Future integrations for telemedicine include lab-on-chip designs for infectious disease diagnosis, EKG monitors, blood pressure and glucose sensors, and temperature probes. For HIV, portable CD4+ cell counters for monitoring viral load would also benefit ART patients that do not have easy access to clinical facilities.
In Africa, it is important to note that the penetration of multimedia message systems (MMS), by which camera photographs can be sent, ranges anywhere from less than 2% to over 90% [79]. Also, access to mobile networks may become an issue for telemedical devices that require remote processing of sent images such lensless, holographic images[53]. Additional infrastructure to support MMS and mobile networks is essential to the widespread use of telemedicine in developing countries.
VI. Sociopolitical Challenges
Equally important to considering the challenges associated with device design, development, and testing are those that are met once the device reaches the end-user. Local resistance to new technologies is often met in developing countries due to social and political circumstances unique to that area [2, 81]. This leads to a number of negative impacts in the fight against HIV, including increased risks of drug resistance and willing refusal to partake in HIV prevention techniques or treatments [12, 75]. These challenges have made global goals of reversing the HIV epidemic difficult. Figure 10 illustrates the current status of people with HIV in sub-Saharan Africa.
Figure 10.
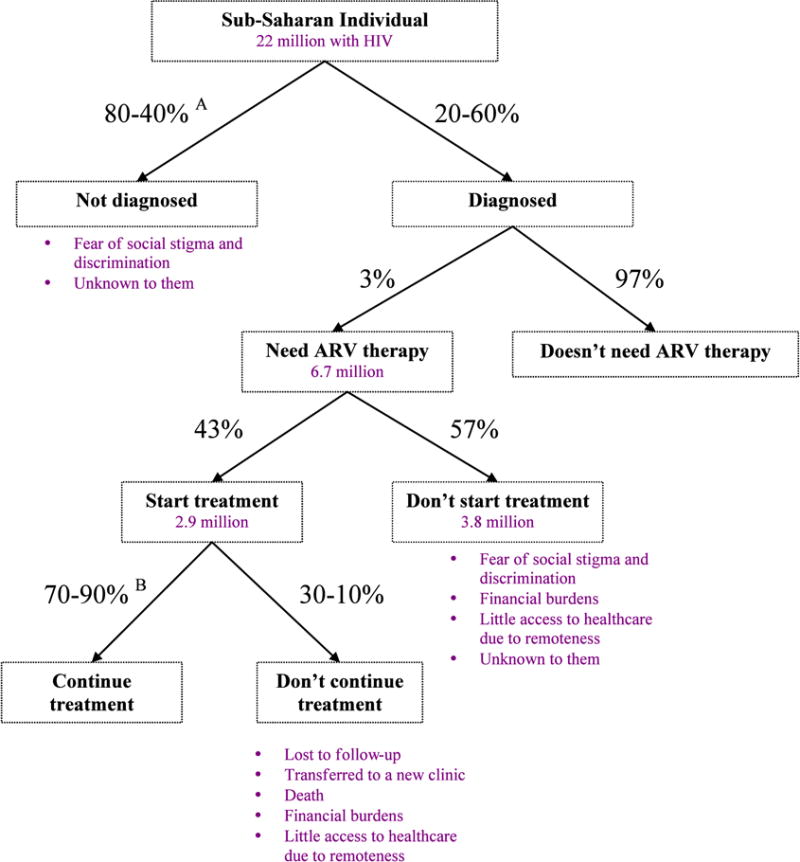
Possible choices and outcomes for HIV-related decisions in sub-Saharan Africa. Percentages are based on averages in sub-Saharan Africa, 2008. Source: WHO, Towards Universal Access, Progress Report 2009.
A Actual percentages depend on which sub-Saharan country; based on a 2007 survey.
B Percentage of patients that are still on ART after 12 months at the same clinic; over two-thirds of sub-Saharan countries report these values.
Resistance to Technology
The question of what makes the acceptance and implementation of HIV technologies so difficult in the developing world is one that often eludes researchers and policy-maker alike. Much of the resistance by villagers stem from sociopolitical challenges such as (i) stigma and discrimination towards HIV, (ii) gender-equality gaps, (iii) lack of anti-HIV discriminatory policies, and (iv) sub-par education among young adults concerning HIV transmission, prevention, and treatment [2]. Healthcare workers themselves may even disregard new technologies if they find them too complicated or are discouraged by the lack of cooperation with the locals to use them [81]. Many groups contribute to this problem and many groups are positioned to overcome it. It has been clear that governments, healthcare workers, and even scientists play important roles in reducing end-user resistance and helping to realize HIV prevention goals.
The Influence of Stigma on People with HIV
Stigma is perhaps the biggest social challenge that the HIV epidemic faces, with discrimination spreading between the low-resource settings in sub-Saharan Africa to more developed countries around the world [2]. Victims of HIV stigmatization are usually ostracized and face social and physical isolation from society. Many that have revealed their HIV status have expressed regret and fear for their economic wellbeing. Property rights of HIV-infected individuals are not well protected in many countries, especially for women. Only one-third of countries have anti-HIV discrimination measures. Some hospitals are known to discriminate against HIV patients and only recently did the U.S. and China lift bans on incoming foreigners with HIV/AIDS [2, 82, 83]. Women often bear the brunt of HIV discrimination, especially as gender equality remains a critical issue in many countries.
Due to this stigma, there is tremendous social pressure that discourages individuals from disclosing their HIV status with sexual partners and seeking Voluntary Counseling and Treatment (VCT). On average, only 39% of sub-Saharan Africans know their HIV status, [84]. In some cases, mothers were uncomfortable and resisted diagnosis of their newborns that are at risk for MTCT. A poll indicated that some pregnant women in Lusaka would rather take nevarapine unconditionally to treat for a potential HIV infection than take a test to determine their HIV status [85]. Many individuals will even choose not to discuss protective healthcare such as condoms in fear of being assumed that they are HIV-positive [2]. These actions perpetuate the cycle of HIV infection and hinder the fight against HIV prevention.
Decreasing Resistance to New Devices or Health Regimens
By 2007, 67% of countries had reached their goals, as set by UNAIDS, for administering ART to people with HIV/AIDS; however, reaching their goals for HIV prevention proved a much more difficult task [2]. Clearly, diagnostic tools and drugs are available for universal HIV healthcare and prevention, but access to these tools and treatments is severely limited by logistical and social issues. In order to increase the availability and acceptance of these technologies, a number of steps must be taken on the part of governments, scientists, and healthcare workers. Only through a combined and synergistic effort can these challenges be overcome in order to expedite efforts to reduce HIV prevalence and eliminate the global HIV pandemic.
The Role of Government
Many of these challenges, especially social stigma, can be curbed by improving the overall education and awareness of HIV [2]. Governments can implement public policies to decrease the resistance to new HIV devices. First, to curb HIV stigmatization, governments can implement anti-HIV discrimination measures. Second, since women often face the bulk of discrimination and are a high-impact group for HIV, efforts must be made to settle the gender-equality gap [2]. Third, overall education of HIV transmission, prevention, and treatment, for both children and adults, is essential.
The success of these measures by countries that have taken them are readily evident [2]. In the two years following 2005, the average number of people who knew their HIV status went from 15% to 39%, globally [84]. In India, educating hospital workers on HIV and prohibiting obvious discrimination have decreased the presence of stigmatized attitudes [86]. Education of young adults on HIV transmission has slightly improved, but still remains critical. It is clear that many efforts are still needed.
The Role of Scientists, Researchers, and Engineers
Recent endeavors to involve the end-user and use their input and insights during the development phase has greatly contributed to successful, long-term implementations of diagnostic devices [81]. These end-users include healthcare workers, doctors, and even the patients themselves. Biomedical devices, especially for telemedicine and mobile health applications, should also be sensitive to the issue of privacy [87]. Due to the concern of stigmatization, privacy of information storage, especially health status, is highly regarded in HIV-prevalent areas. In some cases, failure to address this issue can slow down public or government approval for HIV-related proposals. Thus, for those developing mobile health technologies, the storage site of personal health information should be made clear to the target population.
The Role of the Healthcare Worker
Due to varying levels of education in developing countries, villagers are sometimes wary of healthcare workers bringing new treatments or devices to their community. In some cases, the health technology that once had high hopes for making a positive impact generates mistrust and a bad reputation among the villagers. Healthcare workers become discouraged by this resistance and may even stop using the device [81]. Oftentimes, it is the lack of careful explanation of the benefits, side effects, and workings of a new technology or treatment that can result in its rejection by the community. In India, vaccinations for small pox were administered to a local community who suffered a side effect of developing itchy, red sores. The locals believed that they had been contaminated with a disease, generating mistrust in the clinical team. Therefore, proper education and well-thought out execution is critical to full acceptance and integration of the device into the community. In areas where tribal communities play a strong role in local politics, gaining blessings from a tribal chief can also lead to acceptance by the rest of the community. Clarifying the involvement of the government can further help facilitate acceptance by local villagers.
Careful coordination of efforts by these three groups for reducing rejection of new technologies in developing countries could significantly bolster efforts to reduce the global HIV problem. By working together to increase education and awareness while eliminating social stigmatization, efforts made by scientists and engineers to develop new and effective diagnostic technologies can be fully utilized in underdeveloped regions of the world.
Social and Political Solutions to Reducing Pediatric HIV
Aside from developing diagnostics, encouraging mothers to seek VCT is clearly a major step in reducing child deaths due to HIV [2]. Unfortunately, because women often bear the brunt of HIV discrimination, getting mothers to seek pediatric HIV care has been especially challenging. Only a small fraction (34%) of pregnant mothers choose to attend clinics, either pre- or post-natal, in middle- to low-income countries. Mothers are often wary of consenting to having their child tested, and if they do agree, encouraging mother-baby pairs to attend clinical follow-ups is even more difficult [2, 3, 37]. Interestingly, mothers become more open to diagnosis and HIV care for their child if they know there are drugs and counseling to treat their baby [37]. Fortunately, global awareness of the severity of pediatric HIV in sub-Saharan Africa has encouraged several governments to implement policies to establish guidelines for treatments, services, and counseling tailored exclusively for mothers and/or children. These campaigns are a good start to educate women and assist them financially with ART for themselves and their children.
Creating more effective guidelines for pediatric treatments in developing countries would also be very beneficial. The side effects of improper doses of any drug can be toxic to a child. Some studies have shown that ARV drugs cause children to be more susceptible to morbid infections [88], making infant disease monitoring critical. Mobile health applications that can relay pediatric treatment guidelines could assist the limited number of doctors available in developing countries. Further research on multiplex diagnostics and the effect of drug treatments on infants is greatly needed to understand and address pediatric drug treatments.
VII. Conclusions and future outlook
Since the identification of the disease over thirty years ago, global efforts have come a long way in reducing the rate of HIV infection and developing new and effective anti-retroviral drugs. Prolific research has resulted in the development of many classes of diagnostic technologies that each have advantages and disadvantages in their use throughout vastly different parts of the world. While HIV prevention and treatment has advanced in developed nations, underdeveloped countries still struggle with problems of limited access to appropriate technologies due mainly to tight resources. Technologies such as conventional flow cytometers, RT-PCR tests, ELISA assays, and WB assays all require bulky, expensive, and well-maintained equipment that is scarcely available in developing countries. Consequently, newer research within the field of microfluidics has led to a number of devices that have shown the potential to replace these tests with more compact, robust, and economical alternatives. Even still, sociopolitical challenges often stand in the way of these technologies becoming fully utilized in low-resource regions of the world. A concerted effort by governments, scientists, and healthcare workers to increase education and awareness and thwart social stigma related to HIV would help counteract the rejection of new technologies and facilitate their widespread use. By taking steps to further improve microfluidic diagnostic technologies, effective diagnosis and treatment may finally lead to the eradication of the global health menace that is HIV.
Table 3.
Characteristics of the ideal diagnostic for low-resource settings.
| Ideal characteristic | Motivation |
|---|---|
| High sensitivity and specificity | Must be comparable to current gold standards |
| Reasonable cost | To mitigate the financial burden of having HIV, which can range anywhere from 20% to 85% of a family’s annual income [12] |
| Easy-to-use | To make up for the lack of healthcare workers that deliver health aid to rural communities To have minimally invasive impact on the patient |
| Rapid | To be high-throughput and provide real-time analysis |
| Robust | To limit the costs of device replacement and maintenance To address the limited funds to maintain reagents, device, and equipment under environmental conditions |
| Long shelf-life at ambient temperatures | |
| Compact and Portable | To be portable and suitable for field work in rural communities with little or no access to healthcare |
References
- 1.Consulting, VH. mHealth for Development: The Opportunity of Mobile Technology for Healthcare in the Developing World. UN Foundation-Vodafone Foundation Partnership; Washington, D.C. and Berkshire, UK: 2009. [Google Scholar]
- 2.UNAIDS. 2008 Report on the Global AIDS Epidemic: Executive Summary. 2008 [Google Scholar]
- 3.Dabis F, Ekpini ER. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359(9323):2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 4.UNAIDS. and World Health Organization. AIDS epidemic update December 2009. Geneva: UNAIDS; 2009. p. 99. [Google Scholar]
- 5.Ugochukwu EF, Kanu SO. Early Infant Diagnosis of HIV Infection in Southeastern Nigeria: Prevalence of HIV Infection Among HIV-Exposed Babies. West Afr J Med. 29(1):3–7. doi: 10.4314/wajm.v29i1.55945. [DOI] [PubMed] [Google Scholar]
- 6.Levy JA. Pathogenesis of human immunodeficiency virus infection. Microbiol Rev. 1993;57(1):183–289. doi: 10.1128/mr.57.1.183-289.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss RA. How does HIV cause AIDS? Science. 1993;260(5112):1273–9. doi: 10.1126/science.8493571. [DOI] [PubMed] [Google Scholar]
- 8.Douek DC, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417(6884):95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 9.Prasitsuebsai W, et al. Pediatric HIV Clinical Care Resources and Management Practices in Asia: A Regional Survey of the TREAT Asia Pediatric Network. Aids Patient Care and Stds. 24(2):127–131. doi: 10.1089/apc.2009.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alimonti JB, Ball TB, Fowke KR. Mechanisms of CD4+ T lymphocyte cell death in human immunodeficiency virus infection and AIDS. J Gen Virol. 2003;84(Pt 7):1649–61. doi: 10.1099/vir.0.19110-0. [DOI] [PubMed] [Google Scholar]
- 11.Moon S, et al. Integrating microfluidics and lensless imaging for point-of-care testing. Biosens Bioelectron. 2009;24(11):3208–14. doi: 10.1016/j.bios.2009.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng X, et al. Enhancing the performance of a point-of-care CD4+ T-cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab Chip. 2009;9(10):1357–64. doi: 10.1039/b818813k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez WR, et al. A microchip CD4 counting method for HIV monitoring in resource-poor settings. PLoS Med. 2005;2(7):e182. doi: 10.1371/journal.pmed.0020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lilian RR, Bhowan K, Sherman GG. Early diagnosis of human immunodeficiency virus-1 infection in infants with the NucliSens EasyQ assay on dried blood spots. Journal of Clinical Virology. 48(1):40–43. doi: 10.1016/j.jcv.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Stevens WS, Scott LE, Crowe SM. Quantifying HIV for Monitoring Antiretroviral Therapy in Resource-Poor Settings. Journal of Infectious Diseases. 201:S16–S26. doi: 10.1086/650392. [DOI] [PubMed] [Google Scholar]
- 16.O’Gorman MR, Zijenah LS. CD4 T cell measurements in the management of antiretroviral therapy–A review with an emphasis on pediatric HIV-infected patients. Cytometry B Clin Cytom. 2008;74(Suppl 1):S19–26. doi: 10.1002/cyto.b.20398. [DOI] [PubMed] [Google Scholar]
- 17.Vassall A, et al. Patient costs of accessing collaborative tuberculosis and human immunodeficiency virus interventions in Ethiopia. International Journal of Tuberculosis and Lung Disease. 14(5):604–610. [PubMed] [Google Scholar]
- 18.Hogg R, et al. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO, Ua. AIDS epidemic update. 2009 [Google Scholar]
- 20.UNAIDS and WHO. 2007 AIDS epidemic update. 2007 [Google Scholar]
- 21.Marsden MD, Zack JA. Eradication of HIV: current challenges and new directions. Journal of Antimicrobial Chemotherapy. 2009;63(1):7–10. doi: 10.1093/jac/dkn455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization and UNAIDS. HIV assays: operational characteristics Report 16, Rapid assays. Geneva: World Health Organization; 2009. p. 50. [Google Scholar]
- 23.World Health Organization. Blood Safety Unit. and UNAIDS. Operational characteristics of commercially available assays to detect antibodies to HIV-1 and/or HIV-2 in human sera: report 11. Geneva: World Health Organization; 1999. p. 63. [Google Scholar]
- 24.World Health Organization. Dept. of Essential Health Technologies and UNAIDS. HIV assays: operational characteristics (Phase I) Report 13: Urine specimens, oral fluid (saliva) specimens. Geneva: World Health Organization; 2002. p. 30. [Google Scholar]
- 25.World Health Organization. Dept. of Essential Health Technologies and UNAIDS. HIV simple/rapid assays: operational characteristics (Phase I) Report 12: Simple rapid tests, whole blood specimens. Geneva: World Health Organization; 2002. p. 26. [Google Scholar]
- 26.World Health Organization. Dept. of Essential Health Technologies. and UNAIDS. HIV assays: operational characteristics (Phase 1) Report 15, Antigen/antibody ELISAs. Geneva: World Health Organization; 2004. p. 57. [Google Scholar]
- 27.World Health Organization. Dept. of Essential Health Technologies., UNAIDS., and WHO Collaborating Centre for HIV/AIDS Diagnostic and Laboratory Support. HIV simple/rapid essays: operational characteristics (Phase 1) Report 14, Simple/rapid tests. Geneva: World Health Organization; 2004. p. 63. [Google Scholar]
- 28.Huh D, et al. Microfluidics for flow cytometric analysis of cells and particles. Physiol Meas. 2005;26(3):R73–98. doi: 10.1088/0967-3334/26/3/R02. [DOI] [PubMed] [Google Scholar]
- 29.Laerum OD, Farsund T. Clinical application of flow cytometry: a review. Cytometry. 1981;2(1):1–13. doi: 10.1002/cyto.990020102. [DOI] [PubMed] [Google Scholar]
- 30.Miller RG, et al. Usage of the flow cytometer-cell sorter. J Immunol Methods. 1981;47(1):13–24. doi: 10.1016/0022-1759(81)90252-0. [DOI] [PubMed] [Google Scholar]
- 31.Mackenzie NM, Pinder AC. The application of flow microfluorimetry to biomedical research and diagnosis: a review. Dev Biol Stand. 1986;64:181–93. [PubMed] [Google Scholar]
- 32.Jennings CD, Foon KA. Flow cytometry: recent advances in diagnosis and monitoring of leukemia. Cancer Invest. 1997;15(4):384–99. doi: 10.3109/07357909709039744. [DOI] [PubMed] [Google Scholar]
- 33.Hengel RL, Nicholson JK. An update on the use of flow cytometry in HIV infection and AIDS. Clin Lab Med. 2001;21(4):841–56. [PubMed] [Google Scholar]
- 34.HIV Testing Implementation Guidance for Correctional Settings. Centers for Disease Control and Prevention; 2009. pp. 1–38. [Google Scholar]
- 35.Lofgren SM, et al. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. Aids. 2009;23(18):2459–2466. doi: 10.1097/QAD.0b013e328331f702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherman GG, Lilian RR, Coovadia AH. ORAL FLUID TESTS FOR SCREENING OF HUMAN IMMUNODEFICIENCY VIRUS-EXPOSED INFANTS. Pediatric Infectious Disease Journal. 29(2):169–172. doi: 10.1097/INF.0b013e3181b9a19c. [DOI] [PubMed] [Google Scholar]
- 37.Early infant diagnosis of HIV through dried blood spot testing: Pathfinder International/Kenya’s prevention of mother to child transmission project. Pathfinder International; Kenya: 2007. [Google Scholar]
- 38.Leelawiwat W, et al. Dried blood spots for the diagnosis and quantitation of HIV-1: Stability studies and evaluation of sensitivity and specificity for the diagnosis of infant HIV-1 infection in Thailand. Journal of Virological Methods. 2009;155(2):109–117. doi: 10.1016/j.jviromet.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 39.van Deursen P, et al. Measuring human immunodeficiency virus type 1 RNA loads in dried blood spot specimens using NucliSENS EasyQ HIV-1 v2.0. Journal of Clinical Virology. 47(2):120–125. doi: 10.1016/j.jcv.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 40.Lee WG, et al. Nano/Microfluidics for diagnosis of infectious diseases in developing countries. Advanced Drug Delivery Reviews. 2010;62(4–5):449–457. doi: 10.1016/j.addr.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Myers FB, Lee LP. Innovations in optical microfluidic technologies for point-of-care diagnostics. Lab on a Chip. 2008;8(12):2015–2031. doi: 10.1039/b812343h. [DOI] [PubMed] [Google Scholar]
- 42.Bhattacharyya A, Klapperich CM. Thermoplastic microfluidic device for on-chip purification of nucleic acids for disposable diagnostics. Analytical Chemistry. 2006;78(3):788–792. doi: 10.1021/ac051449j. [DOI] [PubMed] [Google Scholar]
- 43.Chudy M, et al. Miniaturized tools and devices for bioanalytical applications: an overview. Analytical and Bioanalytical Chemistry. 2009;395(3):647–668. doi: 10.1007/s00216-009-2979-2. [DOI] [PubMed] [Google Scholar]
- 44.Vacarro A, et al. Humanitarian Technology Challenge: Reliable Electric Power for Developing Countries. United Nations Foundation IEEE; [Google Scholar]
- 45.Kuhn W, et al. Usefulness of the paralens fluorescent microscope adaptor for the identification of mycobacteria in both field and laboratory settings. Open Microbiol J. 4:30–3. doi: 10.2174/1874285801004010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patterson KV, et al. Use of UV paralens adaptor for detection of acid-fast organisms. Journal of Clinical Microbiology. 1995;33(1):239–241. doi: 10.1128/jcm.33.1.239-241.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mattia AR, Waldron MA, Sierra LS. Use of the UV paralens adapter as an alternative to conventional fluorescence microscopy for detection of pneumocystis-carinii in direct immunofluorescent monoclonal antibody-stained pulmonary specimens. Journal of Clinical Microbiology. 1993;31(3):720–721. doi: 10.1128/jcm.31.3.720-721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tung YC, et al. PDMS-based opto-fluidic micro flow cytometer with two-color, multi-angle fluorescence detection capability using PIN photodiodes. Sensors and Actuators B-Chemical. 2004;98(2–3):356–367. [Google Scholar]
- 49.Lee GB, Lin CH, Chang GL. Micro flow cytometers with buried SU-8/SOG optical waveguides. Sensors and Actuators a-Physical. 2003;103(1–2):165–170. [Google Scholar]
- 50.Cho SH, et al. Human mammalian cell sorting using a highly integrated micro-fabricated fluorescence-activated cell sorter (microFACS) Lab Chip. 10(12):1567–73. doi: 10.1039/c000136h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozcan A, Demirci U. Ultra wide-field lens-free monitoring of cells on-chip. Lab Chip. 2008;8(1):98–106. doi: 10.1039/b713695a. [DOI] [PubMed] [Google Scholar]
- 52.Stybayeva G, et al. Lensfree holographic imaging of antibody microarrays for high-throughput detection of leukocyte numbers and function. Anal Chem. 2010;82(9):3736–44. doi: 10.1021/ac100142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tseng D, et al. Lensfree microscopy on a cellphone. Lab Chip. 10(14):1787–92. doi: 10.1039/c003477k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seo S, et al. Multi-color LUCAS: Lensfree On-chip Cytometry Using Tunable Monochromatic Illumination and Digital Noise Reduction. Cellular and Molecular Bioengineering. 2008;1(2–3):146–156. [Google Scholar]
- 55.Seo S, et al. Lensfree holographic imaging for on-chip cytometry and diagnostics. Lab Chip. 2009;9(6):777–87. doi: 10.1039/b813943a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su TW, et al. High-throughput lensfree imaging and characterization of a heterogeneous cell solution on a chip. Biotechnol Bioeng. 2009;102(3):856–68. doi: 10.1002/bit.22116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hauck TS, et al. Nanotechnology diagnostics for infectious diseases prevalent in developing countries. Advanced Drug Delivery Reviews. 62(4–5):438–448. doi: 10.1016/j.addr.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Herr AE, et al. Microfluidic immunoassays as rapid saliva-based clinical diagnostics. Proc Natl Acad Sci U S A. 2007;104(13):5268–73. doi: 10.1073/pnas.0607254104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Casanova AM, et al. Battery power comparison to charge medical devices in developing countries. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:931–4. doi: 10.1109/IEMBS.2009.5333717. [DOI] [PubMed] [Google Scholar]
- 60.Martinez AW, et al. Simple telemedicine for developing regions: camera phones and paper-based microfluidic devices for real-time, off-site diagnosis. Anal Chem. 2008;80(10):3699–707. doi: 10.1021/ac800112r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xie J, Lee C, Feng HH. Design Fabrication, and Characterization of CMOS MEMS-Based Thermoelectric Power Generators. Journal of Microelectromechanical Systems. 19(2):317–324. [Google Scholar]
- 62.Leonov V, Vullers RJM. Wearable electronics self-powered by using human body heat: The state of the art and the perspective. Journal of Renewable and Sustainable Energy. 2009;1(6) [Google Scholar]
- 63.Qi Y, et al. Piezoelectric Ribbons Printed onto Rubber for Flexible Energy Conversion. Nano Letters. 10(2):524–528. doi: 10.1021/nl903377u. [DOI] [PubMed] [Google Scholar]
- 64.Watkins N, et al. A robust electrical microcytometer with 3-dimensional hydrofocusing. Lab on a Chip. 2009;9(22) doi: 10.1039/b912214a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Powell D. Building a handheld HIV detector. Inside Science News Service [Google Scholar]
- 66.Zhu H, et al. A miniature cytometry platform for capture and characterization of T-lymphocytes from human blood. Anal Chim Acta. 2008;608(2):186–96. doi: 10.1016/j.aca.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 67.Cheng X, et al. A microfluidic device for practical label-free CD4(+) T cell counting of HIV-infected subjects. Lab Chip. 2007;7(2):170–8. doi: 10.1039/b612966h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lee SH, et al. A polymer lab-on-a-chip for reverse transcription (RT)-PCR based point-of-care clinical diagnostics. Lab on a Chip. 2008;8(12):2121–2127. doi: 10.1039/b811131f. [DOI] [PubMed] [Google Scholar]
- 69.Tang W, et al. Nucleic Acid Assay System for Tier II Laboratories and Moderately Complex Clinics to Detect HIV in Low-Resource Settings. Journal of Infectious Diseases. 201:S46–S51. doi: 10.1086/650388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fang XE, et al. Loop-Mediated Isothermal Amplification Integrated on Microfluidic Chips for Point-of-Care Quantitative Detection of Pathogens. Analytical Chemistry. 82(7):3002–3006. doi: 10.1021/ac1000652. [DOI] [PubMed] [Google Scholar]
- 71.Walling MA, Novak JA, Shepard JRE. Quantum Dots for Live Cell and In Vivo Imaging. International Journal of Molecular Sciences. 2009;10(2):441–491. doi: 10.3390/ijms10020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yun KS, et al. A microfluidic chip for measurement of biomolecules using a microbead-based quantum dot fluorescence assay. Measurement Science & Technology. 2006;17(12):3178–3183. [Google Scholar]
- 73.Liu WT, et al. Microfluidic device as a new platform for immunofluorescent detection of viruses. Lab Chip. 2005;5(11):1327–30. doi: 10.1039/b509086e. [DOI] [PubMed] [Google Scholar]
- 74.Klostranec JM, et al. Convergence of quantum dot barcodes with microfluidics and signal processing for multiplexed high-throughput infectious disease diagnostics. Nano Lett. 2007;7(9):2812–8. doi: 10.1021/nl071415m. [DOI] [PubMed] [Google Scholar]
- 75.Nations, U. The Millennium Development Goals Report 2010. :42–53. [Google Scholar]
- 76.The Global HIV/AIDS Timeline. The Henry J. Kaiser Family Foundation; p. Timeline based on multiple sources. [Google Scholar]
- 77.Curioso WH, Kurth AE. Access, use and perceptions regarding Internet, cell phones and PDAs as a means for health promotion for people living with HIV in Peru. BMC Med Inform Decis Mak. 2007;7:24. doi: 10.1186/1472-6947-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Godse CS, et al. Mobile camera microphotography: a simple but elegant technique for telediagnosis of malaria. JK Science. 2008;10(3) [Google Scholar]
- 79.Bellina L, Missoni E. Mobile cell-phones (M-phones) in telemicroscopy: increasing connectivity of isolated laboratories. Diagn Pathol. 2009;4:19. doi: 10.1186/1746-1596-4-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mudanyali O, et al. Compact, light-weight and cost-effective microscope based on lensless incoherent holography for telemedicine applications. Lab Chip. 10(11):1417–28. doi: 10.1039/c000453g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramachandran D, et al. Mobile-izing health workers in rural India. ACM: CHI 2010:†Proceedings of the 28th international conference on Human factors in computing systems [Google Scholar]
- 82.Carty D. U.S. Lifts Immigration Ban on HIV/Aids Patients. CBSNews.com [Google Scholar]
- 83.Shapiro L. UNAIDS report on the end of the HIV ban. Immigration Equality [Google Scholar]
- 84.WHO. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector. Chapter 2: HIV testing and counseling. 2009 [Google Scholar]
- 85.Sinkala M, et al. Zambian women’s attitudes toward mass nevirapine therapy to prevent perinatal transmission of HIV. Lancet. 2001;358(9293):1611–1612. doi: 10.1016/S0140-6736(01)06662-4. [DOI] [PubMed] [Google Scholar]
- 86.UNAIDS. 2008 Report on the Global AIDS Epidemic: Addressing Societal Causes of HIV Risk and Vulnerability. 2008 [Google Scholar]
- 87.Engelbrecht L. Clearing m-health hurdles. ITWeb 2009 [Google Scholar]
- 88.Mubiana-Mbewe M, et al. Causes of morbidity among HIV-infected children on antiretroviral therapy in primary care facilities in Lusaka, Zambia. Tropical Medicine & International Health. 2009;14(10):1190–1198. doi: 10.1111/j.1365-3156.2009.02360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


