Abstract
Three-dimensional (3D) printing is becoming an increasingly common technique to fabricate scaffolds and devices for tissue engineering applications. This is due to the potential of 3D printing to provide patient-specific designs, high structural complexity, rapid on-demand fabrication at a low-cost. One of the major bottlenecks that limits the widespread acceptance of 3D printing in biomanufacturing is the lack of diversity in “biomaterial inks”. Printability of a biomaterial is determined by the printing technique. Although a wide range of biomaterial inks including polymers, ceramics, hydrogels and composites have been developed, the field is still struggling with processing of these materials into self-supporting devices with tunable mechanics, degradation, and bioactivity. This review aims to highlight the past and recent advances in biomaterial ink development and design considerations moving forward. A brief overview of 3D printing technologies focusing on ink design parameters is also included.
Keywords: rapid prototyping, additive manufacturing, polymers, hydrogel, ceramic, tissue engineering
Graphical Abstract
1. INTRODUCTION
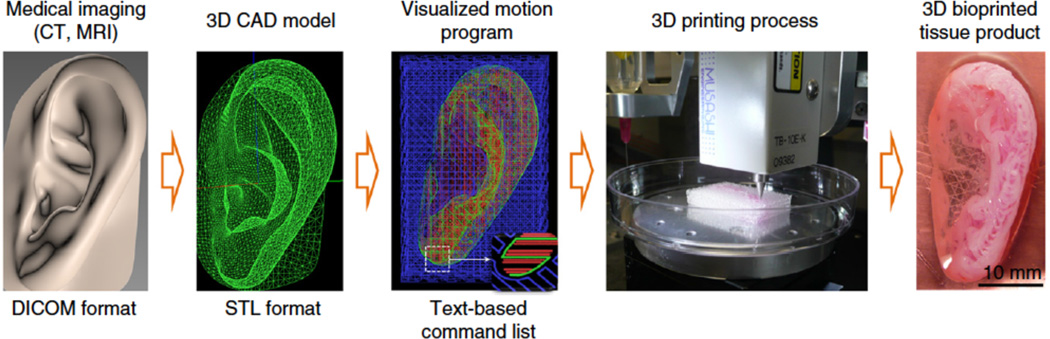
3D printing is an additive manufacturing technique that allows fabrication of modular and patient-specific scaffolds with high structural complexity and design flexibility. This technology enables design and fabrication of constructs based on tissue images captured with commonly used medical imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI) (Figure 1). This is not possible with conventional fabrication techniques. Recently, 3D printing has found a wide range of applications in medicine, including craniofacial implants,1 dental molds, crowns and implants,1,2 prosthetic parts,3 on-demand medical equipment,4,5 surgical models,6–9 scaffolds for tissue regeneration such as skin and bone,10,11 organ printing,12–15 and tissue models for drug discovery.16,17 The ability to make patient-specific devices, control orientation and porosity, and combine multiple materials, both synthetic and biological, has attracted the attention of many curious minds. As a result, this surge in technology has led to the creation of many breakthrough treatments and devices. For instance, recent studies showed the potential of this technology for biofabrication of anatomically shaped and scaled tissue constructs with structural integrity.18,19 All of this is thanks to the recent advances in 3D printing technology and biomaterial design.20,21
Figure 1.
Common stages of the 3D printing process to develop tissue-mimetic devices. A 3D computer assisted design (CAD) model is developed from a medical image of the target tissue. Digitally sliced images that consist of text-based command lists, including ink parameters and printing directions, are generated. A 3D printer generates the tissue mimetic construct. Reprinted with permission from ref 19 Copyright 2016 Nature Publishing Group.
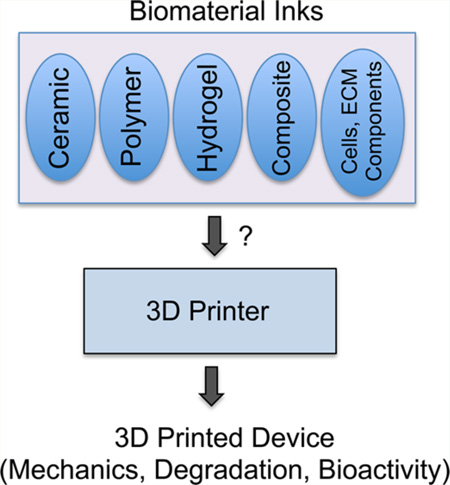
Although 3D printing technology offers great potential, there are still significant issues to overcome before it can be recognized as a common biofabrication technique in medicine. One of the major issues is the limited capabilities of the 3D printers. Processing speed, printing speed and printer resolution have increased vastly over the past years yet still lag behind optimal levels in many cases. The second major issue is the lack of diversity in 3D printable biomaterials. Many printable materials have great properties for external applications, but implantable biomaterials require specific characteristics based on both physiological conditions and interactions with the body that make development much more difficult. In general, printable biomaterials must: (1) be printable, (2) be biocompatible, (3) have appropriate mechanical properties, (4) have good degradation kinetics, (5) form safe degradation byproducts, and (6) exhibit tissue biomimicry. How to fulfill each of these requirements varies slightly depending on which printing method is being used and the projected end application of the device. Furthermore, many of these characteristics can work against each other. For example, in bone tissue, it is desirable to have stiff materials for osteoblast development and load bearing, however, this can lead to either slow to nonexistent degradation. Soft materials can be printable and quicker to biodegrade, however, their ability to be handled and applied to certain tissue types may be a concern. The majority of 3D printed constructs are used in bone or cartilage applications due to the inherent stiffness of most printed biomaterials mimicking the natural stiffness of these tissues, outside of some hydrogel systems. Ultimately, a balance between all these parameters must be struck for creation of an appropriate printable biomaterial. Finally, reproducibility concerns, quality control issues, and regulatory hurdles need to be addressed before 3D printed scaffolds and devices can reach the medical market.
In this review, our goal is to give a detailed summary of currently available 3D printable biomaterials, including advantages and limitations for tissue engineering applications, and design criteria for printability. A brief overview of 3D printing techniques is also included as printability of a biomaterial is strictly determined by the printing technique. We hope that this review will inspire the scientific community to develop novel 3D printable biomaterials, which we believe will eventually ensure the success of 3D printing in tissue engineering and regenerative medicine applications.
2. OVERVIEW OF 3D PRINTING TECHNOLOGIES
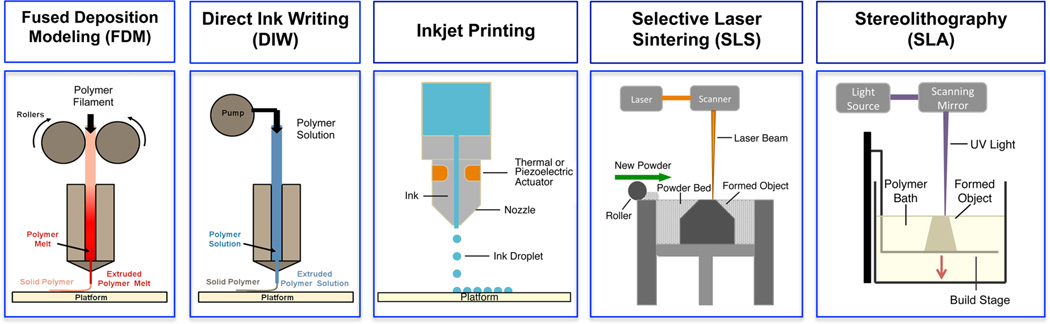
Printability of a biomaterial system is strictly determined by the printing technique. Although it is possible to print the same material using multiple printing techniques “ink formulation”, i.e., the form and composition of the printable material, varies significantly. This review focuses on the most commonly used 3D printing technologies for biomedical applications. 3D printing technologies are classified under four main groups in this review: extrusion-based methods, particle fusion-based methods, light induced (photopolymerization) methods and inkjet printing (Figure 2). Each of these categories contains subgroups that use slight mechanical or chemical variations on each technique, which affect the material properties required for successful design and printing of the “ink” material.
Figure 2.
Schematics depicting 3D printing techniques: extrusion-based methods such as fused deposition modeling (FDM) and direct ink writing (DIW), inkjet printing, particle fusion-based method such as selective laser sintering (SLS), and light-based method stereolithography (SLA).
2.1. Extrusion-Based 3D Printing Methods
Extrusion-based 3D printing methods, such as fused deposition modeling (FDM) and direct ink writing (DIW), are some of the most widespread methods to fabricate devices and scaffolds for tissue engineering applications.22 The idea behind extrusion based additive manufacturing is that an ink is forced through a nozzle as a viscous liquid or melt to form individual lines that solidify onto a build plate. As the material is extruded, the nozzle follows a predefined path determined by a computer model to build up a 3D object layer-by-layer. For traditional FDM, the ink is in the form of a solid filament (typically, 1.50 or 1.75 mm in diameter). The ink is rolled into a hot nozzle (typically temperatures up to 200 °C) where it is melted (to become flowable) at the nozzle, and extruded using a motorized pinch roller system. This requires materials, generally polymers that can be formed into a filament with sharp solid-to-melt transition (i.e., flow and solidify readily upon melting and cooling, respectively).
Solvent-cast direct-writing inks are either polymer solutions in a preferably water-miscible, low boiling point solvent (such as dichloromethane (DCM), dimethyl sulfoxide (DMSO) or tetrahydrofuran (THF)) that evaporates rapidly upon extrusion, leaving a solid polymer matrix behind,23 or hydrogels that maintain structure following extrusion. Structural integrity of 3D printable hydrogels can be maintained postextrusion using thixotropic behavior, temperature sensitivity or cross-linking or a combination thereof. For FDM and DIW, resolution levels for nozzle location of 25 µm in the x, y plane are achievable with line dimensions and layer thickness of 200–500 µm, determined by the nozzle diameter.24 In general, these two methods struggle with creating structures with long, unsupported sections or sharp overhangs due to the fabrication process. Extruded filaments tend to lack the material strength to support themselves immediately upon extrusion, leading to sagging or complete collapse of the unsupported segment. Filler materials that could be removed (dissolved or burned out) postprinting have been developed to eliminate this issue.25 But they need to be printed in parallel with the actual ink, which requires printers with dual nozzles. In summary, FDM and DIW are methods that are easy to use and have been employed effectively for tissue engineering applications.26
2.2. Particle Fusion-Based 3D Printing Methods
Particle fusion printing methods, consisting of selective laser sintering (SLS) and particle binding (PB), have found significant applications in industrial prototyping due to the ability to print polymers, ceramics, metals and composites of these into unique and complex geometries.27 SLS uses a directed laser beam, traditionally from a CO2 laser, to raise the temperature of the polymer or metal particles to above their melting temperature, causing the particles to fuse together.28 The beam is patterned over the cross sectional area of the computer-modeled object to create a single layer, at which point a new layer of particles is applied over the top, and the process is repeated. Therefore, the ink materials suitable for SLS should be processable into a fine powder form (range from 10 to 100 µm), and must have an attainable melting temperature, and bind together when heated (above Tm). It is also important that the particles possess good particle flow dynamics within the bed system, which may require surface functionalization to eliminate electrostatic forces.29 Generally, SLS machines are slow, bulky, expensive, and require a large amount of material. However, the ability to process multiple materials in a single bed has proven useful in many manufacturing industries.
PB (also referred to as indirect SLS) follows the same principles as selective laser sintering but instead of melting particles together with a laser, this technology uses a liquid binding solution to fuse particles together within each layer followed by a high-temperature, sintering step to solidify the final 3D object postproduction.30–32 For both technologies, resolution is in the range of 700–1000 µm in the horizontal axis and 100 µm in the vertical axis.29 Resolution is significantly affected by the particle size (10–100 µm), the particle size distribution, material binding properties, and laser or binder width.27 SLA and PB methods have been used to create devices for hard-tissue engineering applications, such as orthopedics and oral surgeries.32–35
2.3. Light-Assisted 3D Printing Methods
Light-assisted 3D printing, also known as stereolithography (SLA), is considered the original additive manufacturing method after Charles Hull first developed and commercialized the process of curing specific areas of polymer resins in the mid-1980s. SLA involves patterning a beam of light (UV or laser) over a bath of photopolymerizable (viscous) liquid polymer to create a single hardened polymer layer. After polymerization, the build stage lowers further into the solution, allowing new resin to flow over the printed surface, and the next layer is polymerized on top of the previous. Recent advances in the development of more efficient light sources and refined mirror-lens systems have drastically improved SLA regarding both its speed and resolution. For instance, Tumbleston et al. demonstrated the continuous generation of polymeric parts up to tens of centimeters in size with feature resolution below 100 µm within minutes instead of hours.36 They established a continuous liquid interface achieved with an oxygen-permeable window below the ultraviolet image projection plane leading to a persistent liquid interface where photopolymerization is inhibited between the window and the polymerizing part. Of all the printing methods, SLA has one of the highest resolutions with traditional SLA reaching 25 µm resolution, whereas micro SLA (µ-SLA) and high-definition SLA devices reach feature resolutions in the single micrometer range.37 However, SLA has been limited in its biomedical applications by the harsh nature of UV-based cross-linking, extensive postprocessing, inadequate mechanical properties, trapping of liquid resin within the end product and most importantly the lack of available biocompatible and biodegradable materials suitable for SLA. Recent developments in both natural and synthetic biodegradable, cross-linkable polymers, as well as higher-resolution machines have started to open SLA to a wider array of applications, especially in tissue engineering.
2.4. Inkjet Printing
Inkjet printing enables disposition of very small volumes (1–100 picoliters)38 of individual droplets from a nozzle to a printing surface with the goal of forming structures postsolidification. Multinozzle inkjet print heads containing several hundred individual nozzles have been developed to accelerate the printing process. Inkjet printers are classified into two groups based on the droplet generation mechanism: continuous inkjet (CIJ) printing and drop-on-demand (DOD) inkjet printing.38 In CIJ printing continuous stream of drops (around 100 µm in diameter) are produced and unused ink is recycled. In DOD inkjet printing, individual drops (in the range of 25 to 50 µm in diameter) are generated when required. DOD type printers are commonly used for tissue engineering applications. The power of inkjet printing is the spatial resolution, i.e., placement of picoliter drops with positional accuracy (∼10 µm in x-y-axis). There are three important stages in inkjet printing that define and constrain printable ink formulations including drop generation, drop/ substrate interaction, and drop solidification.38–40 The mechanism of drop formation defines the ink (fluid) properties that are required for a given polymer solution to be printable. The most important properties of the ink are the viscosity and the surface tension. The viscosity of the ink should be suitably low usually below 10 cP (mPa s), under high shear rates, between 1 × 105 and 1 × 106 −1. The surface tension determines the shape of the drop emerging from the nozzle and the shape of the drop on the substrate. Surface tension values of the inks generally range from 28 to 350 mN m−1. The resolution and accuracy of the printed object are determined by the interaction between adjacent drops (coalescence)41,42 and between individual drops and the substrate (such as surface tension and wettability).43,44 The liquid-to-solid phase transformation (i.e., solvent evaporation, temperature controlled transition, or gelling of a precursor solution)38 controls the final shape and size of the printed objected.
Inks in the form of photocurable solutions, colloidal suspensions (allowing high-molecular-weight polymers in low viscosity form), and polymer melts are suitable for tissue engineering applications. Ceramic suspensions have also been printed using inkjet printing.45–48 Polymers in solution (or in melt state) can undergo irreversible structural changes due to flow-induced deformations.49–51 This could even lead to chain scission if the rate of chain extension exceeds the rate of chain relaxation.52 Biological materials, including cells, can be incorporated into the ink formulation, forming bioinks.53–56 However, the shear forces and temperature changes at the point of extrusion should also be taken into consideration. Development of open pool, nozzleless bioprinting systems has begun to address some of the problems associated with forces experienced by cells upon extrusion.57 In addition, to maintain droplet formation and reduce clogging and shear stress the achievable cell density is often limited in comparison with physiological cell densities. It is also not possible to create unsupported structures such as overhangs and bridges using inkjet printing. Inkjet printing has been used for tissue engineering (bioadhesives, scaffolds, and living cells) and pharmaceutical applications.53,55,56,58,59
3. INKS: 3D PRINTABLE BIOMATERIALS
The improvements in 3D printing technologies over the past 15 years have brought these technologies to many new fields. Medical devices and tissue engineering are one particular area where 3D printing has garnered significant interest. With the potential to fabricate patient-specific, customizable devices in short time frames and for lower costs, 3D printing is a perfect technology for the coming era of personalized medicine. A wide range of biomaterials has been used as inks forming 3D structures with a wide range of size and stiffness (Figure 3).60,61 However, because of their beginnings in industrial prototyping, most 3D printing methods lack sufficiently developed biocompatible materials that can compete with traditional biomedical treatments. In this section, we will outline the material properties that are key to developing biomaterial inks for each printing method and review currently used biomaterial inks used in 3D printing, including their specific benefits and drawbacks (Figure 3).
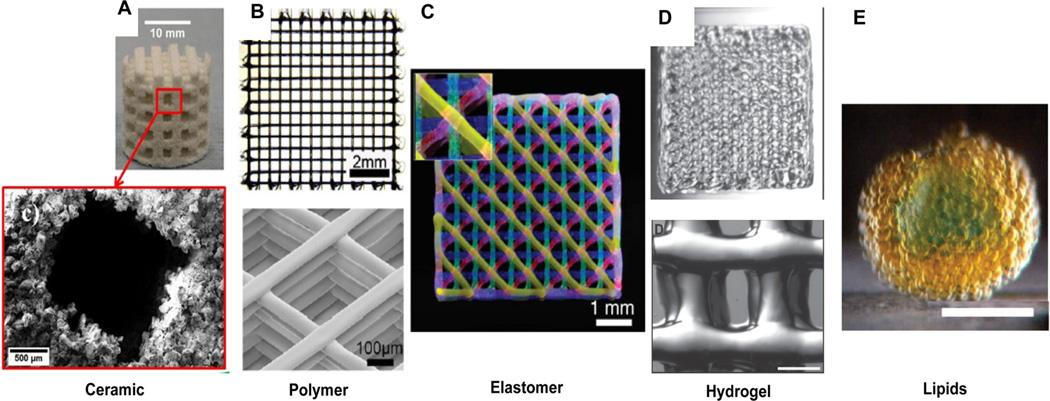
Figure 3.
3D printed constructs from hard (left) to soft (right) in nature. (A) (Top) Structure of 50 wt % hydroxylapatite (HA) scaffold, (bottom) SEM image of the pore and surrounding ceramic particles. Reproduced with permission from ref 146. Copyright 2015 Elsevier. (B) Polycaprolactone scaffold (solvent-cast three-dimensional printing of multifunctional microsystems. Reproduced with permission from ref 23. Copyright 2013 John Wiley and Sons. (C) Fluorescent image of 4-layer lattice printed by sequential depositing of four PDMS inks each dyed with a different fluorophore. Reproduced with permission from ref 60. Copyright 2014 John Wiley and Sons. (D) PEG-based hydrogel with gelatin (15 mm × 15 mm), bottom structure scale bar, 500 µm. Reproduced with permission from ref 113. Copyright 2015 John Wiley and Sons. (E) Picture of a hollow sphere-shaped lipid droplet network (printed in bulk aqueous solution). Scale bar, 200 mm. Reproduced with permission from ref 61. Copyright 2013 AAAS.
3.1. Polymeric Biomaterial Inks
Polymers make up the majority of the biomaterial inks used in 3D printing due to their ease of processability, low cost, and properties such as biocompatibility, degradation and mechanics. Polymer inks come in the form of filaments for FDM, beads (powders) for SLS, solutions and gels for DIW, and solutions for SLA. Each of these techniques requires specific material parameters for printability. For instance, polymer filaments must have a constant diameter of 1.75 mm for most FDM machines, a sharp solid-to-melt transition to facilitate viscous melt formation pre-extrusion and solidification postextrusion, elastic modulus to melt viscosity ratio below 5 × 105 −1 to prevent filament buckling and shear thinning tendencies in liquid form.22
Solutions for DIW are often comprised of a polymer dissolved in a rapidly evaporating organic solvent, such as dichloromethane, tetrahydrofuran or dimethyl sulfoxide that rapidly dissipates upon extrusion, leaving a solid polymer strut behind. These DIW inks must dry in times of seconds to minutes in order to maintain shape integrity, support polymer solubility in excess of 20–30 wt %, achieve complete solvent removal postmanufacturing, have sufficiently low viscosity to facilitate printing at moderate to low pressure, and be shear thinning to prevent clogging and facilitate flow.
SLS of polymer beads requires bead diameters in the range of 10–150 µm to allow for particle flow within the bed while maintaining print resolution,27,35 melt temperature attainable by the laser, generally below 200 °C used, and low melt viscosity. Finally, stereolithography relies heavily on stable, photo-cross-linkable polymers that react and polymerize rapidly when subject to UV radiation. The density and viscosity of the liquid bath must be such that the final 3D printed object is sturdy enough for the desired function and that new material flows rapidly into place when the build stage lowers following each completed layer while cross-linking should be rapid to maintain resolution and print speed. On the basis of these design parameters, many different materials have been fashioned into printable polymer bioinks for 3D printing technologies.
3.1.1. Poly(lactic acid)
Polylactic acid (PLA) is the preeminent polymer for FDM due to its low cost, nontoxicity, biocompatibility, biodegradability, renewable feedstocks and easy processability.62,63 With a melt temperature around 175 °C, PLA can be easily formed into filaments for use with melt based printing systems where it is generally extruded between 200 and 230 °C. A serious concern surrounding PLA is its long-term biocompatibility because of the release of acidic byproducts during degradation, which could lead to tissue inflammation and cell death.64 Degradation occurs through hydrolysis of the ester bond and results in the localized decrease in physiological pH through the release of lactic acid. To remedy this, PLA has been combined with ceramics, primarily calcium phosphates, to create composite scaffolds with increased bone response and reduce formation of a localized acidic environment.65,66 PLA has good mechanical properties for a synthetic polymer but tends to be brittle and have a lower compressive strength compared to bone, which can be problematic because of its primary use in musculoskeletal tissue engineering. Again, composites of PLA with ceramics can be beneficial, this time by increasing the compressive strength and improving mineralization upon implantation. Despite these drawbacks, PLA is one of the best and most commonly used printable materials available for biological applications of FDM.
3.1.2. Poly(d,l-lactide)
(PDLLA) oligomers functionalized with methacrylol chloride have been developed for biomedical applications of SLA.67 Previously, PDLLA had been used successfully in resorbable bone fixation devices, which is indicative of its good biocompatibility and high mechanical strength. In this system, functionalized PDLLA was diluted in nonreactive ethyl lactate to form the resin and photocured with UV light. Following scaffold formation, the structures were extracted with acetone and isopropanol to remove unreacted resin. Mouse preosteoblasts readily adhered to the scaffold surface and proliferated well, showing that the biocompatibility expected from this material was not altered through the chemical modification or printing method.67 In a similar system, PDLLA has been functionalized with fumaric acid monoethyl ester to provide photoionizability and diluted in N-vinyl-2-pyrrolidone (NVP) to form a resin. Porous 3D scaffolds were printed through directed UV cross-linking of the resin and seeded with mouse preosteoblasts after processing. The seeded cells attached to the polymer network and proliferated throughout the scaffold.68 The hydrophilicity of the PDLLA networks can be enhanced by increasing the amount of NVP, which could have beneficial effects on cell attachment and tissue integration. The biocompatibility of PDLLA scaffolds along with the mechanical properties warrants further investigation as a 3D printable scaffolding material.
3.1.3. Poly(caprolactone) (PCL)
PCL, like polylactic acid, is a low cost, biodegradable polyester that has seen a surge in use with the advent of FDM and the recent need for more biocompatible materials.69 Once cast aside by history, this polymer is back at the forefront of science again due to the growth of tissue engineering and 3D printing.69 PCL has excellent rheological and viscoelastic properties upon heating that make it a prime candidate for melt-based extrusion printing. However, the stiffness and extended degradation profile of printed PCL make it useful mostly for hard tissue engineering. PCL is stable in the body for over 6 months and degrades fully and nontoxically in around three years, allowing it to provide support during healing and later be absorbed over time. Because of its history in drug delivery devices, PCL has a shorter regulatory path to market than many other polymer systems, further adding to its benefits. For instance, a custom-designed airway splint device was printed using PCL, and administered to the patient under the emergency-use exemption from the Food and Drug Administration.70
PCL is also the main polymer used in SLS systems in the form of beads in the size range of 10–100 µm, which can be melt-fused with laser heating.71 Scaffolds printed with PCL using SLS showed good porosity with high levels of interconnectedness, beneficial surface roughness and compressive modulus similar to bone.71 When seeded with BMP7-transduced fibroblasts and implanted subcutaneously, these scaffolds show good tissue ingrowth and bone generation. This model was applied to rapid fabrication of a pig condyle defect with a PCL and beta-tricalcium phosphate (β-TCP) mixture to show that CT images of actual defects could be processed and produced in short time frames.72
3.1.4. Poly(propylene fumarate) (PPF)
PPF is one of the most extensively studied biodegradable and photo-cross-linkable polymers used in stereolithography.73 Generally, the printing solution used in SLA consists of PPF as the base polymer combined with diethyl fumarate (DEF) as the solvent and bisacrylphosphrine oxide as the photoinitiator. Solution viscosity and PPF/DEF ratio play an important role in printability and mechanical strength of the final scaffold.74 DEF is required to reduce the viscosity of the polymer so that the material flows over the printed scaffold for construction of each new layer. This must be balanced carefully as mechanical strength of scaffolds decreases dramatically at PPF/DEF ratios below 50% because of changes in the polymer density. In a recent study, scaffolds created using PPF stereolithography were coated with either β-TCP, synthetic bone mineral (SBM) or biphasic calcium phosphate (BCP) along with bone morphogenetic protein (BMP-2) and studied in vitro and in vivo.75 A rabbit model showed bone regeneration throughout the scaffolds with both ingrowth of native bone from the edges and generation of bone at locations on the interior of the scaffold for all three coatings. There was no sign of a persistent inflammatory response in any of the investigated scaffolds indicating no problems with residual solvents. From this study, it is evident the BCP, β-TCP, and SBM loaded with BMP all enhance osteoconductivity and osteointegration of PPF scaffolds but none provides any particular advantages.75
3.1.5. Polyether Ether Ketone (PEEK)
PEEK is a semi-crystalline polymer that has most commonly been used as an ink material for additive manufacturing to create customized craniofacial implants.76 PEEK has many beneficial properties that make it a prime material for bone replacement including bioinertness, biocompatibility, radiolucency, low heat conductivity, and strength and elasticity comparable to cortical bone. Processing conditions for PEEK are more extreme than other polymers because of its high melting point, around 350 °C, which has limited its application to only selective laser sintering.77 However, this same property gives 3D printed PEEK objects superior heat resistance, allowing them to undergo steam sterilization without softening.78 As a biomaterial, PEEK lacks the osteointegrative properties that make for good tissue engineering materials. Because it does not integrate with native tissues well, it is often at risk to trigger foreign body reactions including encapsulation, dislodging, and extrusion. Furthermore, PEEK implants are more expensive than many other polymer implants. Recent advances in FDM printer technology have increased processing temperatures attainable by the hot end of FDM machines, leading to the development of PEEK filaments for this more common 3D printing method. With the greater speed, availability, and reduced cost of FDM printing now in play, further biomedical uses of PEEK are starting to be explored.79
3.1.6. Poly(butylene terephthalate)
Polybutylene terephthalate (PBT) is another thermoplastic polyester, similar to PLA and PCL, and it has been used in FDM printing experiments. However, due to the lack of apparent advantages over either PLA or PCL and higher melting point of 225 °C, it has seen much less investigation as a 3D printable material than the other two polyesters despite its similar biocompatibility. In one experiment, filaments of PBT were used in an FDM process to create bone scaffolds based on micro CT scans of canine trabecular bones.80 These bone scaffolds were able to match the trabecular bone in porosity, indicating the potential for creating biomimetic scaffolds in the future, but cell compatibility was not investigated. Calcium phosphate coated PBT scaffolds have previously been shown to increase bone formation in a canine knee model without significant drawbacks.81 PBT-poly(ethylene oxide) (PEO) coatings have also been used to increase the bone binding properties of titanium alloy implants.82 These beneficial effects could either be a result of the coating and scaffold combination or the coating alone.
3.1.7. Acrylonitrile Butadiene Styrene (ABS)
ABS is a petrochemical-based, triblock copolymer that possesses good strength from the acrylonitrile and butadiene elements while gaining toughness from the styrene units, giving it an advantage over the somewhat brittle polyester materials. This combined with a melting point of 105 °C makes ABS is an attractive candidate for use in FDM and SLA systems. However, ABS has seen limited use as a scaffold material outside of cartilage engineering because it generally performs the same or worse than PLA in areas of cell integration, processability and cost.83,84 In addition, it is not biodegradable, which is a major detriment in an industry that is moving toward resorbable materials.
3.2. Hydrogel Inks
Hydrogels are three-dimensional polymer networks with the ability to hold a large quantity of water. Hydrogels have been exploited in a variety of biomedical applications including 2D and 3D culture scaffolds for tissue engineering applications, as well as cell and/or biomolecule delivery.85–87 Hydrogels provide perfect “soft material” systems to mimic native extracellular matrix (ECM) microenvironments due to their tunable mechanics, degradation and functionalizability.88 Injectable, shear-thinning hydrogels are a subgroup of all hydrogel systems but form the majority of those used in research and clinical biomedical applications.89 Hydrogel inks formulated from injectable hydrogels are required to (1) flow under modest pressures, (2) gel quickly, and (3) maintain sufficient integrity after build up.90 Hydrogel inks are referred to as bioinks when they contain cells and/or biochemical molecules such as ECM components. Inkjet, light-assisted, and extrusion-based 3D printing systems are the most suitable methods for hydrogel printing.91–94 The classical approach to designing a hydrogel ink is to formulate a polymer solution that forms a network immediately after printing. The network could be physically or chemically cross-linked in response to an external stimulus such as temperature, light, or ion concentration. The main advantage of the physically cross-linked hydrogels is the absence of chemical agents, which decreases the material toxicity. On the other hand, chemically cross-linked hydrogels are prepared through covalent bond formation and the resulting hydrogel is more resistant to mechanical forces but it usually undergoes greater volume changes than physically cross-linked networks. There are a limited number of suitable hydrogels that can act as a bioink, and tuning their properties remains a challenge.95
Generally, common hydrogels for 3D bioprinting are made from natural polymers such as alginate, agar, gelatin, cellulose, collagen, fibrinogen, hyaluronic acid,96,97 or from synthetic polymers such as polyacrylamide,98 polyurethane,99 poly-(ethylene glycol) (PEG),100,101 or a synthetic-natural mixture.102,103 Their physicochemical properties and gelation method can be tuned through chemical, physical and enzymatic mechanisms or modulated by thermal/pH sensitivity.104 For instance, alginate is a versatile polysaccharide and its gelation properties can be induced either by addition of divalent cations as gelling agents102,105–109 or through chemical modification of alginate-dialdehyde (ADA) with gelatin.110 A triple network hydrogel composed by alginate and agar was reported.111 In this system, the entanglement of the alginate chains with the agar double network restricted the agar helical chain bundles and formed an extremely tough hydrogel.
Direct-write bioprinting of cell-laden, and chemically and photo-cross-linked gelatin hydrogels has been reported in the literature.112–114 Billiet et al. proposed the alternative use of a biocompatible VA-086 as a photoinitiator compared to the conventional Irgacure 2959.91 Their findings showed a high cell viability (>97%) for 3D printing of cell laden gelatin methacrylamide (GelMa). In another work, Bertassoni et al. showed that a significant pressure was required to dispense the cell-laden GelMa hydrogels from the glass capillary.114 But, cell viability and proliferation were not significantly affected, suggesting that the hydrogel matrix may function as barrier to protect the encapsulated cells from the shear stress resulting from friction with the capillary dispensing system. Novel processing strategies for commercial materials were found by Matrigel115 and Pluronic F-127.116 Matrigel and mixed Pluronic and calcium phosphate cell-laden hydrogels were biofabricated and applied as a microtissue onto a microfluidic device and as a composite hydrogel and ceramic ink for robotic-assisted deposition, respectively.
Usually high polymer concentrations are required to formulate hydrogel inks for printing multiple layers with high shape conformity.111 However, highly dense hydrogel constructs are usually detrimental for cell encapsulation, limit nutrient and waste transportation, and inhibit network remodeling and functional integration of the construct. To overcome some of these problems, Li et al. developed a two-component DNA hydrogel ink system, composed of polypeptide-DNA conjugate and complementary DNA linker, that displays rapid cross-linking because of DNA hybridization.117 They were able to fabricate cell containing 3D structures with tunable hydrogel properties. Villar et al. developed tissue-like structures printing aqueous droplets into a solution of lipids in oil leading to lipid monolayers forming bilayers with droplets in the growing network (Figure 3E).61
Pre- or postprinting cross-linking (chemical or UV-assisted) using multimaterial inks have also been shown to further enhance conformity of printed constructs. For example, two-component hydrogels consisting of a thermoresponsive polymer mixed with poly(ethylene glycol) or a hyaluronic acid were partially cross-linked by a chemoselective oxo-ester reaction before extrusion-based printing.118 The 3D hydrogels were deposited on a heated plate at 37 °C and subsequently chemical cross-linking was conducted to form stable hydrogels. This reinforced construct exhibited an elastic modulus of 9 kPa after 3 h, and showed high cell viability of chondrocytes.118 In another study, a photopolymerizable and thermosensitive A-B-A type triblock copolymer ink was developed.119 The ink was composed of poly(N-(2-hydroxypropyl)methacrylamide lactate) A-blocks partially functionalized with methacrylate groups, and poly(ethylene glycol) B-blocks. A concentrated polymer solution (25 wt %) in the presence of a UV initiator at room temperature was dispensed on a preheated substrate at 37 °C forming a hydrogel construct. The system was then exposed to UV light to further strengthen the construct. In another study, precross-linking was employed to create a lightly cross-linked PEG-based gel as a bioink, which can easily flow under moderate shear but is viscous enough to support itself postprinting.113 Pluronic hydrogels have been widely used in 3D printing. However, on their own, they do not support cells in the long term because of their density and lack of sufficient nutrient transport. To remedy this, Muller et al. mixed acrylated with unmodified Pluronic F127 to maintain printability while adding a cross-linking component to the gel.120 After printing, stable gels were created with UV cross-linking and the unmodified Pluronic F127 was washed away, leaving an open nanostructured hydrogel. These nanostructured gels were mechanically weak; however, the addition of methacrylated hyaluronic acid increased the stiffness.
Direct 3D printing of a shear thinning hydrogel was recently reported by Highley et al.121 Methacrylated hyaluronic acid (MeHA) macromers were either modified with adamantine (Ad-MeHA) or β-cyclodextrin (CD-MeHA). When these macromers were mixed they formed guest-host based, physically cross-linked hydrogels with shear thinning behavior allowing them to flow under modest pressures. The hydrogels were then exposed to UV light in the presence of a photoinitiator to further strengthen the printed structure. To develop self-supporting structures, we printed hydrogel inks into a support gel, which is formed from the same HA-based macromers without the methacrylate functionality. The support gel was removed after UV exposure. Markstedt K. et al, combined the shear thinning properties of nanofibrillated cellulose with the fast cross-linking ability of alginate to formulate a nanocellulose-based bioink.122 Human chondrocytes bioprinted in nanocellulose-based bioink exhibited 86% viability after 7 days of 3D culture.
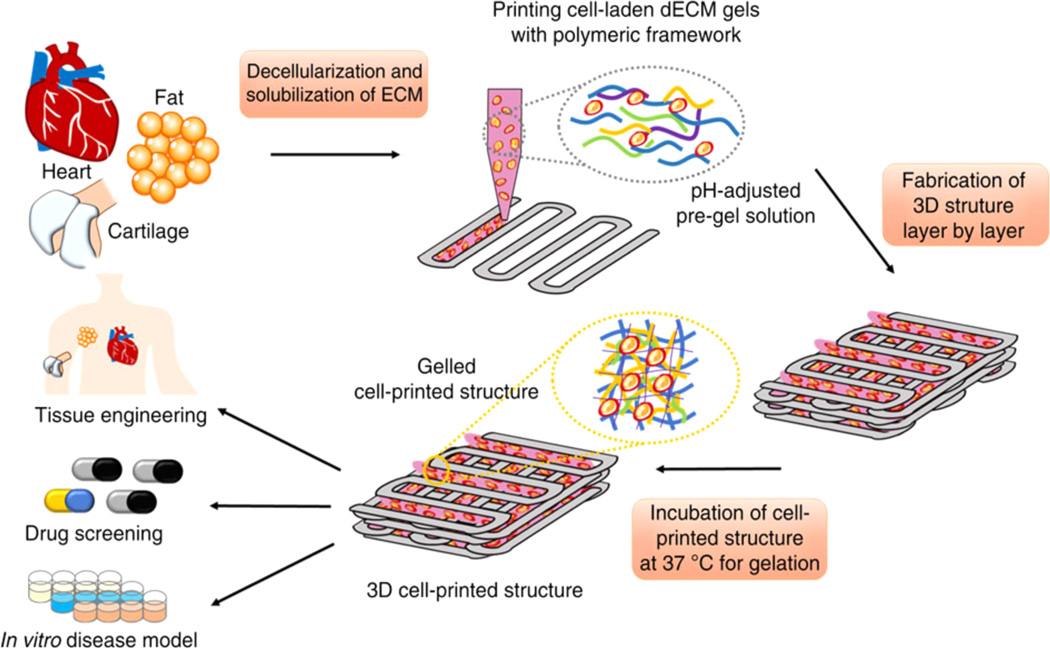
Finally, a novel tissue-specific ECM based bioink has been developed using decellularized ECM from adipose, cartilage and heart tissues.123 These bioinks were prepared as a solution (3%), remained as a solution below 15 °C, and gelled at 37 °C within 30 min. To develop self-supported constructs, we printed a PCL framework in combination with the hydrogel (Figure 4). Following this study, Kang et al. developed clinically relevant sized, vascularized constructs by printing cell-laden hydrogels (composed of gelatin, fibrinogen, and hyaluronic acid) together with biodegradable polymers (PCL).19
Figure 4.
Fabrication of 3D constructs from ECM based bioinks. Bioinks were developed from decellularized tissues after harvesting. Cell-laden ECM bioinks were printed in combination with polymeric framework. The image is printed with permission from ref 123. Copyright 2014 Nature Publishing Group.
3.3. Ceramic-Based Inks
Ceramic materials are commonly used in biomedical applications due to their high stiffness and bioactivity (i.e., their similarity to the mineral phase of natural bone) providing a natural and osteoinductive surface for bone tissue development (potentially useful for orthopedics and dental surgery).45,124,125 Currently available 3D printing methods are limited to provide direct printing of ceramics46,126–129 as liquid phase forming ceramic materials are limited in number, and their melting temperatures are far above the range of FDM printers. SLA is not an option for direct printing of ceramics as they are not responsive to light. It is also difficult to get highly dense and porous structures via SLS of ceramic powders.31,46,130 PB and inkjet printing are the two promising methods for direct printing of ceramics from powder and suspension form. 30,131–134 Another option is to use them as an additive in a composite system, which makes it possible to use FDM and SLA methods.135–140
For use in PB, the ceramic powder must have an appropriate powder size to balance high-resolution printing and particle flow within the bed, usually 10–150 µm in diameter, and an appropriate binding solution to either entrap the particles in a matrix or recrystallize the beads together.141,142 For inkjet printing, ink parameters include solid content, particle size, and viscosity. 38,48,143 Below, we summarize the two most commonly used ceramic-based ink materials in tissue engineering applications.
3.3.1. Hydroxyapatite (HA)
The bulk of the biomedically related 3D printing of ceramics is in the creation of pure ceramic scaffolds that mimic the minerals, structures and mechanical properties found naturally in bone. As a result, HA in powder form is commonly used in 3D printing because of its high presence as a calcium phosphate phase in mineralized bone. 144,145 Powder binding of HA is achieved through the layer-by-layer spraying of poly(acrylic acid) solutions onto HA powders followed by sintering to compete the solidification process.146 After sintering, scaffolds constructed by HA powder binding can reach compressive strengths (0.5 to 12 MPa) in the low end of those found in cancellous bone. When implanted in mouse models, these scaffolds show ingrowth of new bone from the edges of the scaffolds after 8 weeks and osteoid formation in the interior of the scaffolds with some preliminary vascularization as well.147 However, the autograft control treatments run alongside these printed scaffolds had filled the defect site with tissue, bone, and marrow after 8 weeks, so although the artificial scaffold performed well, the results were still far below the standard clinical treatments. Fiertz et al. fabricated HA-based scaffolds with 70% interconnected porosity, and showed the suitability of these scaffolds for potential clinical applications using osteogenic-stimulated progenitor cells.148 Michna et al. developed highly concentrated HA inks for direct printing of periodic scaffolds potentially useful for bone tissue engineering.149 By carefully tailoring the ink composition and viscosity, they fabricated self-supporting HA scaffolds from HA inks with minimal organic content (<1 wt %).
3.3.2. Tricalcium Phosphates
Another class of calcium phosphate powders that is used in 3D printing are variations of α- and β-TCP.150–158 Tricalcium phosphates are the second most common calcium phosphate phase found in the human skeleton behind hydroxyapatite. β-TCP has a faster resorption rate in the body than HA which is often cited as the reason that it is used more than other calcium phosphate phases. The most common binder used with TCP is phosphoric acid, which partially dissolves the calcium salt, allowing it to recrystallize and form new bridges between particles upon drying.159 Resolution, porosity, and strength of printed TCP scaffolds depends strongly on particle size, binder droplet size, depowdering efficiency, and scaffold geometry. As with other powder printing materials, depowdering and sintering are necessary and beneficial post-processing steps to remove excess material and increase scaffold strength. Similarly to HA, β-TCP has been studied with polymer additives that aim to improve the binding properties of the final scaffold.160,161 For instance, β-TCP has been combined with PCL specifically to improve interlayer binding and scaffold mechanical properties while pharmaceutical agents, like alendronate, coated on the surface have been used simultaneously to improve the wound healing response in vivo.162,163 Alternatively, powder binding methods employing mixtures of TCP and alginate powders combined with phosphoric acid as the binding solvent have been used to print three-dimensional scaffolds with improved strength and reduced brittleness.164 Scaffolds containing TCP and 2.5% alginate showed increased mechanical strength as well as improved MG63 osteoblastic cell compatibility and proliferation compared to pure TCP controls.164 Using natural polymers like alginate reduces the chances of side effects associated with some synthetic polymers such as harmful breakdown products and decreased cell attachment.165 Bian et al. developed β-TCP/collagen scaffolds via ceramic SL and gel casting to develop osteochondral scaffolds for tissue engineering.166 On the inorganic chemical side, the addition of strontium and magnesium oxide to TCP scaffolds has been shown to increase mechanical properties significantly.167 Overall, TCP scaffolds perform similarly to HA with slight trade-offs in resorption rate and strength, but both are good base material options depending on the individual applications.
3.4. Composite Inks
3D printing initially focused on processing exclusively pure metals and polymers; however, as the technology grew, the development of composite inks quickly emerged. The main goal of using composite inks is to enhance ink properties such as processability, printability, mechanics (stiffness) and bioactivity (to enhance cellular function and tissue integration). Below is a summary of the most commonly used composite ink systems for tissue engineering applications, which fall under three major groups including polymer-based composites, hydrogel-based composites, and ceramic composites. The additives in these systems include ceramics, biomolecules, carbon nanotubes, and in some rare cases, metals.
3.4.1. Polymer-Based Composites
PLA is one of the most used polymeric inks in 3D printing. As described above, it is not bioactive; it allows cell adhesion but does not support cellular activity. Therefore, it is a common practice to add bioactive ceramics into PLA ink formulations.168 For instance, Serra et al. combined PLA with PEG as a plasticizer and bioactive calcium phosphate glass in chloroform to make a printable polymer-based bioink for DIW bone scaffold construction.62,66 Primary mesenchymal stem cells (MSC), when cultured on those printed scaffolds, showed increased adhesion to the surface over controls, suggesting the potential for a better migratory and healing response in vivo. The addition of bioactive glass changes the chemical and topographical nature of the scaffold surface in ways that seem to benefit cell progression.
Surface erosion of PCL within the body makes it an interesting system for drug and growth factor delivery in printed scaffolds because the constant, slow degradation behavior allows for controlled release of trapped biomolecules.169 PCL fibers made with poloxamine and dexamethasone (DXMS) have been printed using fused deposition modeling and studied to assess the release profile of dexamethasone and its effect on MSC development.170 The degradation and release profile was found to depend on the fiber composition ratio when tested in vivo. DXMS containing scaffolds showed greater cell proliferation at earlier time points than those without DXMS, giving it potential as an additive in scaffolding systems. In another study, PCL pellets mixed with HA particles were extruded to form filaments for FDM, which were printed into goat femoral chondyle replicas.171 To mimic an osteochondral interface, we coated the scaffolds with PGA/PLA and tested in a goat knee model. After 10 weeks, smooth, homogeneous articular cartilage and integrated subchondral bone were observed. PCL dissolved in DCM has been used as a base solution for direct ink writing. To enhance osteogenic behavior of scaffolds after 3D printing, we developed an ink formulation composed of HA powder in DCM and carbon nanotubes and PCL in THF, and printed it on scaffolds with 200–700 µm pores.172,173 The addition or carbon nanotubes was found to increase the mechanical properties and add conductivity to the scaffolds; MG63 cells attached to the scaffold surface and proliferated.
Manufacturing of PEEK-hydroxyapatite composites has been investigated using SLS printing to create tissue engineering scaffolds.174 Assessment of different weight percent powder mixtures by Tan et al. showed that many different powder compositions, from 10 to 40% HA in PEEK, could be printed using SLS by varying the laser power and sintering temperature.175 For this mixture, it was found that 40% HA produced structures with good mechanical integrity while scaffolds created from higher levels of HA became brittle and difficult to handle. Because HA is a natural mineral phase of bone, it is theorized that the inclusion of HA particles into PEEK scaffolds will increase their osteointegrative properties and reduce the drawbacks associated with PEEK’s bioinertness while maintaining its beneficial mechanical and heat-resistant properties.
3.4.2. Hydrogel-Based Composites
Luo et al. experimented with DIW of a system of water-soluble poly(vinyl alcohol) (PVA) and alginate combined with bioactive glass and dexamethasone.176 After printing, the scaffolds were solidified by chemically cross-linking the alginate hydrogel with a calcium chloride solution. The flexibility of this formulation allowed for tailoring of the mechanical properties by changing the bioactive glass levels within the ink. The hollow struts combined with bioactive glass led to increased bone ingrowth in vivo and better control of degradation rate.176 Pluronic hydrogels are not commonly used alone in DIW bone scaffold engineering because of their low strength and inertness with respect to osteoblasts. Bioactive glass is a common additive that provides increased compressive strength to the end stage scaffold as well as increased bioactivity toward osteogenic lineages.177
Co-printing with a second, stiffer polymer is another option for reaching more desirable stiffness levels. In a similar system, natural, biocompatible, biodegradable hyaluronic acid was combined with UV-curable glycidyl methacrylate. When this material was printed into linear porous scaffolds, cross-linked and implanted into a porcine mandibular model, it showed good biocompatibility and supported tissue growth.178 Modified P123 mixtures containing strontium, mesoporous bioactive glass, and PVA have been printed into scaffolds using DIW.179 These scaffolds have shown good cell attachment and proliferation when cultured with MC3T3-E1 cells. Higher proliferation was seen on scaffolds containing strontium than the MBG only controls, along with increased levels of ECM mineralization. Furthermore, dexamethasone could be loaded with these Sr-MBG powders and gave an initial burst release followed by a slow, sustained release in vitro.180 Importantly, these scaffolds showed a high compressive strength comparable to that of human trabecular bone (8–9 MPa vs 2–12 MPa) according to the study. Increased mechanical strength was achieved through the addition of PVA which functions to bind the individual Sr-MBG particles together, decrease the scaffold brittleness and increase stability in solution. The variety of modifications that can be made to pluronics as well as the potential to incorporate a wide variety of additives makes this an interesting system for exploration, but the mechanics will likely hinder its use in hard tissue scaffolds.
Finally, bioinks containing metal powders have been developed for DIW printing of mechanically stiff, porous scaffold systems.181 These powder-based liquid inks consist of a variety of metal powders mixed with polylactic-co-glycolic acid (PLGA) in DCM. Once formulated, these inks could be printed through a 200-µm nozzle to form porous, strut based biomedical scaffolds. Following printing, the products were subjected to H2 thermochemical reduction to sinter the 3D object to its final state. This method allowed for the incorporation of any metal or metal oxide that can be obtained in particle form and the bypassing of traditionally difficult 3D metal printing methods for hard tissue engineering such as SLS.
3.4.3. Ceramic-Based Composites
TCP and HA are not mutually exclusive and can be printed jointly simply by mixing the powder forms together within the printing bed. These biphasic calcium phosphate mixtures have been found to produce scaffolds with similar cell viability and enhanced cell proliferation compared to scaffolds with HA or TCP alone.182 The mechanical properties for biphasic ceramic scaffolds are still lacking compared to bone but increasing TCP content and coating with PLGA has been found to improve scaffold properties.183 It should also be noted that mixtures requiring the use of organic solvent binders, like PLGA and β-TCP, are at an inherent disadvantage based on the potential for solvent to remain in the scaffold in trace amounts. In a different study, PLGA/TCP scaffolds showed less bone formation and osteoconduction in rat models than identically structured pure calcium phosphate sintered scaffolds.144 Further research into optimizing the concentration of each calcium phosphate phase and investigating which additives improve end properties the most will be beneficial for improving performance of powder-bound scaffolds.
In an attempt to improve the mechanical strength and flexibility of the scaffolds, HA-poly(vinyl alcohol) (HA-PVA) composite scaffolds were printed using an aqueous binder followed by drying and sintering.146 These scaffolds exhibited physiological porosities and mechanical strengths on the low end of cancellous bone. The addition of PVA to the mixtures allowed for lower sintering temperatures and better binding between layers, making the final scaffold more robust and easier to handle. There is evidence that the pore size and geometry play a role in scaffold performance and can enhance the ability of HA ceramic scaffolds to accelerate healing.144 Combining HA with polymers and adequate porosity by SLS or PB fabrication methods has the potential to create good artificial scaffolds that may one day compete with autologous bone implants.
4. MOVING FORWARD WITH BIOMATERIAL INK DESIGN
There are only a handful of biodegradable polymeric biomaterial inks that are available, and they have limited independent tunability of processability, degradation and mechanics. Within our knowledge, none of these biomaterial inks offer the ability to be functionalized. They are currently used as space filling applications allowing basic cell function but not promoting biological activity. Therefore, the major hurdle for the coming years will be to create novel biodegradable polymer ink libraries with user-defined and tunable properties. For ceramic-based inks, the focus has been and will be on controlling the powder size and particle flow properties by surface modifications, and developing more efficient binding materials. When compared to polymer and ceramic inks, hydrogel inks are receiving much more attention, and significant progress has already been made to design novel ink formulations. The reasons are 2-fold: First, the hydrogel inks are cell compatible and they can be printed together with cells. Second, they are more amenable to chemical modifications, and small changes in their chemistry usually lead to significant changes in their properties, such as processabilty, degradation, stiffness, and ability to be functionalized. For instance, novel supramolecular hydrogels are offering a wider range of possibilities in ink design.184 Loo et al. developed peptide bioinks (lysine-containing hexapeptides) that self-assemble into stable hydrogels with stiffness up to 40 kPa.185 Supramolecular hydrogel inks are developed for extrusion based printing, and have enabled printing of high-resolution multimaterial structures with cells.121 Non-covalent cross-links enabled direct extrusion of hydrogels into self-supporting structures. Another study showed bioprinting of supramolecular peptide-DNA inks to form self-supporting hydrogel structures due to fast reaction kinetics.117 Supra-molecular interactions also enabled low-molecular-weight polyimide-based inks (with viscosities in the range of 3–11 cP) to be inkjet printed in high resolution via self-assembly through tunable π-π interactions.184,186 In addition, the fundamental design approach for ink design has evolved from single component to multicomponent inks, and from distinct cross-linking mechanism to complementary sequential cross-linking mechanisms (pre- or postprinting). The ultimate goal is to create self-supporting, cell-laden 3D scaffolds. Creating hybrid constructs composed of biodegradable polymer inks with much softer cell-laden hydrogels inks bring this technology one step closer to this goal.187
5. CONCLUSION
Recent developments in mechanical systems and software have vastly improved the resolution, accuracy, speed and flexibility of 3D printing methods. As a result, 3D printing has been gaining traction as a tool for creating the complex constructs required to engineer both hard and soft tissue engineering systems. Objects created using additive manufacturing have already seen use clinically as space-filling permanent, or semi permanent, implants in the face, chest and extremities. However, the lack of printable biomaterials with sufficient chemical and mechanical properties for constructing regenerative implants has been a significant barrier to biomedical progress. Scientists and engineers have been working hard to develop biomaterials that take into account factors such as printability, biocompatibility, mechanical properties, degradation kinetics and byproducts, and tissue biomimicry required for a successful 3D printed tissue implant. Polymeric bioinks have been the focus of much of the investigation, specifically with the low-cost polyesters poly(lactic acid) and poly(caprolactone). The ability of these materials to function in many printing technologies with high biocompatibility and good mechanical properties has made them a common base for many formulations. Future development of printable polymeric biomaterials will need to build on these properties while addressing concerns with degradation, brittleness, and cell compatibility. Ceramics, such as hydroxyapatite and β-TCP, have traditionally seen used with success in hard tissue scaffolds and are now seeing more use in polymer-ceramic composite blends to provide strengthening and osteogenic properties to printed polymer bioinks. Advances in hydrogel bioinks and printing systems are bringing us closer to printing fully functional, cell containing model systems that may 1 day lead to functional organ printing. This process has already started with the investigations into supremolecular hydrogel bioinks. With ongoing studies to understand how tissues and scaffolding materials interact in the body, we will hopefully begin to see transitions from some of these bioink-based 3D printed technologies into clinical trials in the coming years. Overall, 3D printing is a promising and exciting tool for tissue engineering and regenerative medicine but there is still a great deal of work to be done in the area of biomaterial ink development before it reaches its full potential.
Acknowledgments
We are grateful for support from National Institute of Biomedical Imaging and Bioengineering (P41EB001046), from CNPq-Conselho a Nacional de Desenvolvimento Científico e Tecnológico (R.M.D.S), and the New Jersey Center for Biomaterials at Rutgers University. We also acknowledge the editorial contributions of Carole Kantor.
Funding
NIH NIBIB P41EB001046 and discretionary funding from the New Jersey Center for Biomaterials at Rutgers University.
Footnotes
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
REFERENCES
- 1.Obregon F, Vaquette C, Ivanovski S, Hutmacher DW, Bertassoni LE. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015;94(9):143S–152S. doi: 10.1177/0022034515588885. [DOI] [PubMed] [Google Scholar]
- 2.Dawood A, Marti BM, Sauret-Jackson V, Darwood A. 3D printing in dentistry. Br. Dent. J. 2015;219(11):521–529. doi: 10.1038/sj.bdj.2015.914. [DOI] [PubMed] [Google Scholar]
- 3.Yoshikawa M, Sato R, Higashihara T, Ogasawara T, Kawashima N. Rehand: Realistic electric prosthetic hand created with a 3D printer; Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society; IEEE Engineering in Medicine and Biology Society; 2015. pp. 2470–2473. [DOI] [PubMed] [Google Scholar]
- 4.Wong JY. Ultra-Portable Solar-Powered 3D Printers for Onsite Manufacturing of Medical Resources. Aerospace Medicine and Human Performance. 2015;86(9):830–834. doi: 10.3357/AMHP.4308.2015. [DOI] [PubMed] [Google Scholar]
- 5.Wong JY. On-Site 3D Printing of Functional Custom Mallet Splints for Mars Analogue Crewmembers. Aerospace Medicine and Human Performance. 2015;86(10):911–914. doi: 10.3357/AMHP.4259.2015. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs S, Grunert R, Mohr FW, Falk V. 3D–Imaging of cardiac structures using 3D heart models for planning in heart surgery: a preliminary study. Interactive cardiovascular and thoracic surgery. 2008;7(1):6–9. doi: 10.1510/icvts.2007.156588. [DOI] [PubMed] [Google Scholar]
- 7.Pietrabissa A, Marconi S, Peri A, Pugliese L, Cavazzi E, Vinci A, Botti M, Auricchio F. From CT scanning to 3-D printing technology for the preoperative planning in laparoscopic splenectomy. Surgical Endoscopy and Other Interventional Techniques. 2016;30(1):366–371. doi: 10.1007/s00464-015-4185-y. [DOI] [PubMed] [Google Scholar]
- 8.Ripley B, Kelil T, Cheezum MK, Goncalves A, Di Carli MF, Rybicki FJ, Steigner M, Mitsouras D, Blankstein R. 3D printing based on cardiac CT assists anatomic visualization prior to transcatheter aortic valve replacement. Journal of Cardiovascular Computed Tomography. 2016;10(1):28–36. doi: 10.1016/j.jcct.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zein NN, Hanouneh IA, Bishop PD, Samaan M, Eghtesad B, Quintini C, Miller C, Yerian L, Klatte R. Three-Dimensional Print of a Liver for Preoperative Planning in Living Donor Liver Transplantation. Liver Transplantation. 2013;19(12):1304–1310. doi: 10.1002/lt.23729. [DOI] [PubMed] [Google Scholar]
- 10.Do A-V, Khorsand B, Geary SM, Salem AK. 3D Printing of Scaffolds for Tissue Regeneration Applications. Adv. Healthcare Mater. 2015;4(12):1742–1762. doi: 10.1002/adhm.201500168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh D, Singh D, Han SS. 3D Printing of Scaffold for Cells Delivery: Advances in Skin Tissue Engineering. Polymers. 2016;8(1):19. doi: 10.3390/polym8010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fedorovich NE, Alblas J, Hennink WE, Oner FC, Dhert WJA. Organ printing: the future of bone regeneration. Trends Biotechnol. 2011;29(12):601–606. doi: 10.1016/j.tibtech.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Mironov V, Kasyanov V, Markwald RR. Organ printing: from bioprinter to organ biofabrication line. Curr. Opin. Biotechnol. 2011;22(5):667–673. doi: 10.1016/j.copbio.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Mironov V, Visconti RP, Kasyanov V, Forgacs G, Drake CJ, Markwald RR. Organ printing: Tissue spheroids as building blocks. Biomaterials. 2009;30(12):2164–2174. doi: 10.1016/j.biomaterials.2008.12.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ozbolat IT, Yu Y. Bioprinting Toward Organ Fabrication: Challenges and Future Trends. IEEE Trans. Biomed. Eng. 2013;60(3):691–699. doi: 10.1109/TBME.2013.2243912. [DOI] [PubMed] [Google Scholar]
- 16.Park KM, Gerecht S. Polymeric hydrogels as artificial extracellular microenvironments for cancer research. Eur. Polym. J. 2015;72:507–513. [Google Scholar]
- 17.Zhao Y, Yao R, Ouyang L, Ding H, Zhang T, Zhang K, Cheng S, Sun W. Three-dimensional printing of Hela cells for cervical tumor model in vitro. Biofabrication. 2014;6(3):035001. doi: 10.1088/1758-5082/6/3/035001. [DOI] [PubMed] [Google Scholar]
- 18.Visser J, Peters B, Burger TJ, Boomstra J, Dhert WJA, Melchels FPW, Malda J. Biofabrication of multi-material anatomically shaped tissue constructs. Biofabrication. 2013;5(3):035007. doi: 10.1088/1758-5082/5/3/035007. [DOI] [PubMed] [Google Scholar]
- 19.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34(3):312–319. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]
- 20.Tibbitt MW, Rodell CB, Burdick JA, Anseth KS. Progress in material design for biomedical applications. Proc. Natl. Acad. Sci. U. S. A. 2015;112(47):14444–14451. doi: 10.1073/pnas.1516247112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 22.Turner BN, Strong R, Gold SA. A review of melt extrusion additive manufacturing processes: I. Process design and modeling. Rapid Prototyping Journal. 2014;20(3):192–204. [Google Scholar]
- 23.Guo SZ, Gosselin F, Guerin N, Lanouette AM, Heuzey MC, Therriault D. Solvent-Cast Three-Dimensional Printing of Multifunctional Microsystems. Small. 2013;9(24):4118–4122. doi: 10.1002/smll.201300975. [DOI] [PubMed] [Google Scholar]
- 24.Chia HN, Wu BM. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015;9:4. doi: 10.1186/s13036-015-0001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vermeulen M, Claessens T, Van Der Smissen B, Van Holsbeke CS, De Backer JW, Van Ransbeeck P, Verdonck P. Manufacturing of patient-specific optically accessible airway models by fused deposition modeling. Rapid Prototyping Journal. 2013;19(5):312–318. [Google Scholar]
- 26.Peltola SM, Melchels FPW, Grijpma DW, Kellomaki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008;40(4):268–280. doi: 10.1080/07853890701881788. [DOI] [PubMed] [Google Scholar]
- 27.Shirazi SFS, Gharehkhani S, Mehrali M, Yarmand H, Metselaar HSC, Kadri NA, Abu Osman NA. A review on powder-based additive manufacturing for tissue engineering: selective laser sintering and inkjet 3D printing. Sci. Technol. Adv. Mater. 2015;16(3):033502. doi: 10.1088/1468-6996/16/3/033502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kruth JP, Mercelis P, Van Vaerenbergh J, Froyen L, Rombouts M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyping Journal. 2005;11(1):26–36. [Google Scholar]
- 29.Yap CY, Chua CK, Dong ZL, Liu ZH, Zhang DQ, Loh LE, Sing SL. Review of selective laser melting: Materials and applications. Appl. Phys. Rev. 2015;2(4):041101. [Google Scholar]
- 30.Deckers J, Shahzad K, Vleugels J, Kruth JP. Isostatic pressing assisted indirect selective laser sintering of alumina components. Rapid Prototyping Journal. 2012;18(5):409–419. [Google Scholar]
- 31.Bertrand P, Bayle F, Combe C, Goeuriot P, Smurov I. Ceramic components manufacturing by selective laser sintering. Appl. Surf. Sci. 2007;254(4):989–992. [Google Scholar]
- 32.Mazzoli A. Selective laser sintering in biomedical engineering. Med. Biol. Eng. Comput. 2013;51(3):245–256. doi: 10.1007/s11517-012-1001-x. [DOI] [PubMed] [Google Scholar]
- 33.Gmeiner R, Deisinger U, Schonherr J, Lechner B, Detsch R, Boccaccini AR, Stampfl J. Additive Manufacturing of Bioactive Glasses and Silicate Bioceramics. J. Ceram. Sci. Technol. 2015;6(2):75–86. [Google Scholar]
- 34.Bonda DJ, Manjila S, Selman WR, Dean D. The Recent Revolution in the Design and Manufacture of Cranial Implants: Modern Advancements and Future Directions. Neurosurgery. 2015;77(5):814–824. doi: 10.1227/NEU.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duan B, Wang M, Zhou WY, Cheung WL, Li ZY, Lu WW. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010;6(12):4495–4505. doi: 10.1016/j.actbio.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 36.Tumbleston JR, Shirvanyants D, Ermoshkin N, Janusziewicz R, Johnson AR, Kelly D, Chen K, Pinschmidt R, Rolland JP, Ermoshkin A, Samulski ET, DeSimone JM. Continuous liquid interface production of 3D objects. Science. 2015;347(6228):1349–1352. doi: 10.1126/science.aaa2397. [DOI] [PubMed] [Google Scholar]
- 37.Barry JJA, Evseev AV, Markov MA, Upton CE, Scotchford CA, Popov VK, Howdle SM. In vitro study of hydroxyapatite-based photocurable polymer composites prepared by laser stereolithography and supercritical fluid extraction. Acta Biomater. 2008;4(6):1603–1610. doi: 10.1016/j.actbio.2008.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Derby B. Inkjet Printing of Functional and Structural Materials: Fluid Property Requirements, Feature Stability, and Resolution. Annu. Rev. Mater. Res. 2010;40:395–414. [Google Scholar]
- 39.Yoo H, Kim C. Experimental studies on formation, spreading and drying of inkjet drop of colloidal suspensions. Colloids Surf., A. 2015;468:234–245. [Google Scholar]
- 40.Bhardwaj R, Fang XH, Somasundaran P, Attinger D. Self-Assembly of Colloidal Particles from Evaporating Droplets: Role of DLVO Interactions and Proposition of a Phase Diagram. Langmuir. 2010;26(11):7833–7842. doi: 10.1021/la9047227. [DOI] [PubMed] [Google Scholar]
- 41.Thompson AB, Tipton CR, Juel A, Hazel AL, Dowling M. Sequential deposition of overlapping droplets to form a liquid line. J. Fluid Mech. 2014;761:261. [Google Scholar]
- 42.Sprittles JE, Shikhmurzaev YD. The coalescence of liquid drops in a viscous fluid: interface formation model. J. Fluid Mech. 2014;751:480–499. [Google Scholar]
- 43.Tian DL, Song YL, Jiang L. Patterning of controllable surface wettability for printing techniques. Chem. Soc. Rev. 2013;42(12):5184–5209. doi: 10.1039/c3cs35501b. [DOI] [PubMed] [Google Scholar]
- 44.Ahn BY, Lewis JA. Amphiphilic silver particles for conductive inks with controlled wetting behavior. Mater. Chem. Phys. 2014;148(3):686–691. [Google Scholar]
- 45.Zocca A, Colombo P, Gomes CM, Gunster J. Additive Manufacturing of Ceramics: Issues, Potentialities, and Opportunities. J. Am. Ceram. Soc. 2015;98(7):1983–2001. [Google Scholar]
- 46.Travitzky N, Bonet A, Dermeik B, Fey T, Filbert-Demut I, Schlier L, Schlordt T, Greil P. Additive Manufacturing of Ceramic-Based Materials. Adv. Eng. Mater. 2014;16(6):729–754. [Google Scholar]
- 47.Gungor GL, Kara A, Blosi M, Gardini D, Guarini G, Zanelli C, Dondi M. Micronizing ceramic pigments for inkjet printing: Part I . Grindability and particle size distribution. Ceram. Int. 2015;41(5):6498–6506. [Google Scholar]
- 48.Derby B. Inkjet printing ceramics: From drops to solid. J. Eur. Ceram. Soc. 2011;31(14):2543–2550. [Google Scholar]
- 49.Odell JA, Keller A, Miles MJ. A method for studying flow-induced polymer degradation - Verification of chain halving. Polym. Commun. 1983;24(1):7–10. [Google Scholar]
- 50.Odell JA, Keller A. Flow-induced chain fracture of isolated linear macromolecules in solution. J. Polym. Sci., Part B: Polym. Phys. 1986;24(9):1889–1916. [Google Scholar]
- 51.A-Alamry K, Nixon K, Hindley R, Odel JA, Yeates SG. Flow-Induced Polymer Degradation During Ink-Jet Printing. Macro-mol. Rapid Commun. 2011;32(3):316–320. doi: 10.1002/marc.201000521. [DOI] [PubMed] [Google Scholar]
- 52.McIlroy C, Harlen OG, Morrison NF. Modelling the jetting of dilute polymer solutions in drop-on-demand inkjet printing. J. Non-Newtonian Fluid Mech. 2013;201:17–28. [Google Scholar]
- 53.Ferris CJ, Gilmore KG, Wallace GG, Panhuis MIH. Biofabrication: an overview of the approaches used for printing of living cells. Appl. Microbiol. Biotechnol. 2013;97(10):4243–4258. doi: 10.1007/s00253-013-4853-6. [DOI] [PubMed] [Google Scholar]
- 54.Ferris CJ, Gilmore KJ, Beirne S, McCallum D, Wallace GG, Panhuis MIH. Bio-ink for on-demand printing of living cells. Biomater. Sci. 2013;1(2):224–230. doi: 10.1039/c2bm00114d. [DOI] [PubMed] [Google Scholar]
- 55.Saunders RE, Derby B. Inkjet printing biomaterials for tissue engineering: bioprinting. Int. Mater. Rev. 2014;59(8):430–448. [Google Scholar]
- 56.Derby B. Printing and Prototyping of Tissues and Scaffolds. Science. 2012;338(6109):921–926. doi: 10.1126/science.1226340. [DOI] [PubMed] [Google Scholar]
- 57.Ali M, Pages E, Ducom A, Fontaine A, Guillemot F. Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication. 2014;6(4):045001. doi: 10.1088/1758-5082/6/4/045001. [DOI] [PubMed] [Google Scholar]
- 58.Doraiswamy A, Dunaway TM, Wilker JJ, Narayan RJ. Inkjet Printing of Bioadhesives. J. Biomed. Mater. Res. Part B. 2009;89B(1):28–35. doi: 10.1002/jbm.b.31183. [DOI] [PubMed] [Google Scholar]
- 59.Boehm RD, Miller PR, Daniels J, Stafslien S, Narayan RJ. Inkjet printing for pharmaceutical applications. Mater. Today. 2014;17(5):247–252. [Google Scholar]
- 60.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of Vascularized, Heterogeneous Cell-Laden Tissue Constructs. Adv. Mater. 2014;26(19):3124–3130. doi: 10.1002/adma.201305506. [DOI] [PubMed] [Google Scholar]
- 61.Villar G, Graham AD, Bayley H. A Tissue-Like Printed Material. Science. 2013;340(6128):48–52. doi: 10.1126/science.1229495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Serra T, Mateos-Timoneda MA, Planell JA, Navarro M. 3D printed PLA-based scaffolds A versatile tool in regenerative medicine. Organogenesis. 2013;9(4):239–244. doi: 10.4161/org.26048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Garlotta D. A literature review of poly(lactic acid) J. Polym. Environ. 2001;9(2):63–84. [Google Scholar]
- 64.Suganuma J, Alexander H. J. Appl. Biomater. 1993;4(1):13–27. doi: 10.1002/jab.770040109. [DOI] [PubMed] [Google Scholar]
- 65.Schiller C, Epple M. Carbonated calcium phosphates are suitable pH-stabilising fillers for biodegradable polyesters. Biomaterials. 2003;24(12):2037–2043. doi: 10.1016/s0142-9612(02)00634-8. [DOI] [PubMed] [Google Scholar]
- 66.Serra T, Planell JA, Navarro M. High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 2013;9(3):5521–5530. doi: 10.1016/j.actbio.2012.10.041. [DOI] [PubMed] [Google Scholar]
- 67.Melchels FPW, Feijen J, Grijpma DW. A poly(D,L-lactide) resin for the preparation of tissue engineering scaffolds by stereolithography. Biomaterials. 2009;30(23–24):3801–3809. doi: 10.1016/j.biomaterials.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 68.Jansen J, Melchels FPW, Grijpma DW, Feijen J. Fumaric Acid Monoethyl Ester-Functionalized Poly(D,L-lactide)/N-vinyl-2-pyrrolidone Resins for the Preparation of Tissue Engineering Scaffolds by Stereolithography. Biomacromolecules. 2009;10(2):214–220. doi: 10.1021/bm801001r. [DOI] [PubMed] [Google Scholar]
- 69.Woodruff MA, Hutmacher DW. The return of a forgotten polymer-Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35(10):1217–1256. [Google Scholar]
- 70.Zopf DA, Hollister SJ, Nelson ME, Ohye RG, Green GE. Bioresorbable Airway Splint Created with a Three-Dimensional Printer. N. Engl. J. Med. 2013;368(21):2043–2045. doi: 10.1056/NEJMc1206319. [DOI] [PubMed] [Google Scholar]
- 71.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, Hollister SJ, Das S. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 72.Konopnicki S, Sharaf B, Resnick C, Patenaude A, Pogal-Sussman T, Hwang KG, Abukawa H, Troulis MJ. Tissue-Engineered Bone With 3-Dimensionally Printed beta-Tricalcium Phosphate and Polycaprolactone Scaffolds and Early Implantation: An In Vivo Pilot Study in a Porcine Mandible Model. J. Oral Maxillofac. Surg. 2015;73(5):1016E1–1016E11. doi: 10.1016/j.joms.2015.01.021. [DOI] [PubMed] [Google Scholar]
- 73.Childers EP, Wang MO, Becker ML, Fisher JP, Dean D. 3D printing of resorbable poly(propylene fumarate) tissue engineering scaffolds. MRS Bull. 2015;40(2):119–126. [Google Scholar]
- 74.Fisher JP, Dean D, Mikos AG. Photocrosslinking characteristics and mechanical properties of diethyl fumarate/poly-(propylene fumarate) biomaterials. Biomaterials. 2002;23(22):4333–4343. doi: 10.1016/s0142-9612(02)00178-3. [DOI] [PubMed] [Google Scholar]
- 75.Dadsetan M, Guda T, Runge MB, Mijares D, LeGeros RZ, LeGeros JP, Silliman DT, Lu L, Wenke JC, Brown Baer PR, Yaszemski MJ. Effect of calcium phosphate coating and rhBMP-2 on bone regeneration in rabbit calvaria using poly(propylene fumarate) scaffolds. Acta Biomater. 2015;18:9–20. doi: 10.1016/j.actbio.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 76.Parthasarathy J. 3D modeling, custom implants and its future perspectives in craniofacial surgery. Annals of Maxillofacial Surgery. 2014;4(1):9–18. doi: 10.4103/2231-0746.133065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schmidt M, Pohle D, Rechtenwald T. Selective laser sintering of PEEK. CIRP Ann. 2007;56(1):205–208. [Google Scholar]
- 78.Kurtz SM. Chemical and Radiation Stability of PEEK. Peek Biomaterials Handbook. 2012:75–79. [Google Scholar]
- 79.Shah AM, Jung H, Skirboll S. Materials used in cranioplasty: a history and analysis. Neurosurgical Focus. 2014;36(4):E19. doi: 10.3171/2014.2.FOCUS13561. [DOI] [PubMed] [Google Scholar]
- 80.Tellis BC, Szivek JA, Bliss CL, Margolis DS, Vaidyanathan RK, Calvert P. Trabecular scaffolds created using micro CT guided fused deposition modeling. Mater. Sci. Eng., C. 2008;28(1):171–178. doi: 10.1016/j.msec.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szivek JA, Bliss CL, Geffre C, Margolis DS, DeYoung DW, Ruth JT, Schnepp AB, Tellis B, Vaidyanathan RK. Porous polybutylene terephthalate implants allow for bone ingrowth and provide awell-anchored scaffold that can be used to deliver tissue-engineered cartilage. J. Invest. Med. 2006;54(1):S116–S116. [Google Scholar]
- 82.Radder AM, Leenders H, Vanblitterswijk CA. Bone-bonding behavior pf poly(ethylene oxide)-polybutylene terephthalate copolymer coatings and bulk implants - A comparative study. Biomaterials. 1995;16(7):507–513. doi: 10.1016/0142-9612(95)91122-f. [DOI] [PubMed] [Google Scholar]
- 83.Rosenzweig DH, Carelli E, Steffen T, Jarzem P, Haglund L. 3D–Printed ABS and PLA Scaffolds for Cartilage and Nucleus Pulposus Tissue Regeneration. Int. J. Mol. Sci. 2015;16(7):15118–15135. doi: 10.3390/ijms160715118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cai H, Azangwe G, Shepherd DET. Skin cell culture on an ear-shaped scaffold created by fused deposition modelling. Bio-Med. Mater. Eng. 2005;15(5):375–380. [PubMed] [Google Scholar]
- 85.Buwalda SJ, Boere KWM, Dijkstra PJ, Feijen J, Vermonden T, Hennink WE. Hydrogels in a historical perspective: From simple networks to smart materials. J. Controlled Release. 2014;190:254–273. doi: 10.1016/j.jconrel.2014.03.052. [DOI] [PubMed] [Google Scholar]
- 86.DeForest CA, Anseth KS, Prausnitz JM. Advances in Bioactive Hydrogels to Probe and Direct Cell Fate. Annu. Rev. Chem. Biomol. Eng. 2012;3:421–444. doi: 10.1146/annurev-chembioeng-062011-080945. [DOI] [PubMed] [Google Scholar]
- 87.Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr. Opin. Biotechnol. 2013;24(5):841–846. doi: 10.1016/j.copbio.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Seliktar D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science. 2012;336(6085):1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 89.Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8(2):260–272. [Google Scholar]
- 90.Stanton MM, Samitier J, Sanchez S. Bioprinting of 3D hydrogels. Lab Chip. 2015;15(15):3111–3115. doi: 10.1039/c5lc90069g. [DOI] [PubMed] [Google Scholar]
- 91.Billiet T, Gevaert E, De Schryver T, Cornelissen M, Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35(1):49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 92.Billiet T, Vandenhaute M, Schelfhout J, Van Vlierberghe S, Dubruel P. A review of trends and limitations in hydrogel-rapid prototyping for tissue engineering. Biomaterials. 2012;33(26):6020–6041. doi: 10.1016/j.biomaterials.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 93.Pereira RF, Bartolo PJ. 3D bioprinting of photocrosslinkable hydrogel constructs. J. Appl. Polym. Sci. 2015;132(48) [Google Scholar]
- 94.Zhang X, Zhang Y. Tissue Engineering Applications of Three-Dimensional Bioprinting. Cell Biochem. Biophys. 2015;72(3):777–782. doi: 10.1007/s12013-015-0531-x. [DOI] [PubMed] [Google Scholar]
- 95.Malda J, Visser J, Melchels FP, Juengst T, Hennink WE, Dhert WJA, Groll J, Hutmacher DW. 25th Anniversary Article: Engineering Hydrogels for Biofabrication. Adv. Mater. 2013;25(36):5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 96.Lee V, Singh G, Trasatti JP, Bjornsson C, Xu X, Thanh Nga T, Yoo S-S, Dai G, Karande P. Design and Fabrication of Human Skin by Three-Dimensional Bioprinting. Tissue Eng., Part C. 2014;20(6):473–484. doi: 10.1089/ten.tec.2013.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials. 2006;27(19):3580–3588. doi: 10.1016/j.biomaterials.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 98.Ilkhanizadeh S, Teixeira AI, Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28(27):3936–3943. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 99.Hsieh F-Y, Lin H-H, Hsu S-h. 3D bioprinting of neural stern cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48–57. doi: 10.1016/j.biomaterials.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 100.Gao G, Schilling AF, Hubbell K, Yonezawa T, Truong D, Hong Y, Dai G, Cui X. Improved properties of bone and cartilage tissue from 3D inkjet-bioprinted human mesenchymal stem cells by simultaneous deposition and photocrosslinking in PEG-GelMA. Biotechnol. Lett. 2015;37(11):2349–2355. doi: 10.1007/s10529-015-1921-2. [DOI] [PubMed] [Google Scholar]
- 101.Hockaday LA, Kang KH, Colangelo NW, Cheung PYC, Duan B, Malone E, Wu J, Girardi LN, Bonassar LJ, Lipson H, Chu CC, Butcher JT. Rapid 3D printing of anatomically accurate and mechanically heterogeneous aortic valve hydrogel scaffolds. Biofabrication. 2012;4(3):035005. doi: 10.1088/1758-5082/4/3/035005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fedorovich NE, Dewijn JR, Verbout AJ, Alblas J, Dhert WJA. Three-dimensional fiber deposition of cell-laden, viable, patterned constructs for bone tissue printing. Tissue Eng., Part A. 2008;14(1):127–133. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- 103.Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels. Anat. Rec. 2003;272A(2):497–502. doi: 10.1002/ar.a.10059. [DOI] [PubMed] [Google Scholar]
- 104.Fedorovich NE, Swennen I, Girones J, Moroni L, van Blitterswijk CA, Schacht E, Alblas J, Dhert WJA. Evaluation of Photocrosslinked Lutrol Hydrogel for Tissue Printing Applications. Biomacromolecules. 2009;10(7):1689–1696. doi: 10.1021/bm801463q. [DOI] [PubMed] [Google Scholar]
- 105.Boland T, Tao X, Damon BJ, Manley B, Kesari P, Jalota S, Bhaduri S. Drop-on-demand printing of cells and materials for designer tissue constructs. Mater. Sci. Eng., C. 2007;27(3):372–376. [Google Scholar]
- 106.Lee HJ, Kim YB, Ahn SH, Lee J-S, Jang CH, Yoon H, Chun W, Kim GH. A New Approach for Fabricating Collagen/ ECM-Based Bioinks Using Preosteoblasts and Human Adipose Stem Cells. Adv. Healthcare Mater. 2015;4(9):1359–1368. doi: 10.1002/adhm.201500193. [DOI] [PubMed] [Google Scholar]
- 107.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, Ramadan MH, Hudson AR, Feinberg AW. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science advances. 2015;1(9):e1500758–e1500758. doi: 10.1126/sciadv.1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cohen DL, Lipton JI, Bonassar LJ, Lipson H. Additive manufacturing for in situ repair of osteochondral defects. Biofabrication. 2010;2(3):035004. doi: 10.1088/1758-5082/2/3/035004. [DOI] [PubMed] [Google Scholar]
- 109.Luo Y, Lode A, Akkineni AR, Gelinsky M. Concentrated gelatin/alginate composites for fabrication of predesigned scaffolds with a favorable cell response by 3D plotting. RSC Adv. 2015;5(54):43480–43488. [Google Scholar]
- 110.Zehnder T, Sarker B, Boccaccini AR, Detsch R. Evaluation of an alginate-gelatine crosslinked hydrogel for bioplotting. Biofabrication. 2015;7(2):025001. doi: 10.1088/1758-5090/7/2/025001. [DOI] [PubMed] [Google Scholar]
- 111.Wei J, Wang J, Su S, Wang S, Qiu J, Zhang Z, Christopher G, Ning F, Cong W. 3D printing of an extremely tough hydrogel. RSC Adv. 2015;5(99):81324–81329. [Google Scholar]
- 112.Das S, Pati F, Choi Y-J, Rijal G, Shim J-H, Kim SW, Ray AR, Cho D-W, Ghosh S. Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs. Acta Biomater. 2015;11:233–246. doi: 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 113.Rutz AL, Hyland KE, Jakus AE, Burghardt WR, Shah RN. A Multimaterial Bioink Method for 3D Printing Tunable, Cell-Compatible Hydrogels. Adv. Mater. 2015;27(9):1607. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bertassoni LE, Cardoso JC, Manoharan V, Cristino AL, Bhise NS, Araujo WA, Zorlutuna P, Vrana NE, Ghaemmaghami AM, Dokmeci MR, Khademhosseini A. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication. 2014;6(2):024105. doi: 10.1088/1758-5082/6/2/024105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Snyder JE, Hamid Q, Wang C, Chang R, Emami K, Wu H, Sun W. Bioprinting cell-laden matrigel for radioprotection study of liver by pro-drug conversion in a dual-tissue microfluidic chip. Biofabrication. 2011;3(3):034112. doi: 10.1088/1758-5082/3/3/034112. [DOI] [PubMed] [Google Scholar]
- 116.Franco J, Hunger P, Launey ME, Tomsia AP, Saiz E. Direct write assembly of calcium phosphate scaffolds using a water-based hydrogel. Acta Biomater. 2010;6(1):218–228. doi: 10.1016/j.actbio.2009.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li C, Faulkner-Jones A, Dun AR, Jin J, Chen P, Xing YZ, Yang ZQ, Li ZB, Shu WM, Liu DS, Duncan RR. Rapid Formation of a Supramolecular Polypeptide-DNA Hydrogel for In Situ Three-Dimensional Multilayer Bioprinting. Angew. Chem., Int. Ed. 2015;54(13):3957–3961. doi: 10.1002/anie.201411383. [DOI] [PubMed] [Google Scholar]
- 118.Boere KWM, Blokzijl MM, Visser J, Linssen JEA, Malda J, Hennink WE, Vermonden T. Biofabrication of reinforced 3D–scaffolds using two-component hydrogels. J. Mater. Chem. B. 2015;3(46):9067–9078. doi: 10.1039/c5tb01645b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Censi R, Schuurman W, Malda J, di Dato G, Burgisser PE, Dhert WJA, van Nostrum CF, di Martino P, Vermonden T, Hennink WE. A Printable Photopolymerizable Thermosensitive p(HPMAm-lactate)-PEG Hydrogel for Tissue Engineering. Adv. Funct. Mater. 2011;21(10):1833–1842. [Google Scholar]
- 120.Muller M, Becher J, Schnabelrauch M, Zenobi-Wong M. Nanostructured Pluronic hydrogels as bioinks for 3D bioprinting. Biofabrication. 2015;7(3):035006. doi: 10.1088/1758-5090/7/3/035006. [DOI] [PubMed] [Google Scholar]
- 121.Highley CB, Rodell CB, Burdick JA. Direct 3D Printing of Shear-Thinning Hydrogels into Self-Healing Hydrogels. Adv. Mater. 2015;27(34):5075. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 122.Markstedt K, Mantas A, Tournier I, Avila HM, Hagg D, Gatenholm P. 3D Bioprinting Human Chondrocytes with Nano-cellulose-Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules. 2015;16(5):1489–1496. doi: 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- 123.Pati F, Jang J, Ha D-H, Kim SW, Rhie J-W, Shim J-H, Kim D-H, Cho D-W. Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 2014;5 doi: 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yang SF, Yang HY, Chi XP, Evans JRG, Thompson I, Cook RJ, Robinson P. Rapid prototyping of ceramic lattices for hard tissue scaffolds. Mater. Eng. 2008;29(9):1802–1809. [Google Scholar]
- 125.Will J, Melcher R, Treul C, Travitzky N, Kneser U, Polykandriotis E, Horch R, Greil P. Porous ceramic bone scaffolds for vascularized bone tissue regeneration. J. Mater. Sci.: Mater. Med. 2008;19(8):2781–2790. doi: 10.1007/s10856-007-3346-5. [DOI] [PubMed] [Google Scholar]
- 126.Zhang X, Jiang XN, Sun C. Micro-stereolithography of polymeric and ceramic microstructures. Sens. Actuators, A. 1999;77(2):149–156. [Google Scholar]
- 127.Xiang QF, Evans JRG, Edirisinghe MJ, Blazdell PF. Solid freeforming of ceramics using a drop-on-demand jet printer. Proc. Inst. Mech. Eng., Part B. 1997;211(3):211–214. [Google Scholar]
- 128.Slade CE, Evans JRG. Freeforming ceramics using a thermal jet printer. J. Mater. Sci. Lett. 1998;17(19):1669–1671. [Google Scholar]
- 129.Klosterman DA, Chartoff RP, Osborne NR, Graves GA, Lightman A, Han GW, Bezeredi A, Rodrigues S, Pak S, Kalmanovich G, Dodin L, Tu S. Direct fabrication of ceramics and CMCs by rapid prototyping. Am. Ceram. Soc. Bull. 1998;77(10):69–74. [Google Scholar]
- 130.Waetjen AM, Polsakiewicz DA, Kuhl I, Telle R, Fischer H. Slurry deposition by airbrush for selective laser sintering of ceramic components. J. Eur. Ceram. Soc. 2009;29(1):1–6. [Google Scholar]
- 131.Oezkol E, Ebert J, Uibel K, Waetjen AM, Telle R. Development of high solid content aqueous 3Y–TZP suspensions for direct inkjet printing using a thermal inkjet printer. J. Eur. Ceram. Soc. 2009;29(3):403–409. [Google Scholar]
- 132.Mott M, Evans JRG. Zirconia/alumina functionally graded material made by ceramic ink jet printing. Mater. Sci. Eng., A. 1999;271(1–2):344–352. [Google Scholar]
- 133.Song JH, Edirisinghe MJ, Evans JRG. Formulation and multilayer jet printing of ceramic inks. J. Am. Ceram. Soc. 1999;82(12):3374–3380. [Google Scholar]
- 134.Mott M, Song JH, Evans JRG. Microengineering of ceramics by direct ink-Jet printing. J. Am. Ceram. Soc. 1999;82(7):1653–1658. [Google Scholar]
- 135.Kalita SJ, Bose S, Hosick HL, Bandyopadhyay A. Development of controlled porosity polymer-ceramic composite scaffolds via fused deposition modeling. Mater. Sci. Eng., C. 2003;23(5):611–620. [Google Scholar]
- 136.Jafari MA, Han W, Mohammadi F, Safari A, Danforth SC, Langrana N. A novel system for fused deposition of advanced multiple ceramics. Rapid Prototyping Journal. 2000;6(3):161–174. [Google Scholar]
- 137.Griffith ML, Halloran JW. Freeform fabrication of ceramics via stereolithography. J. Am. Ceram. Soc. 1996;79(10):2601–2608. [Google Scholar]
- 138.Griffin EA, Mumm DR, Marshall DB. Rapid prototyping of functional ceramic composites. Am. Ceram. Soc. Bull. 1996;75(7):65–68. [Google Scholar]
- 139.Greco A, Licciulli A, Maffezzoli A. Stereolitography of ceramic suspensions. J. Mater. Sci. 2001;36(1):99–105. [Google Scholar]
- 140.Gentry SP, Halloran JW. Depth and width of cured lines in photopolymerizable ceramic suspensions. J. Eur. Ceram. Soc. 2013;33(10):1981–1988. [Google Scholar]
- 141.McNulty TF, Mohammadi F, Bandyopadhyay A, Shanefield DJ, Danforth SC, Safari A. Development of a binder formulation for fused deposition of ceramics. Rapid Prototyping Journal. 1998;4(4):144–150. [Google Scholar]
- 142.Khalyfa A, Vogt S, Weisser J, Grimm G, Rechtenbach A, Meyer W, Schnabelrauch M. Development of a new calcium phosphate powder-binder system for the 3D printing of patient specific implants. J. Mater. Sci.: Mater. Med. 2007;18(5):909–916. doi: 10.1007/s10856-006-0073-2. [DOI] [PubMed] [Google Scholar]
- 143.Ramakrishnan N, Rajesh RK, Ponnambalam P, Prakasan K. Studies on preparation of ceramic inks and simulation of drop formation and spread in direct ceramic inkjet printing. J. Mater. Process. Technol. 2005;169(3):372–381. [Google Scholar]
- 144.Roy TD, Simon JL, Ricci JL, Rekow ED, Thompson VP, Parsons JR. Performance of hydroxyapatite bone repair scaffolds created via three-dimensional fabrication techniques. J. Biomed. Mater. Res. 2003;67A(4):1228–1237. doi: 10.1002/jbm.a.20034. [DOI] [PubMed] [Google Scholar]
- 145.Leukers B, Gulkan H, Irsen SH, Milz S, Tille C, Schieker M, Seitz H. Hydroxyapatite scaffolds for bone tissue engineering made by 3D printing. J. Mater. Sci.: Mater. Med. 2005;16(12):1121–1124. doi: 10.1007/s10856-005-4716-5. [DOI] [PubMed] [Google Scholar]
- 146.Cox SC, Thornby JA, Gibbons GJ, Williams MA, Mallick KK. 3D printing of porous hydroxyapatite scaffolds intended for use in bone tissue engineering applications. Mater. Sci. Eng., C. 2015;47:237–247. doi: 10.1016/j.msec.2014.11.024. [DOI] [PubMed] [Google Scholar]
- 147.Hoath SD, Vadillo DC, Harlen OG, McIlroy C, Morrison NF, Hsiao WK, Tuladhar TR, Jung S, Martin GD, Hutchings IM. Inkjet printing of weakly elastic polymer solutions. J. Non-Newtonian Fluid Mech. 2014;205:1–10. [Google Scholar]
- 148.Fierz FC, Beckmann F, Huser M, Irsen SH, Leukers B, Witte F, Degistirici O, Andronache A, Thie M, Muller B. The morphology of anisotropic 3D–printed hydroxyapatite scaffolds. Biomaterials. 2008;29(28):3799–3806. doi: 10.1016/j.biomaterials.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 149.Michna S, Wu W, Lewis JA. Concentrated hydroxyapatite inks for direct-write assembly of 3-D periodic scaffolds. Biomaterials. 2005;26(28):5632–5639. doi: 10.1016/j.biomaterials.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 150.Vorndran E, Klarner M, Klammert U, Grover LM, Patel S, Barralet JE, Gbureck U. 3D Powder Printing of beta-Tricalcium Phosphate Ceramics Using Different Strategies. Adv. Eng. Mater. 2008;10(12):B67–B71. [Google Scholar]
- 151.Tarafder S, Balla VK, Davies NM, Bandyopadhyay A, Bose S. Microwave-sintered 3D printed tricalcium phosphate scaffolds for bone tissue engineering. J. Tissue Eng. Regener. Med. 2013;7(8):631–641. doi: 10.1002/term.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Miranda P, Pajares A, Saiz E, Tomsia AP, Guiberteau F. Mechanical properties of calcium phosphate scaffolds fabricated by robocasting. J. Biomed. Mater. Res., Part A. 2008;85A(1):218–227. doi: 10.1002/jbm.a.31587. [DOI] [PubMed] [Google Scholar]
- 153.Gbureck U, Hozel T, Klammert U, Wurzler K, Muller FA, Barralet JE. Resorbable dicalcium phosphate bone substitutes prepared by 3D powder printing. Adv. Funct. Mater. 2007;17(18):3940–3945. [Google Scholar]
- 154.Gbureck U, Hoelzel T, Biermann I, Barralet JE, Grover LM. Preparation of tricalcium phosphate/calcium pyrophosphate structures via rapid prototyping. J. Mater. Sci.: Mater. Med. 2008;19(4):1559–1563. doi: 10.1007/s10856-008-3373-x. [DOI] [PubMed] [Google Scholar]
- 155.Butscher A, Bohner M, Roth C, Ernstberger A, Heuberger R, Doebelin N, von Rohr PR, Muller R. Printability of calcium phosphate powders for three-dimensional printing of tissue engineering scaffolds. Acta Biomater. 2012;8(1):373–385. doi: 10.1016/j.actbio.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 156.Butscher A, Bohner M, Doebelin N, Hofmann S, Muller R. New depowdering-friendly designs for three-dimensional printing of calcium phosphate bone substitutes. Acta Biomater. 2013;9(11):9149–9158. doi: 10.1016/j.actbio.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 157.Butscher A, Bohner M, Doebelin N, Galea L, Loeffel O, Muller R. Moisture based three-dimensional printing of calcium phosphate structures for scaffold engineering. Acta Biomater. 2013;9(2):5369–5378. doi: 10.1016/j.actbio.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 158.Bergmann C, Lindner M, Zhang W, Koczur K, Kirsten A, Telle R, Fischer H. 3D printing of bone substitute implants using calcium phosphate and bioactive glasses. J. Eur. Ceram. Soc. 2010;30(12):2563–2567. [Google Scholar]
- 159.Komlev VS, Popov VK, Mironov AV, Fedotov AY, Teterina AY, Smirnov IV, Bozo IY, Rybko VA, Deev RV. 3D Printing of Octacalcium Phosphate Bone Substitutes. Front. Bioeng. Biotechnol. 2015;3:81. doi: 10.3389/fbioe.2015.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Shanjani Y, De Croos JNA, Pilliar RM, Kandel RA, Toyserkani E. Solid freeform fabrication and characterization of porous calcium polyphosphate structures for tissue engineering purposes. J. Biomed. Mater. Res., Part B. 2010;93B(2):510–519. doi: 10.1002/jbm.b.31610. [DOI] [PubMed] [Google Scholar]
- 161.Gao L, Li C, Chen F, Liu C. Fabrication and characterization of toughness-enhanced scaffolds comprising beta-TCP/POC using the freeform fabrication system with micro-droplet jetting. Biomedical Materials. 2015;10(3):035009. doi: 10.1088/1748-6041/10/3/035009. [DOI] [PubMed] [Google Scholar]
- 162.Tarafder S, Bose S. Polycaprolactone-Coated 3D Printed Tricalcium Phosphate Scaffolds for Bone Tissue Engineering: In Vitro Alendronate Release Behavior and Local Delivery Effect on In Vivo Osteogenesis. ACS Appl. Mater. Interfaces. 2014;6(13):9955–9965. doi: 10.1021/am501048n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davila JL, Freitas MS, Inforcatti Neto P, Silveira ZC, Silva JVL, d’Avila MA. Fabrication of PCL/beta-TCP scaffolds by 3D mini-screw extrusion printing. J. Appl. Polym. Sci. 2016;133(15) [Google Scholar]
- 164.Castilho M, Rodrigues J, Pires I, Gouveia B, Pereira M, Moseke C, Groll J, Ewald A, Vorndran E. Fabrication of individual alginate-TCP scaffolds for bone tissue engineering by means of powder printing. Biofabrication. 2015;7(1):015004. doi: 10.1088/1758-5090/7/1/015004. [DOI] [PubMed] [Google Scholar]
- 165.Diogo GS, Gaspar VM, Serra IR, Fradique R, Correia IJ. Manufacture of beta-TCP/alginate scaffolds through a Fab@home model for application in bone tissue engineering. Biofabrication. 2014;6(2):025001. doi: 10.1088/1758-5082/6/2/025001. [DOI] [PubMed] [Google Scholar]
- 166.Bian WG, Li DC, Lian Q, Li X, Zhang WJ, Wang KZ, Jin ZM. Fabrication of a bio-inspired beta-Tricalcium phosphate/ collagen scaffold based on ceramic stereolithography and gel casting for osteochondral tissue engineering. Rapid Prototyping Journal. 2012;18(1):68–80. [Google Scholar]
- 167.Tarafder S, Dernell WS, Bandyopadhyay A, Bose S. SrO-and MgO-doped microwave sintered 3D printed tricalcium phosphate scaffolds: Mechanical properties and in vivo osteogenesis in a rabbit model. J. Biomed. Mater. Res., Part B. 2015;103(3):679–690. doi: 10.1002/jbm.b.33239. [DOI] [PubMed] [Google Scholar]
- 168.Bellini A, Shor L, Guceri SI. New developments in fused deposition modeling of ceramics. Rapid Prototyping Journal. 2005;11(4):214–220. [Google Scholar]
- 169.Dash TK, Konkimalla VB. Poly-epsilon-caprolactone based formulations for drug delivery and tissue engineering: A review. J. Controlled Release. 2012;158(1):15–33. doi: 10.1016/j.jconrel.2011.09.064. [DOI] [PubMed] [Google Scholar]
- 170.Costa PF, Puga AM, Diaz-Gomez L, Concheiro A, Busch DH, Alvarez-Lorenzo C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015;496(2):541–550. doi: 10.1016/j.ijpharm.2015.10.055. [DOI] [PubMed] [Google Scholar]
- 171.Ding CM, Qiao ZG, Jiang WB, Li HW, Wei JH, Zhou GD, Dai KR. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials. 2013;34(28):6706–6716. doi: 10.1016/j.biomaterials.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 172.Dorj B, Won JE, Kim JH, Choi SJ, Shin US, Kim HW. Robocasting nanocomposite scaffolds of poly(caprolactone)/ hydroxyapatite incorporating modified carbon nanotubes for hard tissue reconstruction. J. Biomed. Mater. Res., Part A. 2013;101(6):1670–1681. doi: 10.1002/jbm.a.34470. [DOI] [PubMed] [Google Scholar]
- 173.Goncalves EM, Oliveira FJ, Silva RF, Neto MA, Fernandes MH, Amaral M, Vallet-Regí M, Vila M. Three-dimensional printed PCL-hydroxyapatite scaffolds filled with CNTs for bone cell growth stimulation. J. Biomed. Mater. Res., Part B. 2015 doi: 10.1002/jbm.b.33432. [DOI] [PubMed] [Google Scholar]
- 174.Kumar S, Kruth JP. Composites by rapid prototyping technology. Mater. Eng. 2010;31(2):850–856. [Google Scholar]
- 175.Tan KH, Chua CK, Leong KF, Cheah CM, Cheang P, Abu Bakar MS, Cha SW. Scaffold development using selective laser sintering of polyetheretherketone-hydroxyapatite biocomposite blends. Biomaterials. 2003;24(18):3115–3123. doi: 10.1016/s0142-9612(03)00131-5. [DOI] [PubMed] [Google Scholar]
- 176.Luo YX, Zhai D, Huan ZG, Zhu HB, Xia LG, Chang J, Wu CT. Three-Dimensional Printing of Hollow-Struts-Packed Bioceramic Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces. 2015;7(43):24377–24383. doi: 10.1021/acsami.5b08911. [DOI] [PubMed] [Google Scholar]
- 177.Fu Q, Saiz E, Tomsia AP. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011;7(10):3547–3554. doi: 10.1016/j.actbio.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Osterbur L. M.S. Thesis. Champaign, IL: University of Illinois at Urbana-Champaign; 2013. 3D printing of hyaluronic acid scaffolds for tissue engineering applications. [Google Scholar]
- 179.Zhao SC, Zhang JH, Zhu M, Zhang YD, Liu ZT, Tao CL, Zhu YF, Zhang CQ. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015;12:270–280. doi: 10.1016/j.actbio.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 180.Zhang JH, Zhao SC, Zhu YF, Huang YJ, Zhu M, Tao CL, Zhang CQ. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10(5):2269–2281. doi: 10.1016/j.actbio.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 181.Jakus AE, Taylor SL, Geisendorfer NR, Dunand DC, Shah RN. Metallic Architectures from 3D–Printed Powder-Based Liquid Inks. Adv. Funct. Mater. 2015;25(45):6985–6995. [Google Scholar]
- 182.Detsch R, Schaefer S, Deisinger U, Ziegler G, Seitz H, Leukers B. In vitro-Osteoclastic Activity Studies on Surfaces of 3D Printed Calcium Phosphate Scaffolds. J. Biomater. Appl. 2011;26(3):359–380. doi: 10.1177/0885328210373285. [DOI] [PubMed] [Google Scholar]
- 183.Gao C, Deng Y, Feng P, Mao Z, Li P, Yang B, Deng J, Cao Y, Shuai C, Peng S. Current Progress in Bioactive Ceramic Scaffolds for Bone Repair and Regeneration. Int. J. Mol. Sci. 2014;15(3):4714–4732. doi: 10.3390/ijms15034714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Hart LR, Harries JL, Greenland BW, Colquhoun HM, Hayes W. Molecular design of a discrete chain-folding polyimide for controlled inkjet deposition of supramolecular polymers. Polym. Chem. 2015;6(41):7342–7352. [Google Scholar]
- 185.Loo YH, Lakshmanan A, Ni M, Toh LL, Wang S, Hauser CAE. Peptide Bioink: Self-Assembling Nanofibrous Scaffolds for Three-Dimensional Organotypic Cultures. Nano Lett. 2015;15(10):6919–6925. doi: 10.1021/acs.nanolett.5b02859. [DOI] [PubMed] [Google Scholar]
- 186.Hart LR, Harries JL, Greenland BW, Colquhoun HM, Hayes W. Supramolecular Approach to New Inkjet Printing Inks. ACS Appl. Mater. Interfaces. 2015;7(16):8906–8914. doi: 10.1021/acsami.5b01569. [DOI] [PubMed] [Google Scholar]
- 187.Kang H-W, Lee SJ, Ko IK, Kengla C, Yoo JJ, Atala A. A 3D bioprinting system to produce human-scale tissue constructs with structural integrity. Nat. Biotechnol. 2016;34:312. doi: 10.1038/nbt.3413. [DOI] [PubMed] [Google Scholar]