Abstract
Background:
The purpose of this systematic review and meta-analysis of randomized controlled trials (RCTs) was to evaluate the efficacy and safety of combined topical with intravenous tranexamic acid (TXA) versus topical, intravenous TXA alone or control for reducing blood loss after a total knee arthroplasty (TKA).
Methods:
In May 2016, a systematic computer-based search was conducted in the PubMed, Embase, Cochrane Library, Web of Science, and Chinese Wanfang database. This systematic review and meta-analysis were performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement criteria. Only patients prepared for primary TKA that administration combined topical with intravenous TXA with topical TXA, intravenous (IV) TXA, or control group for reducing blood loss were included. Eligible criteria were published RCTs about combined topical with intravenous TXA with topical alone or intravenous alone. The primary endpoint was the total blood loss and need for transfusion. The complications of deep venous thrombosis (DVT) were also compiled to assess the safety of combined topical TXA with intravenous TXA. Relative risks (RRs) with 95% CIs were estimated for dichotomous outcomes, and mean differences (MDs) with 95% CIs for continuous outcomes. The Cochrane risk of bias tool was used to appraise a risk of bias. Stata 12.0 software was used for meta-analysis.
Results:
Fifteen studies involving 1495 patients met the inclusion criteria. The pooled meta-analysis indicated that combined topical TXA with intravenous TXA can reduce the total blood loss compared with placebo with a mean of 458.66 mL and the difference is statistically significant (MD = −458.66, 95% CI: −655.40 to 261.91, P < 0.001). Compared with intravenous TXA, combined administrated TXA can decrease the total blood loss, and the difference is statistically significant (MD = −554.03, 95% CI: −1066.21 to −41.85, P = 0.034). Compared with the topical administration TXA, the pooled meta-analysis indicated that combined TXA can decrease the amount of total blood loss with mean 107.65 mL with statistically significant(MD = −107.65, 95% CI: −525.55 to −239.9141.85, P = 0.001). The pooled results indicated that combined topical with intravenous TXA can decrease the need for transfusion (RR = 0.34, 95% CI: 0.23–0.50, P < 0.001). There is no significant difference between combined topical with intravenous TXA with topical or intravenous TXA (P > 0.05) in terms of need for transfusion and the occurrence of DVT.
Conclusion:
Compared with topical, intravenous TXA alone or control group, combined topical with TXA, can decrease the total blood loss and subsequent need for transfusion without increasing the occurrence of DVT. The dose and timing to administration TXA is different, and more randomized controlled trials are warranted to clarify the optimal dosing and time to administration TXA.
Keywords: blood loss, meta-analysis, total knee replacement, tranexamic acid
1. Introduction
Total knee arthroplasty (TKA) is now an excellent surgical procedure for patients with end-stage osteoarthritis or rheumatoid arthritis, consisting of replacing diseased or damaged knee joint surfaces to relieve the pain and disability of patients. Indeed, the number of primary TKR procedures will be reached at 3.48 million in 2030 in the United States and the number will be as 8-fold to the year of 2005.[1] A large amount of blood loss and subsequent blood transfusion is one of the complications around TKA.[2] It is reported that patients who underwent TKA may result in blood loss range from 1450 mL to 1790 mL.[3,4] The current surgical technique in total knee arthroplasty (TKA) usually includes the use of a tourniquet, prevents intraoperative bleeding but not substantial postoperative blood loss. One of the main problems with TKA is the need for blood transfusion (BT) in some patients. Blood transfusion is considered undesirable since it is associated with the risk of immunological reaction, disease transmission, and large economic costs.[5–7] Blood loss control with tranexamic acid (TXA) may be a preferable alternative. The main mechanism of TXA is competitively blocking the lysine binding sites of plasminogen. Many randomized controlled trials and meta-analysis have been identified the efficacy of TXA in TKA over a range of dose regimens without increasing the occurrence of deep venous thrombosis (DVT).[8] There are different administration route of TXA for TKA and all of those have been identified efficient and have not reach consistent.[9] Intravenous TXA of 1 to 3 dose was found to be the appropriate dose for the beneficial blood-saving effect and without increasing the occurrence of DVT. To reduce the potential risk of thromboembolic complications and extended the effect of TXA postoperatively, the topical administration TXA including intra-articular and infusion TXA was evaluated in previous studies and with notable clinical relevant effect compared with intravenous.[10] In addition, topical TXA could reduce the postoperative swelling and thus the pain which caused by swelling can reducing.
Recently, an extended administration TXA by combined topical with intravenous have been investigated; however, there is no consistent conclusion about the different route administration TXA. What's more, no strong evidence has been obtained from large RCTs evaluating combined topical with intravenous TXA for reducing blood loss after TKA. Thus, a meta-analysis was conducted to further analyses the efficacy and safety of combined topical with intravenous TXA versus topical, intravenous TXA alone or control for reducing blood loss, need for transfusion and the occurrence of DVT for patients for TKA.
2. Material and methods
This review is registered in Protocol registration: PROSPERO 2016: CRD42016043244.
2.1. Literature research
The electronic databases as follows were searched: PubMed, Embase, Cochrane Library, Web of Science and Chinese Wanfang database from inception to May 2016. The search strategies were performed using exploded medical subject headings, and the appropriate corresponding terms including “tranexamic acid,” “total knee replacement,” “total knee arthroplasty,” “TKA,” “TKR,” and “Arthroplasty, Replacement, Knee [Mesh].” The searches were limited to human subjects, and no language restriction was limited. The references of relevant reviews and included studies were also checked manually to identify additional potentially eligible studies. This is a systematic review and meta-analysis that collected data from published papers; thus, no ethics approval was not necessary.
2.2. Study selection
Two investigators executed the literature search independently, removed the duplicate records, screened the titles and abstracts for relevance, and tagged the articles as included, excluded, or requiring further assessment. Published RCTs meeting the following criteria were included: adult patients with unilateral or bilateral TKA; intervention including combined intravenous TXA with topical TXA; the control group including topical, intravenous TXA alone, and no TXA; outcomes including one of the follows: total blood loss, need for transfusion, and occurrence of DVT. Inclusion studies have no limitation on language and publication years. TXA, a synthetic antifibrinolytic agent, can competitively bind to the lysine binding sites of plasminogen and thus the blood loss can be decreased.[11] TXA can be administrated by several routes including topical, intravenous, oral, and intramuscular.[12] TXA can take about 2 hours for oral, 30 minutes for intramuscular, and 5 to 15 minutes for intravenous to achieve the maximum plasma concentration.[13,14] Thus, the best suitable method for rapidly increasing the therapeutic concentration of TXA is by the intravenous route. In previous studies, both intravenous and topical administration TXA can decrease total blood loss and blood loss in drainage without increasing the occurrence of DVT after TKA.[15–17] It has been reported that intravenous administration TXA can only decrease the external blood loss but not the hidden blood loss due to the application of tourniquet in a TKA, and this may increase the hidden blood loss due to the aggravation of local fibrinolytic activity. Through the topical administration TXA before wound closure, TXA can be rapidly absorbed and maintain a biological half-time of ∼3 hours in the joint cavity.[4] In addition, the topical administration TXA can decrease the blood concentration of TXA and lower the occurrence of DVT.
2.3. Data extraction and outcome measures
The following information was extracted from each study and recorded in a prepared sheet: first author, year of publication, number of patients, dose of TXA for topical and intravenous, the route to administrated TXA, whether drainage was applied, transfusion criteria, type of prosthesis, and the postoperative thromboprophylaxis. The outcomes: total blood loss, need for transfusion, and the occurrence of DVT were also extracted from the included studies. Extracted data were entered into a pregenerated standard Microsoft Excel (Microsoft Corporation, Redmond, WA) file. The length of follow up in each studies were also recorded. Data extraction was performed by one author and then checked by another. If the paper does not describe the mean value or standard deviation value, an E-mail was sent to obtain the original data. Any disagreement was resolved by discussion and consensus. The efficacy of TXA was measured by the total blood loss and need for transfusion. If the postoperative blood loss was better controlled, the total blood loss decreased. The most concern problem of TXA is the occurrence of DVT.
2.4. Assessment for the risk of bias
The Cochrane risk of bias tool was used to appraise the risk of bias. Two investigators independently reviewed all studies and graded the risk as “high,” “low,” or “unclear” in the following categories: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and baseline imbalance. Trials with a high risk of bias for any 1 or more key domains were considered to be at a high risk of bias, whereas trials with a low risk of bias for all key domains were considered to be at a low risk of bias. Otherwise, they were considered to be at unclear risk of bias. All discrepancies were resolved by consensus. The risk bias for the included studies is drawn by the software of RevMan 5.30 software (The Cochrane Collaboration, Oxford, UK).
2.5. Statistical analysis
Relative risks (RRs) with 95% confidence intervals (CIs) were estimated for dichotomous outcomes (the occurrence of DVT and need for transfusion), and mean differences (MD) with 95% CIs for continuous outcomes (total blood loss). The results were considered statistically significant if the P < 0.05 level or 95% CI did not include 1.00. A random-effects model was used regardless of heterogeneity. Heterogeneity was reported using the I2 statistic; a value of 0% indicated no heterogeneity and over 50% indicated significant heterogeneity. Results were considered as statistically significant if P < 0.05. Funnel plots were created to determine the presence of publication bias, and a sensitivity analysis was conducted if there is large heterogeneity between the included studies. If the sensitivity analysis cannot seek out the source of heterogeneity, then the subgroup analysis was conducted according to the dose of TXA. All statistical analyses were done using Stata 12.0 (Stata Corp., College Station, TX).
3. Results
3.1. Search results and risk bias
The selection of relevant literatures for this meta-analysis is summarized in the flow diagram. Some 13 studies with a total of 1224 patients (605 TXA, 619 control) were finally included in this meta-analysis.[18–30] Study baseline characteristics are presented in Table 1. All studies were published between 2014 and 2016, and the sample size ranged from 20 to 73. The mean age of patients in each study was from 63 to 70.7 years. Two studies compared combined TXA with control, topical, and intravenous TXA.[21,24] Topical administration TXA including directly injected into the joint or through injected into the drain tube. Details of the risk of bias for included RCTs are presented in Figs. 1 and 2. Seven studies give a detailed description of the random sequence generation,[18,20–22,24,26,27,30] and 5 trials perform the allocation concealment.[18,19,22,26,30] TXA is an anti-fibrinolytic agent, and the efficacy of TXA was tested by the total blood loss and patients’ need for transfusion. The antifibrinolytic agents have the potential to increase the occurrence of DVT; thus, the safety of TXA was measured by the occurrence of DVT. The application of topical administration TXA in some trials was through drainage and clamp for several hours to make TXA directly uptake in the joint cavity.
Table 1.
The general characteristic of the included studies.
Figure 1.
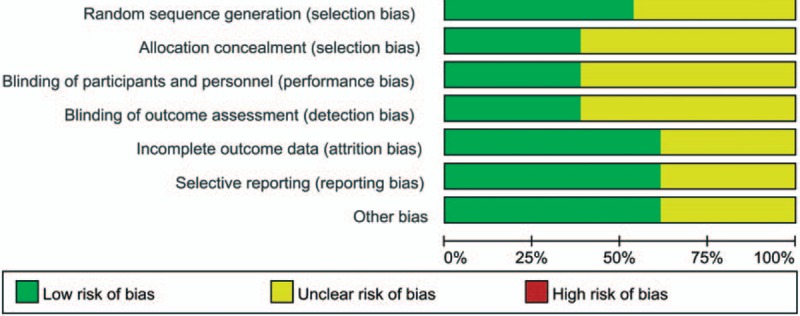
The risk of bias summary of the included studies.
Figure 2.
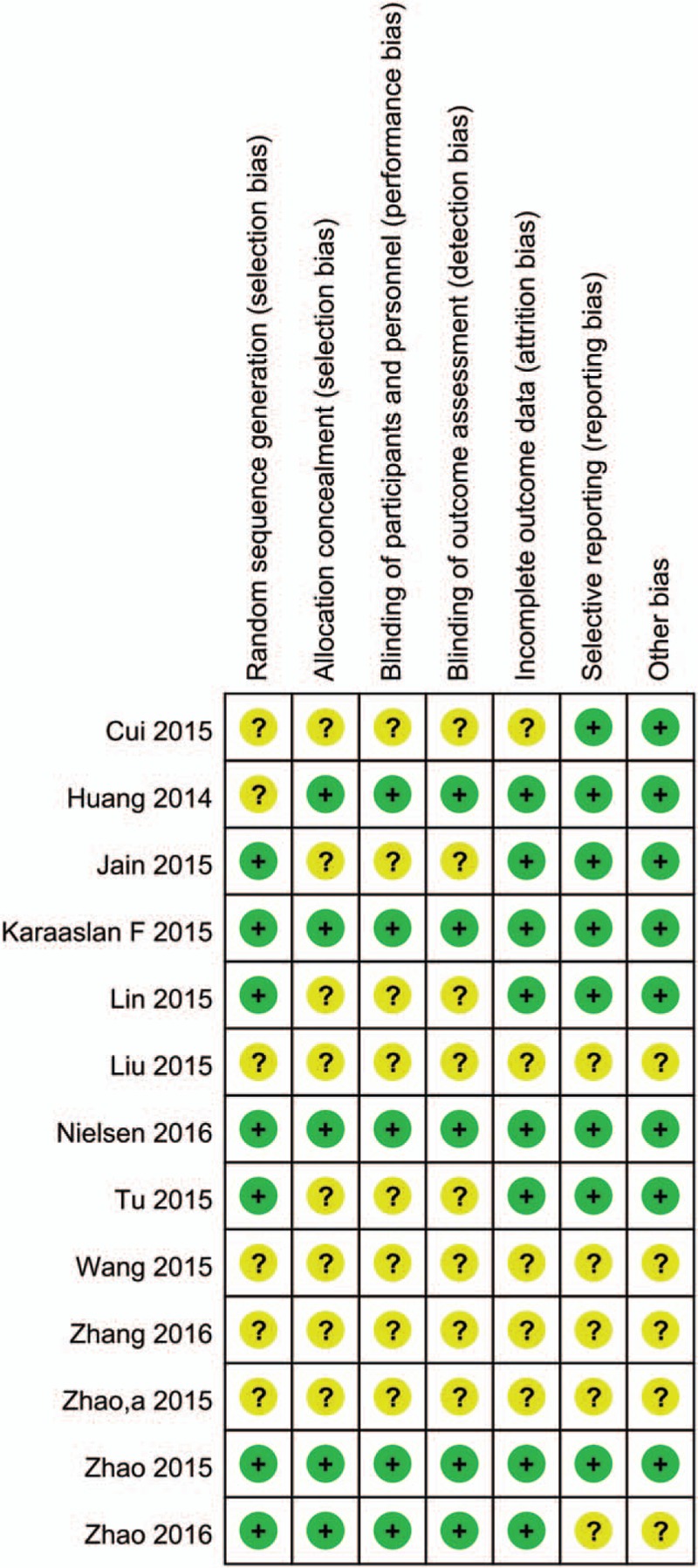
The risk of bias of each included studies.
A total of 7 trials[20–22,24,26,27,30] with 1110 patients perform the total blood loss after TKA, and 7 studies[20–22,24,26,27,30] compared the combined topical TXA with intravenous TXA with control, 3 studies[19,21,28] compared the combined topical TXA with intravenous TXA with intravenous TXA, and 3 studies[18,21,24] compared the combined TXA with the topical administration TXA. The pooled meta-analysis indicated that the combined topical TXA with intravenous TXA can reduce the total blood loss compared with placebo with a mean of 458.66 mL and the difference is statistically significant (MD = −458.66, 95% CI: −655.40 to 261.91, P < 0.001, Fig. 3). A random-model was performed as there is a large heterogeneity between the studies (I2 = 94.9%, P < 0.001).
Figure 3.
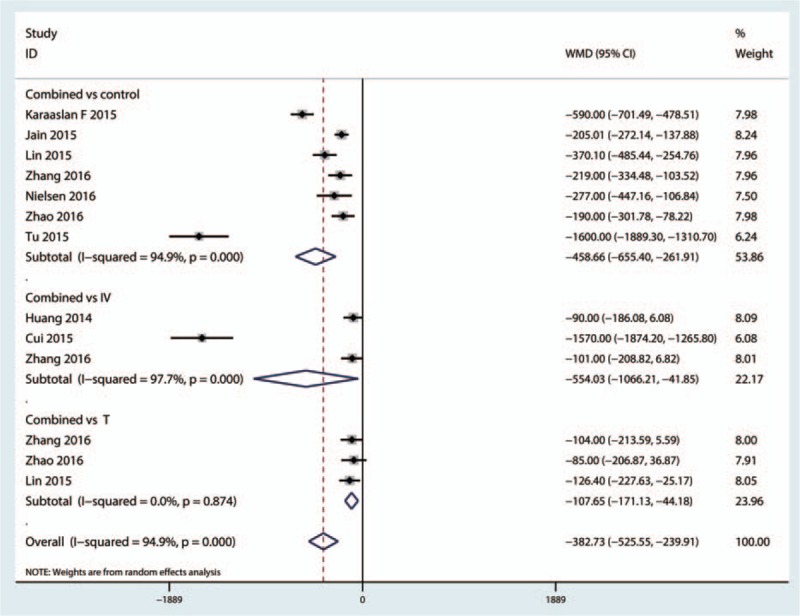
Forest plot of the combined TXA with topical or intravenous or topical for total blood loss. TXA = tranexamic acid.
Three trials[18–30] with 370 patients reported the data of total blood loss between the combined TXA versus intravenous TXA, and since there is a large heterogeneity between the studies (I2 = 97.9%, P < 0.001, Fig. 3), a random-model was adopted and the pooled results indicated that the combined administrated TXA can decrease the total blood loss; the difference is statistically significant (MD = −554.03, 95% CI: −1066.21 to −41.85, P = 0.034, Fig. 3).
A total of 3 trials[18–30] with 167 patients compared the combined TXA versus the topical administration TXA, and the pooled meta-analysis indicated that the combined TXA can decrease the amount of total blood loss with 107.65 mL; the difference is statistically significant (MD = −107.65, 95% CI: −525.55 to −239.91, P = 0.001, Fig. 3).
Since there is a large heterogeneity between the studies, the sensitivity analysis was conducted to seek out the source of heterogeneity, and the results are presented in Fig. 4. There is no studies stride the critical line. Publication bias was tested by the funnel plot and the results indicated that there is publication bias between the studies. The P value drawn from Begg's test is below 0.05, which indicates that there is a large heterogeneity between the included studies (Figs. 5 and 6). The subgroup analysis was conducted based on the dose of intravenous TXA (the high dose is >1 g and the low dose is <1 g). The results show that the intravenous high dose TXA can obtain better blood loss control than the low dose TXA (Table 2).
Figure 4.
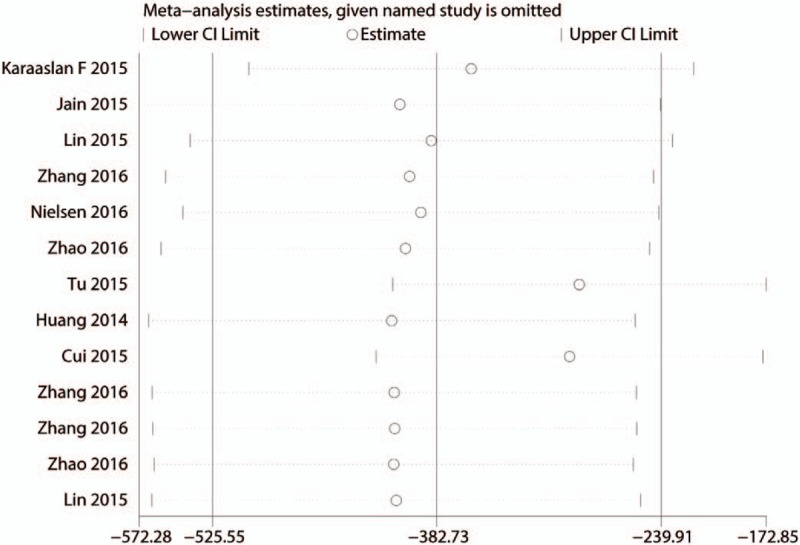
Sensitivity analysis for the total blood loss.
Figure 5.
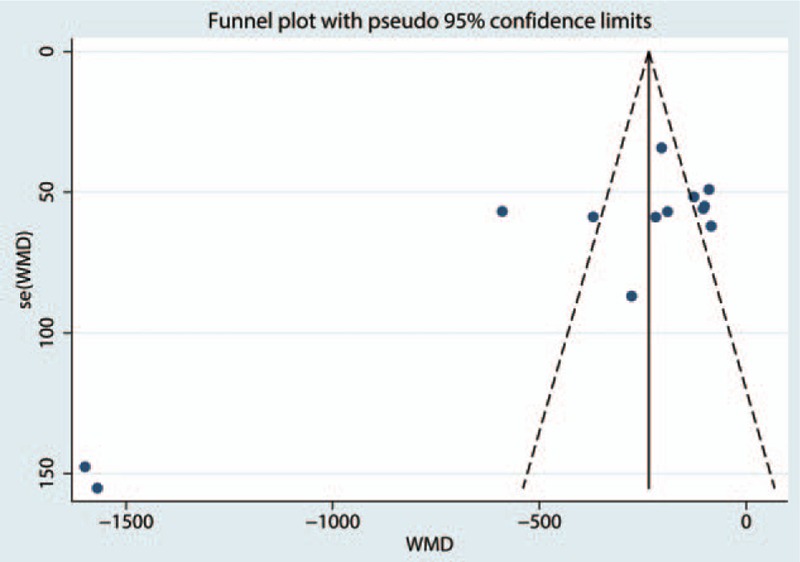
Funnel plot of the total blood loss for the test for the publication bias.
Figure 6.
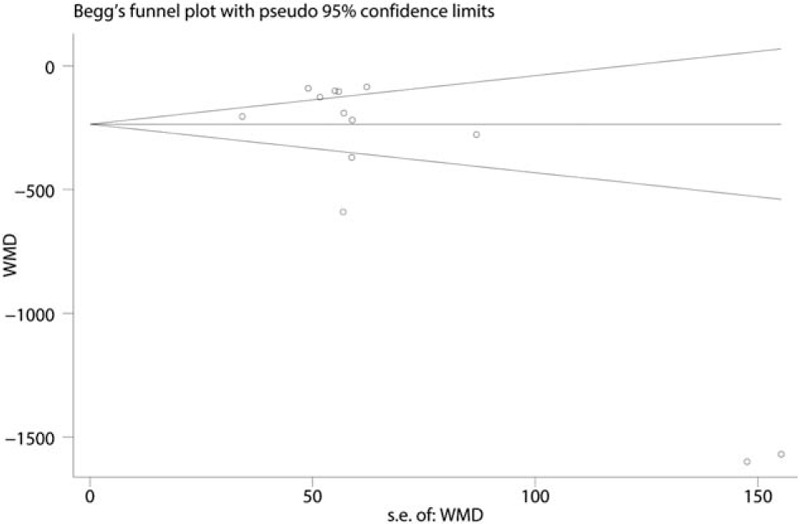
Begg's test for the publication bias.
Table 2.
Subgroup analysis for the total blood loss.

A total of 9[18–22,24,26–28] studies perform the data of the need for transfusion after TKA and pooled results indicated that combined topical with intravenous TXA can decrease the need for transfusion (RR = 0.34, 95% CI: 0.23–0.50, P < 0.001, Fig. 7). Compared with intravenous TXA, combined topical with intravenous TXA can decrease the need for transfusion (RR = 0.76, 95% CI: 0.52–1.11, P = 0.151, Fig. 7). Compared with the topical administration TXA, combined topical with intravenous TXA can decrease the need for transfusion (RR = 0.47, 95% CI: 0.18–1.27, P = 0.136, Fig. 7).
Figure 7.
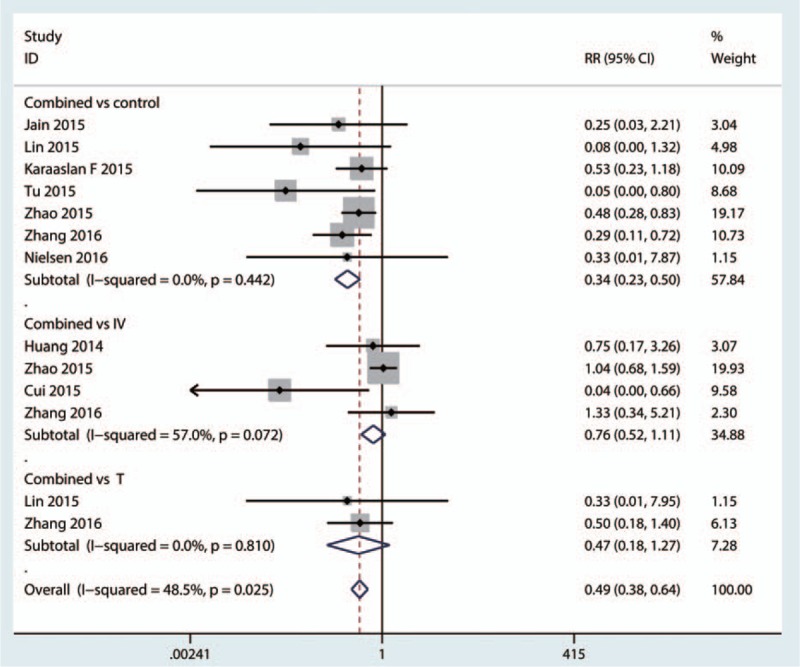
Forest plot comparing the combined TXA for need for transfusion with topical or intravenous or control group. TXA = tranexamic acid.
There was no significant difference between the combined topical with intravenous TXA with topical, intravenous, and control group (P > 0.05, Fig. 8).
Figure 8.
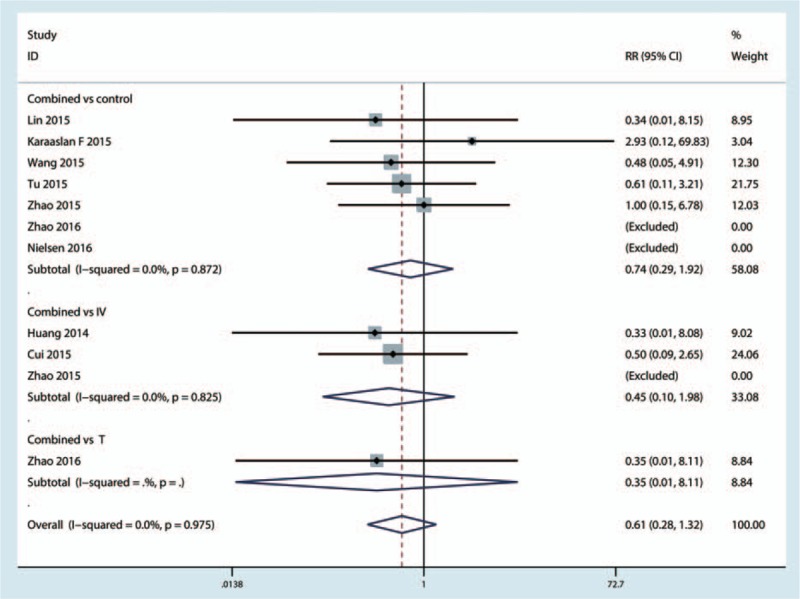
Forest plot comparing the occurrence of DVT of combined TXA with topical or intravenous or control group. DVT = deep venous thrombosis, TXA = tranexamic acid.
4. Discussion
This is the first systematic review and meta-analysis to compare the efficacy and safety of combined topical with intravenous TXA in TKA. It is well established that patients undergoing TKA are at risk of blood loss and blood transfusion.[31] The reason is that blood loss cause not only direct tissue damage but also the activation of local fibrinolysis caused by surgical trauma and the application of tourniquet.[32] The use of tourniquet can prevent blood loss intraoperative but not substantial postoperative blood loss. The results of this meta-analysis indicated that combined topical with intravenous administration TXA can decrease total blood loss and blood transfusion to an extreme compared with intravenous TXA, topical TXA, and control group. Since fibrinolytic activation is an enzymatic cascade process, it is easily inhibited in the early stages. The opportunity to intravenous TXA is usually at 15 minutes before surgery, and thus, it can reach peak plasma concentration at tourniquet release. On the one hand, the sample in each study is small and there is still need for high-quality RCTs to further identify. On the other hand, the transfusion criteria in each study are different.
As for DVT, there is no significant difference between the combined topical with intravenous TXA group with topical TXA or intravenous TXA group. Many studies have been identified that there is no significant difference between intravenous TXA with control group in terms of the occurrence of DVT. Those trials focused on the patients with no thrombosis history and with no long-term follow up. The topical administration TXA have been also identified to not increase the occurrence of DVT. It is widely accepted that TXA does not inhibit fibrinolytic activity in the normal vein wall, and the real impact on the disrupted endothelium remains unknown.[33]
There were several limitations in this meta-analysis: (1) only 13 RCTs were included, and the sample sizes in each trial were not large enough, which affects the final results; (2) the duration of follow-up in some studies was unclear, and long-term follow-up was needed for this analysis; (3) the publication bias that existed in the meta-analysis influenced the results; (4) the dose and the method of TXA administration differ among the included trials, which affects the final conclusion; and (5) the heterogeneity among the studies will also affect the final conclusion, although we tried to use the subgroup analysis to solve it.
In conclusion, based on the current meta-analysis, combined topical with TXA can decrease the total blood loss compared with topical or intravenous TXA without increasing the occurrence of DVT. However, there is no significant difference between combined topical with intravenous TXA with topical or intravenous TXA. Thus, more high-quality RCTs are needed to identify the clinical value of combined topical with intravenous TXA in TKA.
Footnotes
Abbreviations: CI = confidence interval, DVT = deep venous thrombosis, MD = mean differences, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RR = relative risk, TKA = total knee arthroplasty, TXA = tranexamic acid.
CL and YQ have contributed equally to this study, and considered as co-first authors.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [2].Kapadia BH, Banerjee S, Issa K, et al. Preoperative blood management strategies for total knee arthroplasty. J Knee Surg 2013;26:373–7. [DOI] [PubMed] [Google Scholar]
- [3].Good L, Peterson E, Lisander B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Brit J Anaesth 2003;90:596–9. [DOI] [PubMed] [Google Scholar]
- [4].Hiippala ST, Strid LJ, Wennerstrand MI, et al. Tranexamic acid radically decreases blood loss and transfusions associated with total knee arthroplasty. Anesth Analg 1997;84:839–44. [DOI] [PubMed] [Google Scholar]
- [5].Charoencholvanich K, Siriwattanasakul P. Tranexamic acid reduces blood loss and blood transfusion after TKA: a prospective randomized controlled trial. Clin Orthop Relat Res 2011;469:2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oei W, Neslo R, Janssen MP. A consensus-based tool for ranking the risk of blood-transmissible infections. Transfusion 2016;56:2108–14. [DOI] [PubMed] [Google Scholar]
- [7].Douros A, Jobski K, Kollhorst B, et al. Risk of venous thromboembolism in cancer patients treated with epoetins or blood transfusions. Brit J Clin Pharmacol 2016;82:839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Antinolfi P, Innocenti B, Caraffa A, et al. Post-operative blood loss in total knee arthroplasty: knee flexion versus pharmacological techniques. Knee Surg Sports Traumatol Arthrosc 2014;22:2756–62. [DOI] [PubMed] [Google Scholar]
- [9].Aguilera X, Martinez-Zapata MJ, Hinarejos P, et al. Topical and intravenous tranexamic acid reduce blood loss compared to routine hemostasis in total knee arthroplasty: a multicenter, randomized, controlled trial. Arch Orthop Trauma Surg 2015;135:1017–25. [DOI] [PubMed] [Google Scholar]
- [10].Lin ZX, Woolf SK. Safety efficacy and cost-effectiveness of tranexamic acid in orthopedic surgery. Orthopedics 2016;39:119–30. [DOI] [PubMed] [Google Scholar]
- [11].Aggarwal AK, Singh N, Sudesh P. Topical vs intravenous tranexamic acid in reducing blood loss after bilateral total knee arthroplasty: a prospective study. J Arthroplasty 2015;31:1442–8. [DOI] [PubMed] [Google Scholar]
- [12].Pinsornsak P, Rojanavijitkul S, Chumchuen S. Peri-articular tranexamic acid injection in total knee arthroplasty: a randomized controlled trial. BMC Musculoskelet Disord 2016;17:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Benoni G, Björkman S, Fredin H. Application of pharmacokinetic data from healthy volunteers for the prediction of plasma concentrations of tranexamic acid in surgical patients. Clin Drug Invest 1995;10:280–7. [Google Scholar]
- [14].Sano M, Hakusui H, Kojima C, et al. Absorption and excretion of tranexamic acid following intravenous, intramuscular and oral administrations in healthy volunteers. Jpn J Clin Pharmacol Therapeutics 1976;7:375–82. [Google Scholar]
- [15].Wang H, Shen B, Zeng Y. Comparison of topical versus intravenous tranexamic acid in primary total knee arthroplasty: a meta-analysis of randomized controlled and prospective cohort trials. Knee 2014;21:987–93. [DOI] [PubMed] [Google Scholar]
- [16].Wu Q, Zhang HA, Liu SL, et al. Is tranexamic acid clinically effective and safe to prevent blood loss in total knee arthroplasty? A meta-analysis of 34 randomized controlled trials. Eur J Orthop Surg Traumatol 2015;25:525–41. [DOI] [PubMed] [Google Scholar]
- [17].Alshryda S, Sukeik M, Sarda P, et al. A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 2014;96-b:1005–15. [DOI] [PubMed] [Google Scholar]
- [18].Zhao G-H, Ma J-B, Duan H-H, et al. The safety and efficacy of combined administration of tranexamic acid to reduce blood loss on total knee arthroplasty. J Pract Orthop 2015;21:691–4. [Google Scholar]
- [19].Huang Z, Ma J, Shen B, et al. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 2014;29:2342–6. [DOI] [PubMed] [Google Scholar]
- [20].Jain NP, Nisthane PP, Shah NA. Combined administration of systemic and topical tranexamic acid for total knee arthroplasty: can it be a better regimen and yet safe? a randomized controlled trial. J Arthroplasty 2015;31:542–7. [DOI] [PubMed] [Google Scholar]
- [21].Zhang J, Zhao M, Kan Z-K. A clinical study of the effect using locally implemented combined with intravenous tranexamic acid on the blood loss after total knee arthroplasty. Ningxia Med J 2016;38:130–2. [Google Scholar]
- [22].Karaaslan F, Karaoğlu S, Mermerkaya MU, et al. Reducing blood loss in simultaneous bilateral total knee arthroplasty: Combined intravenous–intra-articular tranexamic acid administration. A prospective randomized controlled trial. Knee 2015;22:131–5. [DOI] [PubMed] [Google Scholar]
- [23].Zhao Liang-Hu, Liu Dian-Feng, Huang Jin, et al. Effect of intravenous and topical application of tranexamic acid on bleeding amount in the perioperative period of unilateral total knee replacement and its safety evaluation. Rheum Arthritis 2015;4:14–6. [Google Scholar]
- [24].Liu Lin, Yang Zhi, Yao Jian-Feng, et al. Effect of local injections combined intravenous drip tranexamic acid on blood loss in total knee arthroplasty. J North Sichuan Med Coll 2015;30:611–4. [Google Scholar]
- [25].Lin S-Y, Chen C-H, Fu Y-C, et al. The efficacy of combined use of intraarticular and intravenous tranexamic acid on reducing blood loss and transfusion rate in total knee arthroplasty. J Arthroplasty 2015;30:776–80. [DOI] [PubMed] [Google Scholar]
- [26].Nielsen CS, Jans O, Orsnes T, et al. Combined intra-articular and intravenous tranexamic acid reduces blood loss in total knee arthroplasty: a randomized, double-blind, placebo-controlled trial. J Bone Joint Surg Am 2016;98:835–41. [DOI] [PubMed] [Google Scholar]
- [27].Tu S-L, Xu Huo R, Guo H-M. The effect of blood loss control of combined intravenous with intra-articular tranexamic acid for total knee arthroplasty. Chin J Clin Basic Orthop Res 2015;7:44–7. [Google Scholar]
- [28].Cui X-H, Wu H-B. The nursing effect of combined intravenous with topical administration tranxemic acid for blood loss after total knee arthroplasty. J North Pharm 2015;12:195–6. [Google Scholar]
- [29].Ying-Ming W, Rong K, De Wan S, et al. Effects of intravenous and intra-articular tranexamic acid on postoperative blood loss of primary total knee arthroplasty. Anhui Med J 2015;36:1350–2. [Google Scholar]
- [30].Zhao Z, Tao H, Tan J-X, et al. Efficacy and safety of intravenous and intra-articular sequential application of tranexamic acid during total knee arthroplasty. J Chongqing Med Univ 2015;41:142–6. [Google Scholar]
- [31].Callaghan JJ, O’Rourke MR, Liu SS. Blood management: issues and options. J Arthroplasty 2005;20(4 suppl 2):51–4. [DOI] [PubMed] [Google Scholar]
- [32].Jiang FZ, Zhong HM, Hong YC, et al. Use of a tourniquet in total knee arthroplasty: a systematic review and meta-analysis of randomized controlled trials. J Orthop Sci 2015;20:110–23. [DOI] [PubMed] [Google Scholar]
- [33].Astedt B, Liedholm P, Wingerup L. The effect of tranexamic acid on the fibrinolytic activity of vein walls. Ann Chirurgiae Gynaecol 1978;67:203–5. [PubMed] [Google Scholar]


