Abstract
Although incidental pancreatic cystic neoplasms are being diagnosed with increasing frequency, little is known about the accurate prevalence of pancreatic cysts in the general population. The aims of this study were to evaluate the crude prevalence rate of pancreatic cystic neoplasms in asymptomatic healthy adults, and calculate the age- and sex-adjusted nationwide prevalence rate.
A total of 21,745 asymptomatic individuals who underwent abdominal computed tomography (CT) as a health screening examination were enrolled between 2003 and 2013 at the Seoul National University Hospital Healthcare System Gangnam Center. Nationwide population data of 2010 were collected from the National Statistical Office, Korea.
Incidental pancreatic cystic neoplasms were found in 457 individuals whose mean age was 58.7 years. The types of neoplasms were reviewed by 2 separate designated radiologists and the final diagnosis was made as follows: intraductal papillary mucinous neoplasm: 376 (82%), serous cystic neoplasm: 19 (4%), mucinous cystic neoplasm: 7 (2%), and indeterminate cysts: 55 (12%). Eight cases underwent operation. The crude prevalence rate was 2.1% and the age- and sex-adjusted expected nationwide prevalence was 2.2%. The prevalence increased with age.
Here, we reported the first large-scale study among the healthy population to find out the prevalence rate of pancreatic cystic neoplasms; the age- and sex-adjusted prevalence was 2.2%, and increased with age. Further investigations regarding the clinical implications of incidental pancreatic neoplasms are necessary.
Keywords: incidental, pancreatic cystic neoplasms, prevalence
1. Introduction
Incidental pancreatic cystic neoplasms are one of the most frequently detected diseases as a result of advances in imaging technologies, and the increased use of cross-sectional imaging such as ultrasonography (USG), computed tomography (CT), and magnetic resonance imaging (MRI).[1–6] Pancreatic cystic neoplasms comprise of a vast spectrum of congenital, inflammatory, and neoplastic etiologies.[7] Due to the malignant potential of these neoplastic lesions, early detection and potential treatment of these lesions are crucial. However, little is known about the true prevalence of pancreatic cystic neoplasms and clinical significances in the general population.
To date, only a few studies have been performed investigating the true prevalence of pancreatic cysts.[8] Previous studies on the prevalence of incidental pancreatic cystic neoplasms were performed using abdominal USG, CT, and MRI, which have different sensitivities for cyst detection, and comprised of patients with a wide range of medical indications resulting in a wide range of prevalence ranging from 0.2% to 36.7%.[3,9] In order to investigate the crude and expected nationwide prevalence rate of incidental pancreatic cystic neoplasms in the general population, this study was performed on healthy individuals who underwent a preventive health checkup without medical indication through a unique health screening system in Korea. Through the Korean insurance system, which is called the National Health Insurance Service, general health and cancer surveillance are performed nationwide under national guidelines at the government's expense. Many companies also provide thorough health screening examinations for their employees as part of a welfare program. Therefore, a large-scale asymptomatic healthy population that represents the general population was evaluated in this study. Multidetector CT (MDCT) was selected as a diagnostic tool to maximize the number of samples that could be analyzed, as well as to achieve moderate diagnostic accuracy.[10–12]
2. Materials and methods
2.1. Health screening system in SNUH GC and inclusion criteria
Seoul National University Hospital Healthcare System Gangnam Center (SNUH GC) is a branch of SNUH, a tertiary teaching hospital. SNUH GC specializes in health screening examinations and focuses on preventive medicine. Subjects who were willing to undergo a health checkup for preventive purposes visited SNUH GC. Forty percent of the screening subjects were enrolled in the screening program via their companies or work places. In SNUH GC, there were approximately 30,000 screening subjects per year. Subjects who agreed to join the SNUH GC cohort were monitored and given medical advice with plans for regular future screening examinations based on their health screening results. If a certain medical condition was diagnosed during screening examination, subjects were referred to the SNUH for further evaluation or management.
A total of 25,300 healthy individuals who underwent abdominal MDCT as part of a preventive health checkup in the SNUH GC from October 2003 to June 2013 were included in this study. To ensure that any pancreatic cysts discovered were truly incidental, screening subjects with known or suspected pancreatic disease, abdominal symptoms, or a history of pancreatic or gastrointestinal surgery were excluded. A final total of 21,745 individuals were enrolled in this study. Imaging findings were reviewed for differential diagnosis by 2 specialized radiologists. Nationwide population data of 2010 were collected from the National Statistical Office, Korea. This study was approved by the Institutional Review Board of the Seoul National University Hospital (H-1204-018-403) and written consent from the participants was obtained.
2.2. Imaging technique
In this study, 21,745 individuals underwent MDCT consisting of precontrast, arterial, and portal phases. CT scans were obtained using one of the following commercially available MDCT scanners: LightSpeed Ultra 8-channel CT scanner (GE Healthcare, Milwaukee, WI) and Somatom definition dual-source CT scanner (Siemens Medical Solutions, Malvern, PA). The images with 3-mm thick sections were acquired. Using a power injector (Multilevel CT; Medrad, Pittsburgh, PA), 120 mL of nonionic contrast material (Iopromide, Ultravist 370; Schering, Berlin, Germany) was administered at a rate of 3 mL/s through an 18-gauge, plastic, intravenous catheter placed in an antecubital vein, followed by a 20 mL flush of sterile saline. For arterial phase imaging, the scanning delay was determined using an automatic bolus tracking technique provided by the CT manufacturer. Contrast enhancement was automatically calculated by placing the region-of-interest cursor over the vessel of interest, that is, the abdominal aorta, and the level of the trigger threshold was set at an increase of 100 Hounsfield units.
2.3. Radiologic criteria of differential diagnosis
CT scans were analyzed by 2 different specialized radiologists, and they agreed a consensus for the primary and secondary diagnosis after initial analysis. The primary diagnosis was chosen for the analysis. A diagnosis was made using the following criteria.[13–15] The branch duct type intraductal papillary mucinous neoplasm (IPMN) was diagnosed when the following findings were observed: obvious communication with the pancreatic duct on CT; pleomorphic or clubbed fingerlike cystic shape; downstream pancreatic duct dilatation. Mucinous cystic neoplasm (MCN) was diagnosed according to the following image findings: smooth margin with the internal septation; no obvious ductal communication; thick and enhanced wall; presence of mural nodules. If the pancreatic cystic neoplasms were found especially in women patients, and the location was at the body or tail of the pancreas, the imaging diagnosis of MCN was made with priority. The pancreatic cystic neoplasms were diagnosed as serous oligocystic neoplasm (SCN) if they showed the following findings on CT: lobulating contour with or without septation; no solid mural nodule; no pancreatic duct communication; no wall calcification.
When the pancreatic cystic neoplasms were less than 0.5 cm, and did not show typical imaging findings of specific diagnostic criteria, those lesions were classified as indeterminate. Whenever a low-attenuated lesion in the pancreas showed attenuation as low as the surrounding retroperitoneal fat, the lesion was determined as focal fat invagination.
Pancreatic cystic neoplasms without vascular enhancing internal structure were diagnosed as pseudocysts. In addition, presence of heterogeneous attenuation inside the cysts, wall calcification, underlying chronic pancreatitis, and history of pancreatitis were indicative of pseudocysts. If the cysts evolved on follow-up images, the cysts were also diagnosed as pseudocysts.
2.4. Statistical analysis
The crude prevalence rate is defined as the total number of people in a population who have a disease at a given time. Statistical adjustment in epidemiology is used to eliminate or reduce the confounding effects of extraneous confounding factors such as age. Direct age adjustment methods apply age-specific rates from the study population to an age distribution from a reference population.[16] In this study, age- and sex-specific prevalence rate of incidental pancreatic cystic neoplasms was calculated for the expected nationwide prevalence rate by applying the direct method. For example, the expected nationwide number of men in their 30 seconds (E30) with pancreatic cystic neoplasms was calculated from the age-specific prevalence rate of pancreatic cystic neoplasms in men in their 30 seconds (C30). The expected nationwide prevalence rate in men was then derived from the ratio of the sum of numbers of men in each decade to the total nationwide number of men (Fig. 1).
Figure 1.

Estimation of expected prevalence of pancreatic cystic neoplasm. The expected nationwide number of men in their 30 seconds with pancreatic cystic neoplasms (E30) was calculated from the age-specific prevalence rate of pancreatic cystic neoplasms in men in their 30 seconds (C30). The expected nationwide prevalence rate in men was then derived from the ratio of the sum of numbers of men in each decade to the total nationwide number of men with pancreatic cystic neoplasms.
Descriptive statistics were used regarding demographics. Pearson linear function test was used to examine the correlation between cyst prevalence and age. Statistical analysis was performed using SPSS 21.0 (SPSS, Inc, Chicago, IL) and P values less than 0.05 were considered statistically significant.
3. Results
3.1. Characteristics of incidental pancreatic cystic neoplasms
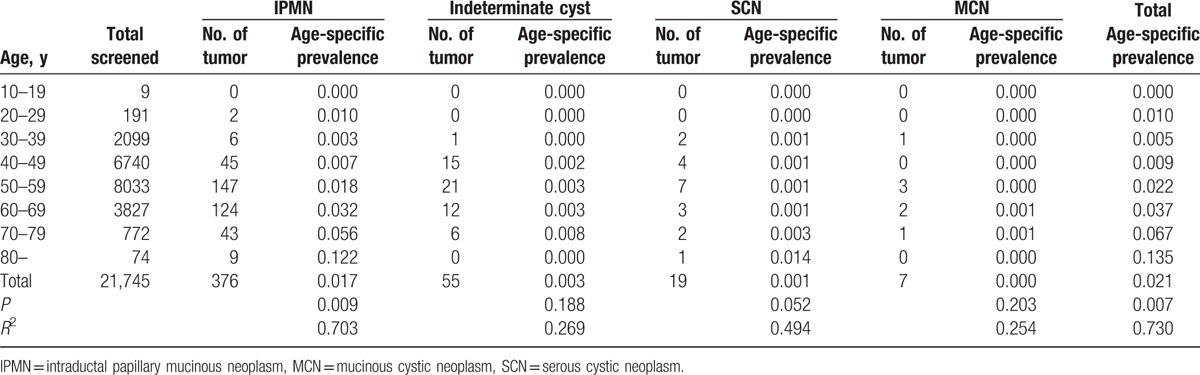
Health screening examinations were most frequently performed in individuals aged 45 to 49 years (Fig. 2). Mean age at the time of CT investigation of the study population was 51.8 years, and male (n = 13,046) to female ratio was 3:2. Pancreatic cystic neoplasms were identified in 457 cases (2.1%) among 21,745 individuals (Table 1). The mean age was 58.7 years at the time of diagnosis and the male to female ratio was 1.1:1.0. The IPMNs were most frequently observed (n = 376, 82%), and the indeterminate cysts, which were too small to be characterized, were the second most common (n = 55, 12%). There were 19 SCNs (4.2%) and 7 MCNs (1.5%). Pseudocysts were found in 6 patients; however, they were not included in the calculation of the prevalence of pancreatic cystic neoplasms. The median size of pancreatic cystic tumors was 10.7 ± 7.1 mm and pancreatic cystic neoplasms were located in the following areas: head (n = 166, 36.3%), body (n = 167, 36.5%), tail (n = 120, 26.3%), and diffuse involvement (n = 4, 0.8%). In addition, 48 pancreatic cystic neoplasms (10.5%) presented as multiple cysts.
Figure 2.

Demographics of the whole study population. Men constituted 60.0% of 21,745 subjects. Subjects within the age 45 to 49 years formed the largest group among both men and women. Values are mean ± standard deviation.
Table 1.
Characteristics of incidental pancreatic cystic neoplasms.

3.2. Follow-up and operated pancreatic cystic neoplasms
Among the subjects who agreed to join the SNUH GC cohort, follow-up imaging studies were performed 2 to 3 years apart if there were no significant changes in individuals with pancreas cystic tumors ≤ 10 mm in diameter. Patients with pancreatic cystic neoplasms > 10 mm in diameter were followed up at 6, 12, and 24 months based on their changes. The final pathologies of surgically resected pancreatic cystic neoplasms were 5 IPMNs with intermediate grade dysplasia, 2 IPMN with an associated invasive carcinoma, and 1 SCN. TNM staging was T1N0 in patients with IPMN with an associated invasive carcinoma.
3.3. Prevalence of pancreatic cystic neoplasms
Including indeterminate cysts, the crude prevalence rate of incidental pancreatic cystic neoplasms among the study population was 2.1% (Table 2). Interestingly, IPMNs were observed in patients in their 20 seconds, and the crude prevalence of IPMNs significantly increased as the age of the patients increased (Table 2). Furthermore, the incidence of SCNs tended to increase as the age of the screening subjects increased. Overall, the crude prevalence significantly increased with the age of the screening subject (r = 0.730, P = 0.007). The prevalence rate among patients in their 80 seconds reached 13.5% (Table 2).
Table 2.
Crude prevalence of incidental pancreatic cystic neoplasms.
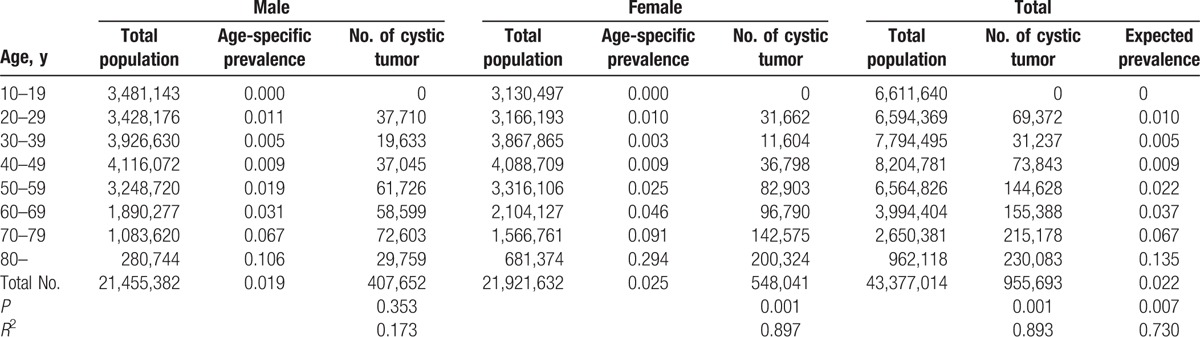
Furthermore, the population data of 2010 from the National Statistical Office of Korea was used for the calculation of the expected nationwide prevalence rate of pancreatic cystic neoplasms. Expected nationwide prevalence rates of men, women, and the whole population were 1.9%, 2.5%, and 2.2%, respectively (Table 3). The prevalence of incidental pancreatic cystic neoplasms also increased with age (r = 0.730, P = 0.007).
Table 3.
Crude prevalence according to sex and expected nationwide prevalence of incidental pancreatic cystic neoplasms.
4. Discussion
The number of incidental pancreatic cystic neoplasms has increased recently due to advances in the use of imaging technologies. However, their true prevalence and clinical significance have not been well studied. In this study, we have used data from a large-scale health screening examination in asymptomatic healthy individuals, which is uniquely generated, and these data are the best tool to investigate incidental pancreatic cystic neoplasms. Incidental pancreatic cystic neoplasms were observed in 457 individuals among the 21,745 health screening subjects. There were 376 IPMNs (82%),19 SCNs (4%), 7 MCNs (2%), and 55 indeterminate cysts (12%). The crude prevalence rate and age- and sex-adjusted expected nationwide prevalence were 2.1% and 2.2%, respectively. The prevalence of incidental pancreatic cystic neoplasms also increased with age. More importantly, we found that 7 (1.5%) out of 457 pancreatic cystic neoplasms required surgical resection.
To date, only a few studies have investigated the prevalence of pancreatic cystic neoplasms, and the reported rate has varied from 0.2% to 36.7%.[3,9] The majority of previous studies have been unable to exclude medical indication and previous medical history, which could cause overestimation of the prevalence or could sometimes result in underestimation during the exclusion process. In addition, the various diagnostic modalities such as USG, CT, MRI, and autopsy which all have different sensitivities in detecting pancreatic cystic neoplasms have contributed to the wide range of prevalence rate which have been studied so far. Ikeda et al[9] performed 30,951 USG examinations and reported a prevalence rate of 0.2%. Using CT scans, Laffan et al[6] reported a prevalence rate of 2.6% from 2832 MDCTs, which was comparable with our results. The prevalence rates evaluated using MRI have a wide range: the lowest rate was 2.4% from 2803 consecutive individuals who underwent MRI during the health screening examinations,[17] 9.3% from 2583 MRIs,[18] and 13.5% from 616 MRIs,[19] while the highest prevalence was 19.6% in 1444 MRIs, which were performed for various indications including pancreatic disorders.[5] In an autopsy of 300 cases, 24.3% (85% were 65 years or older) were found to have pancreatic cystic neoplasms.[20] Among various diagnostic modalities, CT may be a less sensitive tool for differentiating the type of cystic neoplasms of the pancreas compared to MRI or endoscopic ultrasonography[21]; however, current MDCT technology with narrow detector thickness provides spatial and temporal resolution, which facilitates identification of small, nonenhancing lesions in the pancreas.[6] Furthermore, MDCT is more frequently selected for surveillance among healthy asymptomatic individuals than MRI owing to the lower cost, which enables the recruitment of large consecutive series for investigation.
Radiologic differential diagnosis was performed in this study, which was not attempted in previous radiologic studies on the prevalence of incidental pancreatic cystic neoplasms. The diagnostic accuracy of MDCT in differentiation of IPMN from other pancreatic cystic neoplasms are reported as sensitivity of 80.6%, specificity of 86.4%, positive predictive value of 89.3%, and negative predictive value of 76.1% from previous report from our institute.[10] The prevalence of IPMN was 82.3% among incidental pancreatic cystic neoplasms, and 12.0% were too small to be characterized. There was no solid pseudopapillary neoplasm. This may be because the number of young women was relatively small in our study population who underwent health screening examinations on their own initiative and costs.
Similar to the previous studies,[5,6,17,20] the prevalence of pancreatic cystic neoplasms increased with age in this study. The crude prevalence rate in people in their 70 seconds was 6.7%, and the rate increased to 13.5% in people in their 80 seconds. The rate of malignant transformation also increased linearly with advanced age according to previous literature.[22] Spinelli et al[23] reported that age greater than 70 years and the presence of a symptom are predictors of malignant pathology. Although surgical excision was recommended in patients with increasing or symptomatic pancreatic cystic neoplasms in this report, it should be noted that operative risk also increases with age.
Eight cases had surgically resected pancreatic cystic neoplasms among 21,745 subjects in this study. Of the remaining 449 cases with asymptomatic pancreatic cystic neoplasms, the guideline to proceed after detection of a pancreatic cyst remains unclear. Much of the current evidence indicates that the majority of these small, simple, incidentally detected pancreatic cystic neoplasms are benign.[1,3,23–27] On follow-up images, 82% of pancreatic cystic neoplasms were unchanged or smaller in a study by Lee et al.[19] With a median follow-up of 28 months, the patients selected for initial surveillance had a 6.5% likelihood of developing changes that prompted resection and 1% likelihood of developing pancreatic malignancy.[28] Subsequent morphologic changes or development of symptoms prompted an operation in 8% according to Ferrone et al.[29] If the pancreatic cystic neoplasms are assessed to be either benign or of low malignant potential, the decision with which modality and what frequency the follow-up should be made.[30] Previous reports have shown various ways of follow-ups,[31–36] but considerable controversies remain regarding the surveillance of small, asymptomatic pancreatic cystic neoplasms.
According to the international consensus guideline 2012,[37] surveillance of the branch-duct type IPMN without “high-risk stigmata” was recommended according to size stratification. American Gastroenterological Association Institute guidelines suggest that patients with pancreatic cysts <3 cm without a solid component or a dilated pancreatic duct undergo MRI at 1 year, and then every 2 years afterwards for a total of 5 years if there is no change in size or characteristics.[38] In a recent study, incidental pancreatic cystic neoplasms were associated with increased mortality and an overall increased risk of pancreatic adenocarcinoma in patients younger than 65 years whereas there was no change in mortality in patients 65 years or older. Therefore, surveillance was warranted only in patients under 65 years of age.[39] However, there are little data on the natural history of pancreatic cystic neoplasms and very low quality of evidence for surveillance of incidental pancreatic cystic neoplasms to date. Nationwide survey was performed from 1993 to 2005 in 30 university hospitals in Korea on 1064 patients with surgically confirmed pancreatic cystic neoplasms.[40] Data regarding the proportion of each pancreatic cystic neoplasms and factors associated with malignancy were analyzed; however, reported data did not include the prevalence, natural course, and surveillance. Considering that the median size of pancreatic cystic neoplasms was 8.0 mm in this study, MDCT which was the chosen diagnostic tool, was not performed at every follow-up due to the risks associated with radiation. Moreover, since SNUH GC opened in 2004, limited follow-up data are available to investigate the exact rate of neoplasm change or growth in the present study.
The limitation of this study is the representativeness of the individuals included in the study. To calculate the expected nationwide prevalence rate of pancreatic cystic neoplasms, age, sex, and residential area of the study population has to represent the nationwide status. However, individuals who visited the SNUH GC for health screening examinations reside predominantly in the urban area, which was not adjusted in this study. Second, still we have to assume that unresected pancreatic cystic neoplasms are diagnosed based on radiological imaging studies which are not the final pathologic diagnosis.
Here, we reported the first large-scale study among the healthy population to determine the true prevalence rate of pancreatic cystic neoplasms; the age- and sex-adjusted prevalence was 2.2%and increased with age. There were 7 IPMNs (1.5%) out of 457 patients with pancreatic cystic neoplasms; 5 IPMNs with intermediate grade dysplasia, and 2 IPMNs with an associated invasive carcinoma. Pancreatic cystic neoplasms can often be found incidentally among the asymptomatic population, and further investigation with a longer follow-up period would provide more insight into understanding the behavior and clinical significance of pancreatic cystic neoplasms.
Acknowledgment
The authors thank Ji Won Park from Medical Research Collaborating Center, Seoul National University Hospital, Seoul, Korea for the assistance of statistical analysis.
Footnotes
Abbreviations: IPMN = intraductal papillary mucinous neoplasm, MCN = mucinous cystic neoplasm, SCN = serous cystic neoplasm, SNUH GC = Seoul National University Hospital Healthcare System Gangnam Center.
Funding: This study was supported by grants of the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI14C2640) and the Yongang Foundation (30-2014-0180), Republic of Korea.
YRC and JKP contributed equally to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Megibow AJ, Lombardo FP, Guarise A, et al. Cystic pancreatic masses: cross-sectional imaging observations and serial follow-up. Abdom Imaging 2001;26:640–7. [DOI] [PubMed] [Google Scholar]
- [2].Kiely JM, Nakeeb A, Komorowski RA, et al. Cystic pancreatic neoplasms: enucleate or resect? J Gastrointest Surg 2003;7:890–7. [DOI] [PubMed] [Google Scholar]
- [3].Fernandez-del Castillo C, Targarona J, Thayer SP, et al. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg 2003;138:427–3. discussion 424–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Balcom IJ, Fernandez-Del Castillo C, Warshaw AL. Cystic lesions in the pancreas: when to watch, when to resect. Curr Gastroenterol Rep 2000;2:152–8. [DOI] [PubMed] [Google Scholar]
- [5].Zhang XM, Mitchell DG, Dohke M, et al. Pancreatic cysts: depiction on single-shot fast spin-echo MR images. Radiology 2002;223:547–53. [DOI] [PubMed] [Google Scholar]
- [6].Laffan TA, Horton KM, Klein AP, et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am J Roentgenol 2008;191:802–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004;351:1218–26. [DOI] [PubMed] [Google Scholar]
- [8].de Jong K, Bruno MJ, Fockens P. Epidemiology, diagnosis, and management of cystic lesions of the pancreas. Gastroenterol Res Pract 2012;2012:147465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ikeda M, Sato T, Morozumi A, et al. Morphologic changes in the pancreas detected by screening ultrasonography in a mass survey, with special reference to main duct dilatation, cyst formation, and calcification. Pancreas 1994;9:508–12. [DOI] [PubMed] [Google Scholar]
- [10].Song SJ, Lee JM, Kim YJ, et al. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: comparison of multirow-detector CT and MR imaging using ROC analysis. J Magn Reson Imaging 2007;26:86–93. [DOI] [PubMed] [Google Scholar]
- [11].Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. AJR Am J Roentgenol 2007;189:648–56. [DOI] [PubMed] [Google Scholar]
- [12].Sahani DV, Sainani NI, Blake MA, et al. Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol 2011;197:W53–61. [DOI] [PubMed] [Google Scholar]
- [13].Choi BI. Radiology Illustrated: Hepatobiliary and Pancreatic Radiology. 2014;Heidelberg, Germany:Springer-Verlag GmbH, 35. [Google Scholar]
- [14].Kim SY, Lee JM, Kim SH, et al. Macrocystic neoplasms of the pancreas: CT differentiation of serous oligocystic adenoma from mucinous cystadenoma and intraductal papillary mucinous tumor. AJR Am J Roentgenol 2006;187:1192–8. [DOI] [PubMed] [Google Scholar]
- [15].Kim SH, Lim JH, Lee WJ, et al. Macrocystic pancreatic lesions: differentiation of benign from premalignant and malignant cysts by CT. Eur J Radiol 2009;71:122–8. [DOI] [PubMed] [Google Scholar]
- [16].Kasim K. Basic Concepts of Modern Epidemiology: Epidemiology and Research. 2012;Saarbrücken, Germany:LAP LAMBERT Academic Publishing, 63–71. [Google Scholar]
- [17].de Jong K, Nio CY, Hermans JJ, et al. High prevalence of pancreatic cysts detected by screening magnetic resonance imaging examinations. Clin Gastroenterol Hepatol 2010;8:806–11. [DOI] [PubMed] [Google Scholar]
- [18].de Oliveira PB, Puchnick A, Szejnfeld J, et al. Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS ONE 2015;10:e0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lee KS, Sekhar A, Rofsky NM, et al. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol 2010;105:2079–84. [DOI] [PubMed] [Google Scholar]
- [20].Kimura W, Nagai H, Kuroda A, et al. Analysis of small cystic lesions of the pancreas. Int J Pancreatol 1995;18:197–206. [DOI] [PubMed] [Google Scholar]
- [21].Curry CA, Eng J, Horton KM, et al. CT of primary cystic pancreatic neoplasms: can CT be used for patient triage and treatment? AJR Am J Roentgenol 2000;175:99–103. [DOI] [PubMed] [Google Scholar]
- [22].Gardner TB, Glass LM, Smith KD, et al. Pancreatic cyst prevalence and the risk of mucin-producing adenocarcinoma in US adults. Am J Gastroenterol 2013;108:1546–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Spinelli KS, Fromwiller TE, Daniel RA, et al. Cystic pancreatic neoplasms. Ann Surg 2004;239:651–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sahani DV, Saokar A, Hahn PF, et al. Pancreatic cysts 3 cm or smaller: how aggressive should treatment be? Radiology 2006;238:912–9. [DOI] [PubMed] [Google Scholar]
- [25].Lee SH, Shin CM, Park JK, et al. Outcomes of cystic lesions in the pancreas after extended follow-up. Dig Dis Sci 2007;52:2653–9. [DOI] [PubMed] [Google Scholar]
- [26].Matsumoto T, Aramaki M, Yada K, et al. Optimal management of the branch duct type intraductal papillary mucinous neoplasms of the pancreas. J Clin Gastroenterol 2003;36:261–5. [DOI] [PubMed] [Google Scholar]
- [27].Sugiyama M, Izumisato Y, Abe N, et al. Predictive factors for malignancy in intraductal papillary-mucinous tumours of the pancreas. Br J Surg 2003;90:1244–9. [DOI] [PubMed] [Google Scholar]
- [28].Gaujoux S, Brennan MF, Gonen M, et al. Cystic lesions of the pancreas: changes in the presentation and management of 1,424 patients at a single institution over a 15-year time period. J Am Coll Surg 2011;212:590–600. discussion 593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Ferrone CR, Correa-Gallego C, Warshaw AL, et al. Current trends in pancreatic cystic neoplasms. Arch Surg 2009;144:448–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Oh HC, Kim MH, Hwang CY, et al. Cystic lesions of the pancreas: challenging issues in clinical practice. Am J Gastroenterol 2008;103:229–39. quiz 228, 240. [DOI] [PubMed] [Google Scholar]
- [31].Allen PJ, Jaques DP, D’Angelica M, et al. Cystic lesions of the pancreas: selection criteria for operative and nonoperative management in 209 patients. J Gastrointest Surg 2003;7:970–7. [DOI] [PubMed] [Google Scholar]
- [32].Levy P, Jouannaud V, O’Toole D, et al. Natural history of intraductal papillary mucinous tumors of the pancreas: actuarial risk of malignancy. Clin Gastroenterol Hepatol 2006;4:460–8. [DOI] [PubMed] [Google Scholar]
- [33].Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology 2006;6:17–32. [DOI] [PubMed] [Google Scholar]
- [34].Salvia R, Crippa S, Falconi M, et al. Branch-duct intraductal papillary mucinous neoplasms of the pancreas: to operate or not to operate? Gut 2007;56:1086–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Tanaka M. Intraductal papillary mucinous neoplasm of the pancreas: diagnosis and treatment. Pancreas 2004;28:282–8. [DOI] [PubMed] [Google Scholar]
- [36].Handrich SJ, Hough DM, Fletcher JG, et al. The natural history of the incidentally discovered small simple pancreatic cyst: long-term follow-up and clinical implications. AJR Am J Roentgenol 2005;184:20–3. [DOI] [PubMed] [Google Scholar]
- [37].Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012;12:183–97. [DOI] [PubMed] [Google Scholar]
- [38].Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015;148:819–22. quiz 812–813. [DOI] [PubMed] [Google Scholar]
- [39].Chernyak V, Flusberg M, Haramati LB, et al. Incidental pancreatic cystic lesions: is there a relationship with the development of pancreatic adenocarcinoma and all-cause mortality? Radiology 2015;274:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yoon WJ, Lee JK, Lee KH, et al. Cystic neoplasms of the exocrine pancreas: an update of a nationwide survey in Korea. Pancreas 2008;37:254–8. [DOI] [PubMed] [Google Scholar]


