Abstract
The aim of the study was to compare clinical manifestations, disease activity, functional capacity, spinal mobility, and radiological findings between men and women from a multicenter, multiethnic Ibero-American cohort of patients with Spondyloarthritis (SpA).
This observational cross-section study included 1264 consecutive SpA patients who fulfilled the modified New York criteria for ankylosing spondylitis (AS). Demographic, clinical, and radiologic data were evaluated. Categorical data were compared by X2 or Fisher's exact tests and continuous variables by ANOVA with post-hoc tests.
Primary AS was diagnosed in 1072 patients, psoriatic spondylitis in 147, and spondylitis associated to inflammatory bowel disease (IBD) in 45 patients. Overall, male patients were significantly younger, had longer diagnostic delay, lower disease activity, worse spinal mobility, better quality of life, and more severe radiologic damage. Dactylitis and enthesitis, as well as swollen joint count, were significantly more common among women. In primary AS, there was a marked male predominance (76.2%). Among patients with psoriatic spondylitis, male predominance was lower (57.8%), but was also associated with worse spinal mobility and more severe radiologic damage. In the total population, male patients with primary AS referred higher permanent work disability (13.2% vs 6.9%; P < 0.05), although no difference was observed in psoriatic or IBD spondylitis according to the gender.
Among Ibero-American SpA patients, there are some differences in clinical and radiological manifestations, men showing more structural damage, whereas women more active disease. These data suggest that the phenotype of SpA differs between genders. This can influence the subsequent diagnostic approach and therapeutic decisions.
Keywords: ankylosing spondylitis, gender differences, psoriatic spondylitis, spondyloarthritis
1. Introduction
Spondyloarthritis (SpA) comprise a group of diseases that share certain common features such as spinal, sacroiliac and peripheral joint involvement, presence of enthesopathies, familial occurrence, and a frequent genetic association with the HLA-B27 antigen. Primary ankylosing spondylitis (AS), psoriatic arthritis (PsA), SpA associated to inflammatory bowel disease (SpA IBD), and reactive arthritis are included in this group. Furthermore, these diseases may present distinctive articular and extra-articular features.[1]
During the last years, some differences between male and female patients with AS have been described. The early presentation in male AS patients has been discussed,[2,3] and the higher HLA-B27 positivity in the male gender can be associated with this presentation. It has also been observed that female AS patients present higher disease activity, higher fatigue scores, and more frequent peripheral involvement. On the contrary, the male gender is associated with more severe radiologic damage [4] and a faster radiologic progression.[5] Moreover, women are more likely to present cervical spine involvement, whereas men tend to complain more frequently about lumbar pain.[6]
Most studies that have assessed gender differences in patients with PsA found similar findings to AS. Women report more frequent peripheral joint involvement and more functional disability, whereas the male gender is associated with axial involvement and more severe radiographic damage.[7,8] It is important to consider that the clear definition of axial involvement in PsA is still a matter of discussion.[9] Different proposals have been considered, such as unilateral/asymmetrical sacroiliitis, presence of syndesmophytes, and inflammatory back pain in association with limited movement of the spine.[10–12]
As there are few data regarding gender differences in patients with SpA associated with psoriasis and IBD, the present study aims to describe and compare clinical manifestations, disease activity, functional capacity, axial mobility, and radiologic findings between men and women from a multicentric and multiethnic Ibero-American cohort of patients with different SpA.
2. Patients and methods
A total of 2044 consecutive SpA patients fulfilling the European Spondyloarthropathy Study Group (ESSG) classification criteria [13] were included in an observational multicenter international cohort, the RESPONDIA Registry (Registro Iberoamericano de Espondiloartritis), enrolled between June and December 2006. Data were collected by rheumatologists from different Ibero-American countries, including Argentina, Brazil, Costa Rica, Chile, Ecuador, Mexico, Peru, Uruguay, and Portugal, who used a common protocol of investigation and later transmitted the data on-line to be stored on the database of the Spanish SpA Registry (REGISPONSER), supported by the Spanish Society of Rheumatology. The general characteristics of this cohort have been published elsewhere.[14,15]
For this study, a cross-sectional analysis was performed including only those patients that met modified New York criteria.[16] Patients identified as psoriatic spondylitis needed to further have skin disease and fulfill the Chandran et al[12] definition. Patients with SpA associated with IBD were included if they had Crohn's disease or ulcerative colitis plus axial involvement.
Socio-demographic data and information related to the disease, such as disease duration (time since first symptom and inclusion in the study), diagnosis delay (time since first symptom and diagnosis), presence of dactylitis, and enthesitis were recorded. Disease activity was measured by Bath Ankylosing Spondylitis Disease Activity Index (BASDAI),[17] functional capacity by Bath Ankylosing Spondylitis Functional Index (BASFI),[18] quality of life assessment was carried out with the Ankylosing Spondylitis Quality of Life (ASQoL) [19] questionnaire, and axial mobility was measured by the Bath Ankylosing Spondylitis Metrology Index (BASMI).[20] For enthesitis, the following anatomic regions were assessed: bilateral first and seventh costochondral joints, fifth lumbar spinous process, anterior superior iliac spines, iliac crests, posterior superior iliac spines, and Achilles tendons. Clinical enthesitis was defined as the presence or the absence of local tenderness.[21] Radiography studies of pelvis for sacroiliac joints (Ferguson view), lumbar, dorsal, and cervical spine were performed at entry, and the radiologic involvement was assessed according to the Bath Ankylosing Spondylitis Radiology Index (BASRI).[22] Special training and reliability exercise workshops for clinimetric, radiologic, and protocol procedures were performed by site investigators at a national or regional level, and were coordinated by the same experts (EC-E and JV-M). Radiography studies of sacroiliac joints were performed according to the routine techniques, but radiograph reading was not centralized. Consensus about radiograph reading was reached during the training workshops, prior to the onset of the study. Blood samples were obtained from all patients for determination of erythrocyte sedimentation rate (ESR) by Westergren's method. Finally, work disability was evaluated by direct questioning of the patients and was graded into 3 broad categories: absent, and if present as partial (able to do at least 50% of their work) or total (unable to do any work or permanent labor disability). Patients gave written consent and the study was approved by the ethics board of the hospital (named Bioethics Committee Instituto de Rehabilitación Psicofísica).
2.1. Statistical analysis
Categorical data were compared using the X2 or Fisher's exact test, and continuous variables by ANOVA with Levene variance homogeneity test and post-hoc analyses. Multiple logistic regressions were performed using the gender as the dependent variable including those variables that had significance <0.01. Models with a classification capacity greater than 80% were included. A value of P < 0.05 was considered significant.
3. Results
From a total of 2044 patients included, 1264 met modified New York criteria; 73% were male (mean age 42.9 years, SD = 15.8), and 27% female (mean age 45.8, SD = 12.6) (Table 1). Specifically, 1072 patients had primary AS, 147 had psoriatic spondylitis, and 45 had IBD spondylitis. A significant male predominance was detected in AS patients (76.2%), whereas no difference was observed in psoriatic or IBD spondylitis (Fig. 1).
Table 1.
Clinical characteristics in the total spondyloarthritis population and according to specific diagnosis (n = 1264).
Figure 1.
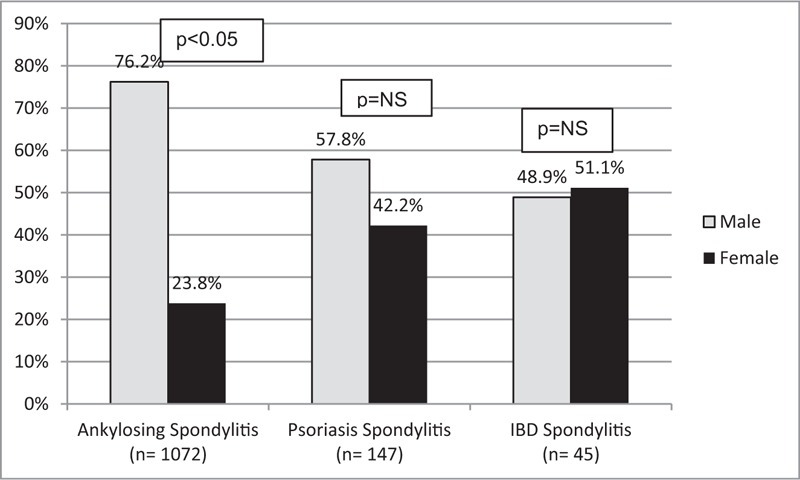
Gender differences according to diagnosis. IBD = inflammatory bowel disease, NS = non-significant.
Overall, male patients were significantly younger, had longer diagnostic delay, lower disease activity (BASDAI), and better quality of life (ASQoL), but worse spinal mobility (BASMI) and higher radiologic score (BASRI). Presence of dactylitis and enthesitis, as well as swollen joint count, was significantly more frequent among women (Table 1). ESR was evaluated in 1081 patients and was significantly higher among women compared to men (28.98 mm/h vs 22.84 mm/h, P < 0.001), no additional associations were observed between ESR and other disease parameters, including BASDAI and other patients’ reported outcomes. Determination of C-reactive protein (CRP) was not analyzed due to the small number of patients tested and different methods used for measurement.
Among primary AS patients, besides the common characteristics shown by the whole male SpA group, BASRI spine scores were also higher in men compared to women (mean BASRI spine 7.3 vs 5.8; P = < 0.001). Analyzing only psoriatic spondylitis, male gender presented less frequent peripheral involvement and worse spinal mobility (BASMI), as well as higher BASRI total and BASRI spine. Patients with IBD spondylitis only differed regarding lateral flexion of the lumbar spine (49.9 ± 10.5 in females vs 35.2 ± 11.1 in males; P = 0.01).
Regarding treatment, patients with psoriatic spondylitis used disease-modifying antirheumatic drugs with more frequency (mostly methotrexate and leflunomide), IBD patients used sulfasalazine, and patients with primary AS used nonsteroidal anti-inflammatory drugs. Given the fact that only 5.7% of the total population was being treated with tumor necrosis factor inhibitors (anti-TNF), no further analysis was considered to be made concerning this topic.
Regarding work disability in the total SpA population, men presented higher permanent work disability (13.2% vs 6.9%; P < 0.05). This difference persisted among AS patients, but no difference was observed between psoriatic or IBD spondylitis (Fig. 2).
Figure 2.
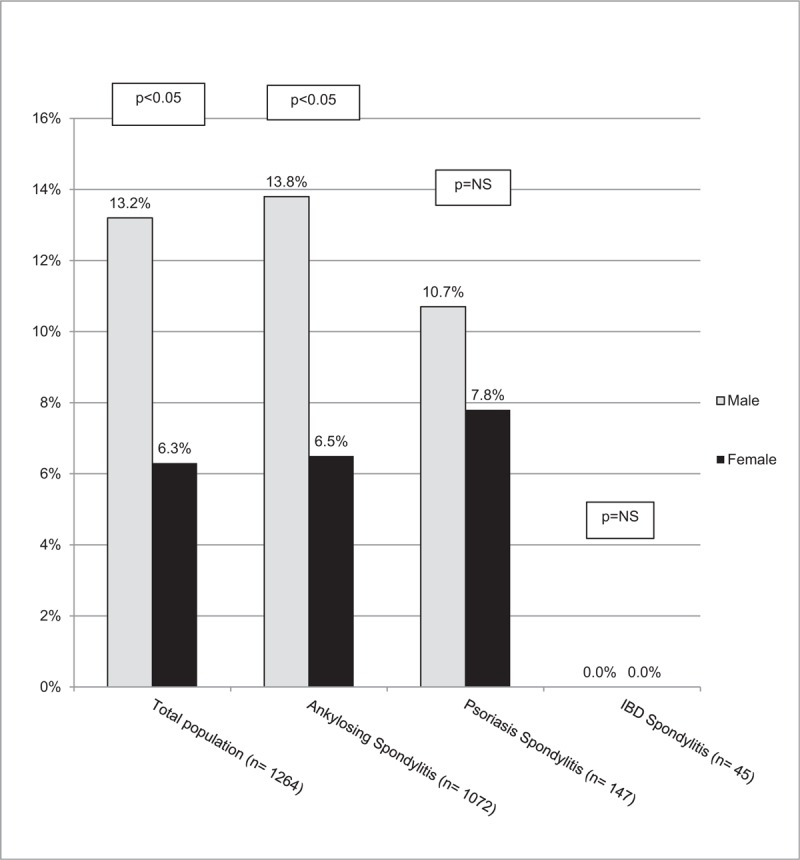
Permanent work disability according to gender. IBD = inflammatory bowel disease, NS = non-significant.
4. Discussion
Spondyloarthritis comprise a group of chronic inflammatory diseases with axial and peripheral involvement which were originally considered as a group of diseases that primarily affected men. In 1949, the male:female ratio was estimated to be 10:1 for AS patients; yet, recent data have established a new 2–3:1 ratio.[23] According to recent publications and consistent with our findings, there are no differences in the prevalence of psoriatic or IBD spondylitis among men and women; only a significant male predominance is detected in primary AS, associated with more severe axial involvement and radiologic progression.[24,25] In the present study, the male: female ratio was 3:1 for AS, 1.3:1 for psoriatic spondylitis and 1:1 for IBD spondylitis.
An interesting finding is that there are significant differences among the genders concerning clinical and radiologic presentation and disease indexes. Men were significantly younger, presenting longer diagnostic delay and more severe radiologic damage (measured by total BASRI). On the other hand, women referred higher involvement of peripheral joints, dactylitis and enthesitis, associated with higher disease activity (BASDAI) and worse quality of life (ASQoL). Similar data were observed in 1505 patients from the Brazilian Registry of Spondyloarthritis (RBE) [26] and 1514 patients from the Spanish SpA Registry (REGISPONSER),[27] as well as in a British study [28] and in the French DESIR cohort, which included patients with axial SpA of recent onset.[29]
The striking observation of a shorter diagnosis delay in females is in contrast to other publications and requires further investigation.[27,30,31] However, some factors should be taken into consideration. First, family history of SpA was not analyzed, having been shown that its presence is associated with lower delay, and second, in this cohort women presented worse disease activity (BASDAI) and quality of life (ASQoL), which may have driven in a prompt medical consultation.
It is worth to note that women reported significant higher indexes of disease activity (BASDAI), disease functionality (BASFI), and quality of life (ASQoL). A recent meta-analysis about different pain scores associated to the gender in inflammatory arthritis also found a significant higher peripheral involvement in women and axial involvement in men, recommending that future randomized trials and cohort studies recognize the need to evaluate for differential baseline pain levels and responses to treatment according to gender.[32–34]
In the present study, although women referred worse functional involvement and quality of life, male patients presented higher work disability, similar to what has been observed in the North-American PSOAS cohort.[35] Worth to note, although men showed higher permanent work disability in the overall population, they had a higher employment rate at study entry (data not shown). It might be of interest to assess differences in employment rates in these countries according to the gender and if there is a larger interest in men to apply for work in comparison to women.
Similar to what has been observed in AS, female patients with psoriatic spondylitis experienced better spinal mobility and lower radiologic damage. Different risk factors have been associated with the development of axial involvement in patients with psoriasis, such as male sex, nail dystrophy, periostitis, radiologic joint involvement, and high ESR and CRP levels.[36] Some of those features were not included in our analysis. Therefore, it is possible that we had a selection bias in our population and that such bias could be due to the fact that we only used modified New York criteria in the absence of a unified consensus to define axial involvement in psoriatic patients. The new ASAS (Assessment of SpondyloArthritis International Society) criteria were not available at the time that this study was carried out.
Regarding work disability, no gender differences were observed in patients with psoriatic spondylitis in this study. It has been proven in other studies that women with psoriatic arthritis experience higher work disability; however, a small percentage of patients with axial involvement were included.[37] In a recent study with patients with early PsA, short diagnostic delay, preserved function, and male gender were the best predictors of good clinical outcome at 5-year follow-up.[38] A recent systematic review has shown that work disability is high in PsA, but the small number of reports and the heterogeneity of the obtained data limit its interpretation.[39] Genetic factors may also be associated with the different presentations in PsA.[40] Unfortunately, we did not analyze genetic associations among Latin-American patients with SpA, where native populations are quite heterogeneous in origin.
Although a small number of patients with IBD spondylitis were included, there were no significant gender differences among them, except for a lesser lateral flexion of the lumbar spine among men.
One limitation of our study is that we employed self-administered questionnaires that limited a direct analysis of the impact of work disability and did not give further conclusion. Moreover, as determination of HLA-B27 was not mandatory for registration into the database and not all centers in Latin America had easy access to HLA testing, few patients had such test, precluding further analysis. A small number of patients with IBD spondylitis were recruited, probably due to the low prevalence of this disease with axial involvement. This small patient's sample impedes further analysis and conclusions regarding this disease. The cross section study design did not allow to further gather prospective data of this cohort, which could have resulted in valuable information regarding gender differences in SpA along the time. Finally, only patients that met modified New York criteria were included. Hence, patients with non-radiograhic axial SpA, where some gender differences in other studies were recently detected, were not included.
It could be argued why BASRI scoring method was used instead of the modified Stoke Ankylosing Spondylitis (mSASS) score. However, the first takes into consideration sacroiliac and hip joint involvement. Furthermore, at the moment of the study the mSASS scoring method had recently been developed and was not yet worldwide used to assess radiologic involvement. As this study was a cross sectional study, where no longitudinal data were going to be evaluated, the investigators considered that the BASRI method was a suitable tool.
Finally, it is important to note that the vast majority of this population was TNF naive, factor that could explain some differences with other recent cohorts that include only anti-TNF-treated patients.
We conclude that SpA may present different disease expressions according to gender in Iberoamerican patients. Further longitudinal studies are required to assess genetic background of gender differences in disease expression, especially in multiethnic cohorts like this.
Footnotes
Abbreviations: Anti-TNF = tumor necrosis factor inhibitors, AS = ankylosing spondylitis, ASAS = Assessment of SpondyloArthritis International Society, ASQoL = Ankylosing Spondylitis Quality of Life, BASDAI = Bath Ankylosing Spondylitis Disease Activity Index, BASFI = Bath Ankylosing Spondylitis Functional Index, BASMI = Bath Ankylosing Spondylitis Metrology Index, BASRI = Bath Ankylosing Spondylitis Radiology Index, CRP = C-reactive protein, ESR = erythrocyte sedimentation rate, ESSG = European Spondyloarthropathy Study Group, IBD = inflammatory bowel disease, mSASS = modified Stoke Ankylosing Spondylitis score, PsA = psoriatic arthritis, RBE = Brazilian Registry of Spondyloarthritis, REGISPONSER = Database of the Spanish Spondyloarthritis Registry, RESPONDIA Registry = “Registro Iberoamericano de Espondiloartritis,” SpA = spondyloarthritis, SpA IBD = spondyloarthritis associated to inflammatory bowel disease.
Funding: This was supported in part by clinical research grants from Fundacion Reumatologica Argentina “Dr. Osvaldo García Morteo.”
The authors have no conflicts of interest to disclose.
References
- [1].Stolwijk C, Boonen A, van Tubergen A, et al. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am 2012;38:441–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ciurea A, Scherer A, Weber U, et al. Age at symptom onset in ankylosing spondylitis: is there a gender difference? Ann Rheum Dis 2014;73:1908–10. [DOI] [PubMed] [Google Scholar]
- [3].Van der Horst-Bruinsma IE, Zacke DJ, Szumski A, et al. Female patients with ankylosing spondylitis: analysis of the impact of gender across treatment studies. Ann Rheum Dis 2013;72:1221–4. [DOI] [PubMed] [Google Scholar]
- [4].Ibn Yacoub Y, Amine B, Laatiris A, et al. Gender and disease features in Moroccan patients with ankylosing spondylitis. Clin Rheumatol 2012;31:293–7. [DOI] [PubMed] [Google Scholar]
- [5].Baraliakos X, Listing J, von der Recke A, et al. The natural course of radiographic progression in ankylosing spondylitis: differences between genders and appearance of characteristic radiographic features. Curr Rheumatol Rep 2011;13:383–7. [DOI] [PubMed] [Google Scholar]
- [6].Gran JT, Husby G, Hordvik M, et al. Radiological changes in men and women with ankylosing spondylitis. Ann Rheum Dis 1984;43:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eder L, Thavaneswaran A, Chandran V, et al. Gender difference in disease expression, radiographic damage and disability among patients with psoriatic arthritis. Ann Rheum Dis 2013;72:578–82. [DOI] [PubMed] [Google Scholar]
- [8].Queiro R, Sarasqueta C, Torre JC, et al. Comparative analysis of psoriatic spondyloarthropathy between men and women. Rheumatol Int 2001;21:66–8. [DOI] [PubMed] [Google Scholar]
- [9].Taylor WJ, Zmierczack HG, Helliwell PS. Problems with the definition of axial and peripheral diseases patterns in psoriatic arthritis. J Rheumatol 2005;32:974–7. [PubMed] [Google Scholar]
- [10].Gladman DD. Axial disease in psoriatic arthritis. Curr Rheumatol Rep 2007;9:455–60. [DOI] [PubMed] [Google Scholar]
- [11].Fernandez-Sueiro JL, Willisch A, Pertega-Diaz S, et al. Evaluation of ankylosing spondylitis spinal mobility measurements in the assessment of spinal involvement in psoriatic arthritis. Arthritis Rheum 2009;61:386–92. [DOI] [PubMed] [Google Scholar]
- [12].Chandran V, Barrett J, Schentag CT, et al. Axial psoriatic arthritis: update on a long-term prospective study. J Rheumatol 2009;36:2744–50. [DOI] [PubMed] [Google Scholar]
- [13].Dougados M, van der Linden S, Juhlin R, et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondyloarthropathy. Arthritis Rheum 1991;34:1218–27. [DOI] [PubMed] [Google Scholar]
- [14].Vázquez-Mellado J, Ugalde PF, Gomáriz EM, et al. Iberoamerican Spondyloarthritis Registry (RESPONDIA): what is it, who are we, what we do? General methods. Reumatol Clin 2008;4:17–22.21794548 [Google Scholar]
- [15].Pérez Alamino R, Maldonado Cocco JA, Citera G, et al. Differential features between primary ankylosing spondylitis and spondylitis associated with psoriasis and inflammatory bowel disease. J Rheumatol 2011;38:1656–60. [DOI] [PubMed] [Google Scholar]
- [16].van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum 1984;27:361–8. [DOI] [PubMed] [Google Scholar]
- [17].Garret S, Jenkinson T, Kennedy LG, et al. A new approach to defining disease status in ankylosing spondylitis: the Bath Ankylosing Spondylitis Disease Activity Index. J Rheumatol 1994;21:2286–91. [PubMed] [Google Scholar]
- [18].Calin A, Garret S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Functional Index. J Rheumatol 1994;21:2281–5. [PubMed] [Google Scholar]
- [19].Doward LC, Spoorenberg A, Cook SA, et al. Development of the ASQoL: a quality of life instrument specific to ankylosing spondylitis. Ann Rheum Dis 2003;62:20–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jones SD, Porter J, Garret SL, et al. A new scoring system for the Bath Ankylosing Spondylitis Metrology Index (BASMI). J Rheumatol 1995;22:1609. [PubMed] [Google Scholar]
- [21].Heuft-Dorenbosch L, Spoorenberg A, van Tubergen R, et al. Assessment of enthesitis in ankylosing spondylitis. Ann Rheum Dis 2003;62:127–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wanders AJ, Landewé RB, Spoorenberg A, et al. What is the most appropriate radiologic scoring method for ankylosing spondylitis? A comparison of the available methods based on the Outcome Measures in Rheumatology Clinical Trials filter. Arthritis Rheum 2004;50:2622–32. [DOI] [PubMed] [Google Scholar]
- [23].Lee W, Reveille JD, Weisman MH. Women with ankylosing spondylitis: a review. Arthritis Rheum 2008;59:449–54. [DOI] [PubMed] [Google Scholar]
- [24].Haglund E, Bremander AB, Petersson IF, et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann Rheum Dis 2011;70:943–8. [DOI] [PubMed] [Google Scholar]
- [25].Onen F, Akar S, Birlik M, et al. Prevalence of ankylosing spondylitis and related spondyloarthritides in an urban area of Izmir, Turkey. J Rheumatol 2008;35:305–9. [PubMed] [Google Scholar]
- [26].de Carvalho HM, Bortoluzzo AB, Gonçalves CR, et al. Gender characterization in a large series of Brazilian patients with spondyloarthritis. Clin Rheumatol 2012;31:687–95. [DOI] [PubMed] [Google Scholar]
- [27].Castro RO, Ugalde PF, Villegas MC, et al. Different clinical expression of patients with ankylosing spondylitis according to gender in relation to time since onset of disease. Data from REGISPONSER. Reumatol Clin 2013;9:221–5. [DOI] [PubMed] [Google Scholar]
- [28].Roussou E, Sultana S. Spondyloarthritis in women: differences in disease onset, clinical presentation, and Bath Ankylosing Spondylitis Disease Activity and Functional indices (BASDAI and BASFI) between men and women with spondyloarthritides. Clin Rheumatol 2011;30:121–7. [DOI] [PubMed] [Google Scholar]
- [29].Tournadre A, Pereira B, Lhoste A, et al. Differences between women and men with recent-onset axial spondyloarthritis: results from a prospective multicenter French cohort. Arthritis Care Res 2013;65:1482–9. [DOI] [PubMed] [Google Scholar]
- [30].Dincer U, Cakar E, Kiralp MZ, et al. Diagnosis delay in patients with ankylosing spondylitis: possible reasons and proposals for new diagnostic criteria. Clin Rheumatol 2008;27:457–62. [DOI] [PubMed] [Google Scholar]
- [31].Feldtkeller E, Bruckel J, Khan MA. Scientific contributions of ankylosing spondylitis patient advocacy groups. Curr Opin Rheumatol 2000;12:239–47. [DOI] [PubMed] [Google Scholar]
- [32].Aloush V, Ablin JN, Reitblat T, et al. Fibromyalgia in women with ankylosing spondylitis. Rheumatol Int 2007;27:865–8. [DOI] [PubMed] [Google Scholar]
- [33].Azevedo VF, Paiva ES, Felippe LR, et al. Ocurrence of fibromyalgia in patients with ankylosing spondylitis. Braz J Rheumatol 2010;50:646–50. [PubMed] [Google Scholar]
- [34].Barnabe C, Bessette L, Flanagan C, et al. Sex differences in pain scores and localization in inflammatory arthritis: a systematic review and metaanalysis. J Rheumatol 2012;39:1221–30. [DOI] [PubMed] [Google Scholar]
- [35].Lee W, Reveille JD, Davis JC, Jr, et al. Are there gender differences in severity of ankylosing spondylitis? Results from the PSOAS cohort. Ann Rheum Dis 2007;66:633–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chandran V, Tolusso DC, Cook RJ, et al. Risk factors for axial inflammatory arthritis in patients with psoriatic arthritis. J Rheumatol 2010;37:4. [DOI] [PubMed] [Google Scholar]
- [37].Wallenius M, Skomsvoll JF, Koldingsnes W, et al. Work disability and health-related quality of life in males and females with psoriatic arthritis. Ann Rheum Dis 2009;68:685–9. [DOI] [PubMed] [Google Scholar]
- [38].Theander E, Husmark T, Alenius GM, et al. Early psoriatic arthritis: short symptom duration, male gender and preserved physical functioning at presentation predict favourable outcome at 5-year follow-up. Results from the Swedish Early Psoriatic Arthritis Register (SwePsA). Ann Rheum Dis 2014;73:407–13. [DOI] [PubMed] [Google Scholar]
- [39].Tillett W, de-Vries C, McHugh NJ. Work disability in psoriatic arthritis: a systematic review. Rheumatology (Oxford) 2012;51:275–83. [DOI] [PubMed] [Google Scholar]
- [40].Queiro R, Tejón P, Alonso S, et al. Age at disease onset: a key factor for understanding psoriatic disease. Rheumatology (Oxford) 2014;53:1178–85. [DOI] [PubMed] [Google Scholar]


