Supplemental Digital Content is available in the text
Keywords: spatial-temporal variations, breast cancer, colorectal cancer, cancer screening, mammography
Abstract
Cancer screening tests are important tools to combat cancer-related morbidity and mortality. There is limited up-to-date research on spatial and temporal variations of colorectal and breast cancer screening in the United States.
County-level data of cancer screening adherence rates were generated from 2008 to 2012 Behavioral Risk Factor Surveillance System. We performed the univariate local indicators for spatial analyses (LISA) for the geographic differences of screening adherence rate and the differential LISA for the change of screening adherence rate from 2008 to 2012.
In the univariate LISA, low-to-low clusters were consistently identified in counties of New Mexico, Wyoming, and Mississippi (P < 0.05) for both screenings. In the differential LISA, we found low-to-low clusters in Indiana counties (P < 0.05) for mammography screening, which implied that counties with a below-average difference in mammography adherence were surrounded by counties of below-average difference in adherence rates. A high-to-high cluster was also identified in the southern Appalachian counties for mammography screening (P < 0.05). No obvious spatial pattern was found for the colorectal cancer screening adherence rate across the United States.
We found low-to-low clusters over time in adherence to screening guidelines for both cancer types in New Mexico, Wyoming, and Mississippi, and clusters of potential decrease in adherence to mammography screening guideline in counties of Indiana. The study also showed improvement on mammography screening clustered in southern Appalachia. The methodology adopted in this study identified areas with clusters of consistent low adherence to screening and a decrease in adherence, which implies that further research and intervention is warranted.
1. Introduction
Breast cancer and colorectal cancer (CRC) are 2 common types of cancers. In 2016, the estimated new cases for breast cancer and colon cancer among Americans were over 379,000.[1] In addition, 40,450 estimated deaths for breast cancer (among females) and 49,190 for CRC might be expected in 2016.[2] Moreover, the medical cost associated with these 2 types of cancer is the highest among all types of cancer, with breast cancer at around $16 billion, followed by CRC at $14 billion in 2010.[3]
Cancer screening tests are important tools to combat cancer-related morbidity and mortality. Mammography has been shown to be associated with significant reduction in late breast cancer diagnosis and increases in overall survival.[4,5] According to the American Cancer Society (ACS), women without breast symptoms aged 45 to 54 years are recommended to receive mammography screening every year, and those who are aged 55 years and older should have mammography screening every 2 years.[6] The United States Preventive Services Task Force (USPSTF) recommends biannual screening for females aged between 50 and 74 years (see Table 1, Supplemental Content, which illustrates the recommendations for breast cancer screening). According to National Cancer Institute (NCI), the overall rate of breast cancer screening among average-risk women aged 50 to 74 years was 72.6% in 2013.[7] Additionally, mammogram screening was associated with reduced risk of breast cancer death among women aged 74 years and older with mild or moderate level of comorbidity as compared with those with severe comorbidity. The factors associated with adherence to mammography screening included age, income, health insurance coverage, available medical resource, family history of breast cancer, and breast problems in the past.[8,9]
Colorectal cancer screenings include, but are not limited to, colonoscopy, sigmoidoscopy, guaiac-based fecal occult blood test (FOBT), fecal immunochemical test, and multitargeted stool DNA test (see Table 1, Supplemental Content, which illustrates the recommendations for CRC screening). Colonoscopy is widely recommended and offered as a primary screening for CRC. It is more commonly used among individuals in the United States.[10,11] Screenings with sigmoidoscopy and FOBT have also consistently demonstrated prevention of deaths from CRC.[11,12] Screenings by FOBT every year, flexible sigmoidoscopy every 5 years, or colonoscopy every 10 years are recommended by the USPSTF and the ACS, and also the American College of Gastroenterology (ACG). The USPSTF recommends CRC screening for individuals aged between 50 and 75 years.[13] Relative to breast cancer screening, the CRC screening rate in the United States was much lower at 58% reported by a 2013 study.[7,14]
A decreasing trend in self-reported use of mammography was observed from 1999 to 2005 in the United States,[15,16] whereas an ascending trend was observed for the screening rate of CRC in the United States from 2000 to 2003.[17] In addition, geographic disparities on CRC screenings were reported by Lian et al[18] in Missouri. They found considerable geographic variations of CRC screening in Missouri on the 5-digit zip code level. However, there is a lack of updated report on spatial-temporal variations of adherence to screening guidelines for breast cancer and CRC across the United States. Understanding these variations can help evaluate relevant interventions, initiatives, or policies in different regions, and identify target areas and population for the further development of strategies and policies to advance the adherence to guideline recommendations. This may significantly improve population health and alleviate health disparities arising from the geographic location.
With the advancement of geo-techniques in epidemiology and availability of software tools, it would also be beneficial to explore and illustrate these spatial-temporal variations by applying advanced spatial analysis tools to better influence healthcare planning. The local indicators for spatial analyses (LISA)[19] can be used to help identify the spatial patterns, such as low-to-low clusters, high-to-high clusters, low-to-high outliers, and high-to-low outliers. It includes the univariate LISA for exploring geographic variations such as the areas with clusters of low adherence to screening guidelines; and the differential LISA for incorporating the temporal changes such as identifying the areas with clusters of potential decreases and increases in adherence. Therefore, the study objective was to explore the spatial and temporal variations of adherence to breast and CRC screening guidelines, and narrow the focus of these variations on the county level in the United States from 2008 to 2012.
2. Methods
2.1. Study design
We conducted an ecological study using aggregated data to study the spatial-temporal variations of adherence to mammography and CRC (sigmoidoscopy/colonoscopy/FOBT) screening guideline at the county level across the United States. Ethical approval was waived since we used public available de-identified data.
2.2. Data source
Data were derived from the Behavioral Risk Factor Surveillance System (BRFSS) 2008 and 2012.[20] The BRFSS collects data from all 50 states and the District of Columbia through standardized and random-digit dialed telephone survey on the health-related risk behaviors, chronic health conditions, and use of preventive services from noninstitutionalized US adults every year.[20] The BRFSS questionnaires are composed of core questions, optional modules, and state-specific questions. Cancer screening questions were core questions that were asked every even year.
2.3. Study population
We utilized the most recent screening guideline (until May 2016) for breast cancer from the ACS[6] and for CRC from the USPSTF.[13] The eligible populations were: females between the age 46 and 55 years having mammography screening no less than once within the last year; and females aged 56 and over getting mammography screening no less than once within the past 2 years; individuals between the age 51 and 75 years who had at least 1 colonoscopy in the past 10 years, 1 sigmoidoscopy in the past 5 years, or 1 FOBT in the past year for CRC screening; who had no missing data on mammography screening, or sigmoidoscopy, colonoscopy, and FOBT, and also no missing data on the Federal Information Processing Standards (FIPS) codes of the county; and completed the interview in 2008 and 2012, respectively (unweighted N = 165,118 for CRC in 2008; N = 195,164 for CRC in 2012; unweighted N = 147,487 for breast cancer in 2008; N = 160,820 for breast cancer in 2012). Individual data were weighted and adjusted for the unequal probability of selection, differential nonresponse, and possible deficiencies in the sampling frame. Adherence rates to screening guidelines for both cancers were aggregated at the county level. Counties with relative standard error (RSE) over 30% were excluded from analyses, according to the suggestions from the data provider.[20]
2.4. Measurement
The main variables of this study were county-level adherence rates to the mammography guideline and the CRC screening (sigmoidoscopy/colonoscopy/FOBT) guideline. We first obtained individual-level data, which included mammography screening guideline adherence (yes/no), and CRC screening guideline adherence (yes/no). The guideline adherence was based on the questions asked in BRFSS, including “Have you ever had a mammography screening?” and “How long has it been since you had your last mammography screening?”; and “Have you ever had either of these exams (sigmoidoscopy or colonoscopy) or FOBT?,” “Was your MOST RECENT exam a sigmoidoscopy/colonoscopy?,” and “How long has it been since you had your last sigmoidoscopy/colonoscopy/FOBT?” Second, we derived the weighted percent of individuals who adhered to the guideline in 2008 and 2012, respectively, and obtained the county-level adherence rate to the guideline for mammography and CRC screening.
2.5. Geo-mapping
Geo-mapping using ArcGIS 10.3 (ESRI, Redlands, CA) was applied to visualize the rates of mammography and CRC screening for counties in the United States. We used 60%, 70%, 80%, and 90% as cut-off points for the classification scheme in the choropleth maps for mammography screening, and 50%, 60%, 70%, and 80% for CRC screening. The cut-off points were chosen based on the data distributions and the facilitation of a better comparison between 2008 and 2012. Lighter color represented lower rates, whereas deeper color represented higher rates. Counties with missing values or excluded were filled with white color.
2.6. Spatial analyses
We exported the shape files from ArcGIS to GeoDa 1.8 software[19] to conduct LISA to study the spatial-temporal variations on adherence. First, we conducted the univariate LISA to identify where the spatial clusters were located for both the years 2008 and 2012. In the analyses of univariate LISA, a low-to-low cluster indicated the county with a below-average adherence rate that was also surrounded by the counties of below-average adherence rates. If a low-to-low cluster was identified in the same area in both years, it may imply a cluster of counties with consistently low adherence. The patterns of consistently low and high adherence to screening guidelines were also presented in maps.
We also used GeoDa 1.8 software to map differential LISA clusters[19,21] for mammography and CRC screening. Differential LISA studies spatial autocorrelation on change over time. In this study, the spatial patterns of county-level difference in adherence from 2008 to 2012 were mapped by using differential LISA. The county-level difference was calculated by subtracting the adherence rate in 2008 from the rate in 2012. A positive value of the difference indicates the possible increase in adherence from 2008 to 2012, a negative value means the possible decrease in adherence, and a 0 value refers to no change. In addition, a high-to-high cluster in differential LISA suggested a county with an above-average difference (mean for CRC screening = 3.08; mean for mammography = −1.95) was also surrounded with counties of above-average difference.
We utilized the Queen Contiguity weights matrix that defined those counties sharing a border or vertex/corner as a county's neighbors. The LISA statistic was calculated for each observation and cluster, with the significant level at P < 0.05. In addition, global Moran I and differential Moran I statistics were employed to assess spatial autocorrelation. Moran I varies from −1 to 1. “+1” indicates a strong positive spatial correlation (ie, spatial clustering), and “−1” indicates a strong negative spatial correlation that implies spatial dispersion, and a 0 value means a random spatial pattern.[16] A significant level at P < 0.05 was used to assess spatial autocorrelation.
Sensitivity analyses using ACS guideline for colorectal cancer and USPSTF recommendation for mammography screening were conducted.
3. Results
3.1. Adherence to mammography screening guideline
A total of 2101 counties in 2008 and 2077 counties in 2012 were included for analyzing mammography screening. Figure 1 shows the maps of county-level adherence to mammography screening guideline in years 2008 and 2012. The average county-level adherence rates to mammography were 77.5% in 2008 (standard deviation [SD] 10.9%, range 38.0%–100%) and 75.6% in 2012 (SD 11.2%, range 42.2%–100%), respectively. The mean ± SD of the difference between the 2 years was −2.0 ± 12.4% (range –47.4% to 42.3%).
Figure 1.
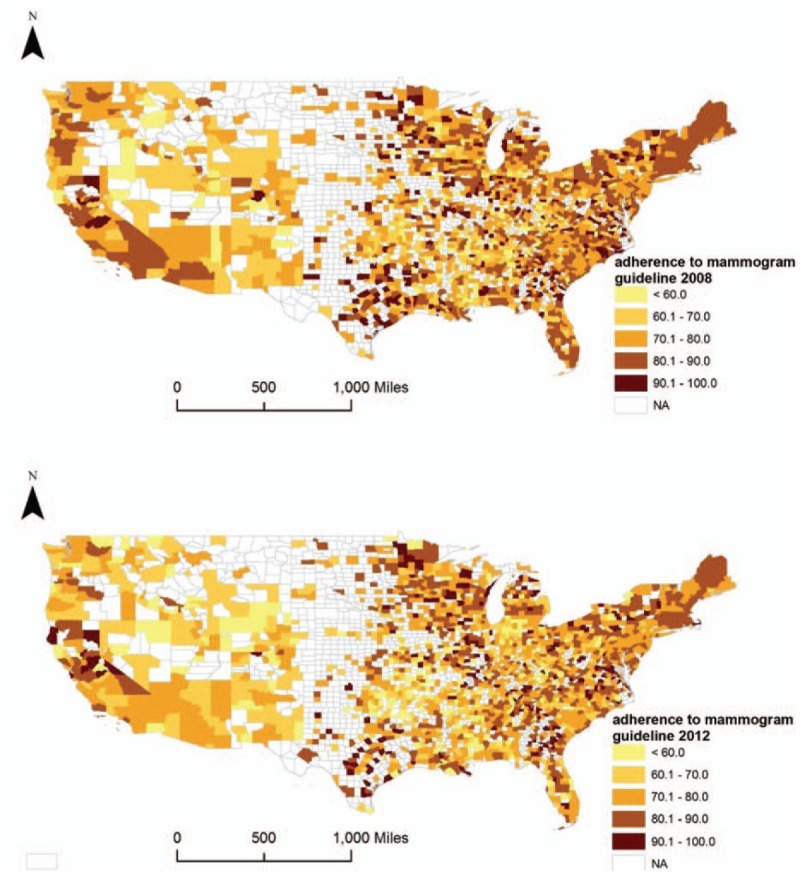
County-level adherence to mammography screening guideline in 2008 and 2012. The choropleth maps illustrate the county-level adherence to mammography screening guideline in 2008 and 2012, using cut-off points at 60%, 70%, 80%, and 90%. Counties with missing values or excluded are filled with white color.
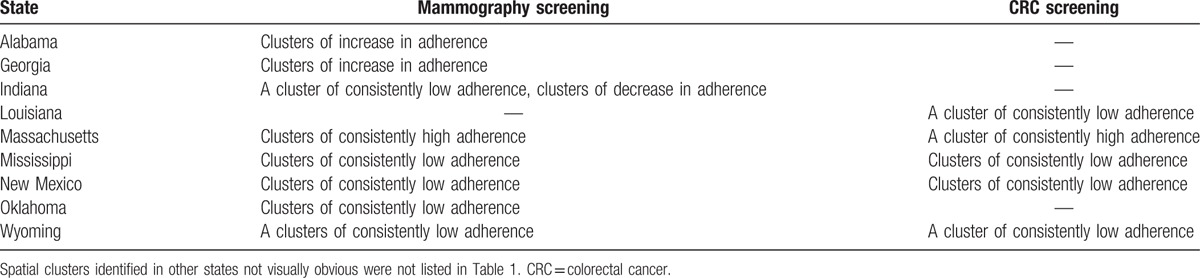
Figure 2 (A–C) illustrates the results of univariate LISA for county-level adherence to mammography. Moran I of the univariate LISA in 2008 and 2012 were 0.17 (P = 0.002) and 0.22 (P = 0.002), respectively, suggesting potential spatial clustering. We also identified and mapped areas of low-to-low and high-to-high clusters in both years, which indicate consistently low or high adherence to mammography screening guideline. We consistently found low-to-low clusters in New Mexico, Wyoming, Oklahoma, Mississippi, and northern Indiana (P < 0.05), and high-to-high clusters in Massachusetts (P < 0.05). We summarized the US states with the clusters of consistently low and high adherence (Table 1), and clusters of decreased and increased adherence to screening guidelines for breast cancer and CRC (see Table 2, Supplemental Content that presents counties identified as low-to-low clusters and high-to-high cluster in the LISAs).
Figure 2.
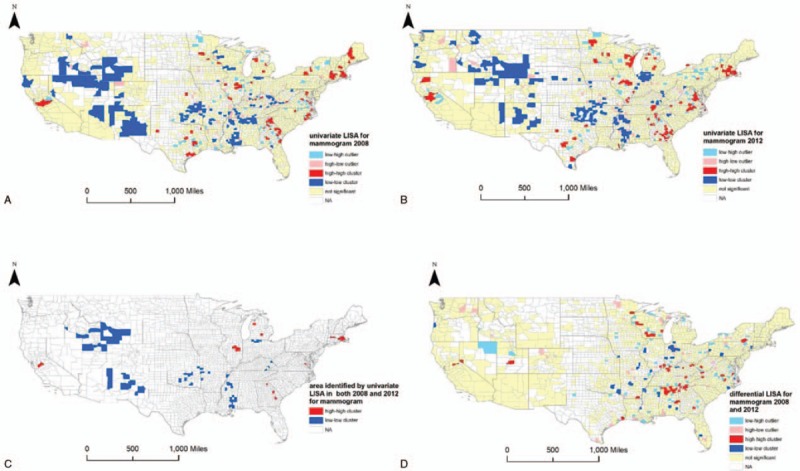
Results of local indicators for spatial analyses for mammography screening. A, Results of univariate local indicators for spatial analyses (LISA), which illustrate the spatial patterns of adherence to mammography screening guidelines in 2008, are shown. B, Results of univariate LISA for adherence to mammography screening guidelines in 2012 are shown. C, Maps the clusters that indicate consistently low or high adherence to mammography screening guidelines. D, Results of differential LISA that depict the spatial autocorrelation on the change of adherence from 2008 to 2012.
Table 1.
Spatial patterns identified in the states of the US.
Furthermore, differential Moran I was 0.002 (P = 0.42) for the adherence to the mammography screening guideline, which suggested a random spatial pattern of the United States. In the differential LISA cluster map (Fig. 2D), we can see low-to-low clusters in northern Indiana (P < 0.05), indicating a possible decrease in adherence to mammography screening guideline from 2008 to 2012. On the contrary, a high-to-high cluster implying a possible increase in adherence was identified in south Appalachia (northern Alabama and Georgia), which was also confirmed with the exact values in those counties (data not provided).
3.2. Adherence to colorectal cancer screening guideline
A total of 1969 counties in 2008 and 2124 counties in 2012 were included for the analysis of CRC screening (sigmoidoscopy/colonoscopy/FOBT). The average county-level adherence to the CRC screening guideline was 64.9% (SD 12.9%, range 30.4%–100%) in 2008 and 67.9% (SD 12.0%, range 29.7%–100%) in 2012, respectively. The mean ± SD for the difference of county-level adherence rate from 2008 to 2012 was 3.1 ± 12.9% (range −47.9% to 58.37%). We mapped the rates of adherence to the CRC screening guideline in 2008 and 2012 in Fig. 3.
Figure 3.
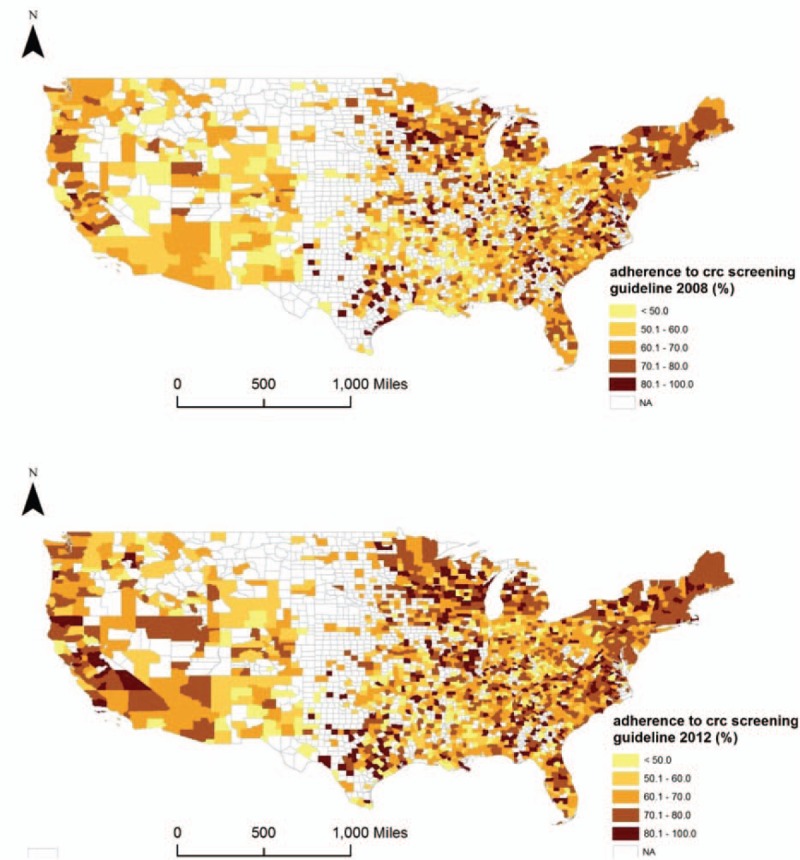
County-level adherence to colorectal cancer screening guideline in 2008 and 2012, These maps show the county-level adherence to colorectal cancer screening guideline in 2008 and 2012, using cut-off points at 50%, 60%, 70%, and 80%. Counties with missing values or excluded are filled with white color.
The Moran I of univariate LISA was 0.28 (P = 0.002) in 2008 and 0.29 (P = 0.002) in 2012, which indicated spatial clusters in both years. Figure 4 (A–C) displays the spatial patterns by the univariate LISA, and also areas identified as low-to-low cluster and high-to-high cluster in both years. We found clusters of consistently low adherence to the CRC screening guideline in counties of New Mexico, Wyoming, Mississippi, and Louisiana (P < 0.05), and also clusters of consistently high adherence in Massachusetts (P < 0.05) from 2008 to 2012. The differential Moran I was −2.6 × 10–5 (P = 0.49) for the adherence to the CRC screening guideline, which also indicated a random spatial pattern in the United States. Figure 4D presents the results of differential LISA for CRC screening.
Figure 4.
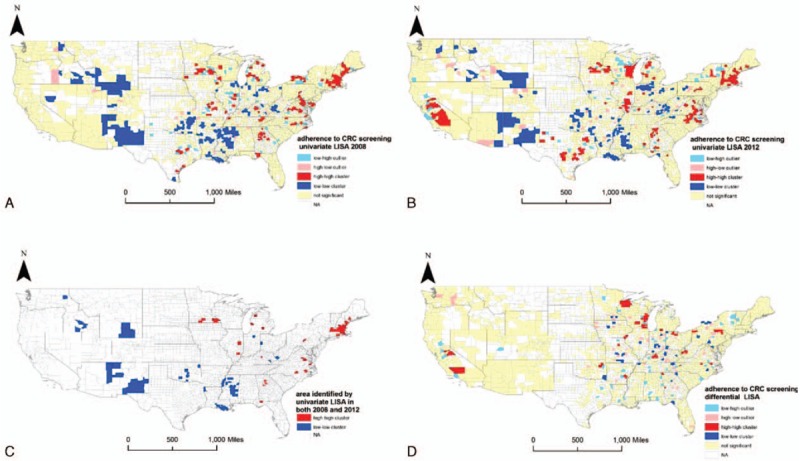
Results of local indicators for spatial analyses for colorectal cancer screening. A, Results of univariate local indicators for spatial analyses (LISA), which illustrate the spatial patterns of adherence to colorectal cancer (CRC) screening guidelines in 2008, are shown. B, Results of univariate LISA for adherence to CRC screening guidelines in 2012 are shown. C Clusters of consistently low or high adherence to CRC screening guidelines in both years of 2008 and 2012 are shown. D, Random spatial pattern in the United States on the change of adherence to CRC screening from 2008 to 2012, by using differential LISA, is shown.
In addition, we have applied the sensitivity analyses using both guidelines (ACS and USPSTF) for both county-level cancer screening adherence. Most of the results remained the same for the univariate LISA. We still found significant low-to-low clusters in regard to the adherence to screening guidelines over time in New Mexico, Wyoming, and Mississippi for the adherence to both cancer screening guidelines. The low-to-low clusters by the differential LISA in counties of Indiana for mammography screening still existed; however, the high-to-high clusters were not very obvious in southern Appalachia, which were possibly effected by the inclusion criteria (women over 75 years were not included in USPSTF guideline) (figures not shown).
4. Discussion
The study is amongst the first to explore spatial-temporal variations in the adherence to breast cancer and CRC screening guidelines, using the updated data from a US nationally representative sample. This study also demonstrated the feasibility of using advanced spatial techniques such as the LISA to identify spatial clusters of cancer screening guidelines adherence across the US and examine how these spatial clusters may change over time. Significant geographical disparities across the United States on the county-level adherence to mammogram and CRC screening guidelines were found in both 2008 and 2012.
Consistently, low adherence to mammography screening guideline was identified and clustered in areas of New Mexico, Wyoming, Mississippi, Oklahoma, and Indiana. Northern Indiana showed interesting results, in that not only was low adherence to guidelines consistently found, but also clusters of decrease in adherence to mammography screening were identified. However, there seems to be a lack of research on the geographic disparity and potential targeted interventions to improve mammography screening in this region. However, clusters of an increase in adherence in southern Appalachia including northern Alabama and Georgia were observed. This may possibly be attributed to the continuous efforts to improve mammography screening in this region. For example, the Alabama Breast and Cervical Cancer Early Detection Program[22] offers free breast and cervical cancer screenings, which include mammograms for women who meet the eligibility guidelines (eg, income ≤200% of the federal poverty guidelines, without insurance or underinsured). There are also other interventions and initiatives occurring in the Appalachian region to improve breast cancer screenings, for example, mobile mammography screening programs.[23,24,21] In West Virginia, for instance, “Bonnie Bus,” a mobile mammography unit, travels across West Virginia and offers breast cancer screenings to women who qualify for the program since 2009.[24] These programs may serve as good examples for the health underserved areas identified in our study.
In terms of CRC screening, consistently low adherence to the CRC screening guideline was identified in New Mexico, Wyoming, Louisiana, and Mississippi. The finding in the southeast parts of New Mexico was consistent with previous studies.[25–27] These studies found that low CRC screening rate in New Mexico may be associated with transformative and/or stigmatizing embarrassment, fear of pain and discomfort, low CRC knowledge, insufficient patient–physician discussion, and lack of health insurance. At the US national level, the lack of health insurance, area-level poverty rate, and out-of-pocket expenditures were reported as significant predictors of low screening rate for CRC screenings.[25] Furthermore, although primary care physicians play a critical role in implementing CRC screening guidelines to their patients,[11] there were only 19.1% of the primary care physicians in the United States adhering to the screening recommendations.[28] In addition, there exist geographical variations in physicians’ nonadherence to the CRC screening guideline. Yabroff et al found that primary care physicians reported better guideline adherence on CRC screenings in the north central and the west regions of the United States as compared with the southern region.[28] State variations in the insurance coverage of CRC screenings may also impact the participation rate. For example, coverage of CRC screening was required in at least 29 states and the District of Columbia by 2010, which included New Mexico, Wyoming, Louisiana, but not Mississippi.[29]
Several limitations in this study needs to be noted. First, we were unable to cover all the counties in the United States, since we used the data available in the BRFSS and only included data with RSE <30%. Furthermore, using telephone surveys by the BRFSS might lead to under-representation of racial/ethnic minorities, women and younger individuals, with state variations in the response rates, particularly in those states with lower response rates.[30] This method also generally includes people with above-average health awareness. Second, we used only 2 iterations of the BRFSS in this study. More iterations may provide us with the ability to capture more accurate temporal variations, which may warrant future research to verify our findings. In addition, since the BRFSS design is cross-sectional, the outcomes of independent survey iterations might not be directly comparable. Still, the BRFSS data are representative of the total noninstitutionalized population over 18 years of age, given that it uses stratified random sampling design in each state of the United States among over 0.4 million adults every year. Third, we were not able to exclude those patients who had already been diagnosed with breast cancer or CRC from the analyses. Fourth, we did not include all CRC screening tests due to the limited available data. The recommendations used in this study may not be applied to individuals with positive BRCA gene test, family history of breast cancer, inflammatory bowel disease, and prior history of colon polyps; however, we cannot differentiate participants with these conditions in this analysis by using the BRFSS data. Finally, we used up-to-date guidelines by the ACS and USPSTF in this study, but variations on adherence rate could be expected if other guidelines or versions were utilized. The recommendations from the ACS mammography screening guideline 2015 version were less strict than those from previous versions, so we expect mammography screening adherence rate would be lower if we had applied previous versions (eg, 2008 version). Nevertheless, given our focus on the spatial-temporal variations, we need to choose 1 guideline for each cancer screening to facilitate comparisons over time.
5. Conclusion
We found low-to-low clusters over time in adherence to screening guidelines for both cancer types in New Mexico, Wyoming, and Mississippi, and clusters of potential decrease in adherence to mammography screening guideline in counties of Indiana. Our study findings also showed improvement on mammography screening in southern Appalachia. Overall, further research, targeted intervention, or policy may be warranted in the areas with clusters of consistently low adherence to screening and decrease in adherence. Reducing structural barriers, providing one-to-one education, increasing provider assessment and feedback, and reducing out-of-pocket costs may be effective strategies to improve breast cancer and/or CRC screening.[31,32]
Supplementary Material
Footnotes
Abbreviations: ACG = American College of Gastroenterology, ACS = American Cancer Society, BRFSS = Behavioral Risk Factor Surveillance System, CRC = colorectal cancer, FIPS = Federal Information Processing Standards, FOBT = fecal occult blood test, LISA = local indicators for spatial analyses, SD = standard deviation, USPSTF = United States Preventive Services Task Force.
There is no conflict of interest.
Supplemental Digital Content is available for this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
References
- [1].National Cancer Institute. Common Cancer Types: updated 2016. Available at: http://www.cancer.gov/types/common-cancers Accessed October 20, 2016. [Google Scholar]
- [2].American Cancer society. Cancer facts and Figures 2016: updated 2016. Available at: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-047079.pdf Accessed October 21, 2016. [Google Scholar]
- [3].National Cancer Institute 1/12/2011. Cancer costs projected to reach at least $158 billion in 2020; updated 2011. Available at https://www.cancer.gov/news-events/press-releases/2011/CostCancer2020 Accessed by October 21, 2016 [Google Scholar]
- [4].Tabar L, Yen M, Vitak B, et al. Mammography service screening and mortality in breast cancer patients: 20-year follow-up before and after introduction of screening. Lancet 2003;361:1405–10. [DOI] [PubMed] [Google Scholar]
- [5].Gøtzsche PC, Jørgensen KJ. Screening for Breast Cancer With Mammography. The Cochrane Library 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].American Cancer Society. History of ACS recommendations for the early detection of cancer in people without symptoms: updated 2015. Available at: http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/chronological-history-of-acs-recommendations Accessed May 30, 2016. [Google Scholar]
- [7].National Cancer Institute. Screening Rates for Several Cancers Miss Their Targets. https://www.cancer.gov/news-events/cancer-currents-blog/2015/screening-targets Accessed October 20, 2016. [Google Scholar]
- [8].Henry KA, McDonald K, Sherman R, et al. Association between individual and geographic factors and nonadherence to mammography screening guidelines. J Women Health 2014;23:664–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vyas A, Madhavan S, LeMasters T, et al. Factors influencing adherence to mammography screening guidelines in Appalachian women participating in a mobile mammography program. J Commun Health 2012;37:632–46. [DOI] [PubMed] [Google Scholar]
- [10].Brenner H, Stock C, Hoffmeister M. Effect of screening sigmoidoscopy and screening colonoscopy on colorectal cancer incidence and mortality: Systematic review and meta-analysis of randomised controlled trials and observational studies. BMJ 2014;348:g2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sabatino SA, White MC, Thompson TD, et al. Centers for Disease Control and Prevention (CDC). Cancer screening test use: United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:464–8. [PMC free article] [PubMed] [Google Scholar]
- [12].Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale: update based on new evidence. Gastroenterology 2003;124:544–60. [DOI] [PubMed] [Google Scholar]
- [13].U.S. Preventive Services Task Force. Colorectal Cancer: Screening: updated 2008. Available at: http://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/colorectal-cancer-screening#Pod2 Accessed May 29, 2016. [Google Scholar]
- [14].Meester RG, Doubeni CA, Zauber AG, et al. Public health impact of achieving 80% colorectal cancer screening rates in the united states by 2018. Cancer 2015;121:2281–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Centers for Disease Control and Prevention (CDC) Use of mammograms among women aged >or=40 years: United States, 2000–2005. MMWR Morb Mortal Wkly Rep 2007;56:49–51. [PubMed] [Google Scholar]
- [16].US Cancer Statistics Working Group. 1999–2013 Cancer Incidence and Mortality Data; updated 2016. Available at: https://nccd.cdc.gov/uscs/ Accessed May 29, 2016. [Google Scholar]
- [17].Meissner HI, Breen N, Klabunde CN, et al. Patterns of colorectal cancer screening uptake among men and women in the united states. Cancer Epidemiol Biomarkers Prev 2006;15:389–94. [DOI] [PubMed] [Google Scholar]
- [18].Lian M, Schootman M, Yun S. Geographic variation and effect of area-level poverty rate on colorectal cancer screening. BMC Public Health 2008;8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Anselin L. Local indicators of spatial association: LISA. Geogr Anal 1995;27:93–115. [Google Scholar]
- [20].Centers for Disease Control and Prevention. Overview: Brfss 2008: updated 2013. Available at: http://www.cdc.gov/brfss/annual_data/2008/pdf/overview_08.pdf Accessed May 29, 2015. [Google Scholar]
- [21].Luc Anselin. GeoDa Workshop 2: updated 2016. Available at: https://geodacenter.org/downloads/pdfs/geoda_1.8_2.pdf Accessed July 20, 2016. [Google Scholar]
- [22].Alabama Department of Public Health. The Alabama Breast and Cervical Cancer Early Detection Program: updated 2015. Available at: http://www.adph.org/earlydetection/ Accessed May 29, 2016. [Google Scholar]
- [23].Bencivenga M, DeRubis S, Leach P, et al. Community partnerships, food pantries, and an evidence-based intervention to increase mammography among rural women. J Rural Health 2008;24:91–5. [DOI] [PubMed] [Google Scholar]
- [24].West Virginia University Cancer Institute. Bonnie's bus serving the women of WV: updated 2016. Available at: http://wvucancer.org/cancer-prevention-control/bonnies-bus/ Accessed August 20, 2016. [Google Scholar]
- [25].Hoffman RM, Rhyne RL, Helitzer DL, et al. Barriers to colorectal cancer screening: physician and general population perspectives, New Mexico, 2006. Prev Chronic Dis 2011;8:A35. [PMC free article] [PubMed] [Google Scholar]
- [26].Getrich CM, Sussman AL, Helitzer DL, et al. Expressions of machismo in colorectal cancer screening among New Mexico Hispanic subpopulations. Qual Health Res 2012;22:546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Sanchez JI, Palacios R, Thompson B, et al. Assessing colorectal cancer screening behaviors and knowledge among at-risk Hispanics in southern New Mexico. J Cancer Ther 2013;4:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yabroff KR, Klabunde CN, Yuan G, et al. Are physicians’ recommendations for colorectal cancer screening guideline-consistent? J Gen Intern Med 2011;26:177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].National Conference of State Legislatures. Colorectal Cancer Screening: What Are States Doing? Updated 2011. Available at: http://www.ncsl.org/research/health/colorectal-cancer-screening-laws-by-state.aspx Accessed October 21, 2016. [Google Scholar]
- [30].Schneider KL, Clark MA, Rakowski W, et al. Evaluating the impact of non-response bias in the Behavioral Risk Factor Surveillance System (BRFSS). J Epidemiol Commun Health 2012;66:290–5. [DOI] [PubMed] [Google Scholar]
- [31].Johnson MR, Grubber J, Grambow SC, et al. Physician non-adherence to colonoscopy interval guidelines in the veterans affairs healthcare system. Gastroenterology 2015;149:938–51. [DOI] [PubMed] [Google Scholar]
- [32].Baron RC, Rimer BK, Coates RJ, et al. Client-directed interventions to increase community access to breast, cervical, and colorectal cancer screening: a systematic review. Am J Prev Med 2008;35:S56–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


