Abstract
There is still controversy on the management of septic arthritis in neonates. This study aims to investigate the treatment of septic arthritis in neonates.
We reviewed 52 neonates (37 males and 15 females) with septic arthritis in our hospital during 2004 to 2015. The mean age at onset of infection was 17.5 ± 7.6 days, mean age at admission was 32.6 ± 10.7 days. A total of 56 joints were involved (22 knees, 18 shoulders, 13 hips, and 3 other joints). Thiryt-six patients underwent surgical drainage, 14 patients were treated nonoperatively, 2 families refused treatment. Forty-four patients (48 joints) were followed for 4.5 ± 1.2 years. Based on treatment, these 48 joints were divided into an operative group and a nonoperative group. Clinical presentations, imaging examination results, treatments, and outcomes were analyzed.
Among the patients who were followed-up, the time from onset to treatment in the operatively managed group (12.7 ± 8.1 days) was significantly shorter than that in the conservatively managed group (20.0 ± 8.2 days). There were no significant differences between both groups on the age at onset, age at admission, imaging score, length of hospital stay, WBC counts, and intravenous medication time. Thirty-five sites (72.9%) recovered completely. There was no significant difference on recovery rate between operative and nonoperative group. Only 33.3% of the hips recovered, this was significantly lower than that of knee/ankle (85.0%) and shoulder/elbow (78.9%). Sequels were found in 13 joints. Logistic regression indicated that sex, imaging score, and hip joint involvement were predictors of sequel. One point of imaging score increased the risk of sequels by a factor of 2.960, and hip joint involvement increased the risk of sequels by a factor of 12.712. Females were more likely to have sequels than males.
Surgical drainage is recommended for early diagnosed neonatal septic arthritis and hip infections. A conservative approach may be more efficient for patients whose diagnosis and treatment had been delayed for more than 2 weeks. Antibiotics should be administered intravenously for 2 weeks and then orally for another 2 weeks. First-generation cephalosporin and clindamycin are recommended empirical antibiotics before causative agent and its resistance pattern are known.
Keywords: neonate, osteomyelitis, septic arthritis
1. Introduction
Septic arthritis refers to infection of a joint caused by bacteria; this may occur in isolation, or occur as a consequence of osteomyelitis with spread into the adjacent joint.[1] The incidence of septic arthritis in children ranges between 5 and 12 cases per 100,000 persons.[2] The symptoms and signs of septic arthritis includes fever, swelling, pain, and impaired range of movement. If without proper treatment, it could lead to sequelae such as joint destruction, growth failure, and death of the patient. At present, surgery and antibiotics are the main options in the management of septic arthritis in children.[3,4]
Septic arthritis in the neonate is a serious condition which results in permanent dysfunction or deformity of the limbs in many children affected.[5,6] The incidence of septic arthritis in the neonates varies in different regions. Ho et al[7] reported an incidence of 0.12 per 1000 livebirths or 0.67 per 1000 admissions to the neonatal nursery in Singapore; Narang et al[8] reported an incidence of 1/1500 in India. Due to the low rate of incidence and paucity of signs and symptoms in the neonates, there are still difficulties to diagnose and manage it in an early stage.[9,10] Early diagnosis and proper treatment are essential to obtain good outcomes and avoid sequelae.
Previously, drainage was recommended in most patients diagnosed with septic arthritis. However, in our clinical practice, a lot of neonates with septic arthritis not undergo operative treatment. A group of patients who undergo nonoperative treatment experience good outcomes. Additionally, some studies indicated that nonoperative treatment could result in good outcomes in many patients.[9] Thus, there is still a controversy regarding the management of septic arthritis in neonates.
In the present study, we retrospectively investigated the patients diagnosed with septic arthritis in the neonatal period. The patients were divided into 2 groups based on treatment (operative and nonoperative). We compared the clinical presentation, imaging examination result, treatment, and outcome in 2 groups. Logistic regression was performed to evaluate the risk factors of sequel, including age at onset, sex, time from onset of infection to treatment, imaging changes before treatment, and treatment methods including antibiotic therapy and operation. The aim of this study is to investigate the treatment of delayed septic arthritis in the neonate.
2. Materials and methods
We reviewed 52 patients diagnosed with septic arthritis started during the neonatal period (age < 28 days) in our hospital from January 2004 to June 2015. This study was approved by the Ethical Committee of GuangZhou Women and Children's Medical Center (no: 2015020902). There were 37 boys and 15 girls. The average age at presentation was 17.5 ± 7.6 days, average age at admission was 32.6 ± 10.7 days. The mean birth weight was 3.01 ± 0.48 kg and the mean weight at admission was 3.8 ± 0.45 kg. Seven patients had a gestational age < 37 weeks, 4 babies had a birth weight <2500 g.
A diagnosis of septic arthritis was made if the patient fulfilled 3 of the following 5 criteria: symptoms of infection, such as a fever more than 38°C, local swelling, pain, abnormal posture, or pseudoparesis; ultrasonography (USG), computed tomography (CT), or magnetic resonance imaging (MRI) examination indicating fluid in articular cavity; X-ray examination showing joint dislocation, or damage to the bone; an increase of white blood cell (WBC) count, C-reactive protein (CRP), or erythrocyte sedimentation rate (ESR); joint aspiration demonstrating pus in articular cavity.
A total of 56 joints were involved (Table 1). The most frequently involved joints were knee, shoulder, and hip; 3 patients had multiple sites involved. The commonest early signs of septic arthritis were pseudoparesis (26 cases, 50%), local swelling (19 cases, 36.5%), and fever (7 cases, 13.5%). The average delay time (the time from presentation of infection to treatment) was 15.1 ± 9.3 days (1–42 days). Eleven neonates had significant risk factors before onset of infection, including 4 soft tissue infections, 3 pneumonias, and 4 other risk factors. Four patients showed more than 1 risk factor. Twenty-two patients (42.3%) had a temperature of 38°C or higher, whereas 30 patients (57.7%) never had fever. Fifteen cases (28.8%) had significant risk factors before onset of infection. WBC count greater than 12.0 × 109/L was observed in 39 patients (75%). Blood cultures were drawn in 34 patients, pus or local tissue cultures were obtained in 34 children.
Table 1.
Septic arthritis at different sites.
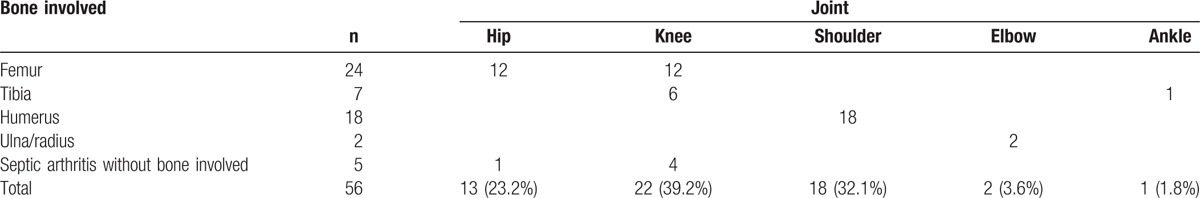
Before treatment, we performed X-ray and USG examination in all patients. Due to the limited conditions in our hospital, we performed CT or MRI examinations not in all patients. We scored each site according to imaging changes, each point recorded 1 score: USG, CT, or MRI examination indicating fluid in articular cavity; X-ray indicated joint dislocation or subluxation; X-ray or CT indicated damage to the metaphysis or epiphysis; USG, CT, or MRI examination indicated damage to the epiphyseal plate.
Most patients received open surgical drainage, except the patients who had a delay time for more than 2 weeks; or those babies whose families refused to undergo an operation. Antibiotics were used in all patients according to practical experience before the causative agent and its resistance pattern was known. Generally, first-generation cephalosporin and clindamycin were used. Once the results of bacterial cultures were known, the antibiotics were adjusted according to sensitivity reports. The time of intravenous medication was 2 weeks, with a switch to oral medication for another 2 weeks. And then stop or continue oral medication depending on the results of WBC count and CRP. If WBC and CRP returned to normal, antibiotics were then stopped. Otherwise, antibiotics were continued until normal WBC and CRP were detected.
X-ray examination was performed at 3 months, 6 months, and each year postdischarge until final follow-up. We performed X-ray examination once a year, because some abnormalities may be detected on X-ray imaging even the patient appeared no deformity or dysfunction on the joint. At final follow-up, the local appearance and function were recorded. We divided the patients those acquired follow-up into 2 groups: recovered and healed with sequels. In the recovered group, all patients must meet the following 4 points: no pain in the joint; joint activity was not limited when compared with the normal side; there was no significant deformity; X-ray examination indicated no changes of the bones and joints.
Statistical analyses were performed using the statistics package SPSS 13.0 (SPSS, Chicago, IL). Independent samples t tests were used to compare the age at admission, age of onset, time from onset of infection to treatment, imaging scores before treatment, time of antibiotic therapy. Chi-square test (Fisher exact test) was used to compare the recovery rate between the operatively treated group and the nonoperatively treated group. Logistic regression was performed to evaluate the risk factors of sequelae.
3. Results
Of these 52 patients, 2 families refused any treatment of their baby. These babies were excluded from analysis. Of the 50 patients who received treatment, 6 patients were lost to follow-up, 44 patients (48 joints) obtained follow-up for 1 to 10 years (mean 4.5 ± 1.2 years). The mean length of hospital stay was 17.2 ± 8.5 days. Of these 50 patients, 36 patients (72%) received open surgical drainage; 14 patients were treated with antibiotics without operation, and 1 patient of them was operated on after 6 months.
The results of blood cultures and pus or local tissue cultures are shown in Table 2. Staphylococcus and Klebsiella were the most common bacteria. From blood cultures we cultivated Staphylococcus epidermidis (S epidermidis) in 5 children, Staphylococcus aureus (S aureus) in 3 children, coagulase negative Staphylococci in 2, and Staphylococcus hominis (S hominis) in 2 children. From pus or local tissue cultures we cultivated S aureus in 10 patients, Klebsiella pneumoniae (KPN) in 3, and Klebsiella oxytoca in 1 children. Pus or local tissue cultures were performed in these 7 patients whose blood cultures indicated S epidermidis or S hominis, we got 5 negative results, 1 Escherichia coli and 1 KPN. In the positive cultures, 52.9% of cases were multiple drug resistance bacteria.
Table 2.
Results of blood and pus/local tissue culture.
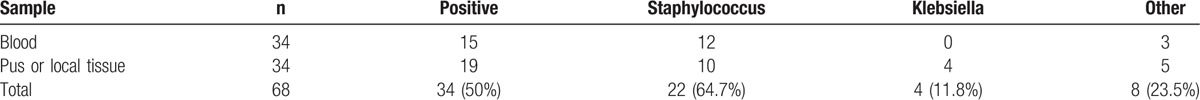
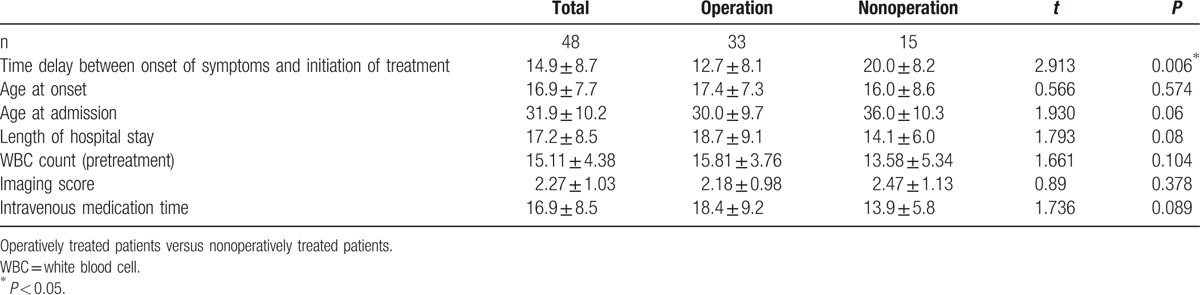
The patients with follow-up were divided into 2 groups, the details of both groups are listed in Table 3. The time from onset of symptoms to treatment in the operatively treated group was significantly shorter than that in the nonoperatively treated group (P = 0.006). There were no significant differences between the 2 groups regarding age at onset of symptoms, age at admission, imaging score, length of hospital stay, WBC counts, and intravenous medication time (Table 3).
Table 3.
Details of 2 groups in 48 sites.
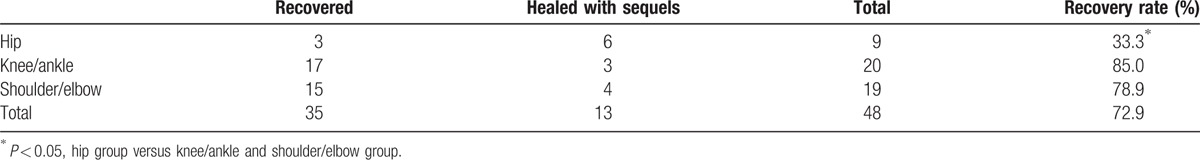
In the 48 joints who underwent follow-up, 35 joints (72.9%) recovered completely. Chi-square test (Fisher exact test) indicated no significant different rate of recovery between the operatively treated group and the nonoperatively treated group (P = 1.000) (Table 4). 33.3% of the hips recovered, 85.0% of knee/ankle joints recovered, and 78.9% of shoulder/elbow joints, there was significant difference between hip and nonhip joint on the rate of recovery (P = 0.002) (Table 5).
Table 4.
Recovery rate of operatively treated patients and nonoperatively treated patients (48 joints).

Table 5.
Recovery rate of different joints.
Sequels were found in 13 joints (27.1%). Six hip joints were affected, all of them developed severe avascular necrosis (AVN) of the femoral head. Four of these joints presented with joint dislocation; although 2 of these joints received open reduction and Salter osteotomy, unfortunately both hip joints developed recurrent dislocation at final follow up. Regarding other joints, 3 joints showed mild deformation on X-ray images but with good function; 3 children presented with joint stiffness; 1 joint showed significant deformation and dysfunction.
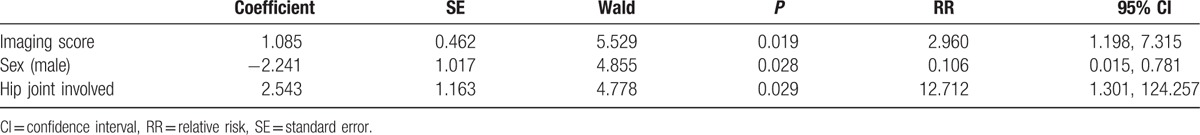
We investigated the factors that may influence the rate of sequelae, including: sex, age at onset, age at admission, neonatal risk factors, delay time from onset to treatment, imaging score before treatment, hip joint involvement, presence of multidrug resistant bacteria, operation, and intravenous medication time. Regression analysis indicated that only sex, imaging score, and hip joint involvement were predictors of sequelae. One point of imaging score before treatment increased the risk of subsequent sequels by a factor of 2.960 and a hip joint involvement increased the risk of subsequent sequels by a factor of 12.712. Females are more likely to develop sequels than males. Other factors had no influence on the rate of sequels (Table 6).
Table 6.
Predictors of sequel by logistic regression.
4. Discussion
Surgical drainage is considered very critical for the treatment of septic arthritis in neonates.[3,4] Surgery makes it possible to get biological samples and then guide the selection of antibiotics according to the causative organisms. It also reduces the intraarticular pressure of the joint, subsequently decreases the risk of complications such as AVN of the bone and permanent cartilage damage.[11,12] However, our study showed that the patients did not gain advantages from surgery. In the 48 joints who underwent follow-up, the total rate of recovery was 72.9%, there was no significant difference between the operative and the nonoperative group. Although surgical drainage has been widely used to treat septic arthritis, there is still no comparative study of surgery and conservative treatment on septic arthritis in neonates. Most studies just reported a total recovery rate. Frederiksen et al[9] reported 25 neonates with septic arthritis treated by nonoperation, 66.7% of the cases recovered without squeal. Kabak et al[13] reviewed 14 neonates who developed septic arthritis and treated by operation, 12 (85.7%) cases improved without squeal. Similar results were reported by Knudsen and Hoffman[14] and Bergdahl et al.[15] We are the first to directly compare the outcomes between the operatively treated and nonoperatively treated patients, we found no differences between them. Furthermore, our logistic regression model also indicates that operation is not a predictor of recovery rate.
Similar results between the operatively treated group and nonoperatively treated group may be partially attributed to the delay of treatment in both groups. Therapeutic delay is considered an important risk factor of sequelae, a 4-day delay has been suggested as acceptable in the treatment of uncomplicated osteomyelitis.[16] In this series, the average delay time was 14.9 days, only 7 patients (13.5%) had delay time for less than 4 days. Most patients sought treatment too late, when significant damage to bone and epiphysis had already happened, even surgical drainage could not change the final outcomes. Another reason may be the similar damages to the bone and epiphysis before treatment. In our regression model, imaging score before treatment was an important predictor of final recovery rate. In this study, there was no difference between the operatively treated group and the nonoperatively treated group on imaging scores, which might result in similar outcomes between them. Additionally, the delay time in the nonoperatively treated group (20.0 days) was significantly longer than that in the operatively treated group (12.7 days), which indicated that the damage to bone and joint might not continue to deteriorate if the patient had a delay time for more than about 2 weeks. Thus, for these patients, nonoperation might be more suitable. It not only avoids the surgery, but also decreases the hospitalization and intravenous medication time. However, we cannot conclude that surgical drainage is unnecessary in managing all septic arthritis in the neonates. In the early stage of septic arthritis, surgical drainage is very important to prevent damages to the bone and growth plate.
In this series, recovery rates varied in different sites. In the 9 hips who followed up, only 33.3% of the hips recovered, which was significantly lower than other sites (88.6%). Similar results were reported by Knudsen and Hoffman[14] and Bergdahl et al.[15] Their recovery rates of hip joints were 68.4% and 52.9% respectively, which were significantly lower than other sites. In addition, our regression model also indicated that hip joint involvement was a predictor of sequels, it increased the risk of subsequent sequels by a factor of 12.712. The low recovery rate of hip may be attributed to the relative narrow space of the hip joint and the special blood supply of the femoral head, which will potentially predispose the joint capsular and the vascularization of the femoral head to be destroyed by the hyper pressure in the hip joint, subsequently leading to dislocation of the hip and AVN of the femoral head.[16] Thus, we recommend surgery for septic arthritis of the hip, even if the delay time is more than 2 weeks.
At present, there is still no consensus on the duration of intravenous therapy in septic arthritis of neonates. Some infection society and specialists have recommended 4 to 6 weeks of antibiotics by intravenous.[17,18] However, most studies pertain to osteomyelitis and septic arthritis in neonates have reported shorter intravenous regimens (from approximately 1–4 weeks).[9,13,18] In this study, the duration of intravenous therapy was similar to previous studies. Recently, shortened regimens of antibiotics were advocated by Peltola and Pääkkönen in treating acute osteomyelitis and septic arthritis in children. Their work was published on The New England Journal of Medicine in 2014.[19] They have suggested to reduce the duration of intravenous antibiotic therapy to 2 to 4 days and then continuing the therapy orally. Another 3 studies also showed no change in outcomes when the intravenous phase was shorter than a week.[20–22] However, septic arthritis in the neonates differ from those in the older child. Neonates are relatively immunocompromised, which may require prolonged treatment.[12,23] At present, an intravenous phase for 2 weeks, followed by another 2 weeks of oral therapy is used in our hospital to treat septic arthritis in the neonates. Then oral therapy is stopped depending on the results of WBC and CRP.
Antibiotic therapy is usually selected to cover the most likely pathogens. In the present study, S aureus was the most common pathogen, first-generation cephalosporin or clindamycin was used as initial treatment. When the culture's results were available, the antibiotic therapy was modified according to the organism and the susceptibility pattern. In our study, 52.9% of positive results were multiple drug resistance bacteria. Thus, we use vancomycin as the empirical antibiotic agent for septic arthritis in neonates recently. At present, consensus has been reached that vancomycin should be an empirical antibiotics for osteomyelitis or septic arthritis if prevalence of methicillin-resistant strains of S aureus (MRSA) in community ≥10% and prevalence of clindamycin-resistant S aureus ≥10%.[3,24]
It should be noted that there are some limitations in the present study. We compared the outcomes between operatively treated and nonoperatively treated groups, but this was a retrospective study, there was some bias attributed to the nonrandom selection of treatment (operation or nonoperation). Additionally, the time delay between onset of symptoms and initiation of treatment in these 2 groups were different, someone may doubt that the comparison between these 2 groups is justified.
In conclusion, surgical drainage is recommended for early diagnosed neonatal septic arthritis and hip infections. A conservative approach may be more efficient for patients whose diagnosis and treatment had been delayed for more than 2 weeks. Antibiotics should be administered intravenously for 2 weeks and then orally for another 2 weeks. Then oral therapy should be stopped depending on the results of WBC and CRP. First-generation cephalosporin and clindamycin are recommended empirical antibiotics before causative agent and its resistance pattern are known.
Footnotes
Abbreviations: AVN = avascular necrosis, CRP = C-reactive protein, CT = computed tomography, ESR = erythrocyte sedimentation rate, KPN = Klebsiella pneumoniae, MRI = magnetic resonance imaging, MRSA = methicillin-resistant strains of S aureus, S aureus = Staphylococcus aureus, S epidermidis = Staphylococcus epidermidis, S hominis = Staphylococcus hominis, USG = ultrasonography, WBC = white blood cell.
The authors have no conflicts of interest to disclose.
References
- [1].Offiah AC. Acute osteomyelitis, septic arthritis and discitis: differences between neonates and older children. Eur J Radiol 2006;60:221–32. [DOI] [PubMed] [Google Scholar]
- [2].García-Arias M, Balsa A, Mola EM. Septic arthritis. Best Pract Res Clin Rheumatol 2011;25:407–21. [DOI] [PubMed] [Google Scholar]
- [3].Agarwal A, Aggarwal AN. Bone and joint infections in children: septic arthritis. Indian J Pediatr 2016;83:825–33. [DOI] [PubMed] [Google Scholar]
- [4].Castellazzi L, Mantero M, Esposito S. Update on the management of pediatric acute osteomyelitis and septic arthritis. Int J Mol Sci 2016;17:855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Halder D, Seng QB, Malik AS, et al. Neonatal septic arthritis. Southeast Asian J Trop Med Public Health 1996;27:600–5. [PubMed] [Google Scholar]
- [6].Kumari S, Bhargava SK, Baijal VN, et al. Neonatal osteomyelitis: a clinical and follow-up study. Indian J pediatr 1978;15:393–7. [PubMed] [Google Scholar]
- [7].Ho NK, Low YP, See HF. Septic arthritis in the newborn—a 17 years’ clinical experience. Singapore Med J 1989;30:356–8. [PubMed] [Google Scholar]
- [8].Narang A, Mukhopadhyay K, Kumar P, et al. Bone and joint infection in neonates. Indian J Pediatr 1998;65:461–4. [DOI] [PubMed] [Google Scholar]
- [9].Frederiksen B, Christiansen P, Knudsen FU. Acute osteomyelitis and septic arthritis in the neonate, risk factors and outcome. Eur J Pediatr 1993;152:577–80. [DOI] [PubMed] [Google Scholar]
- [10].Pittard WB, III, Thullen JD, Fanaroff AA. Neonatal septic arthritis. J Pediatr 1976;88(4 Pt 1):621–4. [DOI] [PubMed] [Google Scholar]
- [11].Ceroni D, Kampouroglou G, Valaikaite R, et al. Osteoarticular infections in young children: what has changed over the last years? Swiss Med Wkly 2014;144:w13971. [DOI] [PubMed] [Google Scholar]
- [12].Pääkkönen M, Peltola H. Management of a child with suspected acute septic arthritis. Arch Dis Child 2012;97:287–92. [DOI] [PubMed] [Google Scholar]
- [13].Kabak S, Halici M, Akcakus M, et al. Septic arthritis in patients followed-up in neonatal intensive care unit. Pediatr Int 2002;44:652–7. [DOI] [PubMed] [Google Scholar]
- [14].Knudsen CJ, Hoffman EB. Neonatal osteomyelitis. J Bone Joint Surg Br 1990;72:846–51. [DOI] [PubMed] [Google Scholar]
- [15].Bergdahl S, Ekengren K, Eriksson M. Neonatal hematogenous osteomyelitis: risk factors for long-term sequelae. J Pediatr Orthop 1985;5:564–8. [PubMed] [Google Scholar]
- [16].Ilharreborde B. Sequelae of pediatric osteoarticular infection. Orthop Traumatol Surg Res 2015;101(1 suppl):S129–37. [DOI] [PubMed] [Google Scholar]
- [17].Overturf GD. Remington JS, Klein JO. Bacterial infections of the bones and joints. Infectious Diseases of the Fetus and Newborn Infant. Philadelphia, PA:WB Saunders; 2006. 319–33. [Google Scholar]
- [18].Ish-Horowicz MR, McIntyre P, Nade S. Septic arthritis caused by multiply resistant Staphylococcus aureus in a neonatal intensive care unit. Pediatr Infect Dis J 1992;11:82–7. [DOI] [PubMed] [Google Scholar]
- [19].Peltola H, Pääkkönen M. Acute osteomyelitis in children. N Engl J Med 2014;370:352–60. [DOI] [PubMed] [Google Scholar]
- [20].Jagodzinski NA, Kanwar R, Graham K, et al. Prospective evaluation of a shortened regimen of treatment for acute osteomyelitis and septic arthritis in children. J Pediatr Orthop 2009;29:518–25. [DOI] [PubMed] [Google Scholar]
- [21].Peltola H, Pääkkönen M, Kallio P, et al. Short-versus long-term antimicrobial treatment for acute hematogenous osteomyelitis of childhood: prospective, randomized trial on 131 culture-positive cases. Pediatr Infect Dis J 2010;29:1123–8. [DOI] [PubMed] [Google Scholar]
- [22].Prado SMA, Lizama CM, Peña DA, et al. Short duration of initial intravenous treatment in 70 pediatric patients with osteoarticular infections. Rev Chilena Infectol 2008;25:30–6. (In Spanish). [PubMed] [Google Scholar]
- [23].Morrissy RT. Bone and joint infection in the neonate. Pediatr Ann 1989;18: 33–4, 36–8, 40–4. [DOI] [PubMed] [Google Scholar]
- [24].Harik NS, Smeltzer MS. Management of acute hematogenous osteomyelitis in children. Expert Rev Anti Infect Ther 2010;8:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]


