Abstract
Parenting strategies can be flexible within a species, and may have varying fitness effects. Understanding this flexibility and its fitness consequences is important for understanding why parenting strategies evolve. Here, we investigate the fitness consequences of flexible parenting in the burying beetle Nicrophorus orbicollis, a species known for its advanced provisioning behaviour of regurgitated vertebrate carrion to offspring by both sexes. We show that even when a parent is freely allowed to abandon the carcass at any point in time, biparental post-hatching care is the most common pattern of care adopted in N. orbicollis. Furthermore, two parents together raised more offspring than single parents of either sex, showing that the presence of the male can directly influences parental fitness even in the absence of competitors. This contrasts with studies in other species of burying beetle, where biparental families do not differ in offspring number. This may explain why biparental care is more common in N. orbicollis than in other burying beetles. We suggest how fitness benefits of two parents may play a role in the evolution and maintenance of flexible biparental care in N. orbicollis.
Keywords: parental care, biparental care, offspring performance, behavioural flexibility, plasticity
Introduction
Parental care occurs in a wide variety of taxa (Clutton-Brock 1991; Royle, Smiseth, and Kölliker 2012). However, parental care is far from a singular phenomenon. Parental care may consist, solely or in any combination, of a variety of behaviours, such as nest construction, egg incubation, guarding of offspring or resources, thermoregulation, and indirect or direct provisioning of food (Tallamy and Wood 1986; Székely et al. 2013). Furthermore, the pattern of parental care is known to vary in several ways. Uniparental female care is typical across taxa (Clutton-Brock 1991; Royle, Smiseth, and Kölliker 2012), but male-only care is most prevalent in fish (Gross and Sargent 1985) and biparental care predominates in birds (Cockburn 2006). A few species exhibt flexible forms of care, with any of the three forms possible (Parker et al. 2015).
One oft-asked question in the field of parental care considers the evolutionary transitions between different patterns of care (Székely and Reynolds 1995; Beck 1998; Kutschera and Wirtz 2001; Reynolds, Goodwin, and Freckleton 2002; Mank, Promislow, and Avise 2005; Klug, Bonsall, and Alonzo 2013; Gilbert and Manica 2015). There appears to be no singular explanations, even in related groups (Andersson 2005). Explanations for the prevalence of female care typically invoke Trivers (1972) arguments for the role of anisogamy (Royle et al. 2012), although more recently factors such as sexual selection, sex-specific mortality, and polyandry have been implicated in maintaining classic sex roles (Kokko and Jennions 2008; Royle et al. 2012). Exclusive male care is sometimes attributed to selection on paternity assurance, although risk of paternity can also result in reduced their parental investment (Royle et al. 2012). Biparental social groups also have variable causes (Vincze et al. 2013). Although biparental care is generally expected to evolve when males joining females provides a fitness benefit to offspring rather than the parent (McNamara et al. 2003; Gilbert and Manica 2015), males joining may arise for multiple reasons. Males may contribute to direct provisioning of offspring alongside the female, or may perform an altogether different role allowing task specialization (Barta et al. 2014).
There are species that display multiple forms of parenting and fine-grained studies of the benefits of parenting mode, where the form of parenting is clear but varies intraspecifically, can provide a compliment to phylogenetic studies. Burying beetles (Silphidae: Nicrophorinae: Nicrophorus) provide a useful system to examine the evolution of parental care modes because of the flexibility of parenting behaviour within the genus and within species. All Nicrophorus species display advanced parental behaviour, in which adults prepare a vertebrate carcass where developing larvae are housed and are fed regurgitated flesh by their parents (Eggert and Müller 1997). However, within a single Nicrophorus species, the mode of parental care can vary broadly. In N. vespilloides Herbst and N. pustulatus Herschel, for example, offspring can develop under the care of both parents, female or male only, or no parent at all (Eggert, Reinking, & Müller 1998; Rauter and Moore 2002, 2004; Smiseth et al. 2005, Schrader, Jarrett, & Kilner 2015a,b). Furthermore, the large number of species displaying similar behaviour in the genus allows for potential comparative analysis informing the evolution of parental transitions. However, more detailed analysis on parental mode and its consequences are needed; Nicrophorus are generally described as biparental (e.g. Gilbert and Manica 2015) despite the likelihood that parenting strategies in this group are more nuanced (Parker et al. 2015).
Here, we investigated parenting mode and its consequences in the North American burying beetle species N. orbicollis Say. Mate removal studies found no obvious benefits to offspring from biparental care (Trumbo and Fernandez 1995). However, alternative approaches may be informative. For example, Parker et al. (2015) show that in N. vespilloides fitness effects are different if desertion is allowed to proceed naturally rather than experimentally. Therefore, to complement previous mate-removal studies, we followed Parker et al. (2015) and allowed parents to freely assort themselves into bi- or uniparental care. Although this experimental design is potentially limited by parents deserting in a non-random manner, it also has two benefits. First, it allowed us to estimate the prevalence of each care mode in our population. Second it ensured that our biparental “treatment” truly consisted of broods being actively cared for by both parents. We found that biparental care was not only common, but also that biparental broods produced more larvae than uniparental broods when allowed to freely adopt their family composition.
Material and Methods
We collected N. orbicollis from Athens, GA and maintained an outbred colony as previously described (Benowitz, Moody, & Moore 2015). We supplemented the original colony with collections in the spring and late summer of 2014, so that some individuals used were the progeny of wild caught parents.
Study pairs were randomly chosen non-sibling males and females of at least 14 days post-eclosion. On the day of pairing, we measured the pronotum width of individuals and weighed them to 1 mg using an electronic balance (Mettler-Toledo, Columbus, OH, USA). We then placed pairs in plastic boxes (17.2 × 12.7 × 6.4 cm; Pioneer Plastics, Dixon, KY, USA) with a thawed 22-25 g mouse on top of about 2 cm of potting soil. All pairings were done at 3:00 pm.
After pairing, we checked each box three times per day at eight-hour intervals, starting at 9:00 am the day after pairing. We recorded the time of eggs laying, hatching, and larval dispersal from the carcass. At dispersal, larvae were counted and weighed individually to 1 mg. At each check we recorded the location of the parent. Cessation of parental care by a beetle is characterized by continuous absence and location away from the carcass, and is a readily recognizable behaviour regardless of the type of observation chamber used (Benowitz et al. 2013, 2015; Hopwood et al. 2015; Parker et al. 2015). As in these previous works, when a beetle was observed to be away from the carcass for three consecutive observations and thus persistently absent over a 24-hour period, we defined it as abandoned. After abandonment, beetles were removed from the breeding box to prevent their later return to the carcass to eat larvae, as is common in N. orbicollis (K.M. Benowitz pers. obs.). We removed beetles once they had abandoned. If both beetles remained on the carcass past the point of larval hatching, the pair was defined as biparental. If the either the male or the female abandoned before hatching, the pair was classified as either female or male uniparental, respectively. Even in uniparental care, both parents most likely contributed to carcass preparation and therefore indirect care; thus, out definition of biparental focuses on direct interactions with larvae. No pairs in which both parents abandoned before hatching produced surviving offspring, consistent with previous work showing that N. orbicollis need parental provisioning to survive (Trumbo 1992). From a total of 150 initial pairs 112 reared at least one larva to dispersal, and were used in analyses of parental care.
Our first goal was simply to compare the frequency of biparental care to male and female uniparental care. We next considered the possibility that beetles may be more likely to abandon the carcass based on its own quality or that of its partner. To examine this, we used logistic regression to measure the effects of body condition on the pattern of parental care adopted, with condition measured as the residual of body weight over pronotum width. Next, we asked whether the mode of parental care (biparental, uniparental female, uniparental male) had any effect on duration of parental care or offspring performance. We answered this question by performing type I ANOVAs using male and female duration of care, number of offspring, average mass of offspring, and development time as dependent variables. We then used Tukey's HSD to compare means post-hoc between the three modes of parental care. To examine the independence of offspring size and number, we then analysed the effect of parental care pattern on offspring size using offspring number as a covariate. Our final question was whether the duration of care directly affected offspring performance. To address this question, we split our analysis by the mode of parental care and ran separate regressions for each mode. For the uniparental pairs, we ran separate univariate regressions of male and female care, respectively, on offspring size. For the biparental pairs, because male and female duration of care were only moderately correlated (r = -0.353; Sokal and Rohlf 2012 p. 650), we ran a multiple regression testing whether female duration of care, male duration of care, or their interaction affected offspring mass. We used JMP (v11.0.0; SAS Institute, Cary, NC) for all analyses.
Results
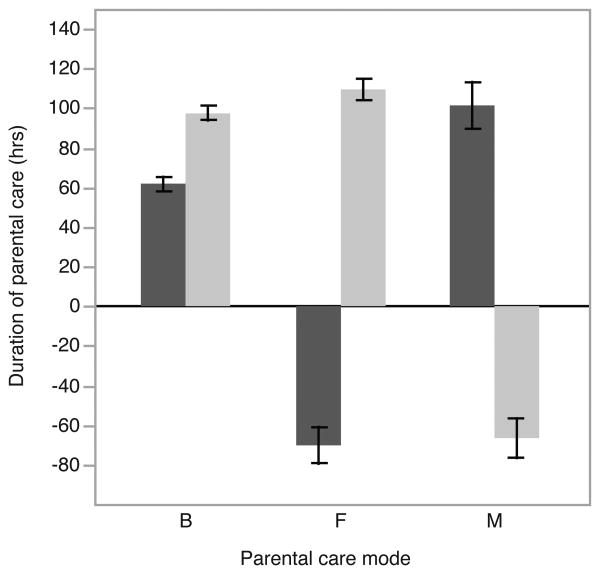
In our sample of 112 breeding pairs, we found N. orbicollis to be a largely biparental species, with 66.1% of all broods cared for by both sexes. About a quarter (25.9%) of broods were cared for uniparentally by the mother, with the remaining 8.0% cared for by uniparental males. Neither the body condition of males (χ22,110 = 1.597, p = 0.450), females (χ22,110 = 1.561, p = 0.458) nor their interaction (χ22,110 = 1.912, p = 0.384) affected the observed pattern of parental care. We next examined how the duration of male and female parental care differed between care patterns. Males provided significantly longer parental care under uniparental conditions than they did under biparental conditions (qs = 3.093, p = 0.007; Fig. 1) whereas females did not differ in their duration of parental care when uniparental or biparental (qs = 1.839, p = 0.162; Fig. 1)). Of those individuals that dispersed and left the care to their partner, both males and females deserted several days before hatching with no statistically significant difference between the two (F1,37 = 0.043, p = 0.837; Fig. 1)).
Figure 1.
The duration of male (dark bars) and female (light bars) care under different parental care modes. Time zero represents hatching; therefore, negative values show how long before larvae arrived that a parent abandoned the carcass. B = biparental care, F = female only care, M = male only care.
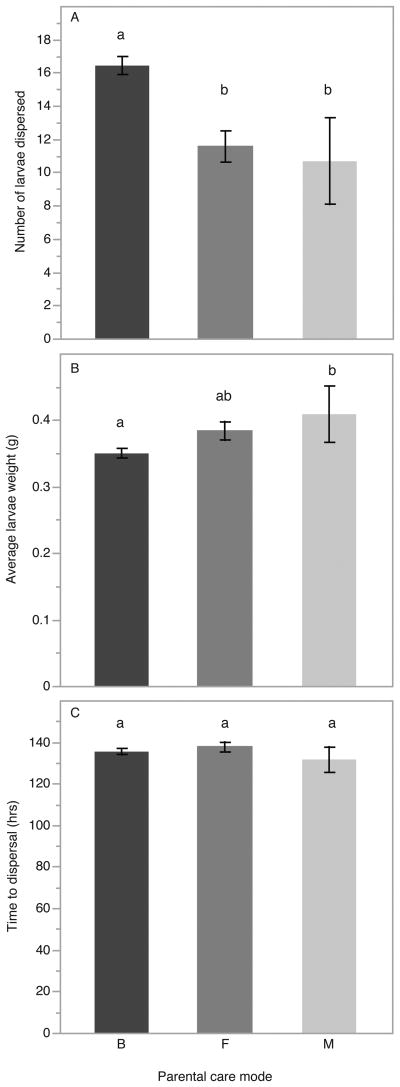
In examining the effects of different modes of care, we found a significant effect of the pattern of care on number of offspring dispersed (F2,110 = 12.578, p < 0.001). Biparental pairs raise significantly more offspring than either single female (qs = 4.362, p < 0.001) or single male (qs = 3.199, p = 0.005) parents (Fig. 2A). There was also an overall effect of pattern of care on size of offspring reared (F2,110 = 4.466, p = 0.014). Single parents reared larger offspring. This was statistically significant for offspring raised by single male parents, where offspring were marginally larger than those raised biparentally (qs = 2.377, p = 0.050), while those raised by single female parents were not statistically significantly different in size than larvae reared by pairs (qs = 2.199, p = 0.0758; Fig. 2B). However, when offspring number was added as a covariate in the analysis of offspring size, pattern of care did not affect average larva weight (F2,110 = 0.933, p = 0.397). Thus, the marginal differences in larva weight between care modes is a reflection of the observed differences in number of larvae reared, rather than increased parental effectiveness. There was no statistically significant difference in development time by parental care mode (F2,110 = 0.752, p = 0.474; Fig. 2C).
Figure 2.
The effects of parental care mode on: A, number of larvae dispersed; B, average larva weight; C, brood development time until dispersal. B = biparental care, F = female only care, M = male only care. Statistically significant differences, determined by Tukey's HSD, are denoted by different letters above the bars.
Lastly, we determined the effects of duration of parental care on offspring performance. Duration of care in both male (r2 = 0.492, F1,8 = 6.791, p = 0.035) and female (r2 =0.200, F1,27 = 6.506, p = 0.017) uniparental conditions had a positive effect on average offspring size. However, under biparental conditions, neither male care (F3,73 = 0.007, p = 0.934), female care (F3,73 = 3.451, p = 0.067, or their interaction (F3,73 = 0.001, p = 0.983) affected offspring size.
Discussion
Understanding how patterns of parental care evolve requires a detailed understanding of variation both within a species and between species (Székely and Reynolds 1995). As a phenotype with social effects, parental care is expected to evolve at an especially fast rate (Moore, Brodie, & Wolf 1997), and thus may differ in surprising ways even amongst closely related species and populations. Yet most studies have focused on between species comparisons (Székely and Reynolds 1995; Beck 1998; Kutschera and Wirtz 2001; Reynolds et al. 2002; Mank et al. 2005; Cockburn 2006; Gilbert and Manica 2015. As a potential first step in examining how parental care has evolved on a fine scale, we have assessed the parenting strategies naturally expressed by the burying beetle Nicrophorus orbicollis and the consequences of variation in those strategies. Placed in the context of data from other burying beetles, we hope to illuminate a more nuanced view of how parental strategy may evolve in this group.
Using a large sample size, and allowing individuals to assort into patterns of care, we found that biparental care was common in N. orbicollis. This finding contrasts with an earlier study of a more northerly population (Wilson and Fudge 1984), where uniparental care was more common, but confirms findings of Scott and Gladstein (1993). In N. vespilloides, using an identical experimental approach, biparental care was seen in 44% of 269 broods, and uniparental female care in 51% (Parker et al. 2015). In burying beetles in general, it appears that males that do stay are typically more variable and show greater differences in participation in care than do females in biparental and uniparental conditions in a variety of burying beetle species (Rauter and Moore 2004; Smiseth et al. 2005; Suzuki and Nagano 2009). In terms of duration of care, our results mirror those of N. vespilloides (Parker et al. 2015) and removal studies in N. orbicollis (Rauter and Moore 2004). Uniparental females and males, and biparental females spend similar amounts of time with larvae but biparental males typically abandon the family early. Males and females that abandon before the larvae hatch do so equally early.
The fitness effects of different modes of care depended on whose fitness we measured and was not necessarily a direct reflection of the number of parents present. Biparental families dispersed more larvae, indicating a fitness benefit to parents. As is the case with other burying beetle species (Smiseth, Ward, & Moore 2006; House et al. 2008, 2009), females of N. orbicollis lay more eggs than the number of larvae that are eventually reared (K. M. Benowitz pers. obs.). Brood size is known to be a consequence of parental culling behaviour during the first larval instar (Trumbo and Fernandez 1995). Thus, it appears that parents caring as a pair cull fewer offspring than single parents, suggesting that direct contributions from both parents allow the rearing of more offspring. However, the larvae in uniparental families were marginally larger than those dispersed from biparental families. Larger body size likely affects burying beetle fitness because there is strong competition for resources, and larger adults are more successful in defending or taking over carcasses (Hopwood, Moore, and Royle 2013, 2014; Lee et al. 2014). The difference we see in size, however, is small and may be offset by an increased probability of being culled under uniparental conditions. Therefore, the fitness consequences to offspring of developing under different patterns of care remain unclear.
Previous work in N. orbicollis (reviewed in Trumbo and Fernandez 1995) has led to the conclusion that biparental care in this species has evolved largely due to task specialization, with males primarily functioning to defend the carcass (Trumbo 2006). Our results also suggest that biparental care can sometimes have direct effects on parental fitness. Our results do not exclude the possibility that male N. orbicollis also provide indirect benefits for offspring in the form of defence. Instead, we suggest that N. orbicollis paternal behaviour varies flexibly between defensive and provisioning, as is observed in avian systems (Mutzel et al. 2013; Vincze et al. 2013; Ghalambor, Peluc, and Martin 2014), although in burying beetles this flexibility appears to be limited to males (Rauter and Moore 2004; Smiseth et al. 2005). Furthermore, a defensive function of males may allow parents to rear more offspring if one parent can respond to the brood more quickly after a threat (S.T. Trumbo pers. comm.). Therefore, our experimental design, with potential disturbances of breeding pairs by our observations, may have created an environment in which biparental care is especially beneficial.
The variation in the propensity to form families with both parents present is consistent with theoretical expectations about the stability of biparental care strategies (McNamara et al. 2003). Where fitness benefits are lacking, uniparental care predominates as in N. vespilloides. Where fitness benefits are greater with two parents, even where subtle, biparental care is seen more often. These differences may further be associated with the fact that in N. orbicollis offspring require parental care to survive at early instars (Trumbo 1992) unlike N. vespilloides (Eggert et al. 1998; Schrader et al. 2015a,b). In both species, however, parental care strategies are highly flexible.
The value of biparental care depends on the burying beetle species studied as well as the experimental design used. The differences between N. orbicollis and N. vespilloides highlights the dangers of generalizing behavioural patterns, even between species that appear to display the same phenotypes. Our data suggests that even minor ecological differences between closely related species can result in social and behavioural effects that differ in important ways. This reflects the idea that parental care strategies, like other social traits, may evolve especially rapidly (Moore et al. 1997).
Acknowledgments
We thank Brice Hsu for assistance with maintenance of the N. orbicollis colony, and Steve Trumbo, Per Smiseth, John Allen, and two anonymous reviewers for helpful comments on the manuscript. This work was supported by funding from an NIH Training Grant (T32GM007103 to KMB), and an NSF grant (IOS-1354358 to AJM).
References
- Andersson M. Evolution of classical polyandry: three steps to female emancipation. Ethology. 2005;111:1–23. [Google Scholar]
- Barta Z, Székely T, Liker A, Harrison F. Social role specialization promotes cooperation between parents. American Naturalist. 2014;183:747–761. doi: 10.1086/676014. [DOI] [PubMed] [Google Scholar]
- Beck CW. Mode of fertilization and parental care in anurans. Animal Behaviour. 1998;55:439–449. doi: 10.1006/anbe.1997.0619. [DOI] [PubMed] [Google Scholar]
- Benowitz KM, Head ML, Williams CA, Moore AJ, Royle NJ. Male age mediates reproductive investment and response to paternity assurance. Proceedings of the Royal Society B-Biological Sciences. 2013;280:20131124. doi: 10.1098/rspb.2013.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz KM, Moody KJ, Moore AJ. Are species differences in maternal effects arising from maternal care adaptive? Journal of Evolutionary Biology. 2015;28:503–509. doi: 10.1111/jeb.12573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clutton-Brock TH. The evolution of parental care. Princeton, N.J: Princeton University Press; 1991. [Google Scholar]
- Cockburn A. Prevalence of different modes of parental care in birds. Proceedings of the Royal Society B-Biological Sciences. 2006;273:1375–1383. doi: 10.1098/rspb.2005.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert A-K, Müller JK. Biparental care and social evolution in burying beetles: lessons from the larder. In: Choe JC, Crespi JB, editors. The evolution of social behavior in insects and arachnids. Cambridge: Cambridge University Press; 1997. pp. 216–236. [Google Scholar]
- Eggert A-K, Reinking M, Müller JK. Parental care improves offspring survival and growth in burying beetles. Animal Behaviour. 1998;55:97–107. doi: 10.1006/anbe.1997.0588. [DOI] [PubMed] [Google Scholar]
- Ghalambor CK, Peluc SI, Martin TE. Plasticity of parental care under the risk of predation: how much should parents reduce care? Biology Letters. 2013;9:20130154. doi: 10.1098/rsbl.2013.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JD, Manica A. The evolution of parental care in insects: a test of current hypotheses. Evolution. 2015;69:1255–1270. doi: 10.1111/evo.12656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross MR, Sargent RC. The evolution of male and female parental care in fishes. American Zoologist. 1985;25:807–822. [Google Scholar]
- Hopwood PE, Moore AJ, Royle NJ. Nutrition during sexual maturation affects competitive ability but not reproductive productivity in burying beetles. Functional Ecology. 2013;27:1350–1357. [Google Scholar]
- Hopwood PE, Moore AJ, Royle NJ. Effects of resource variation during early life and adult social environment on contest outcomes in burying beetles. Proceedings of the Royal Society B. 2014;281:20133102. doi: 10.1098/rspb.2013.3102. doi.org/10.1098/rspb.2013.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood PE, Moore AJ, Tregenza T, Royle NJ. Male burying beetles extend, not reduce, parental care duration when reproductive competition is high. Journal of Evolutionary Biology. 2015;28:1394–1402. doi: 10.1111/jeb.12664. [DOI] [PubMed] [Google Scholar]
- House CM, Evans GMV, Smiseth PT, Stamper CE, Walling CA, Moore AJ. The evolution of repeated mating in the burying beetle, Nicrophorus vespilloides. Evolution. 2008;62:2004–2014. doi: 10.1111/j.1558-5646.2008.00422.x. [DOI] [PubMed] [Google Scholar]
- House CM, Walling CA, Stamper CE, Moore AJ. Females benefit from multiple mating but not multiple mates in the burying beetle Nicrophorus vespilloides. Journal of Evolutionary Biology. 2009;22:1961–1966. doi: 10.1111/j.1420-9101.2009.01800.x. [DOI] [PubMed] [Google Scholar]
- Klug H, Bonsall MB, Alonzo SH. Sex differences in life history drive evolutionary transitions among maternal, paternal and bi-parental care. Ecology and Evolution. 2013;3:792–806. doi: 10.1002/ece3.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokko H, Jennions MD. Parental investment, sexual selection, and sex ratios. Journal of Evolutionary Biology. 2008;21:919–948. doi: 10.1111/j.1420-9101.2008.01540.x. [DOI] [PubMed] [Google Scholar]
- Kutschera U, Wirtz P. The evolution of parental care in freshwater leeches. Theory in Biosciences. 2001;120:115–137. [Google Scholar]
- Lee VE, Head ML, Carter MJ, Royle NJ. Effects of age and experience on contest behavior in the burying beetle, Nicrophorus vespilloides. Behavioral Ecology. 2014;25:172–179. doi: 10.1093/beheco/art101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mank JE, Promislow DEL, Avise JC. Phylogenetic perspectives in the evolution of parental care in ray-finned fishes. Evolution. 2005;59:1570–1578. [PubMed] [Google Scholar]
- McNamara JM, Houston AI, Barta Z, Osorno JL. Should young ever be better off with one parent than with two? Behavioral Ecology. 2003;14:301–310. [Google Scholar]
- Moore AJ, Brodie ED, III, Wolf JB. Interacting phenotypes and the evolutionary process .1. Direct and indirect genetic effects of social interactions. Evolution. 1997;51:1352–1362. doi: 10.1111/j.1558-5646.1997.tb01458.x. [DOI] [PubMed] [Google Scholar]
- Mutzel A, Blom MPK, Spagopoulou F, Wright J, Dingemanse NJ, Kempenaers B. Temporal trade-offs between nestling provisioning and defence against nest predators in blue tits. Animal Behaviour. 2013;85:1459–1469. [Google Scholar]
- Parker DJ, Cunningham CB, Walling CA, Stamper CE, Head ML, Roy-Zokan EM, McKinney EC, Ritchie MG, Moore AJ. Transcriptomes of parents identify parenting strategies and sexual conflict in a subsocial beetle. Nature Communications. 2015;6:8449. doi: 10.1038/ncomms9449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauter CM, Moore AJ. Evolutionary importance of parental care performance, food resources, and direct and indirect genetic effects in a burying beetle. Journal of Evolutionary Biology. 2002;15:407–417. [Google Scholar]
- Rauter CM, Moore AJ. Time constraints and trade-offs among parental care behaviours: effects of brood size, sex and loss of mate. Animal Behaviour. 2004;68:695–702. [Google Scholar]
- Reynolds JD, Goodwin NB, Freckleton RP. Evolutionary transitions in parental care and live bearing in vertebrates. Philosophical Transactions of the Royal Society B-Biological Sciences. 2002;357:269–281. doi: 10.1098/rstb.2001.0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle NJ, Smiseth PT, Kölliker M. The evolution of parental care. Oxford: Oxford University Press; 2012. [Google Scholar]
- Schrader M, Jarrett BJM, Kilner RM. Using experimental evolution to study adaptations for life within the family. American Naturalist. 2015a;185:610–619. doi: 10.1086/680500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader M, Jarrett BJM, Kilner RM. Parental care masks a density-dependent shift from cooperation to competition among burying beetle larvae. Evolution. 2015b;69:1077–1084. doi: 10.1111/evo.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott MP, Gladstein DS. Calculating males: an empirical and theoretical examination of the duration of paternal care in burying beetles. Evolutionary Ecology. 1993;7:651–651. [Google Scholar]
- Smiseth PT, Dawson C, Varley E, Moore AJ. How do caring parents respond to mate loss? Differential response by males and females. Animal Behaviour. 2005;69:551–559. [Google Scholar]
- Smiseth PT, Ward RJS, Moore AJ. Asynchronous hatching in Nicrophorus vespilloides, an insect in which parents provide food for their offspring. Functional Ecology. 2006;20:151–156. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. New York, NY: W.H. Freeman and Co.; 2012. [Google Scholar]
- Suzuki S, Nagano M. To compensate or not? Caring parents respond differentially to mate removal and mate handicapping in the burying beetle, Nicrophorus quadripunctatus. Ethology. 2009;115:1–6. [Google Scholar]
- Székely T, Remes V, Freckleton RP, Liker A. Why care? Inferring the evolution of complex social behaviour. Journal of Evolutionary Biology. 2013;26:1381–1391. doi: 10.1111/jeb.12148. [DOI] [PubMed] [Google Scholar]
- Székely T, Reynolds JD. Evolutionary transitions in parental care in shorebirds. Proceedings of the Royal Society B-Biological Sciences. 1995;262:57–64. [Google Scholar]
- Tallamy DW, Wood TK. Convergence patterns in subsocial insects. Annual Review of Entomology. 1986;31:369–390. [Google Scholar]
- Trivers RL. Parental investment and sexual selection. In: Campbell B, editor. Sexual selection and the descent of man 1871-1971. Chicago, IL: Aldine-Atherton; 1972. pp. 136–179. [Google Scholar]
- Trumbo ST. Monogamy to communal breeding: exploitation of a broad resource base by burying beetles (Nicrophorus) Ecological Entomology. 1992;17:289–298. [Google Scholar]
- Trumbo ST. Infanticide, sexual selection and task specialization in a biparental burying beetle. Animal Behaviour. 2006;72:1159–1167. [Google Scholar]
- Trumbo ST, Fernandez AG. Regulation of brood size by male parents and cues employed to assess resource size by burying beetles. Ethology Ecology & Evolution. 1995;7:313–322. [Google Scholar]
- Vincze O, Székely T, Kupper C, AlRashidi M, Amat JA, Ticó AA, Burgas D, Burke T, Cavitt J, Figuerola J, Shobrak M, Montalvo T, Kosztolányi A. Local environment but not genetic differentiation influences biparental care in ten plover populations. PLoS ONE. 2013;8:e60998. doi: 10.1371/journal.pone.0060998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DS, Fudge J. Burying beetles: intraspecific interactions and reproductive success in the field. Ecological Entomology. 1984;9:195–203. [Google Scholar]