Abstract
Leptin is an adipokine involved in regulating energy balance which has been identified as a potential biological link in development of obesity-associated cancers, such as pancreatic cancer. In this prospective, nested case-control study of 470 cases and 1094 controls from five U.S. cohorts, we used conditional logistic regression to evaluate pancreatic cancer risk by prediagnostic plasma leptin, adjusting for race/ethnicity, diabetes, body-mass index, physical activity, plasma C-peptide, adiponectin and 25-hydroxyvitamin D. Due to known differences in leptin levels by gender, analyses were conducted separately for men and women. We also evaluated associations between 32 tagging single nucleotide polymorphisms (SNPs) in the leptin receptor (LEPR) gene and pancreatic cancer risk. Leptin levels were higher in female versus male control participants (median, 20.8 vs. 6.7µg/mL; P<0.0001). Among men, plasma leptin was positively associated with pancreatic cancer risk, and those in the top quintile had a multivariable-adjusted odds ratio (OR) of 3.02 (95% CI, 1.27–7.16; Ptrend=0.02) compared to men in the bottom quintile. Among women, circulating leptin was not associated with pancreatic cancer risk (Ptrend=0.21). Results were similar across cohorts (Pheterogeneity=0.88 for two male cohorts and 0.35 for three female cohorts). In genetic analyses, rs10493380 in LEPR was associated with increased pancreatic cancer risk among women, with an OR per minor allele of 1.54 (95% CI, 1.18–2.02; multiple hypothesis-corrected P=0.03). No SNPs were significantly associated with risk in men. In conclusion, higher prediagnostic levels of plasma leptin were associated with an elevated risk of pancreatic cancer among men, but not among women.
Keywords: pancreatic cancer, obesity, leptin
INTRODUCTION
Pancreatic cancer is the third-leading cause of cancer-related death in the United States (1). Obesity is associated with increased pancreatic cancer risk (2,3), but the underlying mechanisms are poorly understood. Leptin was initially discovered in studies of obese mice, which were noted to have increased caloric intake. Subsequent studies demonstrated that the underlying cause of the obese phenotype in these mice was a truncating mutation in the gene encoding for leptin (4). Leptin is a hormone synthesized almost exclusively in adipocytes, and plasma levels of leptin in humans are proportional to total body adipose tissue (5). When circulating levels of leptin are increased, downstream signaling is activated through the ObR (HUGO Gene Nomenclature Committee [HGNC] Symbol LEPR) transmembrane receptor on target cells (6). The leptin receptor is present on cells within the hypothalamus, which is the mechanism by which leptin is thought to regulate caloric intake (7). Additionally, leptin receptor expression is distributed widely throughout the body, including on cells within the pancreas (8). Leptin receptors are also present on tumor cells and activation of these receptors increases cancer cell proliferation and reduces rates of cancer cell apoptosis (9).
Given its essential role in regulating energy balance, leptin may be an important biological link in the development of obesity-associated malignancies, including pancreatic cancer. To further investigate the role of leptin in pancreatic carcinogenesis, we examined the association between prediagnostic plasma leptin and subsequent pancreatic cancer risk in five prospective cohorts with up to 26 years of follow-up since blood collection. We additionally evaluated the association between polymorphisms in LEPR with risk of pancreatic cancer.
METHODS
Study Participants
We pooled blood samples and data from five U.S. prospective cohorts. The Health Professionals Follow-up Study (HPFS) enrolled 51,529 male health professionals aged 40–75 in 1986. The Nurses’ Health Study (NHS) enrolled 121,700 female nurses aged 30–55 in 1976. The Physicians’ Health Study I (PHS) was a randomized clinical trial of aspirin and β-carotene that enrolled 22,071 healthy male physicians aged 40–84 in 1982. The aspirin component of the trial ended in 1988, while the β-carotene component ended in 1995, and PHS I participants continue follow-up as an observational cohort. The Women’s Health Initiative (WHI)-Observational Study enrolled 93,676 postmenopausal women aged 50–79 between 1994 and 1998. The Women’s Health Study (WHS) was a randomized clinical trial of low-dose aspirin and vitamin E that enrolled 39,876 healthy female health professionals aged ≥45 in 1992. The trial was completed in 2004 and WHS participants continue to be followed as an observational cohort.
Individual characteristics and lifestyle factors were obtained from baseline questionnaires at enrollment in PHS, WHI, and WHS and from the questionnaires preceding blood draw in HPFS and NHS. Details of these cohorts have been described previously (10–14). The current study was approved by the Human Research Committee at the Brigham and Women’s Hospital, Boston, MA, and participants provided informed consent.
Blood Collection and Plasma Assays
Blood samples were collected from 18,225 men in HPFS from 1993–1995, 32,826 women in NHS from 1989–1990, 14,916 men in PHS from 1982–1984, 93,676 women in WHI from 1994–1998, and 28,345 women in WHS from 1992–1995. Details on blood draw, transportation, and storage have been described previously (12,14–16).
Plasma leptin was assayed in the laboratory of Dr. Nader Rifai (Children’s Hospital, Boston, MA), using reagents from R&D Systems (Minneapolis, MN). Measurement of plasma adiponectin and C-peptide were described previously (17). All samples for leptin and adiponectin were handled identically in a single batch and C-peptide was handled in two batches. Laboratory personnel were blinded to case or control status. The mean intra-assay coefficients of variance for quality control samples were ≤10% for each biomarker.
Pancreatic cancer cases and matched controls
We included cases of pancreatic adenocarcinoma diagnosed through 2008 with prediagnostic blood and no prior history of cancer, except non-melanoma skin cancer. Incident cases were identified by self-report or during follow-up of a participant’s death. Deaths were ascertained from next-of-kin or the U.S. postal service and by searching the National Death Index. Medical records of the cases were requested and reviewed by study physicians blinded to exposure data.
Eligible controls were cohort participants who provided blood and were alive and free of cancer at the date of the case’s diagnosis. We randomly selected 2–3 controls for each case, matching on year of birth (± 5 years), prospective cohort (which concurrently matched on sex), smoking status (never, past, current), fasting status (fasting, non-fasting), and month/year of blood draw.
For the present analysis, 488 pancreatic cancer cases and 1132 matched controls with plasma were available. Due to concern regarding the possible influence of subclinical malignancy, we excluded pancreatic cancer cases diagnosed within 1 year of blood draw (n=19) and their matched controls (n=38), resulting in a total of 470 cases and 1094 controls (Supplementary Table 1). Of these 470 cases, 465 (99%) were confirmed by review of medical records, tumor registry data, or death certificates.
Single Nucleotide Polymorphisms (SNP) selection and Genotyping
A total of 39 SNPs in the LEPR gene +/− 20kb were selected with the tagger algorithm in Haploview, using r2=0.8 and minor allele frequency (MAF) ≥5% among Whites from the HapMap Project database. Five SNPs associated with: serum amyloid A (rs1275319, (18)), soluble Ob-R (rs2767485, (19)), and CRP: (rs4420065 (20), rs6700896 (21) and rs1892534 (22) were forced in. From 412 cases (Supplementary Table 1), DNA was extracted from buffy coat using QIAGEN QIAmp and whole genome amplified using GE Healthcare GenomiPhi. Genotyping was performed at Partners HealthCare Center for Personalized Genetic Medicine using a custom-designed Illumina Golden Gate genotyping assay. Seven tagging SNPs were not supported by the platform. One SNP (rs913199) deviated from Hardy-Weinberg Equilibrium at P=0.008. Replicate samples tested for quality control (N=44 groups) had a mean genotype concordance of 98.2%.
Statistical Analysis
Median leptin levels among cases and controls were compared using the Wilcoxon rank-sum test. Since men and women have different distributions of leptin levels (23), we performed separate analyses by gender using pooled gender-specific quintiles from controls. To compute odds ratios (ORs) and 95% confidence intervals (CIs), we used conditional logistic regression. In multivariate models, we adjusted for potential confounding factors, including race (White, Black, other), multivitamin use (yes, no), diabetes (yes, no), body mass index (BMI, kg/m2), physical activity (MET-hr/wk), plasma C-peptide (continuous), plasma adiponectin (quartiles, as previous analysis demonstrated non-linear association of adiponectin and pancreatic cancer risk (17)), and plasma 25(OH)D (continuous). P-trends were calculated by the Wald test of a score variable that contained median values of quintiles. We also conducted a meta-analysis of cohort-level data among men and women. We calculated ORs for each cohort and then pooled the ORs to compute a summary OR by gender using the random effects model (24). Heterogeneity across studies was tested using the Q statistic (24). To evaluate whether the association between leptin and pancreatic cancer risk was log-linear, we compared the model fit including linear and cubic spline terms to the model fit with only the linear term using the likelihood ratio test (25). We conducted subgroup analyses using unconditional logistic regression adjusted for the matching factors and covariates. Tests for interaction were performed by the Wald test of cross-product terms. We conducted sensitivity analyses excluding diabetics or cases diagnosed within 2 or 4 years from blood draw.
We examined the association between LEPR SNPs and pancreatic cancer risk by modeling each genotype as number of copies of the minor allele (additive model) using conditional logistic regression. We used R software (version R.3.2.2.) to calculate the corrected P-value by taking into account the total number of comparisons, as well as correlations between 32 SNPs(26). We used HaploReg v4.1 to explore the noncoding functional characteristics of identified and highly correlated SNPs (r2 > 0.6 in 1000G CEU data). Statistical analyses were performed with SAS 9.1 (SAS Institute, Cary, North Carolina), and all P values are two sided.
RESULTS
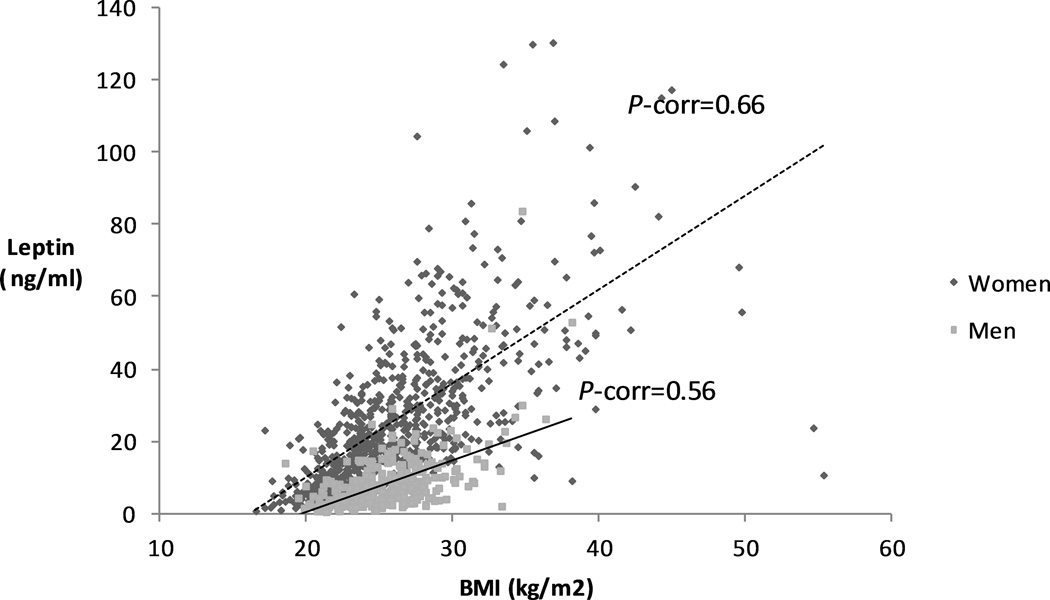
The median time between blood collection and cancer diagnosis was 7.1 years among cases. Among controls, median plasma leptin was 20.8 µg/mL for women and 6.7 µg/mL for men. Leptin levels were comparable across studies for men (HPFS and PHS) and for women (NHS, WHI, and WHS) (Supplementary Table 2). Individuals with higher leptin levels had higher BMI, plasma C-peptide, and prevalence of diabetes (Table 1). After adjusting for age, fasting status and cohort, Spearman correlation coefficients for plasma leptin and BMI were 0.50 (P<0.0001) among men and 0.73 (P<0.0001) among women (Figure 1) (Supplementary table 3), similar to those reported in other populations (27,28).
Table 1.
Age- and study-standardized baseline characteristics according to plasma leptin levels among controls
| Quintiles of plasma leptin | |||||
|---|---|---|---|---|---|
| Characteristic* | 1 | 2 | 3 | 4 | 5 |
| Men | |||||
| Plasma leptin level, ng/mL | ≤3.0 | 3.1–4.8 | 4.9–7.8 | 7.9–11.7 | ≥11.8 |
| No. of controls | 69 | 71 | 71 | 72 | 70 |
| Age at blood draw, years | 60.7 (10.1) | 60.1 (8.9) | 60.2 (10.0) | 60.8 (8.9) | 61.0 (9.1) |
| Race, % | |||||
| White | 94.0 | 93.2 | 85.5 | 93.7 | 91.3 |
| Black | 0 | 1.0 | 0 | 0 | 0 |
| Other | 6.0 | 5.9 | 14.5 | 6.3 | 8.7 |
| Body-mass index, kg/m2 | 23.4 (2.6) | 24.7 (2.1) | 24.8 (2.3) | 26.0 (2.5) | 27.6 (3.6) |
| Physical activity, MET- hr/week |
34.6 (34.9) | 29.1 (31.6) | 30.6 (48.6) | 27.2 (34.6) | 18.1 (22.6) |
| Cigarette smoking, % | |||||
| Never | 37.0 | 36.9 | 37.3 | 40.9 | 33.6 |
| Past | 52.9 | 50.4 | 40.7 | 41.1 | 47.8 |
| Current | 10.2 | 12.7 | 22.0 | 18.1 | 18.6 |
| Missing | 0 | 0 | 0 | 0 | 0 |
| History of diabetes mellitus, % |
1.0 | 2.5 | 2.2 | 1.6 | 7.3 |
| Regular multivitamin use, % |
37.8 | 37.1 | 36.0 | 30.2 | 30.2 |
| Plasma 25(OH)D, nmol/L | 76.9 (28.9) | 74.5 (32.0) | 72.3 (20.7) | 70.2 (20.9) | 66.3 (19.5) |
| Plasma C-peptide levels, ng/mL |
1.8 (1.1) | 2.2 (1.4) | 2.5 (1.6) | 2.8 (3.4) | 3.1 (2.9) |
| Plasma adiponectin levels, µg/mL |
6.0 (3.2) | 5.9 (2.8) | 6.0 (3.3) | 5.8 (3.2) | 5.7 (4.1) |
| Women | |||||
| Plasma leptin level, ng/mL | ≤9.5 | 9.6–17.7 | 17.8–24.8 | 24.9–37.7 | ≥37.8 |
| No. of controls | 148 | 146 | 150 | 148 | 149 |
| Age at blood draw, years | 63.3 (8.3) | 63.2 (7.9) | 64.3 (8.3) | 63.1 (7.3) | 63.1 (7.8) |
| Race, % | |||||
| White | 89.2 | 95.8 | 94.9 | 92.1 | 93.9 |
| Black | 2.4 | 0.6 | 0.9 | 4.6 | 4.1 |
| Other | 8.4 | 3.6 | 4.2 | 3.4 | 2.1 |
| Body-mass index, kg/m2 | 21.8 (2.6) | 24.2 (3.9) | 25.7 (3.7) | 27.4 (3.2) | 31.3 (5.2) |
| Physical activity, MET- hr/week |
21.7 (20.4) | 17.4 (18.2) | 15.3 (16.8) | 13.0 (13.8) | 12.0 (11.8) |
| Cigarette smoking, % | |||||
| Never | 41.1 | 43.6 | 52.9 | 37.7 | 50.7 |
| Past | 42.8 | 44.3 | 38.5 | 48.7 | 42.4 |
| Current | 14.7 | 10.7 | 8.1 | 13.0 | 6.0 |
| Missing | 1.4 | 1.5 | 0.5 | 0.7 | 1.0 |
| History of diabetes mellitus, % |
1.7 | 0 | 3.9 | 3.9 | 7.7 |
| Regular multivitamin use, % |
47.3 | 42.6 | 45.2 | 43.6 | 36.3 |
| Plasma 25(OH)D, nmol/L | 70.0 (25.3) | 65.7 (21.1) | 62.7 (20.3) | 57.4 (32.4) | 54.6 (18.3) |
| Plasma C-peptide levels, ng/mL |
1.3 (0.6) | 1.6 (0.7) | 2.0 (1.2) | 2.2 (1.0) | 2.5 (1.2) |
| Plasma adiponectin levels, µg/mL |
11.1 (6.0) | 9.6 (4.8) | 8.7 (4.7) | 7.7 (4.7) | 8.0 (4.6) |
Mean (standard deviation) for all continuous variables
Abbreviations: 25(OH)D, 25-hydroxyvitamin D ; MET-hr, metabolic equivalent of task-hour
Figure 1. Correlation between body-mass index and plasma leptin in men and women.
Scatterplot of body-mass index vs. plasma leptin for women (blue squares) and men (red squares). Trendline (line of best fit) is shown as full (men) or dashed line (women).
Abbreviations: BMI, body-mass index ; P-corr, P correlation
We observed a positive association between plasma leptin and pancreatic cancer risk among men, but not among women (Pheterogeneity=0.02; Table 2). In the base model conditioned on matching factors, compared to the bottom quintile, men in the top quintile had an OR of 2.77 (1.37–5.61), (Ptrend=0.01; Table 2). In comparison, women in the top quintile had an OR of 1.27 (0.84–1.91) (Ptrend=0.64; Table 2). Further adjustment for race, multivitamin use, plasma 25(OH)D, history of diabetes, BMI, physical activity, plasma C-peptide, and plasma adiponectin yielded similar results (Table 2). Similar associations were observed in sensitivity analyses when we excluded cases with diabetes or cases diagnosed within 2 or 4 years of blood collection and their matched controls (Supplementary Table 4).
Table 2.
Odds ratios (ORs) and 95% confidence intervals (CIs) for pancreatic cancer according to quintiles of plasma leptin
| Quintiles of plasma leptin | Ptrend* | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Men | ||||||
| Leptin levels, ng/mL | ||||||
| Range | ≤3.0 | 3.1–4.8 | 4.9–7.8 | 7.9–11.7 | ≥11.8 | |
| Median | 2.2 | 4.0 | 6.7 | 9.2 | 15.3 | |
| No. of cases | 17 | 26 | 32 | 27 | 42 | |
| No. of controls | 69 | 71 | 71 | 72 | 70 | |
| Base model1 | 1.0 | 1.57 (0.78–3.16) | 1.90 (0.95–3.80) | 1.61 (0.80–3.24) | 2.77 (1.37–5.61) | 0.01 |
| Adjusted model I2 | 1.0 | 1.48 (0.72–3.05) | 1.80 (0.89–3.64) | 1.59 (0.78–3.25) | 2.55 (1.23–5.27) | 0.02 |
| Adjusted model II3 | 1.0 | 1.48 (0.72–3.05) | 1.80 (0.88–3.67) | 1.60 (0.77–3.33) | 2.54 (1.13–5.72) | 0.03 |
| Adjusted model III4 | 1.0 | 1.68 (0.79–3.54) | 2.03 (0.96–4.29) | 1.84 (0.86–3.95) | 3.02 (1.27–7.16) | 0.02 |
| Women | ||||||
| Leptin levels, ng/mL | ||||||
| Range | ≤9.5 | 9.6–17.7 | 17.8–24.8 | 24.9–37.7 | ≥37.8 | |
| Median | 6.2 | 14.0 | 20.8 | 30.6 | 51.5 | |
| No. of cases | 60 | 80 | 45 | 60 | 81 | |
| No. of controls | 148 | 146 | 150 | 148 | 149 | |
| Base model1 | 1.0 | 1.36 (0.91–2.03) | 0.76 (0.49–1.19) | 0.98 (0.64–1.50) | 1.27 (0.84–1.91) | 0.64 |
| Adjusted model I2 | 1.0 | 1.36 (0.91–2.03) | 0.74 (0.48–1.16) | 0.95 (0.61–1.47) | 1.19 (0.78–1.82) | 0.90 |
| Adjusted model II3 | 1.0 | 1.27 (0.84–1.92) | 0.68 (0.43–1.09) | 0.82 (0.50–1.33) | 0.94 (0.55–1.62) | 0.37 |
| Adjusted model III4 | 1.0 | 1.26 (0.82–1.93) | 0.63 (0.38–1.03) | 0.72 (0.43–1.21) | 0.84 (0.46–1.51) | 0.21 |
Ptrend values were calculated by the Wald test of a score variable that contained median values of quintiles.
ORs and 95% CI were estimated by conditional logistic regression conditioned on the matching factors including year of birth, prospective cohort (HPFS, NHS, PHS, WHI, WHS), smoking status (never, past, current), fasting status (fasting, non-fasting), and month/year of blood draw.
Further adjusted for race (White, Black, other), history of diabetes mellitus (yes, no), current multivitamin use (yes, no), and plasma 25(OH)D (continuous).
Further adjusted for BMI (continuous) and physical activity (continuous).
Further adjusted for plasma C-peptide (continuous) and plasma adiponectin (quartiles).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D ; BMI, body mass index; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; PHS, Physicians’ Health Study; WHI, Women’s Health Initiative; WHS, Women’s Health Study
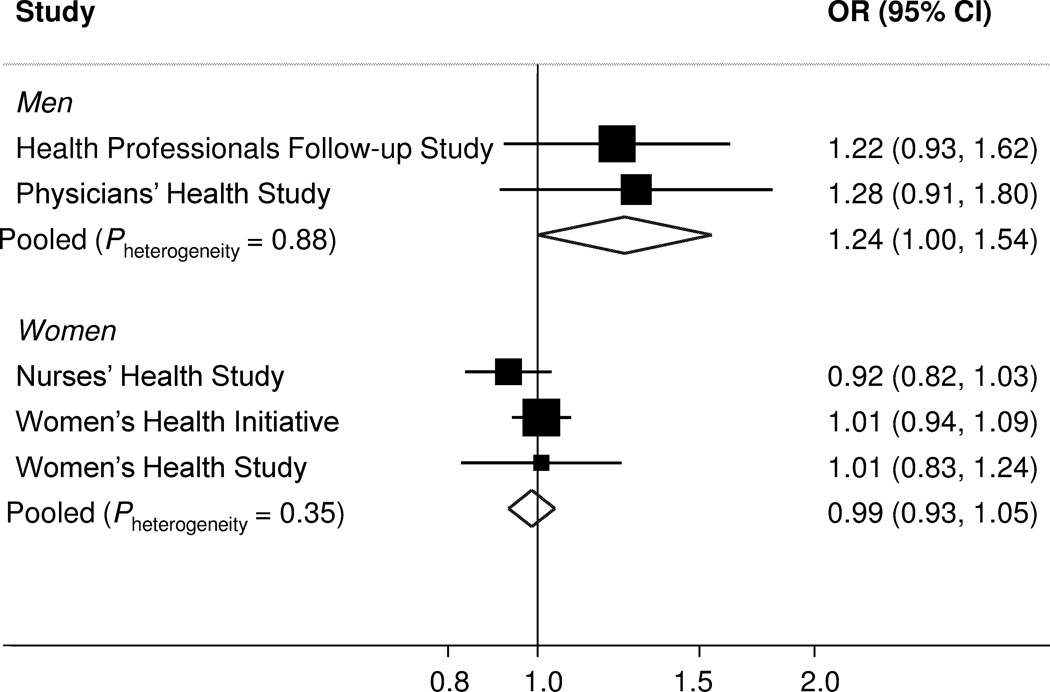
Spline curves were consistent with log-linear associations (Pnonlinear=0.81 for men; Pnonlinear=0.14 for women). Therefore, in subsequent meta-analyses and subgroup analyses, we modeled leptin as a continuous variable. The multivariate ORs for an increment of 5 ng/mL in plasma leptin were 1.25 (1.02–1.54) for men and 0.98 (0.93–1.03) for women (Supplementary Table 5). ORs were similar within the two male and the three female cohorts (Figure 2; Pheterogeneity=0.88 for HPFS and PHS, and Pheterogeneity=0.35 for NHS, WHI, and WHS). In stratified analyses, no statistically significant effect modification was observed (Supplementary Table 5).
Figure 2. Cohort-specific and meta-analysis of pooled odds ratios for pancreatic cancer according to plasma leptin levels (per 5 ng/mL increase in plasma leptin).
Cohort-specific multivariate odds ratios conditioned on matching factors including year of birth, prospective cohort (HPFS, NHS, PHS, WHI, WHS), smoking status (never, past, current), fasting status (fasting, non-fasting), and month/year of blood draw, and adjusted for covariates including race (White, Black, other), history of diabetes mellitus (yes, no), current multivitamin use (yes, no), plasma 25(OH)D (continuous), body-mass index (continuous), physical activity (continuous), plasma C-peptide (continuous), and plasma adiponectin (quartiles). The pooled odds ratio is calculated by the DerSimonian and Laird random-effects model. The solid squares and horizontal lines correspond to the cohort-specific multivariate odds ratios and 95% confidence intervals, respectively. The area of the solid square reflects the cohort-specific weight (inverse of the variance). The open diamond represents the pooled multivariate odds ratio and 95% confidence interval. The solid vertical line indicates an odds ratio of 1.0.
Abbreviations: 25(OH)D, 25-hydroxyvitamin D ; CI, confidence ratio ; HPFS, Health Professionals Follow-up Study; OR, odds ratio; NHS, Nurses’ Health Study; PHS, Physicians’ Health Study; WHI, Women’s Health Initiative; WHS, Women’s Health Study
Several SNPs at the LEPR gene were associated with pancreatic cancer risk among women to P<0.05 in an additive genetic model (Table 3, Supplementary Figure 1). After adjusting for multiple comparisons, rs10493380 located intronic to LEPR remained statistically significantly associated with increased risk of pancreatic cancer (OR per minor allele=1.54; 95% CI=1.18–2.02, multiple hypothesis-corrected P=0.03; Table 3). This association was consistent across the three female cohorts (Pheterogeneity=0.28). The association for rs10493380 was not statistically significant among men (OR=1.19, 95% CI=0.79–1.78, multiple hypothesis-corrected P=1.00; Supplementary Table 6). Analysis of rs10493380 and highly correlated SNPs using HaploReg identified multiple transcription factor binding altered by these SNPs (Supplementary Table 7). Furthermore, in a blood eQTL database (29), rs10493380 (index SNP) and rs3790429 (in high LD with rs10493380, r2=0.90 in 1000G EUR) were found to have cis eQTL effects on LEPR gene expression. No statistically significant association was identified between SNPs at the LEPR gene and plasma leptin levels among controls (Supplementary Table 8).
Table 3.
Association between SNPs in the leptin receptor (LEPR) gene and risk of pancreatic cancer among women
| Controls | Cases | Additive Model | ||||||
|---|---|---|---|---|---|---|---|---|
| SNP | Minor allele |
N | MAF (%) |
N | MAF (%) |
ORa (95% CI) |
Raw P- value |
Corrected P-value |
| rs10493380 | C | 646 | 0.16 | 272 | 0.21 | 1.54 (1.18–2.02) | 0.001 | 0.032 |
| rs3790424 | G | 655 | 0.31 | 280 | 0.24 | 0.69 (0.55–0.89) | 0.003 | 0.069 |
| rs6673324 | G | 650 | 0.48 | 277 | 0.55 | 1.38 (1.11–1.71) | 0.003 | 0.072 |
| rs2154381 | G | 653 | 0.34 | 281 | 0.27 | 0.72 (0.57–0.90) | 0.004 | 0.088 |
| rs6662904 | A | 648 | 0.42 | 278 | 0.48 | 1.32 (1.07–1.62) | 0.009 | 0.170 |
| rs9436746 | A | 653 | 0.45 | 280 | 0.39 | 0.75 (0.61–0.93) | 0.010 | 0.180 |
| rs9436747 | A | 653 | 0.42 | 278 | 0.35 | 0.75 (0.60–0.93) | 0.010 | 0.184 |
| rs11801408 | A | 657 | 0.18 | 283 | 0.23 | 1.36 (1.06–1.75) | 0.014 | 0.243 |
| rs41459646 | C | 654 | 0.16 | 279 | 0.20 | 1.36 (1.05–1.77) | 0.021 | 0.329 |
| rs2767485 | G | 650 | 0.18 | 280 | 0.22 | 1.27 (0.99–1.63) | 0.059 | 0.647 |
| rs9436301 | G | 650 | 0.23 | 280 | 0.27 | 1.25 (0.99–1.58) | 0.063 | 0.672 |
| rs7524834 | G | 659 | 0.42 | 282 | 0.46 | 1.21 (0.98–1.49) | 0.074 | 0.724 |
| rs12025906 | G | 657 | 0.21 | 280 | 0.18 | 0.79 (0.61–1.03) | 0.084 | 0.770 |
| rs9436748 | A | 654 | 0.39 | 278 | 0.43 | 1.18 (0.95–1.46) | 0.134 | 0.901 |
| rs10128072 | C | 650 | 0.15 | 277 | 0.18 | 1.19 (0.90–1.58) | 0.210 | 0.975 |
| rs7602 | A | 655 | 0.20 | 279 | 0.23 | 1.17 (0.91–1.50) | 0.222 | 0.981 |
| rs1887285 | G | 657 | 0.09 | 277 | 0.11 | 1.23 (0.88–1.72) | 0.233 | 0.984 |
| rs3828033 | A | 647 | 0.37 | 276 | 0.35 | 0.89 (0.72–1.10) | 0.293 | 0.996 |
| rs913199 | A | 652 | 0.45 | 279 | 0.49 | 1.09 (0.89–1.33) | 0.420 | 1.000 |
| rs1892534 | A | 653 | 0.39 | 278 | 0.41 | 1.08 (0.87–1.34) | 0.474 | 1.000 |
| rs3790431 | G | 651 | 0.20 | 279 | 0.19 | 0.91 (0.71–1.18) | 0.482 | 1.000 |
| rs3806318 | G | 654 | 0.26 | 279 | 0.28 | 1.08 (0.86–1.36) | 0.519 | 1.000 |
| rs2148683 | G | 655 | 0.47 | 282 | 0.49 | 1.06 (0.87–1.30) | 0.537 | 1.000 |
| rs2148682 | G | 655 | 0.35 | 280 | 0.37 | 1.05 (0.85–1.30) | 0.649 | 1.000 |
| rs12753193 | G | 659 | 0.39 | 279 | 0.40 | 1.05 (0.85–1.30) | 0.663 | 1.000 |
| rs4420065 | A | 651 | 0.39 | 279 | 0.40 | 1.04 (0.84–1.29) | 0.691 | 1.000 |
| rs4655537 | A | 654 | 0.36 | 275 | 0.37 | 1.04 (0.84–1.29) | 0.736 | 1.000 |
| rs6700896 | A | 654 | 0.39 | 280 | 0.40 | 1.03 (0.83–1.27) | 0.776 | 1.000 |
| rs11585329 | A | 655 | 0.16 | 279 | 0.14 | 0.97 (0.72–1.29) | 0.817 | 1.000 |
| rs9436737 | G | 650 | 0.14 | 274 | 0.14 | 1.02 (0.76–1.36) | 0.900 | 1.000 |
| rs3790436 | C | 653 | 0.45 | 277 | 0.46 | 1.01 (0.82–1.25) | 0.920 | 1.000 |
| rs17127601 | G | 649 | 0.13 | 278 | 0.13 | 1.01 (0.75–1.37) | 0.926 | 1.000 |
ORs and 95% CI were estimated using conditional logistic regression, conditioning on matching factors including year of birth, prospective cohort (HPFS, NHS, PHS, WHI, WHS) which also conditions on gender, smoking status (never, past, current), fasting status (fasting, non fasting), and month/year of blood draw, and adjusted for race (White, Black, other), history of diabetes mellitus (yes, no), current multivitamin use (yes, no), plasma 25(OH)D (continuous), BMI (continuous), physical activity (continuous), plasma C-peptide (continuous) and plasma adiponectin (quartiles).
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, body mass index; CI, confidence ratio; HPFS, Health Professionals Follow-up Study; OR, odds ratio; MAF, minor allele frequency; NHS, Nurses’ Health Study; PHS, Physicians’ Health Study; SNP, single nucleotide polymorphism; WHI, Women’s Health Initiative; WHS, Women’s Health Study
DISCUSSION
As seen in prior studies (23), plasma leptin levels were higher in our female compared with male control subjects, with a median level approximately three times higher in women versus men. Interestingly, higher prediagnostic plasma leptin was associated with an increased risk of pancreatic cancer in men, with no increase in risk was observed in women. This positive association among men was independent of other known risk factors for pancreatic cancer, including characteristics and plasma markers associated with obesity and insulin resistance. Furthermore, the association was highly consistent across two cohorts with male participants (positive association in HPFS and PHS) and three cohorts with female participants (no association in NHS, WHI, and WHS). In contrast, single nucleotide variants at the leptin receptor (LEPR) gene were associated with pancreatic cancer risk only in women, but not in men. This association was consistent across the three female cohorts. Notably, the most statistically significant SNP (rs10493380) and highly correlated variants at LEPR may alter leptin receptor gene expression based on bioinformatic analyses. In aggregate, these data support the importance of adipokines and adipokine signaling in pancreatic cancer risk in men and women, even though baseline circulating leptin levels differ greatly by gender.
A previous nested case-control study evaluated the association of prediagnostic plasma leptin with risk of pancreatic cancer (30). In this pooled analysis of three cohorts, plasma leptin was not associated with risk of pancreatic cancer during the first 10 years of follow-up, using gender-specific quintiles of plasma leptin. However, a statistically significant positive association was observed among men (OR, 2.94; 95% CI, 1.64–6.46; Ptrend=0.001; comparing extreme quintiles) with longer follow-up time (≥10 years), while the association among women for this duration could not be evaluated due to small sample size. Similarly, in our cohorts, a positive association of plasma leptin with pancreatic cancer risk was observed in men. However, in stratified analyses by time between blood collection and cancer diagnosis, statistically significant effect modification was not seen. This difference may be partly due to our a priori exclusion of cases with blood collected within 12 months of cancer diagnosis, the time period during which cancer-associated weight loss most commonly occurs (31). The stratified analyses in men were also limited by smaller sample sizes within strata. Three previous cross-sectional studies observed that plasma leptin levels were lower in pancreatic cancer patients compared to controls (32–34). However, in these retrospective studies, hypoleptinemia may have been due to the weight loss that is commonly experienced by patients with pancreatic cancer (5). Therefore, it is difficult to determine whether the observed low leptin levels contributed to pancreatic carcinogenesis or were a consequence of the cancer.
Several lines of evidence support a biological link between leptin and pancreatic carcinogenesis. Leptin plays a central role in the regulation of insulin sensitivity (35), and studies have demonstrated associations between hyperglycemia, insulin resistance, and future risk of pancreatic cancer (17,36–38). Therefore, one mechanism by which leptin may influence pancreatic cancer risk is through its modulation of insulin sensitivity (39). Leptin is synthesized by adipose tissue, and its concentration correlates with total body fat. Leptin may therefore act as a better marker for the relevant states of adiposity than BMI, which does not discriminate between fat and muscle mass (40). Target tissue effects of circulating leptin are not solely mediated centrally on cells within the hypothalamus, as leptin receptors are widely distributed in the body (7). Leptin receptors have been identified on the surface of tumor cells, including pluripotent cells thought to function as tumor initiating cells (41). Therefore, leptin signaling may have an important direct role in promoting tumor initiation and growth, independent of its role in insulin sensitivity (42). As a consequence of this potential direct effect, inhibitors of the leptin receptor are being explored as novel therapeutics to inhibit tumor growth in patients (43).
We observed a positive association between plasma leptin and pancreatic cancer risk only in men. Interestingly, leptin levels are considerably lower in men than in women, even for the same age and body-mass index (44). Although the underlying reasons for this difference in circulating leptin are unclear, sex-differences in reproductive hormones and body fat distribution have been proposed as possible etiologies. Particularly, women tend to have higher total and subcutaneous fat, while men have a greater percentage of visceral fat, which may influence circulating levels of leptin (40). Given the substantially lower leptin levels and differing metabolic environment in men, the actions of leptin may be sex-specific, with implications for disease development. Alternatively, physiologic differences related to circulating leptin may be detectable only when compared in the lower ranges of circulating leptin, which are seen predominantly in men. Notably, a number of prospective cohort studies have examined the association of prediagnostic plasma leptin and the subsequent diabetes risk. Similar to the current study of pancreatic cancer, most of these studies demonstrated positive associations of leptin and diabetes in men, but not in women (45). Of note, crosstalk between estrogen and leptin signaling has been shown previously (46). Other than modulating synthesis of leptin (47), and leptin receptor (48), estrogen receptor alpha enhances leptin-induced activation of downstream signaling pathways, including the JAK/STAT pathway (46). Alternatively, the different association between leptin levels and pancreatic cancer risk between genders in our study could be due to chance, and these findings should be confirmed in future studies.
The current study has several strengths. The prospective design and exclusion of cases diagnosed within 1 year of blood collection reduced the potential impact of reverse causation on our results. Furthermore, similar associations were observed when we excluded cases diagnosed within 2 or 4 years of blood collection. Leptin was measured in a single laboratory as a single batch, with low coefficients of variance for quality control samples. In our analyses, we adjusted for BMI, physical activity, and other biomarkers related to insulin resistance, including C-peptide and adiponectin, to rigorously control for confounding. We evaluated not only circulating leptin levels, but also genetic variants in the leptin receptor, which may affect signal transduction after ligand binding. Additional strengths included a large sample size, long follow-up period, and inclusion of men and women.
Plasma leptin was measured at only one point in time, so leptin levels may not fully reflect long-term plasma concentrations. However, leptin levels are relatively stable over time in healthy subjects; repeated plasma leptin measurements 1-year apart demonstrated a high intra-class correlation coefficient of 0.74 (21). We cannot rule out that residual confounding by adiposity not captured by BMI may be present; however, adjustment for plasma adiponectin, 25(OH)D and C-peptide as markers of adiposity and insulin resistance did not materially alter our results. We identified an association of rs10493380 with pancreatic cancer risk in women. This SNP was not identified as genome-wide significant in two recent pancreatic cancer genome-wide association studies (49,50). However, these studies were required to meet a stringent multiple-hypothesis testing threshold for statistical significance due to testing of >500,000 SNPs, included approximately 60% men, and included a majority of patients from tertiary center cases-control studies. Finally, our study population consisted primarily of White participants and further studies are required to evaluate circulating leptin in participants of different race/ethnicity.
In conclusion, we identified a positive association between prediagnostic circulating leptin levels and pancreatic cancer risk in men, independent of obesity and other risk factors of pancreatic cancer. Although this association was not observed in women, single nucleotide variants in the leptin receptor were associated with pancreatic cancer risk in women, and bioinformatic analyses suggested differences in leptin receptor expression with these variants. Our data provide additional evidence for a biological link between obesity, insulin resistance, and pancreatic cancer risk, specifically focusing attention on adipokines and adipokine signaling in pancreatic cancer development.
Supplementary Material
Acknowledgments
The authors would like to thank the participants and staff of the Health Professionals Follow-up Study, Nurses’ Health Study, Physicians’ Health Study, Women’s Health Initiative and Women’s Health Study, for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Grant Support
HPFS is supported by NIH grant UM1 CA167552. NHS is supported by NIH grants UM1 CA186107, P01 CA87969, and R01 CA49449. PHS is supported by NIH grants CA 97193, CA 34944, CA 40360, HL 26490, and HL 34595. The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The WHS is supported by grants CA047988, HL043851, HL080467, HL099355, and UM1 CA182913 from the National Institutes of Health (Bethesda, MD).
Additional support from NIH KL2 TR001100, NIH U54 CA155626 and NIH to P30 DK046200 to Y. Bao; NIH K07 CA148894 to K. Ng; from NCI R35 CA197735 to S. Ogino; from the Robert T. and Judith B. Hale Fund for Pancreatic Cancer, Perry S. Levy Fund for Gastrointestinal Cancer Research, Pappas Family Research Fund for Pancreatic Cancer, NIH R01 CA124908, and NIH P50 CA127003 to C.S. Fuchs; and from NIH/NCI U01 CA210171, Department of Defense CA130288, Lustgarten Foundation, Pancreatic Cancer Action Network, Noble Effort Fund, Peter R. Leavitt Family Fund, Wexler Family Fund, and Promises for Purple to B.M. Wolpin.
The study sponsors had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301(24):2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michaud DS, Giovannucci E, Willett WC, Colditz GA, Stampfer MJ, Fuchs CS. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA. 2001;286(8):921–929. doi: 10.1001/jama.286.8.921. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 5.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 6.Somasundar P, McFadden DW, Hileman SM, Vona-Davis L. Leptin is a growth factor in cancer. J Surg Res. 2004;116(2):337–349. doi: 10.1016/j.jss.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Baskin DG, Blevins JE, Schwartz MW. How the brain regulates food intake and body weight: the role of leptin. J Pediatr Endocrinol Metab. 2001;14(Suppl 6):1417–1429. [PubMed] [Google Scholar]
- 8.Morioka T, Asilmaz E, Hu J, Dishinger JF, Kurpad AJ, Elias CF, et al. Disruption of leptin receptor expression in the pancreas directly affects beta cell growth and function in mice. J Clin Invest. 2007;117(10):2860–2868. doi: 10.1172/JCI30910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garofalo C, Surmacz E. Leptin and cancer. J Cell Physiol. 2006;207(1):12–22. doi: 10.1002/jcp.20472. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med. 1995;122(5):327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 11.Colditz GA, Hankinson SE. The Nurses' Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5(5):388–396. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women's Health Initiative study design. Ann Epidemiol. 2003;13(9 Suppl):S5–S17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 13.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, et al. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294(1):47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 14.Gann PH, Ma J, Giovannucci E, Willett W, Sacks FM, Hennekens CH, et al. Lower prostate cancer risk in men with elevated plasma lycopene levels: results of a prospective analysis. Cancer Res. 1999;59(6):1225–1230. [PubMed] [Google Scholar]
- 15.Hankinson SE, Willett WC, Manson JE, Hunter DJ, Colditz GA, Stampfer MJ, et al. Alcohol, height, and adiposity in relation to estrogen and prolactin levels in postmenopausal women. J Natl Cancer Inst. 1995;87(17):1297–1302. doi: 10.1093/jnci/87.17.1297. [DOI] [PubMed] [Google Scholar]
- 16.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 17.Bao Y, Giovannucci EL, Kraft P, Stampfer MJ, Ogino S, Ma J, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2013;105(2):95–103. doi: 10.1093/jnci/djs474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzi C, Albrecht E, Hysi PG, Lagou V, Waldenberger M, Tonjes A, et al. Genome-wide association study identifies two novel regions at 11p15.5-p13 and 1p31 with major impact on acute-phase serum amyloid A. PLoS Genet. 2010;6(11):e1001213. doi: 10.1371/journal.pgen.1001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Q, Cornelis MC, Kraft P, Qi L, van Dam RM, Girman CJ, et al. Genome-wide association study identifies polymorphisms in LEPR as determinants of plasma soluble leptin receptor levels. Hum Mol Genet. 2010;19(9):1846–1855. doi: 10.1093/hmg/ddq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghan A, Dupuis J, Barbalic M, Bis JC, Eiriksdottir G, Lu C, et al. Meta-analysis of genome-wide association studies in >80 000 subjects identifies multiple loci for C-reactive protein levels. Circulation. 2011;123(7):731–738. doi: 10.1161/CIRCULATIONAHA.110.948570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott P, Chambers JC, Zhang W, Clarke R, Hopewell JC, Peden JF, et al. Genetic Loci associated with C-reactive protein levels and risk of coronary heart disease. JAMA. 2009;302(1):37–48. doi: 10.1001/jama.2009.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu Y, McDade TW, Kuzawa CW, Borja J, Li Y, Adair LS, et al. Genome-wide association with C-reactive protein levels in CLHNS: evidence for the CRP and HNF1A loci and their interaction with exposure to a pathogenic environment. Inflammation. 2012;35(2):574–583. doi: 10.1007/s10753-011-9348-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havel PJ, Kasim-Karakas S, Dubuc GR, Mueller W, Phinney SD. Gender differences in plasma leptin concentrations. Nat Med. 1996;2(9):949–950. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8(5):551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 26.Conneely KN, Boehnke M. So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am J Hum Genet. 2007;81(6):1158–1168. doi: 10.1086/522036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun Q, van Dam RM, Meigs JB, Franco OH, Mantzoros CS, Hu FB. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes. 2010;59(3):611–618. doi: 10.2337/db09-1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li H, Stampfer MJ, Mucci L, Rifai N, Qiu W, Kurth T, et al. A 25-year prospective study of plasma adiponectin and leptin concentrations and prostate cancer risk and survival. Clin Chem. 2010;56(1):34–43. doi: 10.1373/clinchem.2009.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Westra HJ, Peters MJ, Esko T, Yaghootkar H, Schurmann C, Kettunen J, et al. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat Genet. 2013;45(10):1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stolzenberg-Solomon RZ, Newton CC, Silverman DT, Pollak M, Nogueira LM, Weinstein SJ, et al. Circulating Leptin and Risk of Pancreatic Cancer: A Pooled Analysis From 3 Cohorts. Am J Epidemiol. 2015;182(3):187–197. doi: 10.1093/aje/kwv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pannala R, Leibson CL, Rabe KG, Timmons LJ, Ransom J, de Andrade M, et al. Temporal association of changes in fasting blood glucose and body mass index with diagnosis of pancreatic cancer. Am J Gastroenterol. 2009;104(9):2318–2325. doi: 10.1038/ajg.2009.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezzilli R, Barassi A, Corsi MM, Morselli-Labate AM, Campana D, Casadei R, et al. Serum leptin, but not adiponectin and receptor for advanced glycation end products, is able to distinguish autoimmune pancreatitis from both chronic pancreatitis and pancreatic neoplasms. Scand J Gastroenterol. 2010;45(1):93–99. doi: 10.3109/00365520903358907. [DOI] [PubMed] [Google Scholar]
- 33.Dalamaga M, Migdalis I, Fargnoli JL, Papadavid E, Bloom E, Mitsiades N, et al. Pancreatic cancer expresses adiponectin receptors and is associated with hypoleptinemia and hyperadiponectinemia: a case-control study. Cancer Causes Control. 2009;20(5):625–633. doi: 10.1007/s10552-008-9273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown DR, Berkowitz DE, Breslow MJ. Weight loss is not associated with hyperleptinemia in humans with pancreatic cancer. J Clin Endocrinol Metab. 2001;86(1):162–166. doi: 10.1210/jcem.86.1.7104. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 36.Gapstur SM, Gann PH, Lowe W, Liu K, Colangelo L, Dyer A. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283(19):2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 37.Stolzenberg-Solomon RZ, Graubard BI, Chari S, Limburg P, Taylor PR, Virtamo J, et al. Insulin, glucose, insulin resistance, and pancreatic cancer in male smokers. JAMA. 2005;294(22):2872–2878. doi: 10.1001/jama.294.22.2872. [DOI] [PubMed] [Google Scholar]
- 38.Wolpin BM, Bao Y, Qian ZR, Wu C, Kraft P, Ogino S, et al. Hyperglycemia, insulin resistance, impaired pancreatic beta-cell function, and risk of pancreatic cancer. J Natl Cancer Inst. 2013;105(14):1027–1035. doi: 10.1093/jnci/djt123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology. 2007;132(6):2208–2225. doi: 10.1053/j.gastro.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum M, Nicolson M, Hirsch J, Heymsfield SB, Gallagher D, Chu F, et al. Effects of gender, body composition, and menopause on plasma concentrations of leptin. J Clin Endocrinol Metab. 1996;81(9):3424–3427. doi: 10.1210/jcem.81.9.8784109. [DOI] [PubMed] [Google Scholar]
- 41.Feldman DE, Chen C, Punj V, Tsukamoto H, Machida K. Pluripotency factor-mediated expression of the leptin receptor (OB-R) links obesity to oncogenesis through tumor-initiating stem cells. Proc Natl Acad Sci U S A. 2012;109(3):829–834. doi: 10.1073/pnas.1114438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khandekar MJ, Cohen P, Spiegelman BM. Molecular mechanisms of cancer development in obesity. Nat Rev Cancer. 2011;11(12):886–895. doi: 10.1038/nrc3174. [DOI] [PubMed] [Google Scholar]
- 43.Surmacz E. Leptin and adiponectin: emerging therapeutic targets in breast cancer. J Mammary Gland Biol Neoplasia. 2013;18(3–4):321–332. doi: 10.1007/s10911-013-9302-8. [DOI] [PubMed] [Google Scholar]
- 44.Saad MF, Damani S, Gingerich RL, Riad-Gabriel MG, Khan A, Boyadjian R, et al. Sexual dimorphism in plasma leptin concentration. J Clin Endocrinol Metab. 1997;82(2):579–584. doi: 10.1210/jcem.82.2.3739. [DOI] [PubMed] [Google Scholar]
- 45.Chen GC, Qin LQ, Ye JK. Leptin levels and risk of type 2 diabetes: gender-specific meta-analysis. Obes Rev. 2014;15(2):134–142. doi: 10.1111/obr.12088. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt S, Monk JM, Robinson LE, Mourtzakis M. The integrative role of leptin, oestrogen and the insulin family in obesity-associated breast cancer: potential effects of exercise. Obes Rev. 2015;16(6):473–487. doi: 10.1111/obr.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machinal F, Dieudonne MN, Leneveu MC, Pecquery R, Giudicelli Y. In vivo and in vitro ob gene expression and leptin secretion in rat adipocytes: evidence for a regional specific regulation by sex steroid hormones. Endocrinology. 1999;140(4):1567–1574. doi: 10.1210/endo.140.4.6617. [DOI] [PubMed] [Google Scholar]
- 48.Bennett PA, Lindell K, Wilson C, Carlsson LM, Carlsson B, Robinson IC. Cyclical variations in the abundance of leptin receptors, but not in circulating leptin, correlate with NPY expression during the oestrous cycle. Neuroendocrinology. 1999;69(6):417–423. doi: 10.1159/000054444. [DOI] [PubMed] [Google Scholar]
- 49.Childs EJ, Mocci E, Campa D, Bracci PM, Gallinger S, Goggins M, et al. Common variation at 2p13.3, 3q29, 7p13 and 17q25.1 associated with susceptibility to pancreatic cancer. Nat Genet. 2015;47(8):911–916. doi: 10.1038/ng.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wolpin BM, Rizzato C, Kraft P, Kooperberg C, Petersen GM, Wang Z, et al. Genome-wide association study identifies multiple susceptibility loci for pancreatic cancer. Nat Genet. 2014;46(9):994–1000. doi: 10.1038/ng.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.