Abstract
Stathmin1, a microtubule-destabilizing phosphoprotein, is considered to play a crucial role in regulating cellular microtubule dynamics and controlling mitosis. Previous studies have showed that STMN1 is highly expressed in many human malignancies and is related to development, invasion and metastasis of tumors. However, its expression pattern, clinical performance and functional roles in hypopharyngeal squamous cell carcinoma (HSCC) have not been addressed. In this study, we found that STMN1 was significantly elevated in HSCC and its expression level was correlated with poor differentiation (P<0.001), clinical stage (P<0.001), large tumor size (P=0.001) and lymph node metastasis (P=0.008). A positive correlation between STMN1 and Ki-67 expression was also exhibited. High STMN1 expression predicted poor survival. Furthermore, we found that knockdown of STMN1 by siRNAs inhibited the FaDu cell proliferation and migration. Moreover, decreased STMN1 expression in FaDu cells reversed the acquisition of EMT phenotype by upregulating E-cadherin, as well as reduced vimentin expression at protein and mRNA levels. These results suggested that STMN1 plays an important role in proliferation and migration of HSCC and may be used as a potential prognostic biomarker or therapeutic target of HSCC.
Keywords: hypopharyngeal squamous cell cancer, stathmin1, Ki-67, epithelial-mesenchymal transition
Introduction
Hypopharyngeal squamous cell carcinoma (HSCC), which accounts for 3–5% of all head and neck cancers, is the most aggressive head and neck cancer and has very poor prognosis (1). More than three-quarters of patients afflicted with this cancer are at an advanced stage at the time of diagnosis (1). In the course of the disease, because of the early high rates of cervical lymph nodes metastasis (2), the overall survival is relatively poor, with 5-year survival rates <40%, despite aggressive surgical and adjuvant treatment (3,4). Smoking cigarettes and drinking alcohol are the main risk factors for HSCC (5). However, the molecules involved in tumorigenesis of HSCC are not clearly revealed. Therefore, it is overwhelmingly urgent to discover new biomarkers to advance stratification and development of novel therapeutic targets for HSCC.
The phosphoprotein stathmin1 (STMN1), also known as oncoprotein 18 (OP18), is a 17-kDa cytoplasmic protein (6). Reports have shown that STMN1 could regulate microtubule dynamics by participating in microtubule catastrophe and/or in the sequestering of α/β-tubulin heterodimers, thereby impacting cell cycle progression, proliferation, motility and survival (7). Previous studies have also shown that STMN1 is overexpressed across many different human cancers, including some hematological malignancy, digestive system tumors, genitourinary neoplasms, lung cancer, mesothelioma and some head and neck cancers such as oral squamous-cell carcinoma, laryngeal squamous-cell carcinoma and primary nasopharyngeal carcinoma (8). Furthermore, abundant evidence has shown that STMN1 knockdown could significantly influence cell cycle progression, apoptosis, metastasis, and chemoresistance of tumor cells (8). Our previous study confirmed that STMN1 is significant highly expressed in non-melanoma skin cancers (NMSCs), and its expression is closely related to the proliferation, migration, invasion and apoptosis of cSCC cells (9). However, the effects of STMN1 on human HSCC remain unclear.
In this study, we investigated the expression pattern of STMN1 in HSCC tissues by immunohistochemistry and its correlation to clinicopathological characteristics. In addition, the application of STMN1, a prognostic factor, was assessed. Furthermore, we examined the biological function of STMN1 in HSCC FaDu cells. The data revealed that elevated STMN1 could promote HSCC proliferation and migration and enhance the EMT process, and it may be used as a prognostic biomarker and therapeutic target of HSCC.
Materials and methods
Tissue specimens
A total of 51 HSCC biopsy samples with complete clinical and pathological data were obtained from patients who underwent operations from 2009 to 2015 in the Affiliated Hospital of Nantong University. Sixteen non-cancer HSCC tissue samples were recruited as controls. Prior to surgery, none of the patients received adjuvant chemotherapy or radiotherapy. The pathological analysis was carried out according to the TNM system of the American Joint Committee on Cancer (AJCC 2010). All the detailed information of these 51 HSCC patients and clinicopathological parameters in this series are shown in Table I. Furthermore, 7 HSCC and corresponding 7 para-carcinoma tissues were collected to confirm the expression of STMN1 protein. This study was approved by the Ethics Committee of the Affiliated Hospital of Nantong University and all the participants gave their informed consent.
Table I.
The clinicopathological characteristics and IHC staining for STMN1 of the HSCC patients.
| N | STMN1 IHC score | P-value | |
|---|---|---|---|
| Age | |||
| <60 years | 21 | 7.24±3.11 | 0.430 |
| ≥60 years | 30 | 6.46±3.61 | |
| Gender | |||
| Male | 47 | 6.79±3.36 | 0.824 |
| Female | 4 | 6.33±4.93 | |
| Tobacco smoking | |||
| None or limited | 20 | 6.02±3.46 | 0.214 |
| Excessive | 31 | 7.25±3.35 | |
| Alcohol consumption | |||
| None or limited | 14 | 5.85±3.94 | 0.242 |
| Excessive | 37 | 7.11±3.18 | |
| Differentiation | |||
| High differentiation | 22 | 4.70±2.01 | <0.001** |
| Moderate/poor differentiation | 29 | 8.33±3.45 | |
| Tumor size (cm) | |||
| ≤2 | 24 | 5.22±2.62 | 0.001** |
| >2 | 27 | 8.14±3.48 | |
| Clinical stage | |||
| I–II | 19 | 4.59±2.23 | <0.001** |
| III–IV | 32 | 8.06±3.35 | |
| Lymph node metastasis | |||
| Negative | 25 | 5.50±3.13 | 0.008** |
| Positive | 26 | 7.98±3.27 | |
| Treatment | |||
| Surgery only | 20 | 4.98±2.89 | 0.010* |
| Surgery and radiation | 17 | 7.85±2.58 | |
| Surgery, radiation, chemotherapy | 11 | 7.40±4.16 | |
| Other treatment | 3 | 10.13±3.23 | |
STMN1 IHC score shown as mean ± SD.
Student's t-test for two groups or one-way ANOVA for more than two groups.
The number of fields by microscopy.
Statistically significant
P<0.05,
P<0.01.
Immunohistochemical staining
Immunohistochemical staining was performed on 4-µm paraffin tissue sections mounted on slides and dried at 60°C for 8 h. The slides were deparaffinized in xylene and dehydrated conventionally, then pressure-cooked in sodium citrate buffer (Sangon Biotech, Shanghai, China) (pH 6.0) to facilitate antigen retrieval. After natural cooling, endogenous peroxidase was blocked with 3% hydrogen peroxide (ZSGB-BIO, Beijing, China). Non-specific bindings were blocked by treating slides with normal goat serum (ZSGB-BIO) for 40 min, the sections were subsequently incubated with rabbit anti-STMN1 polyclonal antibody (Abcam, Cambridge, MA, USA, dilution 1:250) or rabbit anti-Ki-67 polyclonal antibody (dilution 1:100, sc-15402; Santa Cruz Biotechnology, Inc. Santa Cruz, CA, USA), overnight at 4°C. After being washed with PBS, the sections were incubated for 30 min with the 2-step plus poly-HRP anti-mouse/rabbit IgG detection system (ZSGB-BIO). DAB detection kit (enhanced polymer) (ZSGB-BIO) was used for 3 min to show immunolabeling, resulting in a brown precipitate. Following the above, sections were counterstained with hematoxylin. Positive and negative controls were performed in parallel.
The immunostaining results were blindly evaluated by two experienced pathologists without knowledge of the clinicopathologic outcomes of these patients. Immunostaining for STMN1 was localized in the cytoplasm and, partly, in the nucleus, and immunostaining for Ki-67 was limited to the nucleus. The proportion of epithelial cells positively-stained was divided into two groups: category A scores from 1 to 4 [(A = 0 (0–4%); 1 (5–25%); 2 (26–50%); 3 (51–75%); 4 (76–100%)]. Intensity of staining was category B, scores 0 (negative); 1 (weak); 2 (moderate); 3 (strong). For the IHC-score assessment, all fields were observed at ×400 magnification. A final score was calculated by multiplying A by B (minimum 0, maximum 12). Moreover, the average score of each case was obtained. Total scores were devided into 4 grades: negative, 0–3; weak, 3–6 (contain 3, 6); moderate, 6–9; strong, 9–12 (contain 9).
Cell culture and siRNA transfection
Human HSCC cell line, FaDu cells, was purchased from ATCC, Shanghai, China. FaDu cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum at 37°C in a humid atmosphere containing 5% CO2. To silence the expression of STMN1 (accession no. NM 203401 form NCBI GenBank), FaDu cells were transfected with four siRNAs which were designed and obtained from Biomics Biotechnologies Co., Ltd. (Nantong, China). The sequence alignment (BLAST) was carried out to ensure no homology with other genes in human. Furthermore, the negative control siRNA (si-NC) was also designed. The targeted sequences are shown in Table II.
Table II.
Sequences of siRNAs targeting STMN1.
| SiRNAs | Sequence (5′-3′) |
|---|---|
| STMN1-si1 | Sense: GCUUCAGAAGGCAAUAGAAdTdT |
| Antisense: UUCUAUUGCCUUCUGAAGCdTdT | |
| STMN1-si2 | Sense: GGAGGAAAUUCAGAAGAAAdTdT |
| Antisense: UUUCUUCUGAAUUUCCUCCdTdT | |
| STMN1-si3 | Sense: GCACGAGAAAGAAGUGCUUdTdT |
| Antisense: AAGCACUUCUUUCUCGUGCdTdT | |
| STMN1-si4 | Sense: GAACAACAACUUCAGUAAAdTdT |
| Antisense: UUUACUGAAGUUGUUGUUCdTdT | |
| Si-NC | Sense: UUCUCCGAACGUGUCACGUdTdT |
| Antisense: ACGUGACACGUUCGGAGAAdTdT |
Immunofluorescence
FaDu cells were cultured in 24-well chamber slides, washed three times with cold 1X PBS, fixed with 4% paraformaldehyde for 1 h at 4°C, washed three times in PBS, permeabilized with 0.1% Triton X-100 for 10 min (except for staining the cell membrane-bound E-cadherin), and blocked with Immunol Staining Blocking Buffer (Beyotime, China) for 1 h at room temperature. Cells were then incubated overnight at 4°C with anti-STMN1 (Abcam, 1:100 dilution), anti-E-cadherin (Ruiying Biological, Suzhou, China, 1:100 dilution) or anti-vimentin (Ruiying Biological, 1:100 dilution) primary antibody and subsequently with Alexa Fluor-conjugated secondary antibodies (1:1,000; Invitrogen Life Technologies, Carlsbad, CA, USA) and Hoechst (Sigma-Aldrich Co., St. Louis, MO, USA) for 2 h at room temperature. The immunofluorescence images were acquired with a fluorescence microscope.
Real-time quantity PCR (RT-qPCR)
The mRNA expression of STMN1, E-cadherin, vimentin in FaDu cells after STMN1-siRNA transfection were quantified by real-time RT-PCR. According to the manufacturer's instructions, total RNA was extracted using a TRIzol® reagent (Vazyme, USA) and 2 µg RNA was reverse-transcribed into cDNA samples by using a Transcriptor First Strand cDNA Synthesis kit (Roche, Germany). Then AceQ® qPCR SYBR Green Master Mix kit (Vazyme) was used for RT-qPCR. The primers used were obtained from Biomics Biotechnologies Co. Ltd. and are shown in Table III. GAPDH was used as the reference gene. The PCR conditions consisted of 10 min at 95°C 1 cycle, 15 sec at 95°C, 30 sec at 60°C and 30 sec at 72°C 40 cycles. The experiment was performed in triplicate. The 2−ΔΔCT method was implemented to analyze the results (10).
Table III.
The primers.
| The molecule | Sequence (5′-3′) |
|---|---|
| STMN1 | Forward: TGGAGAAGCGTGCCTCAG |
| Reverse: TTCATGGGACTTGCGTCTTTC | |
| E-cadherin | Forward: CCAGGAACCTCTGTGATGGA |
| Reverse: TTTTGTCAGGGAGCTCAGGA | |
| Vimentin | Forward: AAATGGCTCGTCACCTTCGT |
| Reverse: CAGCTTCCTGTAGGTGGCAA | |
| GAPDH | Forward: GAAGGTGAAGGTCGGAGTC |
| Reverse: GAAGATGGTGATGGGATTTC |
Protein extraction western blot analysis
The cells or tissue samples were lysed in lysis buffer (PMSF:RIPA = 1:100, Beyotime) and then centrifuged at 10,000 rpm for 10 min at 4°C. Protein concentrations were measured with BCA protein assay kit (Pierce, USA). The supernatant was diluted in 5X SDS-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer [50 mM Tris-HCl (pH 6.8), 10% (w/v) SDS, 0.5% (w/v) bromophenol blue, 50% (w/v) glycerol, 5% (w/v) β-mercaptoethanol] and boiled. Protein samples (20 µg) were separated by 10% (E-cadherin), 12% (vimentin, β-actin) and 15% (STMN1) polyacrylamide gels and electrophoresed, then electroblotted onto polyvinylidine difluoride filter (PVDF) membranes (Millipore, USA), followed by blocking with 5% skim milk in TBST (20 mM Tris, 150 mM NaCl, 0.05% Tween-20, pH 7.5) for 2 h at room temperature. The membranes were incubated with primary antibody overnight at 4°C (anti-STMN1: 1:5,000, Abcam), anti-E-cadherin: 1:300, anti-vimentin: 1:300 (Ruiying Biological), anti-β-actin: 1:2500 (Santa Cruz). After being washed three times with TBST, horseradish peroxidase (HRP)-conjugated secondary antibodies (goat anti-rabbit IgG-HRP with 1:1,000 dilution for STMN1, E-cadherin and vimentin; goat anti-mouse IgG-HRP with 1:2,000 dilution for β-actin) were used for 1.5 h at room temperature. After three additional washes with TBST for 45 min. The immunoreactive bands were detected with ECL plus kit (ZSbio, Beijing, China).
Cell viability studies by CCK-8 assays
FaDu cells (5×103 cells/well) were plated into a 96-well plate containing complete medium and incubated for 24 h before transfection. After treatment with siRNAs for 0, 12, 24, 48 and 72 h, 10 µl Cell Counting Kit-8 (CCK-8) kit solution (Beyotime) was added to each well containing original medium in a final volume of 100 µl and the plate was incubated for 1.5 h in an incubator at 37°C. Then the optical density (OD) was measured at 450 nm by using a Fluorescence Spectrophotometer (Hitachi, Japan). Three wells were used for each experimental condition and all the experiments were independently repeated three times.
Flow cytometric cell cycle analysis
For cell cycle analysis, FaDu cells were harvested at 48 h post-transfection, 6-cm plates. Cells were collected using cold PBS and fixed with 70% cold ethanol over 24 h in −20°C and treated with 1 ng/ml RNaseA for 20 min at 4°C. Cellular DNA was stained with 0.5 mg/ml propidium iodide (PI, 50 mg/ml; Becton-Dickinson, San Jose, CA, USA) for 20 min at 4°C in the dark. The cells were then sorted by FACSCalibur Flow Cytometer (Becton-Dickinson, San Jose, CA, USA) and CellQuest acquisition and analysis programs (Becton-Dickinson, San Diego, CA, USA). The experiments were replicated three times.
Transwell assay
A Transwell chamber was used (8 µm, 24-well format; BD Biosciences) to perform cell migration assays. For the migration assay, 5×104 cells in 500 µl serum-free media were added to the upper chamber after transfection for 48 h, while medium containing 10% FBS was added to the lower chambers to act as a chemoattractant. The upper surfaces of the chamber were coated with a growth factor-reduced Matrigel matrix. Cells were incubated at 37°C with 5% CO2 for 24 h, and the medium was removed from the upper chamber. The non-invaded cells on the upper side of the chamber were gently scraped off with a cotton swab, whereas the cells that had migrated through the membrane were stained with 4% paraformaldehyde and 0.1% crystal violet, imaged, and counted using an inverted microscope and analyzed by visualizing five random fields at a magnification of ×200. Each experiment was replicated three times.
Observation of morphological changes
Morphological changes of the FaDu cells were observed using an inverted microscope. The image was taken using a Leica microscope image system (Leica, Mannheim, Germany) at a magnification of ×200.
Statistical analysis
Statistical analyses were performed using SPSS statistical software package, version 22.0. The STMN1 IHC scores of each subgroup of clinicopathological parameters in Table I are shown as mean ± standard deviation (SD). Statistical significance was determined using t-test. Kaplan-Meier and log-rank tests were used for calculating survival curves. In addition, statistical analysis was performed using GraphPad Prism 5.0 statistical software, sigmaplot 10.0 and Adobe Photoshop CC. Statistical significance was assigned for P<0.05.
Results
Relationship between STMN1 expression and clinicopathologic parameters in HSCC specimens
We first sought to investigate the role of STMN1 in HSCC. Sixteen human normal cases and 51 HSCC cases were observed by us through immunohistochemistry staining. STMN1 protein was localized in the cytoplasm and, partly, in the nucleus in all tumor tissues, while negative in all of the normal tissues.
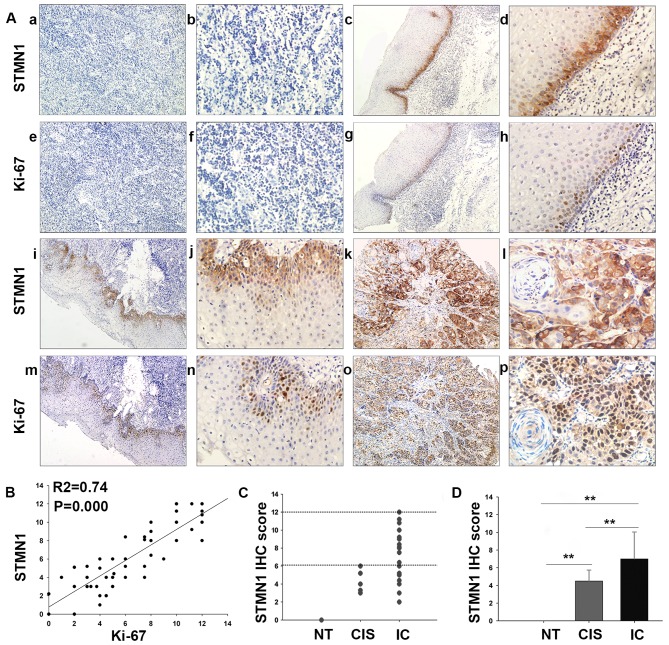
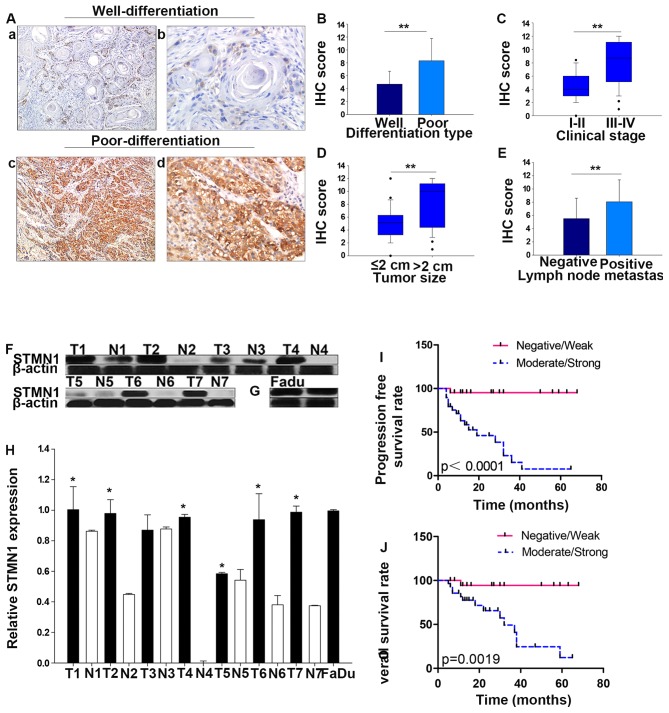
With respect to the staining intensity, normal tissue (NT), cancer in situ (CIS), and invasive cancer (IC) displayed negative, moderate and strong immunoreactivity for STMN1 protein, respectively (Fig. 1A). As showed in Fig. 1C and D, STMN1 IHC score was statistically increased in IC tissue (7.05±3.13) compared with that in the CIS (4.13±1.38, P=0.009) or normal tissue (0, P<0.001). Moreover, the correlations between the expression of STMN1 and clinicopathological characteristics of the patients with HSCC are summarized in Table I (Fig. 2). Interestingly, IHC staining showed that the intensity of STMN1 expression was much stronger in poorly differentiated than well-differentiated samples (P<0.001, Fig. 2A and B). In addition, overexpression of STMN1 was significantly correlated with advanced clinical stage (stage III and IV compared with stage I and II, P<0.001, Fig. 2C), large tumor size (size >2 cm compared with those ≤2 cm, P=0.001, Fig. 2D), lymph node metastasis (metastasis to the lymph nodes compared with non-metastasis, P=0.008, Fig. 2E) and treatment (P=0.01), but not correlated with age (P=0.430), gender (P=0.824), tobacco smoking (P=0.242), or alcohol consumption (P=0.242).
Figure 1.
Expression of STMN1 and Ki-67 in HSCC or normal tissues. (A) STMN1 versus Ki-67 was differentially expressed between HSCC and normal tissues as proved by immunohistochemical staining. (a and b) Negative STMN1; (c and d) weak STMN1; (e and f) negative Ki-67; (g and h) weak Ki-67; (i and j) moderate STMN1; (k and l) strong STMN1; (m and n) moderate Ki-67; (o and p) strong Ki-67 expression in normal tissue (NT), cancer in situ (CIS), early invasive tumor and advanced tumor, respectively (magnification, ×100 and ×400). (B) Scatterplot of Ki-67 versus STMN1 with regression line showed a correlation using the Spearman's correlation coefficient (R2=0.74, P=0.000). (C and D) Distribution of STMN1 IHC score in samples. *P<0.05, **P<0.01, statistically significant. Data were expressed as the mean ± SD.
Figure 2.
Correlation of STMN1 expression with clinicopathological parameter and prognosis of HSCC patients. (A) Representative images of STMN1 IHC staining in various differentiation types. (a and b) Well-differentiated, (c and d) Poor-differentiation (magnification, ×100 and ×400). (B) STMN1 IHC score was significantly higher in poor-differentiation type than that in well-differentiated type. Moreover, STMN1 IHC score was significantly correlated with clinical stages (C) tumor size (D) lymph node metastasis (E). (F) Relative STMN1 protein levels in HSCC tumor (T) and adjacent tissues (N) were explored by western blot analysis. (G) Relative STMN1 protein levels in FaDu cells. (H) Quantitative results of western blot analysis. (I and J) Kaplan-Meier survival curves showed that patients in the moderate/strong STMN1 expression group had significantly poorer prognosis than those in the negative/weak expression group (P=0.0019, P<0.0001). *P<0.05 **P<0.01, statistically significant. Data are expressed as the mean ± SD.
STMN1 is overexpressed in human HSCC tissues and FaDu cells
To further verify the results of immunohistochemistry staining, STMN1 expression at protein levels in 7 HSCC tumor tissues (T1–T7) and their adjacent tissues (N1–N7) were detected using western blot analysis. Results showed that, compared with their adjacent tissues, the expression of STMN1 in HSCC tumor was significantly higher (Fig. 2F and H). Besides, the basic expression and distribution of STMN1 in FaDu cells were examined at protein levels. As is showed in Fig. 2G, the results of western blot analysis suggested that STMN1 is highly expressed in FaDu cells. Simultaneously, strong immunoreactivity for STMN1 protein in cytoplasm in FaDu cells was found by immunocytochemistry (Fig. 3D).
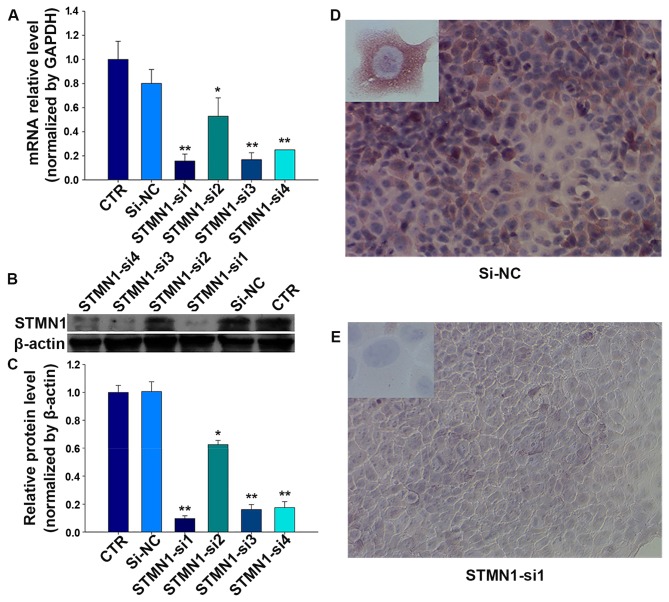
Figure 3.
The expression of STMN1 is suppressed by siRNAs. (A) The mRNA expression of STMN1 in HSCC cells was inhibited by treating with STMN1 siRNAs. (B) siRNAs downregulated the protein expression of STMN1 in HSCC cells. (C) Quantitative results of western blot analysis. (D and E) Strong immunoreactivity of STMN1 in FaDu cells as validated by immunocytochemistry; STMN1 in FaDu cells was weakly stained after treatment with STMN1-si1 (magnification, ×200 and ×400). *P<0.05, **P<0.01 compared with control (CTR) and si-NC.
STMN1 expression significantly associates with tumor proliferation and poor survival of HSCC patients
Furthermore, specimens with positive STMN1 staining showed significantly higher frequencies of Ki-67 positivity (Fig. 1A). Spearman correlation analysis indicates a positive correlation between STMN1 expression and Ki-67 based on proliferative activity (R2=0.74, P=0.000; Fig. 1B). Thus, these findings indicate that overexpression of STMN1 is likely to be involved in the progression of HSCC.
In addtion, in light of our results that displayed a multifaceted expression of STMN1 in HSCC patients with different malignancy grade, we investigated the prognostic significance of STMN1 in HSCC using Kaplan-Meier analysis. Indeed, increased expression of STMN1 was significantly associated with worse prognoses. The choice of treatment is usually associated with clinical stage and the presence of lymph node metastasis, thus the expression of STMN1 was different in patients under different therapy. STMN1 samples highly or moderately stained indicated shorter overall survival and progression-free survival rate than those with STMN1 weakly or negatively stained in the 51 HSCC specimens (P=0.0019, P<0.0001; Fig. 2I and J).
Establishment of siRNAs targeting STMN1
To investigate the effect of STMN1 on HSCC, four siRNAs were designed to knock down the STMN1 expression in FaDu cells. The mRNA and protein levels were determined in treated FaDu cells after 48 h. As shown in Fig. 3A, compared with the control (CTR) and si-NC, after treatments with FaDu cells for 72 h, STMN1 expression was inhibited by siRNAs at the mRNA level achieving 87% (STMN1-si1), and the protein level achieving 90% (STMN1-si1) (Fig. 3B and C). Furthermore, the results of immunofluorescence indicated that the staining intensity of STMN1 in FaDu cells significantly diminished after treatment with STMN1-si1 (Fig. 3D and E).
STMN1 knockdown inhibits cellular proliferation and promotes cell cycle arrest
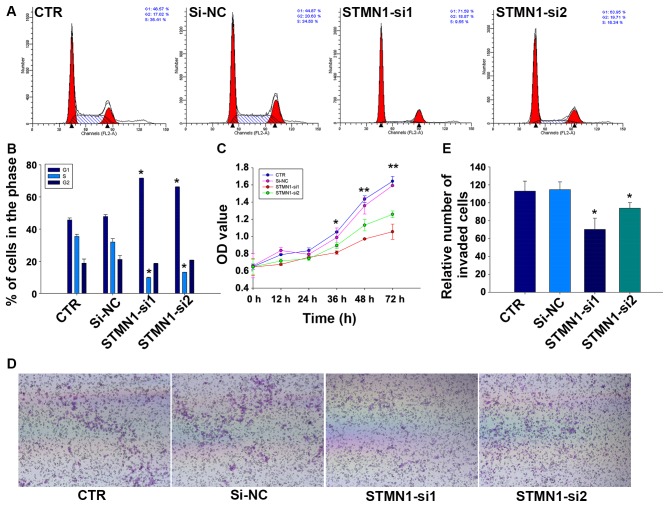
To assess the effect of knocking down STMN1 in proliferation of FaDu cells, CCK-8 and flow cytometry assays were implemented. Results of CCK-8 assay showed that the cell proliferation of FaDu could be greatly reduced by treating with STMN1 siRNAs in comparison with si-NC or control (CTR) (Fig. 4C). We further examined the effect of STMN1 siRNAs on cell cycle distribution of FaDu cells. Flow cytometry analysis revealed that the knockdown of STMN1 expression in FaDu increased the percentage of cells in G1 phase (45.72±1.2 and 47.88±1.13 vs. 71.67±0.12 and 66.18±0.20%, respectively; P<0.05) whereas decreased the percentage of cells in S phase (35.46±1.35 and 31.96±2.14 vs. 9.77±0.31 and 13.15±0.09%, respectively; P<0.05; Fig. 4A and B). These results indicated that reduction of STMN1 expression in FaDu cells could inhibit cellular proliferation and promote cell cycle arrest.
Figure 4.
Knockdown of STMN1 inhibits proliferation and migration of FaDu cells. (A) Cell cycle analysis by flow cytometry. (B) STMN1 knockdown induces a G1 phase arrest and reduced S phase in FaDu cells. (C) Cell proliferation was measured by CCK-8 assay with STMN1 knockdown. (D) Knockdown of STMN1 inhibited cell migration by Transwell assays. (E) The number of invading FaDu cells by cell migration assay was counted manually. The graph displays the mean ± SD of three independent experiments. *P<0.05 versus CTR or si-NC, statistically significant.
STMN1 knockdown reduces the migration activity and induces a change of EMT-related protein expressions in FaDu cells
The influence of STMN1 knockdown on the migration activity of FaDu cells was detected by Transwell assays. As is shown in Fig. 4E, the results indicated that there were fewer cells migrated to the bottom chambers in FaDu/STMN1-si1 (70.33±12.50). FaDu/STMN1-si2 (94.00±6.25) compared with FaDu/si-NC (114.67±8.74) and CTR (113.00±11.14). The representative micrographs are shown in Fig. 4D.
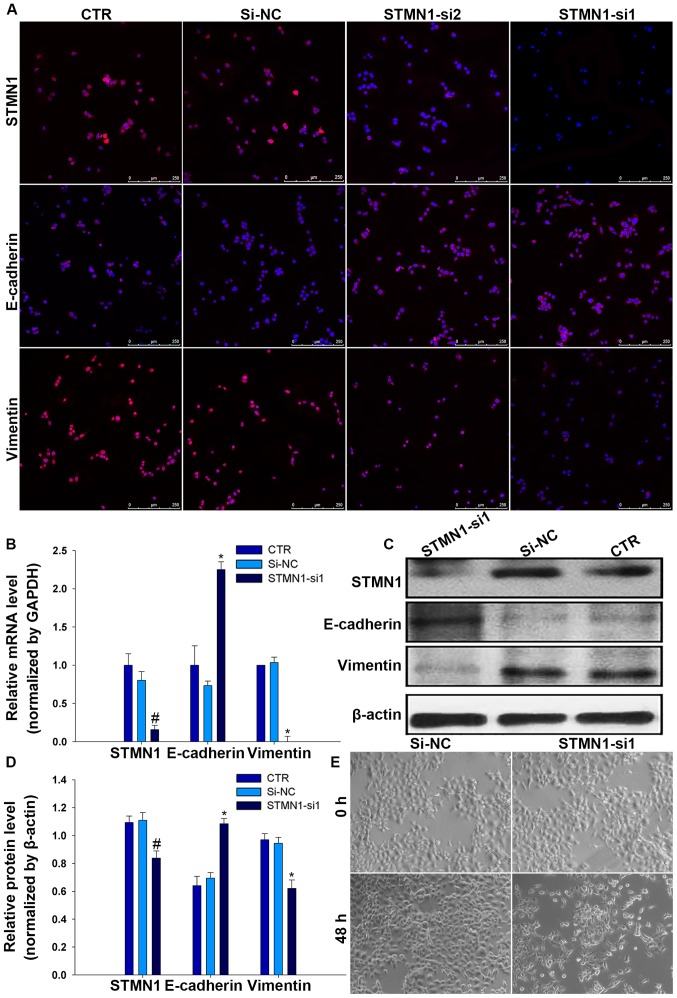
EMT is a crucial mechanism for migration of multiple tumor cells, with epithelial cells usually losing the capability of intercellular adhesion and acquiring a mesenchyme phenotype (11). To assess whether STMN1 promotes FaDu cell migration via stimulating EMT, EMT-associated proteins were detected by immunofluorescence in FaDu/STMN1-siRNA and FaDu/si-NC and CTR. Interestingly, results showed that the expression level of E-cadherin decreased while the expression level of vimentin increased by depleting STMN1 levels in FaDu cells (Fig. 5A), suggestive of a less epithelial phenotype and a more mesenchyme phenotype. These changes in mRNA and protein expression were confirmed by RT-qPCR and western blot analysis (Fig. 5B–D). During the EMT process, this is usually accompanied by a change in cell morphology (12). To explore whether knockdown of STMN1 has an impact on the morphology of FaDu cells, cell morphological changes were evaluated by an inverted microscope after treated with STMN1-si1 or si-NC. The results showed that cells treated with STMN1-si1 were changed from a more rounded, cell polarity to an elongated, with loss of cell-cell contacts, but there was no effect on cells treated with si-NC (Fig. 5E).
Figure 5.
FaDu cells with STMN1 knockdown underwent morphological change and STMN1 has effects on the process of EMT in FaDu cells. FaDu cells treated with STMN1 siRNAs or si-NC or and incubated for 48 h. (A) Immunofluorescence analysis of STMN1 and EMT markers (E-cadherin, vimentin) expression in FaDu cell lines (scale bar, 250 µm). (B) Relative mRNA expression level of STMN1 and EMT markers (E-cadherin and vimentin) was determined by RT-qPCR. (C and D) Relative protein expression level of STMN1, E-cadherin, vimentin was determined by western blot analysis, β-actin as a loading control. (E) Phase contrast microscopy suggested FaDu cells suffered morphologic changes under STMN1-si1 (magnification, ×100). Mean ± SD of three independent experiments. #*P<0.05, statistically significant.
Discussion
In this study, we found that STMN1 expression was linked to tumor grade, differentiation, size, stage and proliferation (Ki-67 index) of HSCC. Also, higher protein level of STMN1 was discovered in HSCC fresh tissues compared with non-cancer tissues. The results of Kaplan-Meier analysis suggested that STMN1 overexpression was closely related to progression-free survival (PFS) and overall survival (OS) of HSCC patients. Furthermore, studies in vitro indicated the vital role of STMN1 in FaDu cell proliferation and migration after STMN1 knockdown.
Previous studies showed that high-expressing and abnormal activation of STMN1 in multiple human cancers along with its overexpression usually indicated an unfavorable prognosis in patients with malignancies (8). It is suggested that STMN1 overexpression is independently predictive of DSS (disease-specific survival), DMeFS (distal metastasis-free survival) and LRFS (local recurrence-free survival) in nasopharyngeal carcinoma (13). The study of Trovik et al (14) also showed that STMN1 immunohistochemical staining identified with high grade, lymph node metastases and poor survival through studies on 1,076 endometrial cancer patients. In breast cancer, high STMN1 and phospho-STMN1 levels predicted poor survival of patients (15). Similar notions were verified in non-small cell lung cancer (16), colorectal cancer (17) and pheochromocytomas (18). Our data were in favour of this observation as well. We demonstrated that STMN1 was differentially expressed in normal, CIS, IC HSCC tissues. Elevated STMN1 was significantly associated with tumor differentiation, tumor size, clinical stage, lymph node metastasis and showed a positive correlation with Ki-67 index (19). Patients with moderate/strong STMN1 staining intensity always underwent poor prognosis. All the above implied that STMN1 may be used as a potential prognostic marker for survival in patients with HSCC.
Microtubule is an essential part of the cytoskeleton, which is required for a wide variety of fundamental cellular functions, including the maintenance of cell differentiation, cell morphology, motility, and polarity (20). With proven destabilizing activity (7), the abnormal activation or increased expression of STMN1 has enhanced the proliferation, cell cycle progression, migration and invasion in human cancer cell lines, including gastric (21), ovarian (22), and hepatoma cell lines (23). In hepatoma HCCLM3 cells, studies found that STMN1 siRNA could obviously inhibit cell proliferation and migration (24). In human endometrial carcinoma (EC) cell line Ishikawa, STMN1 was further identified showing a positive effect on cell viability and migration (25). In agreement with previous reports, our studies in vitro suggested that reduced expression of STMN1 in FaDu cells obviously induced a cell cycle arrest in G1 phase, accompanied by inhibited cell viability. Our investigations also suggested that overexpressed STMN1 could accelerate migration in FaDu cells. Interestingly, high level of STMN1 was noted especially in the tumor fronts and invading tumor islets of HSCC tissue sections. These results may account for this higher expression level of STMN1 in large tumor size, lymph node metastasis and poor prognosis of HSCC patients, simultaneously, informing us that STMN1 may participate in the progression of HSCC.
Metastasis of tumor is a multistep and complex process, yet it remains the most poorly understood component of cancer pathogenesis (26). A handful of studies suggest that EMT facilitates cancer epithelial cells to enter into a mesenchymal-like state, which endows them with migratory and invasive properties (27). EMT process is accompanied by a loss of epithelial marker proteins and dissolution of adherent junction proteins, such as E-cadherin, cytokeratin, γ-catenin and β-catenin which play a significant role in cell-cell adhesion. Concomitantly, mesenchymal marker proteins, such as vimentin, N-cadherin, fibronectin and P-cadherin which contribute to cell migration, are frequently overexpressed (12,28). E-cadherin as the best characterized cadherins, in particular, has a key role in epithelial cell-cell adhesion (29). Decreased E-cadherin expression usually induces cell migration, morphological changes and cancer development (30), while upregulated vimentin are in cancer cells resulting in epithelial cells to acquire a mesenchymal shape and increased motility (31). Indeed, EMT has been reported as a pivotal program in numerous human solid cancers, including pancreas cancer (32), prostate cancer (33) and breast cancer (34), ultimately resulting in tumor metastasis. Lu et al (35) performed a series of evidence to elaborate the role of stathmin1 and microtubule dynamics in promoting EMT. In addition, via the inhibition of stathmin1, Li et al (36) found Siva1 could enhance the formation of microtubules and impedes focal adhesion assembly, cell migration, and EMT. Our present findings are consistent with these previous results. We demonstrated that reducing the expression of STMN1 would result in activation of epithelial marker E-cadherin along with blocked mesenchyme markers vimentin expression in HSCC. These investigations suggested that overexpressed STMN1 may enhance metastasis and aggressiveness in HSCC, at least, in part, via facilitating the EMT process.
In conclusion, our results indicate that STMN1 possesses higher degree of malignancy and shorter PFS and OS of HSCC, suggesting a potential diagnostic biomarker for HSCC. Furthermore, the results from the present work emphasize the vital role of STMN1 in promoting proliferation, enhancing migration and encouraging EMT in FaDu cells. Thus, our data revealed that STMN1 may contribute to the aggressive phenotype of human HSCC, and, perhaps, act as a promising molecular target for controlling cancer progression.
References
- 1.Hall SF, Groome PA, Irish J, O'Sullivan B. The natural history of patients with squamous cell carcinoma of the hypopharynx. Laryngoscope. 2008;118:1362–1371. doi: 10.1097/MLG.0b013e318173dc4a. [DOI] [PubMed] [Google Scholar]
- 2.Chung EJ, Lee SH, Baek SH, Park IS, Cho SJ, Rho YS. Pattern of cervical lymph node metastasis in medial wall pyriform sinus carcinoma. Laryngoscope. 2014;124:882–887. doi: 10.1002/lary.24299. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Ho HC, Hsiao SH, Hwang JH, Lee CC, Hung SK. Treatment results and prognostic factors in locally advanced hypopharyngeal cancer. Acta Otolaryngol. 2008;128:103–109. doi: 10.1080/00016480701387116. [DOI] [PubMed] [Google Scholar]
- 4.Chan JY, Wei WI. Current management strategy of hypopharyngeal carcinoma. Auris Nasus Larynx. 2013;40:2–6. doi: 10.1016/j.anl.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Zhou L. Standardization of the management of laryngeal and hypopharyngeal carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;44:705–706. In Chinese. [PubMed] [Google Scholar]
- 6.Curmi PA, Gavet O, Charbaut E, Ozon S, Lachkar-Colmerauer S, Manceau V, Siavoshian S, Maucuer A, Sobel A. Stathmin and its phosphoprotein family: General properties, biochemical and functional interaction with tubulin. Cell Struct Funct. 1999;24:345–357. doi: 10.1247/csf.24.345. [DOI] [PubMed] [Google Scholar]
- 7.Gupta KK, Li C, Duan A, Alberico EO, Kim OV, Alber MS, Goodson HV. Mechanism for the catastrophe-promoting activity of the microtubule destabilizer Op18/stathmin. Proc Natl Acad Sci USA. 2013;110:20449–20454. doi: 10.1073/pnas.1309958110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nemunaitis J. Stathmin 1: A protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2012;16:631–634. doi: 10.1517/14728222.2012.696101. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Wang L, Li T, You B, Shan Y, Shi S, Qian L, Cao X. STMN1 overexpression correlates with biological behavior in human cutaneous squamous cell carcinoma. Pathol Res Pract. 2015;211:816–823. doi: 10.1016/j.prp.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 10.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 11.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: The importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalluri R. EMT: When epithelial cells decide to become mesenchymal-like cells. J Clin Invest. 2009;119:1417–1419. doi: 10.1172/JCI39675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu HP, Li CF, Lee SW, Wu WR, Chen TJ, Chang KY, Liang SS, Tsai CJ, Shiue YL. Overexpression of stathmin 1 confers an independent prognostic indicator in nasopharyngeal carcinoma. Tumour Biol. 2014;35:2619–2629. doi: 10.1007/s13277-013-1345-3. [DOI] [PubMed] [Google Scholar]
- 14.Trovik J, Wik E, Stefansson IM, Marcickiewicz J, Tingulstad S, Staff AC, Njolstad TS, Vandenput I, Amant F, Akslen LA, et al. MoMaTec Study Group: Stathmin overexpression identifies high-risk patients and lymph node metastasis in endometrial cancer. Clin Cancer Res. 2011;17:3368–3377. doi: 10.1158/1078-0432.CCR-10-2412. [DOI] [PubMed] [Google Scholar]
- 15.Kuang XY, Chen L, Zhang ZJ, Liu YR, Zheng YZ, Ling H, Qiao F, Li S, Hu X, Shao ZM. Stathmin and phospho-stathmin protein signature is associated with survival outcomes of breast cancer patients. Oncotarget. 2015;6:22227–22238. doi: 10.18632/oncotarget.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nie W, Xu MD, Gan L, Huang H, Xiu Q, Li B. Overexpression of stathmin 1 is a poor prognostic biomarker in non-small cell lung cancer. Lab Invest. 2015;95:56–64. doi: 10.1038/labinvest.2014.124. [DOI] [PubMed] [Google Scholar]
- 17.Tan HT, Wu W, Ng YZ, Zhang X, Yan B, Ong CW, Tan S, Salto-Tellez M, Hooi SC, Chung MC. Proteomic analysis of colorectal cancer metastasis: Stathmin-1 revealed as a player in cancer cell migration and prognostic marker. J Proteome Res. 2012;11:1433–1445. doi: 10.1021/pr2010956. [DOI] [PubMed] [Google Scholar]
- 18.Björklund P, Cupisti K, Fryknäs M, Isaksson A, Willenberg HS, Akerström G, Hellman P, Westin G. Stathmin as a marker for malignancy in pheochromocytomas. Exp Clin Endocrinol Diabetes. 2010;118:27–30. doi: 10.1055/s-0029-1202789. [DOI] [PubMed] [Google Scholar]
- 19.Ghanim B, Klikovits T, Hoda MA, Lang G, Szirtes I, Setinek U, Rozsas A, Renyi-Vamos F, Laszlo V, Grusch M, et al. Ki67 index is an independent prognostic factor in epithelioid but not in non-epithelioid malignant pleural mesothelioma: A multicenter study. Br J Cancer. 2015;112:783–792. doi: 10.1038/bjc.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belletti B, Nicoloso MS, Schiappacassi M, Berton S, Lovat F, Wolf K, Canzonieri V, D'Andrea S, Zucchetto A, Friedl P, et al. Stathmin activity influences sarcoma cell shape, motility, and metastatic potential. Mol Biol Cell. 2008;19:2003–2013. doi: 10.1091/mbc.E07-09-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhtar J, Wang Z, Zhang ZP, Bi MM. Lentiviral-mediated RNA interference targeting stathmin1 gene in human gastric cancer cells inhibits proliferation in vitro and tumor growth in vivo. J Transl Med. 2013;11:212. doi: 10.1186/1479-5876-11-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei SH, Lin F, Wang X, Gao P, Zhang HZ. Prognostic significance of stathmin expression in correlation with metastasis and clinicopathological characteristics in human ovarian carcinoma. Acta Histochem. 2008;110:59–65. doi: 10.1016/j.acthis.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Singer S, Ehemann V, Brauckhoff A, Keith M, Vreden S, Schirmacher P, Breuhahn K. Protumorigenic overexpression of stathmin/Op18 by gain-of-function mutation in p53 in human hepatocarcinogenesis. Hepatology. 2007;46:759–768. doi: 10.1002/hep.21736. [DOI] [PubMed] [Google Scholar]
- 24.Gan L, Guo K, Li Y, Kang X, Sun L, Shu H, Liu Y. Up-regulated expression of stathmin may be associated with hepatocarcinogenesis. Oncol Rep. 2010;23:1037–1043. doi: 10.3892/or_00000730. [DOI] [PubMed] [Google Scholar]
- 25.He X, Liao Y, Lu W, Xu G, Tong H, Ke J, Wan X. Elevated STMN1 promotes tumor growth and invasion in endometrial carcinoma. Tumour Biol. 2016;37:9951–9958. doi: 10.1007/s13277-016-4869-5. [DOI] [PubMed] [Google Scholar]
- 26.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–15642. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Deng F, Qin Y, Zhao Z, Wu Z, Xing Z, Ji A, Wang QJ. Long non-coding RNA regulation of epithelial-mesenchymal transition in cancer metastasis. Cell Death Dis. 2016;7:e2254. doi: 10.1038/cddis.2016.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Przybyla L, Muncie JM, Weaver VM. Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu Rev Cell Dev Biol. 2016;32:527–554. doi: 10.1146/annurev-cellbio-111315-125150. [DOI] [PubMed] [Google Scholar]
- 29.van Roy F, Berx G. The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci. 2008;65:3756–3788. doi: 10.1007/s00018-008-8281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan SH, Wang LH. Regulation of cancer metastasis by microRNAs. J Biomed Sci. 2015;22:9. doi: 10.1186/s12929-015-0113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park JS, Lee JH, Lee YS, Kim JK, Dong SM, Yoon DS. Emerging role of LOXL2 in the promotion of pancreas cancer metastasis. Oncotarget. 2016 Jun 7; doi: 10.18632/oncotarget.9918. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bloom JE, McNeel DG. SSX2 regulates focal adhesion but does not drive the epithelial to mesenchymal transition in prostate cancer. Oncotarget. 2016 Jun 2; doi: 10.18632/oncotarget.9802. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karaczyn AA, Adams TL, Cheng RY, Matluk NN, Verdi JM. Human NUMB6 induces epithelial-mesenchymal transition and enhances breast cancer cells migration and invasion. J Cell Biochem. 2016 Jun 15; doi: 10.1002/jcb.25628. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Liu C, Xu YF, Cheng H, Shi S, Wu CT, Yu XJ. Stathmin destabilizing microtubule dynamics promotes malignant potential in cancer cells by epithelial-mesenchymal transition. Hepatobiliary Pancreat Dis Int. 2014;13:386–394. doi: 10.1016/S1499-3872(14)60038-2. [DOI] [PubMed] [Google Scholar]
- 36.Li N, Jiang P, Du W, Wu Z, Li C, Qiao M, Yang X, Wu M. Siva1 suppresses epithelial-mesenchymal transition and metastasis of tumor cells by inhibiting stathmin and stabilizing microtubules. Proc Natl Acad Sci USA. 2011;108:12851–12856. doi: 10.1073/pnas.1017372108. [DOI] [PMC free article] [PubMed] [Google Scholar]