Abstract
Background and objective
Large clinical trials have confirmed the long-term efficacy of inhaled corticosteroid/long-acting β2-agonist combinations in patients with chronic obstructive pulmonary disease (COPD). It was hypothesized that significant treatment effects would already be present within 3 months after the initiation of treatment across a range of clinical outcomes, irrespective of COPD severity.
Methods
Post hoc analysis of 3-month post-randomization outcomes, including exacerbation rates, dropouts, symptoms, reliever use, and lung function, from three studies with similar inclusion criteria of moderate-to-very-severe COPD. Patients (n=1,571) were treated with budesonide/formoterol (B/F) 320/9 μg or placebo, twice daily; in one study, tiotropium 18 μg once daily was also given.
Results
Over the first 3 months of treatment, fewer patients randomized to B/F experienced exacerbations versus the placebo group (111 and 196 patients with ≥1 exacerbation, respectively). This was true in each COPD severity group. Compared with placebo, B/F treatment led to significantly lower 3-month exacerbation rates in the moderate and severe COPD severity groups (46% and 57% reduction, respectively), with a nonsignificant reduction (29%) in very severe COPD. Fewer dropouts occurred among patients treated with B/F versus placebo, this effect being greater with increasing COPD severity. B/F was associated with improved forced expiratory volume in 1 s, morning peak expiratory flow rate, total reliever use, and total symptom score versus placebo.
Conclusion
Treatment with B/F decreased exacerbations in patients with moderate-to-very-severe COPD within 3 months of commencing treatment. This effect was paralleled by improved lung function, less reliever medication use, and fewer symptoms, irrespective of disease severity.
Keywords: bronchodilator agents, clinical respiratory medicine, clinical trials, COPD
Introduction
Chronic obstructive pulmonary disease (COPD) remains a major cause of ill health worldwide and is the third most common cause of death.1,2 In the last decade, a series of large clinical trials have provided the evidence base for current drug treatment of COPD,3–5 and these findings have been incorporated into treatment guidelines.6 These studies resolved many questions about the effect of drugs on individual outcomes. However, there are limited data that indicate how quickly improvement becomes evident following the initiation of treatment, and that describes the factors that influence early responses to treatment.
In many COPD trials lasting ≥1 year, the first clinical visit occurred 3 months after randomization, but results are reported for the whole study duration.3,7 Analysis of diary-card data from the initial long-acting β2-agonist (LABA) and inhaled corticosteroid (ICS) trials in COPD established that treatment improves peak expiratory flow (PEF) rate and symptoms within 2 weeks of initiation, a change that was more rapid when the LABA and ICS were given together.8,9 However, the early impact of treatment on exacerbations and disease control, that is, within 3 months of randomization, was not formally evaluated in these trials. Additionally, in many studies, the impact of disease severity on response to treatment was not considered, for instance, in the 3-month CLIMB study in which patients received budesonide/formoterol in addition to tiotropium.10 The effect of disease severity was assessed in patients receiving salmeterol and fluticasone propionate, alone or in combination, in the TORCH study, where worse disease severity as assessed by spirometry was associated with a greater likelihood of having an exacerbation but was not related to the overall treatment effects.11
It was hypothesized that significant treatment effects would be present across a range of clinical outcomes within 3 months of budesonide/formoterol (B/F) initiation and that these differences would be independent of the severity of spirometric impairment. To test these hypotheses, data were pooled from three previously reported studies confirming the efficacy of the ICS/LABA B/F in patients with COPD.9,10,12
Methods
Study design
A post hoc analysis of 3-month data from three studies of patients with moderate-to-very-severe COPD is reported; complete details of these studies are reported elsewhere.9,10,12 The studies recruited outpatients aged ≥40 years with ≥10 pack-years smoking history, COPD symptoms for ≥2 years, forced expiratory volume in 1 s (FEV1)/vital capacity (VC) ratio ≤70%, pre-bronchodilator FEV1 ≤50% predicted, and history of ≥1 COPD exacerbation within 1–12 months of inclusion. All participants provided written informed consent. Ethical approval was obtained for each of the studies included in this analysis9,10,12 from local Independent Ethics Committees in each of the participating countries.
This analysis included patients treated with B/F (Symbicort® Turbuhaler®; AstraZeneca, Sweden) 320/9 μg twice daily (two inhalations of 160/4.5 μg9,12 or one inhalation of 320/9 μg10) or placebo twice daily. In the CLIMB study,10 study medications were given in addition to tiotropium 18 μg once daily. As-needed terbutaline 0.5 mg/inhalation (Bricanyl® Turbuhaler®; AstraZeneca) was permitted as reliever medication throughout all the studies.
Spirometric severity of COPD was classified as moderate (<80% to ≥50% predicted), severe (<50% to ≥30% predicted), or very severe (<30% predicted), according to GOLD 2016 post-bronchodilator FEV1 grading criteria.6
Study variables
In the studies included in this post hoc analysis, exacerbations were defined as worsening of COPD leading to oral corticosteroids, alone or in combination with antibiotics, and/or hospitalization/emergency room visits. Exacerbations leading to antibiotics alone from two studies9,12 were excluded from the analysis in order to achieve a common definition of exacerbations. The number of patients who dropped out from the studies (for any reason, including exacerbations) was also recorded. Lung function was recorded as pre-dose FEV1 (L) at study visits (at weeks 1, 6, and 1210 or at months 1, 2, and 39,12), and daily morning PEF (L/min) was collected from electronic diaries10 or diary cards9,12 of the patients. Reliever medication use (inhalations/day) and symptoms of dyspnea, cough, chest tightness, and night time awakenings (assessed on a 5-point scale; 0–4, where 4= severe symptoms) were also recorded in patient diaries and collected at clinic visits.
Statistical methods
Patient demographics and baseline characteristics are presented descriptively. All variables were analyzed by disease severity and treatment. The 3-month rates of exacerbations were assessed using Poisson regression analysis, with study and treatment as factors, and the number of days in the study as an offset variable. Over-dispersion was assessed in each model by calculating the ratio of the model deviance to its number of degrees of freedom. These analyses utilized only actual recorded data and did not impute for missing data. The rate (and rate ratios [RR]) of exacerbations within 3 months and corresponding 95% confidence intervals (CIs) were presented. Percentage reductions in exacerbation rates with B/F versus placebo were also presented, which were derived from the rate ratio. The number of patients with at least one exacerbation and dropouts were presented descriptively. Subsequent analyses included change from baseline in FEV1 (L), morning PEF (L/min), reliever use (inhalations/day), and total symptom scores and were analyzed using analysis of covariance to estimate the treatment differences between B/F and placebo by COPD severity group. Least squared means and differences in least squared means with corresponding 95% CIs were presented.
Sensitivity analyses
In addition to the 3-month analyses, the same statistical methods were applied at 12 months using data from the two 12-month studies.9,12
Results
Patient demographics and clinical characteristics
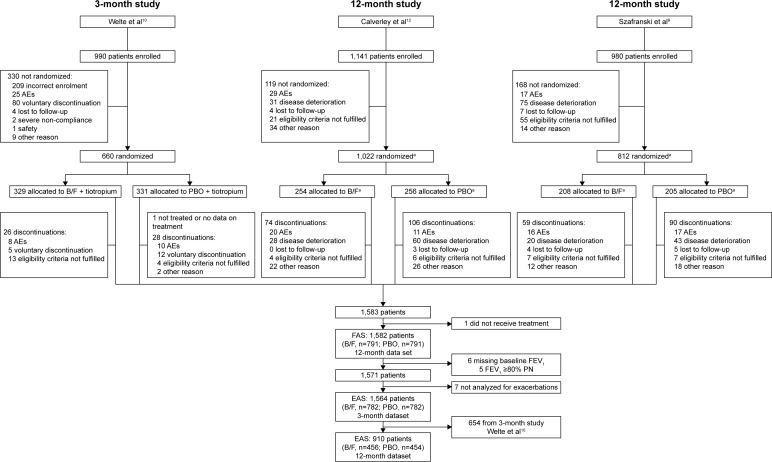
In total, 1,571 patients who received B/F or placebo were identified. Patient flow through the three studies and inclusion in this analysis is shown in Figure 1. Of these, 654 were from the CLIMB study10 and received background tiotropium. Six patients with missing FEV1 data and six patients with post-bronchodilator FEV1 >80% predicted were excluded from all analyses. The mean age of patients was 63.7 years, and 76.6% were male. COPD severity was moderate in 377 (24.0%), severe in 962 (61.2%), and very severe in 232 (14.8%) patients, according to GOLD 2016 criteria.6 Patient demographics and baseline clinical characteristics, by treatment and COPD severity, are summarized in Table 1. Lung function reversibility (% predicted) was higher in those with moderate COPD (10.4%–11.3%) than in those with severe and very severe COPD (4.1% and 2.5%, respectively, Table 1). Baseline characteristics for the 917 patients included in the 12-month sensitivity analysis are summarized in Table S1, and the characteristics by study of all the patients are summarized in Table S2.
Figure 1.
CONSORT patient flow through the three studies and analyses performed.
Notes: aOther patients in the study were randomized to other treatment groups that are not represented in this figure. Reprinted with permission of the American Thoracic Society. Copyright © 2016 American Thoracic Society. Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750.10 The American Journal of Respiratory and Critical Care Medicine is an official journal of the American Thoracic Society.
Abbreviations: AE, adverse event; B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; EAS, efficacy analysis set for pooled analysis; FAS, full analysis set for pooled analysis; PBO, placebo; PN, predicted normal.
Table 1.
Patient demographics and baseline clinical characteristics, by disease severity and treatment (3-month data set: Calverley et al, Szafranski et al, and CLIMB studies)9,10,12
| Characteristic | All patients (n=1,571) |
B/F
|
Placebo
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Moderate COPD (n=199) |
Severe COPD (n=469) |
Very severe COPD (n=118) |
All patients (n=786) |
Moderate COPD (n=178) |
Severe COPD (n=493) |
Very severe COPD (n=114) |
All patients (n=785) |
||
| Age, years | 63.7 (8.9) | 62.8 (9.4) | 63.9 (8.9) | 63.1 (7.9) | 63.5 (8.9) | 63.5 (9.4) | 64.3 (8.8) | 62.8 (8.1) | 63.9 (8.9) |
| Male, n (%) | 1,204 (76.6) | 127 (63.8) | 370 (78.9) | 106 (89.8) | 603 (76.7) | 122 (68.5) | 384 (77.9) | 95 (83.3) | 601 (76.6) |
| Lung function | |||||||||
| Pre-bronchodilator FEV1, L | 1.13 (0.42) | 1.42 (0.49) | 1.11 (0.34) | 0.72 (0.24) | 1.13 (0.43) | 1.46 (0.41) | 1.10 (0.34) | 0.72 (0.22) | 1.12 (0.41) |
| Reversibility, % predicted | 5.51 (5.83) | 11.30 (7.68) | 4.09 (4.07) | 2.50 (2.98) | 5.67 (6.09) | 10.41 (6.51) | 4.15 (4.39) | 2.52 (2.95) | 5.34 (5.55) |
| Post-bronchodilator FEV1, % predicteda | 42.4 (3.2–79.5) | 54.8 (50.0–76.6) | 41.5 (30.1–50.0) | 25.2 (3.2–30.0) | 42.8 (3.2–76.6) | 54.6 (50.0–79.5) | 40.5 (30.0–49.9) | 25.9 (15.8–30.0) | 42.0 (15.8–79.5) |
| Pre-bronchodilator FEV1/VCb ratio, %a | 45.6 (15.7–108.4) | 53.7 (27.7–86.7) | 43.7 (20.2–87.0) | 31.4 (15.7–93.4) | 45.2 (15.7–93.4) | 54.7 (27.9–108.4) | 45.4 (21.6–85.5) | 34.0 (19.6–65.3) | 45.9 (19.6–108.4) |
| Smoking history, pack-yearsa | 38.0 (10.0–280.0) | 35.0 (10.0–160.0) | 38.0 (10.0–280.0) | 38.5 (10.0–132.0) | 37.0 (10.0–280.0) | 35.0 (10.0–150.0) | 40.0 (10.0–156.0) | 37.0 (10.0–160.0) | 39.0 (10.0–160.0) |
| SGRQ total score | 56.34 (18.92) | 53.57 (19.25) | 55.89 (18.58) | 59.43 (18.39) | 55.79 (18.79) | 56.32 (21.30) | 56.49 (18.30) | 59.56 (18.45) | 56.89 (19.05) |
| Reliever use, inhalations/day | 3.40 (3.12) | 2.86 (2.65) | 3.42 (2.95) | 4.00 (3.15) | 3.37 (2.93) | 2.63 (2.40) | 3.34 (3.16) | 5.07 (4.45) | 3.43 (3.31) |
Notes: Data presented as arithmetic mean (standard deviation) unless otherwise stated.
Data are median (range).
FVC in one study10 and VC in two studies.9,12 Moderate COPD was defined as post-bronchodilator FEV1 80%–50% predicted, severe COPD as post-bronchodilator FEV1 50%–30% predicted, and very severe COPD as post-bronchodilator FEV1 <30% predicted.
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SGRQ, St George’s Respiratory Questionnaire; VC, vital capacity.
Exacerbations
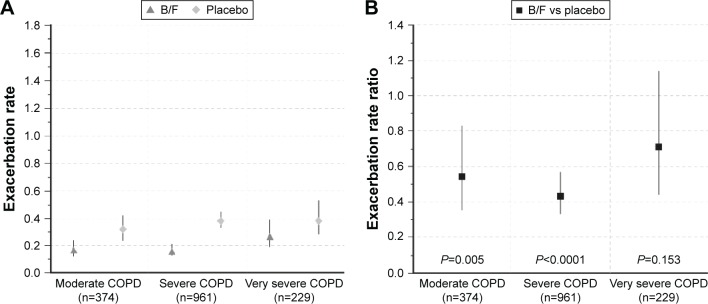
By 3 months, 111 (14.2%) B/F-treated patients and 196 (25.1%) patients in the placebo group had experienced at least one exacerbation (Table 2). B/F treatment for 3 months was associated with lower exacerbation rates (moderate COPD: 0.17 [95% CI 0.12, 0.24]; severe COPD: 0.16 [95% CI 0.13, 0.21]; very severe COPD: 0.27 [95% CI 0.19, 0.39]) than placebo (moderate COPD: 0.32 [95% CI 0.24, 0.42]; severe COPD: 0.38 [95% CI 0.33, 0.45]; very severe COPD: 0.38 [95% CI 0.28, 0.53]). Corresponding exacerbation rate reductions with B/F compared with placebo were significant for moderate (reduction: 46%; RR: 0.54; P=0.005) and severe (reduction: 57%; RR: 0.43; P<0.0001) COPD, although they were not statistically significant in the very severe COPD group (reduction: 29%; RR: 0.71; P=0.153) (Figure 2). There were more patients without exacerbations in the B/F than placebo group (85.8% vs 75.0%), and the proportion of patients with 1, 2, or ≥3 exacerbations was reduced in B/F-treated patients (Figure S1).
Table 2.
Number of patients with at least one exacerbation and dropouts by disease severity in the 3-month data set (Calverley et al, Szafranski et al, and CLIMB studies)9,10,12
| Patients, n (%) | B/F
|
|||
|---|---|---|---|---|
| Moderate (n=196) |
Severe (n=469) |
Very severe (n=117) |
Alla (n=782) |
|
| COPD exacerbation | 24 (12.2) | 60 (12.8) | 27 (23.1) | 111 (14.2) |
| Dropout | 20 (10.2) | 47 (10.0) | 14 (12.0) | 81 (10.4) |
|
| ||||
|
Placebo
|
||||
|
Moderate (n=178) |
Severe (n=492) |
Very severe (n=112) |
Alla (n=782) |
|
|
| ||||
| COPD exacerbation | 44 (24.7) | 122 (24.8) | 30 (26.8) | 196 (25.1) |
| Dropout | 28 (15.7) | 81 (16.5) | 25 (22.3) | 134 (17.1) |
Notes:
The 3-month data set includes 3-month data from the two 12-month studies9,12 as well as that from the 3-month study.10 ITT population includes only patients analyzed for exacerbations (n=1,564). Moderate COPD was defined as post-bronchodilator FEV1 80%–50% predicted, severe COPD as post-bronchodilator FEV1 50%–30% predicted, and very severe COPD as post-bronchodilator FEV1 <30% predicted.
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
Figure 2.
(A) 3-month exacerbation rates in patients with moderate, severe, and very severe COPD receiving B/F or placebo; and (B) 3-month exacerbation rate ratios between B/F and placebo, by disease severity. Vertical lines represent 95% confidence intervals. Moderate COPD was defined as post-bronchodilator FEV1 80%–50% predicted, severe COPD as post-bronchodilator FEV1 50%–30% predicted, and very severe COPD as post-bronchodilator FEV1 <30% predicted, according to GOLD 2016 criteria (3-month data set; Calverley et al, Szafranski et al, and CLIMB studies).9,10,12
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
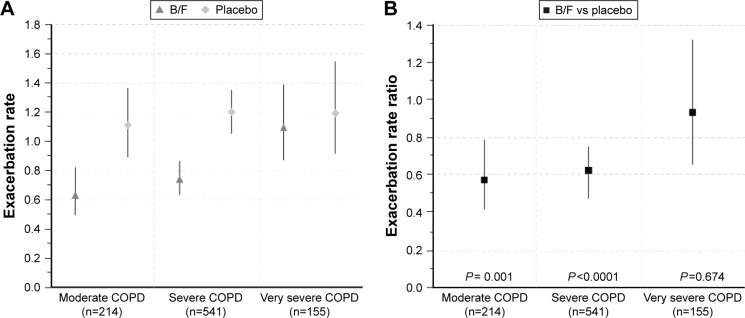
At 12 months, 168 (36.8%) B/F-treated patients and 195 (43.0%) patients in the placebo group had experienced at least one exacerbation (Table 3). B/F was associated with significantly lower exacerbation rates compared with placebo in patients with moderate (reduction: 43%; RR: 0.57; P=0.001) and severe (reduction: 38%; RR: 0.62; P<0.0001) COPD, although rate reductions were not statistically significant in patients with very severe COPD (reduction: 7%; RR: 0.93; P=0.674, Figure 3).
Table 3.
Number of patients with at least one exacerbation and dropouts by disease severity in the 12-month data set (12-month data set; Calverley et al, and Szafranski et al studies only)9,12
| Patients, n (%) | B/F
|
|||
|---|---|---|---|---|
| Moderate (n=106) |
Severe (n=266) |
Very severe (n=84) |
Alla (n=456) |
|
| COPD exacerbation | 30 (28.3) | 96 (36.1) | 42 (50.0) | 168 (36.8) |
| Dropout | 22 (20.8) | 80 (30.1) | 27 (32.1) | 129 (28.3) |
|
| ||||
|
Placebo
|
||||
|
Moderate (n=108) |
Severe (n=275) |
Very severe (n=71) |
Alla (n=454) |
|
|
| ||||
| COPD exacerbation | 43 (39.8) | 121 (44.0) | 31 (43.7) | 195 (43.0) |
| Dropout | 41 (38.0) | 115 (41.8) | 35 (49.3) | 191 (42.1) |
Notes:
The 12-month data set includes data from the two 12-month studies.9,12 ITT population includes only patients analyzed for exacerbations (n=910). Moderate COPD was defined as post-bronchodilator FEV1 80%–50% predicted, severe COPD as post-bronchodilator FEV1 50%–30% predicted, and very severe COPD as post-bronchodilator FEV1 <30% predicted.
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
Figure 3.
(A) Annual exacerbation rates in patients with moderate, severe, and very severe COPD receiving B/F or placebo and (B) annual exacerbation rate ratios between B/F and placebo, by disease severity. Vertical lines represent 95% confidence intervals. Moderate COPD was defined as FEV1 80%–50% predicted, severe COPD as FEV1 50%–30% predicted, and very severe COPD as FEV1 <30% predicted, according to GOLD 2016 criteria (12-month data set; Calverley et al and Szafranski et al studies only).9,12
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s.
Dropouts
Overall, 215 (13.7%) and 320 (35.2%) patients dropped out of the combined 3-month and 12-month study populations, respectively. There were fewer dropouts with B/F than placebo at 3 months (n=81 [10.4%] vs 134 [17.1%]; Table 2) and 12 months (n=129 [28.3%] vs 191 [42.1%]; Table 3). In general, the proportion of dropouts increased with increasing disease severity for both treatments at 3 and 12 months; fewer patients who received B/F than placebo dropped out in all three COPD severity groups (Tables 2 and 3).
Lung function, reliever use, and COPD symptoms
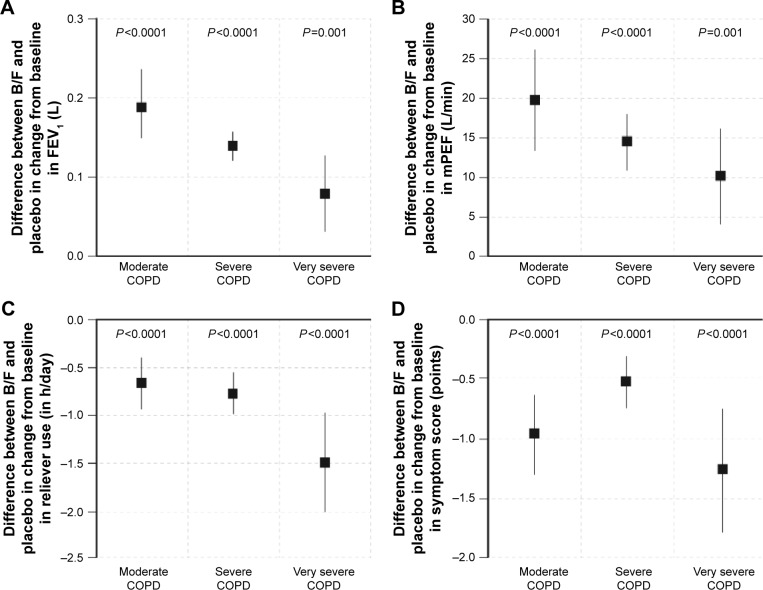
B/F was associated with a greater effect on FEV1 than placebo, irrespective of COPD severity (Figure 4A). The change from baseline in FEV1 (L) after 3 months treatment with B/F was 0.16 (95% CI 0.13, 0.19), 0.10 (95% CI 0.08, 0.12), and 0.09 (95% CI 0.06, 0.12) in moderate, severe, and very severe COPD, respectively. The corresponding values for placebo were −0.03 (95% CI −0.07, 0.00), −0.04 (95% CI −0.06, −0.02), and 0.01 (95% CI −0.03, 0.04).
Figure 4.
Difference in the mean change from baseline in (A) FEV1; (B) morning PEF; (C) daily reliever use; and (D) symptom score between B/F and placebo, by disease severity. Vertical lines represent 95% confidence intervals. Moderate COPD was defined as FEV1 80%–50% predicted, severe COPD as FEV1 50%–30% predicted, and very severe COPD as FEV1 <30% predicted, according to GOLD 2016 criteria (3-month data set; Calverley et al, Szafranski et al, and CLIMB studies).9,10,12
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; inh, inhalations; mPEF, morning peak expiratory flow rate.
B/F also demonstrated a greater effect on morning PEF than placebo (Figure 4B). Changes in morning PEF (L/min) after 3 months treatment with B/F were 15.1 (95% CI 10.6, 19.5), 7.6 (95% CI 4.9, 10.2), and 11.1 (95% CI 6.9, 15.4) in moderate, severe, and very severe COPD, respectively. Corresponding values for placebo were −4.3 (95% CI −9.0, 0.3), −6.8 (95% CI −9.4, −4.2), and 1.1 (95% CI −3.2, 5.4). The improvement with B/F versus placebo was greater in patients with moderate and severe COPD than very severe disease.
B/F-treated patients reported significantly less reliever use than patients in the placebo group, irrespective of COPD severity (all P<0.0001, Figure 4C). The change from baseline in total reliever use (inhalations/day) after 3 months treatment with B/F versus placebo was −0.66 (95% CI −0.94, −0.38), −0.77 (95% CI −0.99, −0.55), and −1.50 (95% CI −2.03, −0.97) in moderate, severe, and very severe COPD, respectively. The cohort of patients with very severe COPD, who experienced the greatest improvement relative to placebo, had the highest reliever use at baseline (4.00 and 5.07 inhalations/day in the B/F and placebo arms, respectively).
Compared with placebo, B/F was also associated with a statistically significant improvement from baseline in the total symptom score, irrespective of COPD severity (all P<0.0001, Figure 4D). The change from baseline in total symptom score after 3 months treatment with B/F was −1.23 (95% CI −1.46, −0.99), −0.57 (95% CI −0.73, −0.41), and −0.92 (95% CI −1.28, −0.56) in moderate, severe, and very severe COPD, respectively. The corresponding values for placebo were −0.26 (95% CI −0.51, −0.01), −0.04 (95% CI −0.19, 0.11), and 0.34 (95% CI −0.03, 0.70).
Safety
Pooled analysis of adverse events (AEs) and serious AEs across the three studies found no difference between B/F and placebo after 3 and 12 months treatment, while the number of discontinuations due to an AE was lower in B/F-treated than placebo-treated patients at both 3 months (5.8% vs 11.5%) and 12 months (17.5% vs 25.8%) (Tables S3 and S4, respectively). Ten patients in each treatment group (2.2%) had a fatal AE. Pooled analysis of the most frequently reported AEs in each treatment group after 3 and 12 months treatment are summarized in Tables S5 and S6. At 3 months, the most frequently reported AEs were COPD (7.1% B/F vs 13.0% placebo), nasopharyngitis (3.8% vs 3.3%), and headache (1.5% vs 0.9%).
Discussion
Previous analyses have shown that ICS/LABA combinations produce improvements in a range of clinical outcomes versus placebo, including prolonged time to first exacerbation, improved lung function, and improved health-related quality of life.9,12–14 These improved outcomes are greater with ICS/LABA combinations than with either agent alone at 12 months. ICS/LABA combinations can be used either as a primary treatment9,12–15 or in addition to therapy with a long-acting antimuscarinic agent in the management of severe COPD.10 However, the early effects (within the first 3 months) of ICS/LABA following treatment initiation are less well understood. This study investigated the early effects of this combination compared with placebo when used either alone9,12 or in addition to tiotropium10 in different COPD severities. Of clinical relevance, the beneficial effects of B/F compared with placebo on exacerbations were evident within 3 months of treatment and were paralleled by improved lung function, reduced need for reliever use, and fewer symptoms compared with placebo, irrespective of the severity of COPD. The beneficial effects of B/F on exacerbation rate, lung function, symptoms, and health-related quality of life have been reported previously in comparison with both budesonide and formoterol alone.9,12
In the three studies included in the analysis,9,10,12 exacerbation rate was assessed in patients with COPD who would fall within grades B, C, and D of the new GOLD classification.6 Conventionally, exacerbations are defined by health care utilization and are reported over 1 year, which increases the number of events and, hence, the statistical power to detect any difference between treatments. This analysis focused on the first 3 months of treatment, thus reducing the total number of exacerbation events. Despite this, one-quarter of those randomized to placebo reported at least one event, and this was significantly more than that seen with combination treatment. Although more patients dropped out in the placebo arm than in the active treatment group, our analyses still had sufficient power to detect treatment-related differences, not only for exacerbation rates, but in lung function improvement, reliever use, and self-reported symptoms.
A consistent reduction in the exacerbation rate over the first 3 months of treatment was observed in patients with spirometrically defined moderate or severe disease. Although there were proportionally more exacerbations in patients with very severe disease, more patients in this group dropped out, which may explain the lack of treatment effect observed. The effect of differential withdrawal on treatment effect has been described previously16 and merits further study.
The analyses were well powered to detect the effects of treatment on spirometry, confirming 1-year spirometry findings in the studied trials9,10,12 and agreeing with results of other studies;13–15 in addition, the analyses showed a treatment effect 3 months after randomization. These data were supported by the PEF recordings from the daily diary cards, which confirmed earlier reports of a treatment effect being evident within 1 week of randomization.9 Statistically significant, although generally smaller, changes in lung function were seen as baseline FEV1 worsened. This accords with observations of the acute bronchodilator effects in COPD, with smaller relative and absolute increases in FEV1 as GOLD severity increases.17 This smaller response is likely to reflect the increased prevalence of tidal expiratory flow limitation in more severe COPD18 and points toward the importance of lung volume change, rather than spirometry in these circumstances. Although the absolute changes in lung function in our data were small, the improvements in terms of rescue medication and their relationship to improved symptom scores were, if anything, greater in patients with worse baseline spirometry. This is important as we have shown that increased use of reliever medication is a marker for exacerbation risk.19 The presence of a statistically significant change in all these outcomes suggests that, for a significant number of individuals, combination therapy offers worthwhile improvements within 3 months of commencement across a range of clinically relevant measures.
This post hoc analysis has focused on changes in efficacy measures at 3 months after initiation of treatment. The effects of combination therapy across a range of safety and tolerability measures have been previously reported in the original publications.9,10,12 In this pooled safety analysis, the observed rates of AEs, serious AEs, and deaths due to AEs were similar in the B/F group to that observed in the placebo group, consistent with the original individual studies,9,10,12 while discontinuations due to AEs occurred less frequently in patients treated with B/F than those receiving placebo. Prior to the analysis, it was determined that when separated out and analyzed by disease severity, the number of individual types of events such as pneumonia would be too small for meaningful interpretation.
This study data have strengths and limitations. The study reports data from a large number of patients, covering a range of spirometric severity, and includes comparisons with placebo. Reductions in 3-month exacerbation rates have previously been reported in prospective studies and were 62% for B/F plus tiotropium versus tiotropium plus placebo10 and 36% for B/F versus formoterol.20 Although the current analysis was post hoc in terms of the primary study outcomes, it adds to the literature, as investigating the effect of an ICS/LABA combination at 3 months in different disease severities is novel. In one study that had been included in the analysis, all patients used tiotropium as a maintenance bronchodilator, and the lower exacerbation rates reported here at 3 months may reflect this.10 However, the behavior of patients in this study did not differ from those in the other two studies in which the baseline exacerbation rate was higher in the placebo arm.9,12 In addition, data were not available on prior exacerbation history or Medical Research Council breathlessness score to use in the analysis by the current GOLD grouping.
This study considered only exacerbations that led to the use of oral corticosteroids (alone or in combination with antibiotics) and/or hospitalization or emergency room visits, a definition currently supported by the GOLD initiative and others. This could, however, identify a more steroid-responsive exacerbation type. Pooled diary card symptom scores could identify treatment effects21 but unlike more recent tools,22,23 they were not validated to identify exacerbations that did not require medical treatment. If we had been able to use this approach, it is likely that the number of events being identified would have been greater. Evidence from other studies suggests that the trend of such events would have been similar to those where patients had sought increases in treatment,24 and hence, these additional episodes might have allowed to draw the same conclusion with fewer patients.
Limitations
Other limitations include the number of patients who dropped out from the study, by both 3 and 12 months. This may have affected the accuracy of the results, including exacerbation rates. High dropout rates can confound the interpretation of randomized controlled trials and can be a common feature in trials involving patients with COPD, with some analyses reporting 27%–53% dropout.25 In addition, the moderate group in our analysis had airway reversibility of 10%–11% (% predicted). It is now recognized that COPD patients with moderate airflow obstruction show a greater absolute change in FEV1 post-bronchodilator than that seen in more severe disease.26 This contributes to the greater degree of reversibility reported in analyses of moderate patients in clinical trials similar to this study. The entry criteria for the three studies included in this analysis are consistent with those of other clinical trials in the area and did not select patients on the basis of known reversibility to bronchodilators.
These data have implications for the use and evaluation of treatment in COPD. It was found that a measurable effect on COPD exacerbations (defined by symptoms necessitating the addition of, or treatment with, oral or systemic corticosteroids) occurred within 3 months of randomization to the ICS/LABA combination in COPD patients who had experienced at least one exacerbation in the past year. This positive effect on exacerbation frequencies was paralleled by improved lung function, lower use of reliever medication, and fewer symptoms. Whether these changes in objective patient-reported outcomes can be used to predict a further course of events remains to be established at an individual patient level. However, they should reassure clinicians that treatment is effective across a range of endpoints, including exacerbation prevention, soon after initiation.
Conclusions
In conclusion, data from this study show that treatment with an ICS/LABA combination has important clinical impact irrespective of disease severity; these effects are evident within 3 months after the initiation of treatment.
Supplementary materials
Proportion of patients with moderate-to-very-severe COPD who received B/F or placebo and who had zero, 1, 2, or ≥3 exacerbations over the 3-month study period.
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease.
Table S1.
Patient demographics and baseline clinical characteristics, by disease severity and treatment (12-month data set: Calverley et al and Szafranski et al studies only)1,2
| Characteristic | B/F
|
Placebo
|
||||||
|---|---|---|---|---|---|---|---|---|
| Moderate COPD (n=109) |
Severe COPD (n=266) |
Very severe COPD (n=85) |
All patients (n=460) |
Moderate COPD (n=108) |
Severe COPD (n=276) |
Very severe COPD (n=73) |
All patients (n=457) |
|
| Age, years | 62.8 (9.4) | 65.4 (9.0) | 62.8 (8.4) | 64.3 (9.0) | 64.5 (9.1) | 65.5 (8.8) | 63.0 (8.2) | 64.9 (8.8) |
| Male, n (%) | 67 (61.5) | 211 (79.3) | 77 (90.6) | 355 (77.2) | 74 (68.5) | 221 (80.1) | 64 (87.7) | 359 (78.6) |
| Lung function | ||||||||
| Pre-bronchodilator FEV1 L | 1.50 (0.50) | 1.08 (0.36) | 0.74 (0.26) | 1.12 (0.45) | 1.48 (0.45) | 1.08 (0.36) | 0.71 (0.18) | 1.11 (0.43) |
| Reversibility, % predicted | 11.56 (7.89) | 4.52 (4.12) | 2.44 (2.93) | 5.80 (6.08) | 10.69 (6.77) | 4.39 (4.55) | 2.62 (2.96) | 5.60 (5.75) |
| Post-bronchodilator FEV1, % predicteda | 54.8 (50.0–76.6) | 40.4 (30.1–50.0) | 25.4 (3.2–30.0) | 42.1 (3.2–76.6) | 54.5 (50.0–79.5) | 39.8 (30.0–49.9) | 25.2 (15.8–30.0) | 40.8 (15.8–79.5) |
| Pre-bronchodilator FEV1/VC ratio, %a | 55.6 (27.7–86.7) | 43.0 (20.2–87.0) | 30.6 (17.5–93.4) | 43.5 (17.5–93.4) | 55.3 (27.9–108.4) | 43.6 (21.6–85.5) | 32.5 (19.6–65.3) | 45.0 (19.6–108.4) |
| Smoking history, pack-yearsa | 33.0 (10.0–160.0) | 38.5 (10.0–240.0) | 40.0 (10.0–132.0) | 37.5 (10.0–240.0) | 39.5 (10.0–150.0) | 40.0 (10.0–124.0) | 36.0 (12.0–160.0) | 39.0 (10.0–160.0) |
| SGRQ total score | 48.73 (18.90) | 51.04 (18.25) | 56.75 (18.72) | 51.45 (18.62) | 51.58 (21.38) | 52.03 (17.79) | 55.30 (18.08) | 52.44 (18.74) |
| Reliever use, inhalations/day | 2.21 (2.44) | 2.85 (2.95) | 3.51 (3.28) | 2.82 (2.93) | 2.00 (2.03) | 2.46 (2.55) | 4.69 (5.04) | 2.70 (3.10) |
Notes: Data presented as arithmetic mean (standard deviation) unless otherwise stated. Moderate COPD was defined as FEV1 80%–50% predicted, severe COPD as FEV1 50%–30% predicted, and very severe COPD as FEV1 <30% predicted.
Data are median (range).
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire; VC, vital capacity.
Table S2.
Patient demographics and baseline clinical characteristics, by study
| Characteristic | Calverley et al2 (n=509) |
Szafranski et al1 (n=408) |
CLIMB 20093 (n=654) |
|---|---|---|---|
| Age, years | 64.5 (9.1) | 64.7 (8.7) | 62.4 (8.7) |
| Male, n (%) | 389 (76.4) | 325 (79.7) | 490 (74.9) |
| Lung function | |||
| Pre-bronchodilator FEV1, L | 1.19 (0.48) | 1.03 (0.37) | 1.14 (0.38) |
| Reversibility, % predicted | 5.91 (6.43) | 5.44 (5.19) | 5.23 (5.70) |
| Post-bronchodilator FEV1, % predicteda | 42.2 (3.2–79.5) | 40.6 (16.9–72.0) | 43.8 (13.7–76.9) |
| Pre-bronchodilator FEV1/VC ratio, %a | 46.1 (18.8–108.4) | 42.0 (17.5–93.4) | 47.1 (15.7–89.3) |
| Smoking history, pack-yearsa | 35.0 (10.0–240.0) | 40.0 (10.0–168.0) | 38.0 (10.0–280.0) |
| SGRQ total score | 49.70 (19.37) | 54.69 (17.44) | 62.30 (17.59) |
| Reliever use, inhalations/day | 1.83 (2.52) | 3.92 (3.18) | 4.29 (3.05) |
Notes: Data presented as arithmetic mean (standard deviation) unless otherwise stated.
Data are median (range).
Abbreviations: B/F, budesonide/formoterol; FEV1, forced expiratory volume in 1 s; SGRQ, St George’s Respiratory Questionnaire; VC, vital capacity.
Table S3.
Number (%) of patients who had an adverse event (AE) in any category in the 3-month budesonide/formoterol vs placebo pool (Welte et al, Calverley et al, and Szafranski et al studies)1,2,4
| Category of AE | Budesonide/formoterol (n=791) |
Placebo (n=791) |
|---|---|---|
| Any AE | 275 (34.8) | 269 (34.0) |
| Serious AEs | 44 (5.6) | 54 (6.8) |
| Serious AEs leading to death | 3 (0.4) | 3 (0.4) |
| Serious AEs not leading to death | 41 (5.2) | 51 (6.4) |
| Discontinuation of study treatment due to AEs | 46 (5.8) | 91 (11.5) |
Notes: Safety population includes all patients who received at least one dose of treatment. This table includes patients with AEs onset during randomized treatment. Patients with multiple events in the same category are counted only once in that category. Patients with events in >1 category are counted once in each of those categories.
Table S4.
Number (%) of patients who had an adverse event (AE) in any category in the 12-month budesonide/formoterol vs placebo pool (Calverley et al and Szafranski et al studies only)1,2
| Category of AE | Budesonide/formoterol (n=462) |
Placebo (n=461) |
|---|---|---|
| Any AE | 284 (61.5) | 270 (58.6) |
| Serious AEs | 93 (20.1) | 88 (19.1) |
| Serious AEs leading to death | 10 (2.2) | 10 (2.2) |
| Serious AEs not leading to death | 85 (18.4) | 80 (17.4) |
| Discontinuation of study treatment due to AEs | 81 (17.5) | 119 (25.8) |
Notes: Safety population includes all patients who received at least one dose of treatment. This table includes patients with AEs onset during randomized treatment. Patients with multiple events in the same category are counted only once in that category. Patients with events in >1 category are counted once in each of those categories.
Table S5.
Number (%) of patients with most common adverse events (AEs) (frequency of ≥2% in any treatment group) in the 3-month budesonide/formoterol vs placebo pool (Welte et al, Calverley et al, and Szafranski et al studies)1,2,4
| Preferred term | Budesonide/formoterol (n=791) |
Placebo (n=791) |
|---|---|---|
| Chronic obstructive pulmonary disease | 56 (7.1%) | 103 (13.0%) |
| Nasopharyngitis | 30 (3.8%) | 26 (3.3%) |
| Headache | 12 (1.5%) | 7 (0.9%) |
| Dysphonia | 14 (1.8%) | 0 (0.0%) |
| Pharyngitis | 4 (0.5%) | 7 (0.9%) |
| Pyrexia | 9 (1.1%) | 1 (0.1%) |
Notes: This table includes patients with AEs onset during randomized treatment. Patients with multiple AEs coded to the same preferred term are counted only once for that preferred term.
Table S6.
Number (%) of patients with most common adverse events (AEs) (frequency of ≥2% in any treatment group) in the 12-month budesonide/formoterol vs placebo pool (Calverley et al and Szafranski et al studies only)1,2
| Preferred term | Budesonide/formoterol (n=462) |
Placebo (n=461) |
|---|---|---|
| Chronic obstructive pulmonary disease | 84 (18.2%) | 125 (27.1%) |
| Nasopharyngitis | 42 (9.1%) | 27 (5.9%) |
| Headache | 15 (3.2%) | 10 (2.2%) |
| Pneumonia | 14 (3.0%) | 11 (2.4%) |
| Chest pain | 16 (3.5%) | 8 (1.7%) |
| Dyspnoea | 8 (1.7%) | 15 (3.3%) |
| Back pain | 9 (1.9%) | 9 (2.0%) |
| Muscle spasms | 14 (3.0%) | 4 (0.9%) |
| Respiratory tract infection | 10 (2.2%) | 7 (1.5%) |
| Diarrhoea | 9 (1.9%) | 6 (1.3%) |
| Influenza | 11 (2.4%) | 2 (0.4%) |
| Rhinitis | 10 (2.2%) | 3 (0.7%) |
| Insomnia | 7 (1.5%) | 5 (1.1%) |
| Myalgia | 8 (1.7%) | 3 (0.7%) |
| Dysphonia | 9 (1.9%) | 1 (0.2%) |
| Pharyngitis | 5 (1.1%) | 5 (1.1%) |
| Pyrexia | 8 (1.7%) | 2 (0.4%) |
| Dyspepsia | 8 (1.7%) | 1 (0.2%) |
| Vomiting | 5 (1.1%) | 1 (0.2%) |
Notes: This table includes patients with AEs onset during randomized treatment. Patients with multiple AEs coded to the same preferred term are counted only once for that preferred term.
References
- 1.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 2.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur RespirJ. 2003;22(6):912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 3.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
Acknowledgments
The authors would like to thank Thomas Similowski and Ian Naya for their contributions to this manuscript. We also thank Katharine Williams and Ash Dunne of inScience Communications, Springer Healthcare, who provided medical writing support funded by AstraZeneca. The sponsor (AstraZeneca) funded this analysis and was involved in the analysis, design, and interpretation of the data, always in conjunction with the authors. The University of Groningen has received money for research by unrestricted educational grants from AstraZeneca and Chiesi. AstraZeneca, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, and Nycomed have provided support for travel to meetings.
The current address of Anders Persson is Centre of Registers, Västra Götaland, Sweden.
Footnotes
Author contributions
PMC, GE, CRJ, ARA, BJM, AP, MF, and DSP all contributed to the conception, writing, and revision of the manuscript, and approved the final version for submission. AP was responsible for statistical analyses. All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
PMC is a board member for Boehringer Ingelheim, the Department of Health Respiratory Programme Board, GlaxoSmithKline, and Nycomed. He has been a consultant for Novartis and provided expert testimony for Forest. PMC has received honoraria for advising on the conduct and analysis of clinical trial data from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, and Nycomed. He has also spoken at meetings supported by these companies. Support for travel to meetings has been provided by AstraZeneca.
GE is an ex-employee of AstraZeneca. He has consulted for Airsonett, ALK and Novartis, participated in advisory board meeting with Almirall, and is a member of the Medicon Village Inhalation Centre (MVIC).
CRJ is an advisory board member for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis, and a consultant for AstraZeneca, GlaxoSmithKline, Pieris, and Chiesi. Educational presentations have been developed for AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis, with honoraria paid to CRJ or her institution. Lectures have been presented on behalf of AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. Support for travel to meetings has been provided by AstraZeneca and Boehringer Ingelheim.
ARA is a consultant and speaker for AstraZeneca, Bayer Pharma, Boehringer Ingelheim, Dey Pharma, GlaxoSmithKline, and Pfizer, and has received honoraria from these companies. Educational presentations have been developed for AstraZeneca, Bayer Pharma, Boehringer Ingelheim, Dey Pharma, GSK, and Pfizer. The University of Texas Health Science Center at San Antonio has received money for research to perform clinical trials. Support for travel to meetings has also been provided by AstraZeneca.
BJM is an advisory board member for Aerocrine, AstraZeneca, Forest, Boehringer Ingelheim, CSL Bering, Forest, Novartis, and Theravance and a consultant for Astellas, Forest, and Chiesi. Clinical trial data have been reviewed for Spiration, with grants received and controlled by National Jewish Health from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Forest, MedImmune, Nabi, National Institutes of Health, Pfizer, and Sunovian. Lectures have been presented on behalf of Boehringer Ingelheim and GlaxoSmithKline. Educational presentations and programs have been developed (Carden Jennings, Cleveland Clinic, Consensus Medical, Foundation for Improving Patient Outcomes, Hybrid Communications, Integrity, Intellisphere, Medscape, National Jewish Health, Projects in Knowledge, SPIRE, Synapse, and WebMD). Royalties have been received from Up-To-Date. BJM has been a speaker for educational programs at Abbott, the American Academy of Family Practice, the American College of Chest Physicians, and the American Thoracic Society. Support for travel to meetings has also been provided by AstraZeneca.
AP and MF are employees of AstraZeneca and own stocks within the company.
The University of Groningen has received honoraria for DSP advising on the conduct and analysis of clinical trial data from AstraZeneca, Nycomed, and Teva, as well as for lectures at meetings supported by AstraZeneca, Chiesi, GlaxoSmithKline, Nycomed, and Teva.
References
- 1.Murray C, Richards M, Newton J, et al. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet. 2013;381(9871):997–1020. doi: 10.1016/S0140-6736(13)60355-4. [DOI] [PubMed] [Google Scholar]
- 2.Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987–2015. doi: 10.1016/S0140-6736(13)61097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calverley P, Anderson J, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 4.Tashkin D, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 5.De Soyza A, Calverley PMA. Large trials, new knowledge: the changing face of COPD management. Eur Respir J. 2015;45(6):1692–1703. doi: 10.1183/09031936.00179714. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Chronic Obstructive Lung Disease (GOLD) From the Global Strategy for the Diagnosis, Management, and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). Updated 2016. [Accessed January 2016]. Available from: http://www.goldcopd.org/2016.
- 7.Wise RA, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl JMed. 2013;369(16):1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 8.Vestbo J, Pauwels R, Anderson J, Jones P, Calverley P, TRISTSAN Group Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax. 2005;60(4):301–304. doi: 10.1136/thx.2004.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Szafranski W, Cukier A, Ramirez A, et al. Efficacy and safety of budesonide/formoterol in the management of chronic obstructive pulmonary disease. Eur Respir J. 2003;21(1):74–81. doi: 10.1183/09031936.03.00031402. [DOI] [PubMed] [Google Scholar]
- 10.Welte T, Miravitlles M, Hernandez P, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(8):741–750. doi: 10.1164/rccm.200904-0492OC. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins CR, Jones PW, Calverley PM, et al. Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: analysis from the randomised, placebo-controlled TORCH study. Respir Res. 2009;10:59. doi: 10.1186/1465-9921-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calverley PM, Boonsawat W, Cseke Z, Zhong N, Peterson S, Olsson H. Maintenance therapy with budesonide and formoterol in chronic obstructive pulmonary disease. Eur RespirJ. 2003;22(6):912–919. doi: 10.1183/09031936.03.00027003. [DOI] [PubMed] [Google Scholar]
- 13.Rennard S, Tashkin D, McElhattan J, et al. Efficacy and tolerability of budesonide/formoterol in one hydrofluoroalkane pressurized metered-dose inhaler in patients with chronic obstructive pulmonary disease: results from a 1-year randomized controlled clinical trial. Drugs. 2009;69(5):549–565. doi: 10.2165/00003495-200969050-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashkin D, Rennard S, Martin P, et al. Efficacy and safety of budesonide and formoterol in one pressurized metered-dose inhaler in patients with moderate to very severe chronic obstructive pulmonary disease: results of a 6-month randomized clinical trial. Drugs. 2008;68(14):1975–2000. doi: 10.2165/00003495-200868140-00004. [DOI] [PubMed] [Google Scholar]
- 15.Sharafkhaneh A, Southard J, Goldman M, Uryniak T, Martin U. Effect of budesonide/formoterol pMDI on COPD exacerbations: a double-blind, randomized study. Respir Med. 2012;106(2):257–268. doi: 10.1016/j.rmed.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 16.Calverley PM, Spencer S, Willits L, Burge PS, Jones PW. Withdrawal from treatment as an outcome in the ISOLDE study of COPD. Chest. 2003;124(4):1350–1356. doi: 10.1378/chest.124.4.1350. [DOI] [PubMed] [Google Scholar]
- 17.Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67(8):701–708. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 18.Dellacà R, Pompilio P, Walker P, Duffy N, Pedotti A, Calverley P. Effect of bronchodilation on expiratory flow limitation and resting lung mechanics in COPD. Eur Respir J. 2009;33(6):1329–1337. doi: 10.1183/09031936.00139608. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins CR, Postma DS, Anzueto AR, et al. Reliever salbutamol use as a measure of exacerbation risk in chronic obstructive pulmonary disease. BMC Pulm Med. 2015;15:97. doi: 10.1186/s12890-015-0077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fukuchi Y, Samoro R, Fassakhov R, et al. Budesonide/formoterol via Turbuhaler(R) versus formoterol via Turbuhaler(R) in patients with moderate to severe chronic obstructive pulmonary disease: phase III multinational study results. Respirology. 2013;18(5):866–873. doi: 10.1111/resp.12090. [DOI] [PubMed] [Google Scholar]
- 21.Calverley P, Pauwels Dagger R, Lofdahl CG, et al. Relationship between respiratory symptoms and medical treatment in exacerbations of COPD. Eur Respir J. 2005;26(3):406–413. doi: 10.1183/09031936.05.00143404. [DOI] [PubMed] [Google Scholar]
- 22.Leidy N, Wilcox T, Jones P, et al. Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Reliability and validity of a patient-reported diary. Am J Respir Crit Care Med. 2011;183(3):323–329. doi: 10.1164/rccm.201005-0762OC. [DOI] [PubMed] [Google Scholar]
- 23.Jones P, Brusselle G, Dal Negro R, et al. Properties of the COPD assessment test in a cross-sectional European study. Eur Respir J. 2011;38(1):29–35. doi: 10.1183/09031936.00177210. [DOI] [PubMed] [Google Scholar]
- 24.Wedzicha J, Decramer M, Ficker J, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 25.Wilt TJ, Niewoehner D, MacDonald R, Kane RL. Management of stable chronic obstructive pulmonary disease: a systematic review for a clinical practice guideline. Ann Intern Med. 2007;147(9):639–653. doi: 10.7326/0003-4819-147-9-200711060-00009. [DOI] [PubMed] [Google Scholar]
- 26.Calverley PM, Albert P, Walker PP. Bronchodilator reversibility in chronic obstructive pulmonary disease: use and limitations. Lancet. 2013;1(7):564–573. doi: 10.1016/S2213-2600(13)70086-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Proportion of patients with moderate-to-very-severe COPD who received B/F or placebo and who had zero, 1, 2, or ≥3 exacerbations over the 3-month study period.
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease.
Table S1.
Patient demographics and baseline clinical characteristics, by disease severity and treatment (12-month data set: Calverley et al and Szafranski et al studies only)1,2
| Characteristic | B/F
|
Placebo
|
||||||
|---|---|---|---|---|---|---|---|---|
| Moderate COPD (n=109) |
Severe COPD (n=266) |
Very severe COPD (n=85) |
All patients (n=460) |
Moderate COPD (n=108) |
Severe COPD (n=276) |
Very severe COPD (n=73) |
All patients (n=457) |
|
| Age, years | 62.8 (9.4) | 65.4 (9.0) | 62.8 (8.4) | 64.3 (9.0) | 64.5 (9.1) | 65.5 (8.8) | 63.0 (8.2) | 64.9 (8.8) |
| Male, n (%) | 67 (61.5) | 211 (79.3) | 77 (90.6) | 355 (77.2) | 74 (68.5) | 221 (80.1) | 64 (87.7) | 359 (78.6) |
| Lung function | ||||||||
| Pre-bronchodilator FEV1 L | 1.50 (0.50) | 1.08 (0.36) | 0.74 (0.26) | 1.12 (0.45) | 1.48 (0.45) | 1.08 (0.36) | 0.71 (0.18) | 1.11 (0.43) |
| Reversibility, % predicted | 11.56 (7.89) | 4.52 (4.12) | 2.44 (2.93) | 5.80 (6.08) | 10.69 (6.77) | 4.39 (4.55) | 2.62 (2.96) | 5.60 (5.75) |
| Post-bronchodilator FEV1, % predicteda | 54.8 (50.0–76.6) | 40.4 (30.1–50.0) | 25.4 (3.2–30.0) | 42.1 (3.2–76.6) | 54.5 (50.0–79.5) | 39.8 (30.0–49.9) | 25.2 (15.8–30.0) | 40.8 (15.8–79.5) |
| Pre-bronchodilator FEV1/VC ratio, %a | 55.6 (27.7–86.7) | 43.0 (20.2–87.0) | 30.6 (17.5–93.4) | 43.5 (17.5–93.4) | 55.3 (27.9–108.4) | 43.6 (21.6–85.5) | 32.5 (19.6–65.3) | 45.0 (19.6–108.4) |
| Smoking history, pack-yearsa | 33.0 (10.0–160.0) | 38.5 (10.0–240.0) | 40.0 (10.0–132.0) | 37.5 (10.0–240.0) | 39.5 (10.0–150.0) | 40.0 (10.0–124.0) | 36.0 (12.0–160.0) | 39.0 (10.0–160.0) |
| SGRQ total score | 48.73 (18.90) | 51.04 (18.25) | 56.75 (18.72) | 51.45 (18.62) | 51.58 (21.38) | 52.03 (17.79) | 55.30 (18.08) | 52.44 (18.74) |
| Reliever use, inhalations/day | 2.21 (2.44) | 2.85 (2.95) | 3.51 (3.28) | 2.82 (2.93) | 2.00 (2.03) | 2.46 (2.55) | 4.69 (5.04) | 2.70 (3.10) |
Notes: Data presented as arithmetic mean (standard deviation) unless otherwise stated. Moderate COPD was defined as FEV1 80%–50% predicted, severe COPD as FEV1 50%–30% predicted, and very severe COPD as FEV1 <30% predicted.
Data are median (range).
Abbreviations: B/F, budesonide/formoterol; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; SGRQ, St George’s Respiratory Questionnaire; VC, vital capacity.
Table S2.
Patient demographics and baseline clinical characteristics, by study
| Characteristic | Calverley et al2 (n=509) |
Szafranski et al1 (n=408) |
CLIMB 20093 (n=654) |
|---|---|---|---|
| Age, years | 64.5 (9.1) | 64.7 (8.7) | 62.4 (8.7) |
| Male, n (%) | 389 (76.4) | 325 (79.7) | 490 (74.9) |
| Lung function | |||
| Pre-bronchodilator FEV1, L | 1.19 (0.48) | 1.03 (0.37) | 1.14 (0.38) |
| Reversibility, % predicted | 5.91 (6.43) | 5.44 (5.19) | 5.23 (5.70) |
| Post-bronchodilator FEV1, % predicteda | 42.2 (3.2–79.5) | 40.6 (16.9–72.0) | 43.8 (13.7–76.9) |
| Pre-bronchodilator FEV1/VC ratio, %a | 46.1 (18.8–108.4) | 42.0 (17.5–93.4) | 47.1 (15.7–89.3) |
| Smoking history, pack-yearsa | 35.0 (10.0–240.0) | 40.0 (10.0–168.0) | 38.0 (10.0–280.0) |
| SGRQ total score | 49.70 (19.37) | 54.69 (17.44) | 62.30 (17.59) |
| Reliever use, inhalations/day | 1.83 (2.52) | 3.92 (3.18) | 4.29 (3.05) |
Notes: Data presented as arithmetic mean (standard deviation) unless otherwise stated.
Data are median (range).
Abbreviations: B/F, budesonide/formoterol; FEV1, forced expiratory volume in 1 s; SGRQ, St George’s Respiratory Questionnaire; VC, vital capacity.
Table S3.
Number (%) of patients who had an adverse event (AE) in any category in the 3-month budesonide/formoterol vs placebo pool (Welte et al, Calverley et al, and Szafranski et al studies)1,2,4
| Category of AE | Budesonide/formoterol (n=791) |
Placebo (n=791) |
|---|---|---|
| Any AE | 275 (34.8) | 269 (34.0) |
| Serious AEs | 44 (5.6) | 54 (6.8) |
| Serious AEs leading to death | 3 (0.4) | 3 (0.4) |
| Serious AEs not leading to death | 41 (5.2) | 51 (6.4) |
| Discontinuation of study treatment due to AEs | 46 (5.8) | 91 (11.5) |
Notes: Safety population includes all patients who received at least one dose of treatment. This table includes patients with AEs onset during randomized treatment. Patients with multiple events in the same category are counted only once in that category. Patients with events in >1 category are counted once in each of those categories.
Table S4.
Number (%) of patients who had an adverse event (AE) in any category in the 12-month budesonide/formoterol vs placebo pool (Calverley et al and Szafranski et al studies only)1,2
| Category of AE | Budesonide/formoterol (n=462) |
Placebo (n=461) |
|---|---|---|
| Any AE | 284 (61.5) | 270 (58.6) |
| Serious AEs | 93 (20.1) | 88 (19.1) |
| Serious AEs leading to death | 10 (2.2) | 10 (2.2) |
| Serious AEs not leading to death | 85 (18.4) | 80 (17.4) |
| Discontinuation of study treatment due to AEs | 81 (17.5) | 119 (25.8) |
Notes: Safety population includes all patients who received at least one dose of treatment. This table includes patients with AEs onset during randomized treatment. Patients with multiple events in the same category are counted only once in that category. Patients with events in >1 category are counted once in each of those categories.
Table S5.
Number (%) of patients with most common adverse events (AEs) (frequency of ≥2% in any treatment group) in the 3-month budesonide/formoterol vs placebo pool (Welte et al, Calverley et al, and Szafranski et al studies)1,2,4
| Preferred term | Budesonide/formoterol (n=791) |
Placebo (n=791) |
|---|---|---|
| Chronic obstructive pulmonary disease | 56 (7.1%) | 103 (13.0%) |
| Nasopharyngitis | 30 (3.8%) | 26 (3.3%) |
| Headache | 12 (1.5%) | 7 (0.9%) |
| Dysphonia | 14 (1.8%) | 0 (0.0%) |
| Pharyngitis | 4 (0.5%) | 7 (0.9%) |
| Pyrexia | 9 (1.1%) | 1 (0.1%) |
Notes: This table includes patients with AEs onset during randomized treatment. Patients with multiple AEs coded to the same preferred term are counted only once for that preferred term.
Table S6.
Number (%) of patients with most common adverse events (AEs) (frequency of ≥2% in any treatment group) in the 12-month budesonide/formoterol vs placebo pool (Calverley et al and Szafranski et al studies only)1,2
| Preferred term | Budesonide/formoterol (n=462) |
Placebo (n=461) |
|---|---|---|
| Chronic obstructive pulmonary disease | 84 (18.2%) | 125 (27.1%) |
| Nasopharyngitis | 42 (9.1%) | 27 (5.9%) |
| Headache | 15 (3.2%) | 10 (2.2%) |
| Pneumonia | 14 (3.0%) | 11 (2.4%) |
| Chest pain | 16 (3.5%) | 8 (1.7%) |
| Dyspnoea | 8 (1.7%) | 15 (3.3%) |
| Back pain | 9 (1.9%) | 9 (2.0%) |
| Muscle spasms | 14 (3.0%) | 4 (0.9%) |
| Respiratory tract infection | 10 (2.2%) | 7 (1.5%) |
| Diarrhoea | 9 (1.9%) | 6 (1.3%) |
| Influenza | 11 (2.4%) | 2 (0.4%) |
| Rhinitis | 10 (2.2%) | 3 (0.7%) |
| Insomnia | 7 (1.5%) | 5 (1.1%) |
| Myalgia | 8 (1.7%) | 3 (0.7%) |
| Dysphonia | 9 (1.9%) | 1 (0.2%) |
| Pharyngitis | 5 (1.1%) | 5 (1.1%) |
| Pyrexia | 8 (1.7%) | 2 (0.4%) |
| Dyspepsia | 8 (1.7%) | 1 (0.2%) |
| Vomiting | 5 (1.1%) | 1 (0.2%) |
Notes: This table includes patients with AEs onset during randomized treatment. Patients with multiple AEs coded to the same preferred term are counted only once for that preferred term.