Abstract
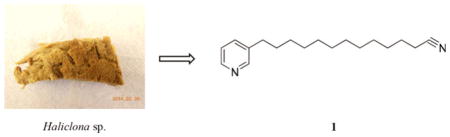
A new alkaloid, 3-dodecyl pyridine containing a terminal cyano group (1), was isolated from the methanol extract of an Indonesia marine sponge Haliclona sp. Its chemical structure was determined by a combination of spectroscopic methods, including 1D and 2D NMR. Bioassay results indicated that compound 1 had moderate cytotoxity against tumour cell lines A549, MCF-7 and Hela with IC50 values of 41.8, 48.4 and 33.2 μM, respectively.
Keywords: 3-alkylpyridine, alkaloid, Haliclona sp, cytotoxicity
Graphical Abstract
1. Introduction
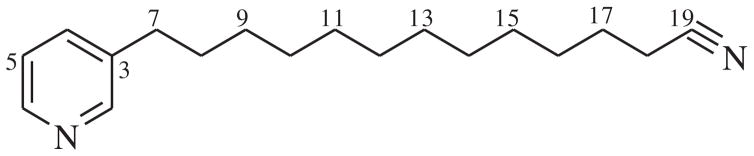
A growing evidence indicates that piperidine and pyridine (pyridinium) alkaloids are widely distributed in marine sponges of five genera, including Amphimedon (Berlinck et al. 1996; Kubota et al. 2007; Nishi et al. 2008), Cribrochalina (Matsunaga et al. 1993; Kariya et al. 2006), Haliclona (Volk & Köck 2003; Schmidt et al. 2012), Niphates (Kobayashi et al. 1990, 1992) and Xestospongia (Sakemi et al. 1990; Oku et al. 2004). Previous bioassay indicated that these alkaloids have a broad spectrum of bioactivities, such as antibacteria, antitumour, antic-holinesterase, haemolysis and neurotoxicity (Berlinck et al. 1996). In our continuous investigation of bioactive secondary metabolites from marine sponges, one new pyridine alkaloid, 3-dodecyl pyridine with a terminal cyano group (1), was isolated from an Indonesia marine sponge Haliclona sp. Here we describe the isolation and structure elucidation of this alkaloid (Figure 1).
Figure 1.
Chemical structure of compound 1.
2. Results and discussion
The sponge Haliclona sp. (no. 95546) was collected from the Indonesian Sea and kept in methanol at 4°C until extraction. A portion of the specimen (13.8 g) was extracted using an accelerated solvent extractor according to reported protocol (Johnson et al. 2010). The resulting methanol extract (286 mg) was quickly fractionated into six fractions (F0–F5) using a preparative column. Bioassay tests indicated that fraction F3 had moderate in vitro cytotoxicity against tumour cell lines L1210, Colon 38 and H-125. F3 was subjected to high performance liquid chromatography (HPLC) with a reversal column to give compound 1.
Compound 1, obtained as colourless oil, had a molecular formula of C18H28N2 by the positive HR-ESI-MS ion at m/z 273.2312 [M + H]+ (calcd for C18H29N2, 273.2325) with six degrees of unsaturation. The 1H and 13C NMR signals for the aromatic region (see Figures S1 and S2) suggested the presence of a mono-3-alkyl-substituted pyridine ring. By careful inspection of 13C NMR and HSQC spectra, a quaternary carbon C-19 at δ 119.1 was present in compound 1. It was assumed that C-19 and the remaining nitrogen atom form a cyano (CN) group at the terminus of the aliphatic chain. This assumption was unambiguously confirmed by the HMBC correlation between H2-18 (δ 2.27, m) and C-19. Since all other aliphatic carbons were shown to be methylene carbons by the HSQC spectrum, the aliphatic chain of 1 would have 12 carbon atoms. The six degrees of unsaturation were accounted for by the pyridine ring and the cyano group. Therefore, compound 1 was determined to be 3-dodecyl pyridine containing a terminal cyano group.
Bioassay results showed that compound 1 had moderate cytotoxity against tumour cell lines A549, MCF-7 and Hela with IC50 values of 41.8, 48.4 and 33.2 μM, respectively.
3. Experimental section
3.1. General
All NMR experiments were run on a Varian Unity INOVA spectrometer (600 and 150 MHz for 1H and 13C, respectively) equipped with a 5 mm triple resonance (HCN) cold probe. LR-and HR-ESI-TOF-MS spectra were recorded on an Applied Biosystems Mariner instrument. The analytical LC-UV-ELSD-MS system was controlled by the Empower software and comprised Waters HPLC components equipped with a reversed-phase column (Phenomenex, Luna C18, 150 mm × 4.6 mm, 5 μm) and ran at 1.0 mL/min. The analytical HPLC system comprised Waters HPLC components (a gradient controller, two 515 pumps and one 2487 UV–Visable detector) and was equipped with a reversed-phase semi-preparative column (Phenomenex, Synergi Hydro-RP, 250 mm × 10.0 mm, 4 μm). All chemicals are of analytical grade.
3.2. Biological material
The marine sponge Haliclona sp. (coll. no. 95546) was collected from Indonesia Sea using scuba at depths of 15–30 m. It had no special skin skeleton and took the form of an encrusting mass of cylindrical to volcano-shaped projections with oscula at the high end. Taxonomic identification was based on comparison of the biological characteristics to another voucher sample in the repository. The voucher specimen and underwater photos are available at Crews laboratory in the University of California Santa Cruz (USA).
3.3. Extraction and isolation
Accelerated solvent extraction was performed on 13.8 g of dry and lightly chopped specimen at high pressure and temperature (1500 psi N2, 70°C) using the solvent series, water, hexane, dichloromethane and methanol. The afforded methanol extract (286 mg) was quickly fractionated into six fractions (F0–F5) using a preparative column (Phenomenex Gemini-NX C18, 50 mm × 21.2 mm, 5 μm). Bioassay test indicated that fraction F3 (38.1 mg) had moderate in vitro cytotoxicity against tumour cell lines L1210, Colon 38 and H-125. Fraction F3 was subjected to HPLC equipped with a reversal column (Phenomenex Synergi Hydro-RP, 250 mm × 4.6 mm, 4 μm) to give compound 1 (1.6 mg, retention time 52 min) utilising a flow rate of 1 mL/min, 254 nm detection and the elution condition: 60 min gradient from 52% to 90% CH3CN in H2O.
Compound 1: colourless oil; 1H NMR (600 MHz, CDCl3) δ 8.46 (2H, br s, H-2, H-6), 7.49 (1H, d, J = 12 Hz, H-4), 7.21 (1H, br s, H-5), 2.60 (2H, t, J = 6 Hz, H-7), 2.27 (2H, m, H-18), 1.25–1.31 (20H, m, H-8-H-17); 13C NMR (150 MHz, CDCl3) δ 150.0 (d, C-6), 147.2 (d, C-2), 135.9 (s, C-3), 130.0 (d, C-4), 123.5 (d, C-5), 119.1 (s, C-19), 36.0 (t, C-7), 27.0–33.1 (t, C-8-C-17), 24.6 (t, C-18); HR-ESI-TOF-MS m/z [M + H]+273.2312 [M + H]+ (calcd for C18H29N2, 273.2325).
3.4. Cytotoxicity assay
Tumour cell lines A549, MCF-7 and Hela were grown in RPMI medium with 10% foetal bovine serum, penicillin (100 U/mL) and streptomycin (50 μg/mL) and cultured in a 96-well plate at a density of 5 × 105 cells per well. Compound 1 was added to each well, respectively, with increasing concentrations. Cell lines without treatment by compound were used as the control. The incubation was performed in a humidified, 37°C, 5% CO2-containing incubator for 24 h. Then 10 μL CCK8 dye (Beyotime Inst. Biotech., Shanghai, China) was added to each well, cells were incubated at 37°C for 1–2 h and plates were read in a Victor-V multilabel counter (Perkin-Elmer, Waltham, USA) using the default europium detection protocol. IC50 value of compound 1 was calculated by comparison with DMSO-treated control wells.
Supplementary Material
Acknowledgments
Funding
This work was co-financially supported from the ‘Oversea Academic Research’ programme from Zhejiang University of Technology (China) and the Qianjiang Talent plan D of Zhejiang (China).
Footnotes
Supplemental data for this article can be accessed at http://dx.doi.org/10.1080/14786419.2015.1054826
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Berlinck RGS, Ogawa CA, Almeida AMP, Sanchez MAA, Malpezzi ELA, Costa LV, Hajdu E, De Freitas JC. Chemical and pharmacological characterization of halitoxin from Amphimedon viridis (Porifera) from the southeastern Brazilian coast. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1996;115:155–163. doi: 10.1016/S0742-8413(96)00107-7. [DOI] [PubMed] [Google Scholar]
- Johnson TA, Morgan MVC, Aratow NA, Estee SA, Sashidhara KV, Loveridge ST, Segraves NL, Crews P. J Nat Prod. 2010;73:359–364. doi: 10.1021/np900565a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariya Y, Kubota T, Fromont J, Kobayashi J, Pyrinadine A. Pyrinadine a, a novel pyridine alkaloid with an azoxy moiety from sponge Cribrochalina sp. Tetrahedron Lett. 2006;47:997–998. doi: 10.1016/j.tetlet.2005.11.163. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Murayama T, Kosuge S, Kanda F, Ishibashi M, Kobayashi H, Ohizumi Y, Ohta T, Nozoe S, Sasaki T. Niphatesines A–D, new antineoplastic pyridine alkaloids from the okinawan marine sponge Niphates sp. J Chem Soci Perkin Trans 1. 1990;12:3301–3303. doi: 10.1039/p19900003301. [DOI] [Google Scholar]
- Kobayashi J, Zeng CM, Ishibashi M, Shigemori H, Sasaki T, Mikami Y. Niphatesines E–H, new pyridine alkaloids from the Okinawan marine sponge Niphates sp. J Chem Soc Perkin Trans 1. 1992;11:1291–1294. doi: 10.1039/p19920001291. [DOI] [Google Scholar]
- Kubota T, Nishi T, Fukushi E, Kawabata J, Fromont J, Kobayashi J. Nakinadine A, a novel bis-pyridine alkaloid with a β-amino acid moiety from sponge Amphimedon sp. Tetrahedron Lett. 2007;48:4983–4985. doi: 10.1016/j.tetlet.2007.05.121. [DOI] [Google Scholar]
- Matsunaga S, Shinoda A, Fusetani N. Cribrochalinamine oxides A and B, antifungal β-substituted pyridines with an azomethine N-oxide from a marine sponge Cribrochalina sp. Tetrahedron Lett. 1993;34:5953–5954. doi: 10.1016/S0040-4039(00)73823-8. [DOI] [Google Scholar]
- Nishi T, Kubota T, Fromont J, Sasaki T, Kobayashi J. Nakinadines B–F: new pyridine alkaloids with a β-amino acid moiety from sponge Amphimedon sp. Tetrahedron. 2008;64:3127–3132. doi: 10.1016/j.tet.2008.01.111. [DOI] [Google Scholar]
- Oku N, Nagai K, Shindoh N, Terada Y, van Soest RWM, Matsunaga S, Fusetani N. Three new cyclostellettamines, which inhibit histone deacetylase, from a marine sponge of the genus Xestospongia. Bioorg Med Chem Lett. 2004;14:2617–2620. doi: 10.1016/j.bmcl.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Sakemi S, Totton LE, Sun HH. Xestamines A, B, and C, three new long-chain methoxylamine pyridines from the sponge Xestospongia wiedenmayeri. J Nat Prod. 1990;53:995–999. doi: 10.1021/np50070a038. [DOI] [Google Scholar]
- Schmidt G, Timm C, Grube A, Volk CA, Köck M. Viscosalines B1,2 and E1,2: challenging new 3-alkyl pyridinium alkaloids from the marine sponge Haliclona viscosa. Chem Eur J. 2012;18:8180–8189. doi: 10.1002/chem.201101362. [DOI] [PubMed] [Google Scholar]
- Volk CA, Köck M. Viscosamine: the first naturally occurring trimeric 3-alkyl pyridinium alkaloid. Org Lett. 2003;5:3567–3569. doi: 10.1021/ol035006i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.