Capsule Summary
The authors identify Natural Killer cell deficiency in post-transplant severe combined immunodeficiency patients who developed severe human papilloma virus infections as a long term complication.
Keywords: HPV infection, NK cell deficiency, stem cell transplant
To The Editor
Hematopoietic stem cell transplantation (HSCT) has significantly improved survival in severe combined immunodeficiency (SCID). Earlier identification and intervention with HSCT is associated with fewer short-term complications and improved long-term survival.1 Long-term follow up studies of post-transplant SCID patients document sustained T cell engraftment that is nonetheless complicated by respiratory disease, growth and neurodevelopmental impairment and susceptibility to chronic infections.2 Severe chronic human papilloma virus (HPV) infections have been noted in subsets of SCID patients post-transplant3,4 This has been confined to only those having γc or JAK-3 mutations. The etiology of severe HPV infections in these patients has not been clearly defined.
Intact T-cell mediated immunity and NK cell function is imperative for host defense against HPV infection.5,6 Fifty-percent of subjects with T−B+NK− SCID due to γc or JAK-3 genetic mutations were identified as having severe HPV infections years post-HSCT.3 No specific differences in transplant-specific or immune parameters were defined between the patients having or not having HPV. While median NK cell counts were lower in SCID patients with γc or JAK-3 mutations as compared to other SCID phenotypes, there were no significant differences in NK cell counts between those having or not having HPV. NK cell cytotoxic function while depressed, did not correlate with HPV infection.
We performed a retrospective review of SCID patients (n=65) treated at our institution between 1981 and 2012. Standard post HSCT evaluation included T, B and NK cell enumeration at 100 days, 6 months and annually. NK cell functional assays were performed in patients with HPV disease. Sixty demonstrated donor engraftment. None had HPV exposure at delivery or any HPV clinical disease prior to HSCT. T−B+NK− SCID phenotype distribution was present in 52% (31/60) children. These were comprised of 20 γc, 3 Jak-3, 2 CD3 δ, and 6 unknown molecular defects. Fifteen (48%) of these 31 patients received no (n=14) or reduced intensity conditioning (n=1), and all others (n=16) received myeloablative conditioning pre-HSCT.
Six (19%) patients died, all within 12 months of transplant and all had serious infections at presentation. Six of the surviving patients developed severe cutaneous HPV infection (2 or more locations and resistant to >3 forms of treatment) but none had genital lesions or epidermodysplasia verruciformis. No patient with HPV disease had other chronic viral infections.
Long term follow up records were not available for data extraction in 5 patients, two of whom had evidence of HPV disease. Extensive medical record review was performed for surviving patients and was possible for 20 patients (15γc, 2JAK3, 2CD3δ, 1 unknown mutation).
Four patients had >10 year post-HSCT follow up and chronic HPV infection (Table I and example of HPV disease in Figure Ia). All had excellent T cell engraftment with normal T cell function and 75% had normal B cell function post-HSCT (see Table E1). None of the HPV affected patients received pre-HSCT conditioning. Mean age at onset of warts was 7.4±1.4 years post-HSCT. All SCID cases without HPV disease (n=16; 12 γC, 2 JAK3, 2CD3δ) received myeloablative conditioning and mean follow-up was 6.5 years post-HSCT.
Table I.
Demographic, clinical and immunologic characteristics of four patients with severe HPV disease.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| SCID Phenotype | T-B+NK− | T-B+NK− | T-B+NK− | T-B+NK− |
| Specific diagnosis | γc | Unknown | γc | γc |
| Age at transplant (days) | 22 | 120 | 14 | 219 |
| Type of Transplant | MMRD mother | MMRD mother | MRD sister | MRD sister |
| Pre-transplant conditioning | None | None | None | None |
| T cell Depletion | None | None | None | None |
| Engraftment | Mixed (T+B−) | Mixed (T+B−) | Mixed (T+B−) | Mixed (T+B−) |
| T cell Engraftment (%) | 100 | 99 | 98 | 100 |
| Time of onset of warts after transplant (years) | 7.75 | 9.4 | 7 | 5.75 |
| Location of warts | Face, trunk | Trunk, arms | Face, hands, feet, arms, legs | Face, hands, feet, arms, legs |
| Other Long Term Comorbidities | PLE, Asthma, On Immunoglobulin replacement | None | None | None |
| Most recent ALC cells/mm3 | 1755 | 2014 | 1650 | 1482 |
| Most recent CD3 cells/mm3 (%) | 1615 (92%) | 1672 (83%) | 1518 (92%) | 1289 (87%) |
| Most recent CD4 cells/mm3 (%) | 562 (32%) | 467 (23%) | 726 (45%) | 888 (60%) |
| Most recent CD19 cells/mm3 (%) | 123 (7%) | 282 (14%) | 99 (6%) | 133 (9%) |
| Most recent IgG mg/dl | 599 | 873 | 1139 | 1690 |
(MRD: Matched Related Transplant, MMRD: Mismatched related transplant, PLE: Protein Losing enteropathy, ALC: Absolute Lymphocyte count)
Figure Ia.
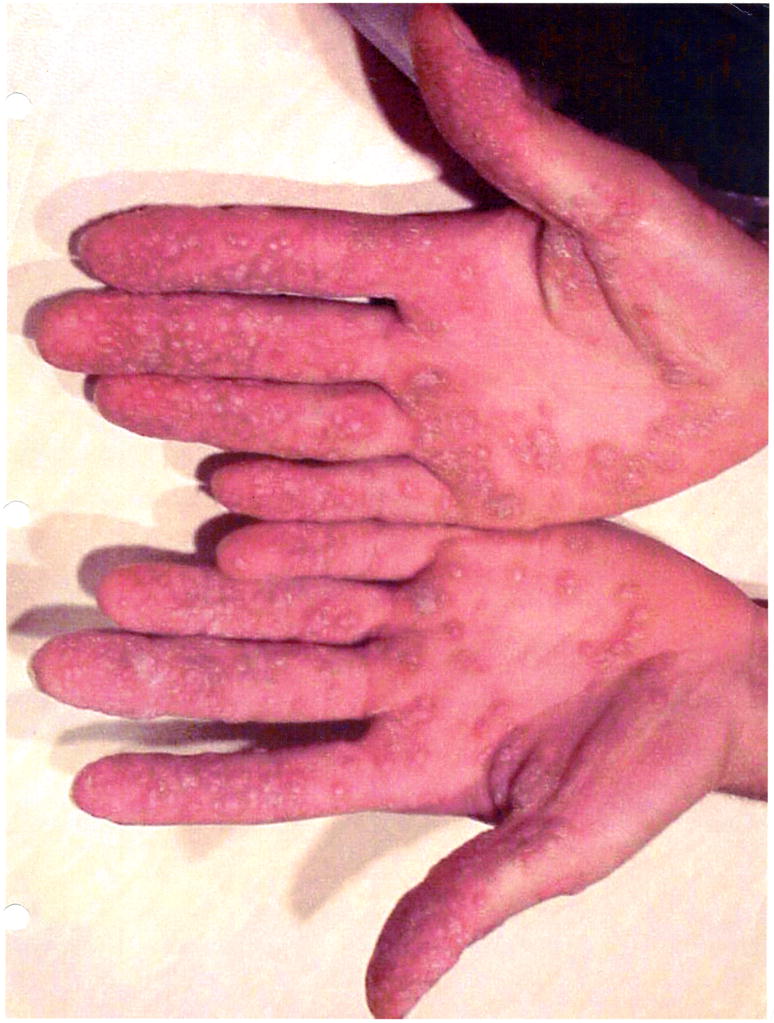
Multiple warts seen on the hands of a patient 7 years after HSCT for γc SCID.
As expected, the pre-transplant NK cells numbers were low, mean 38±75 cells/mm3. In the HPV cohort, the absolute number of NK cells post-transplant remained low and did not change significantly from the pre-transplant NK cell numbers (mean 34±22 cells/mm3, P=.9). In contrast, patients without HPV disease (n=16), had good NK cell restoration post-HSCT with a mean absolute number of NK cells increasing to 209±137 cells/mm3; P <.0001. The difference in NK cell immune reconstitution between patients with and without HPV disease was significant (P=.02, Figure Ib). When the non γc and JAK-3 patients were excluded from the analysis, the NK cell numbers were still significantly lower in patients with HPV disease (mean 34±22 vs 175±105 cells/mm3 p=.02).
Figure Ib. HPV infection in post-HSCT patient is associated with low number of NK cells in PBMCs.
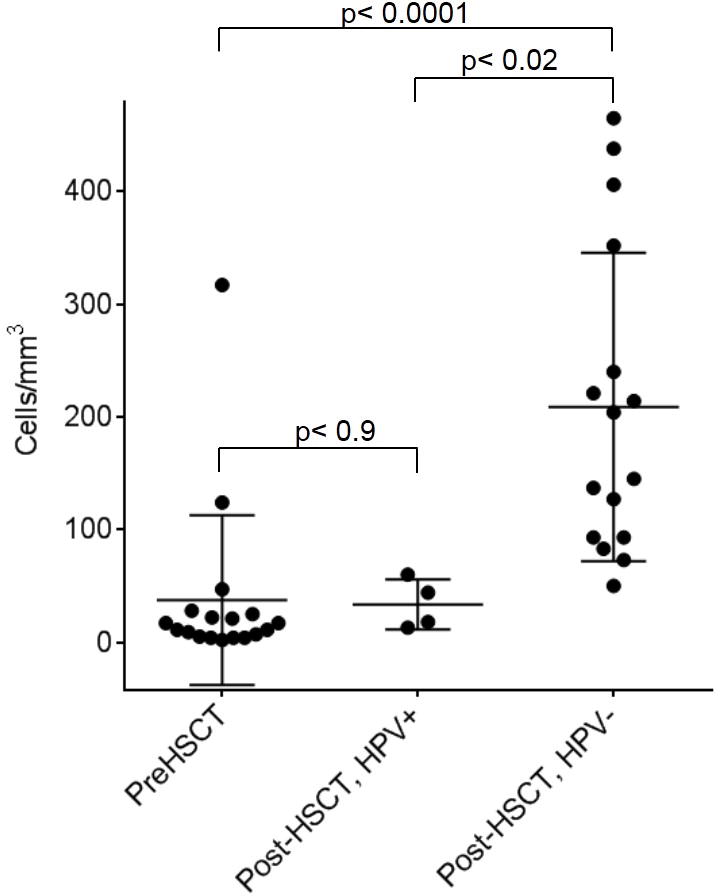
NK cell numbers were compared in patients before (Pre-HSCT, left) and after (Post-HSCT, middle and right) hematopoietic stem cell transplant(HSCT). In comparison to the Pre-HSCT group, a failure to increase NK cell number (p<0.09) after transplant is noted in patients with HPV (middle), whereas patients without HPV show significantly higher number of NK cells (p<0.0001) (right). P values were obtained by 2-tailed T-test.
In the HPV cohort, NK cell cytolytic activity was performed using PBMC freshly isolated from whole blood in 51Cr –release assays against K562 target cells in an effort to functionally confirm the reduction in NK cell counts.7 In-vitro NK cell cytotoxic function was severely depressed in all 4 patients (Figure Ic). Stimulation with IL-2 modestly, but significantly improved cytotoxic function. CD107a mobilization was measured by flow cytometry to assess the NK cell degranulation process in a single patient. Despite having low NK cell counts the existing NK cells demonstrated abnormal degranulation with <50% of normal Mean Channel Fluorescence for induced CD107a levels.
Figure Ic. IL-2 rescues cytotoxic effect of NK cells in HPV+ SCID patients.
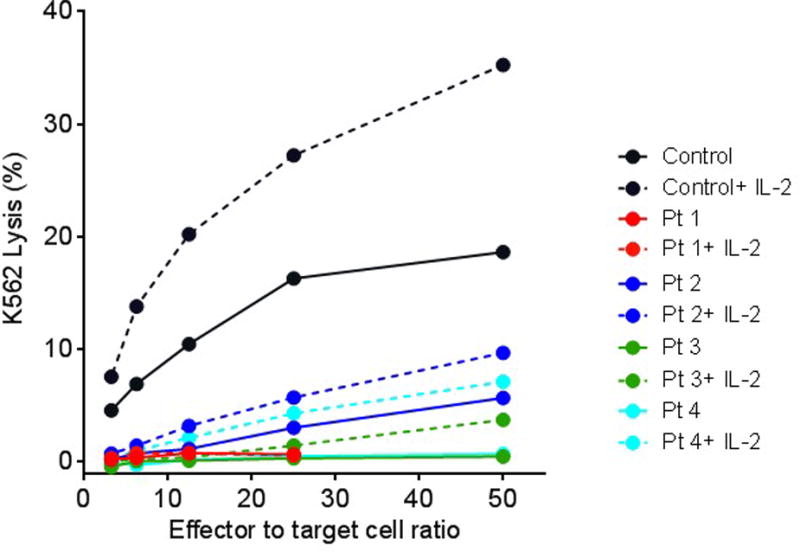
4 hour cytotoxicity assay was performed against K562 target cells using PBMCs as effectors from healthy donors (black) and patients with HPV (red, blue, green and teal) in the presence (dotted line) or absence (solid line) of IL-2. For each pair of IL-2 treated vs untreated group, increases in IL-2 mediated cytotoxicity were significant (P < 0.002, two-way ANOVA).
Our study, consistent with previous reports, demonstrated chronic HPV disease in T−B+NK− SCID patients after HSCT. Severe warts were not observed in any of the cases that received myeloablative conditioning. All T−B+NK− SCID patients without conditioning and with poor evidence of NK cell engraftment developed warts over 7 years of follow up. In contrast to previously reported rates (50–64%) of severe HPV disease, our cohort showed a lower rate (19%) of HPV affected children overall. While our numbers were relatively small, in contrast to other studies, we observed significantly depressed NK cell counts in patients with HPV disease. ‘Orange et al have previously described improved in vivo and ex vivo NK cell function in Wiskott-Aldrich patients with IL-2 administration through F actin reorganization.8 We also noted that IL-2 was able to promote at least some cytotoxic activity from patient cells in-vitro suggesting a potential topic for further therapeutic research.
Study limitations include absence of data for 2 HPV patients who were lost to follow up. We had a shorter follow up period for HPV-negative T−B+NK− SCID, 6.5 years versus >10 years for the HPV cohort. This could have introduced an ascertainment bias for HPV detection and may explain our lower overall rate of identified HPV. However, it is clear that post-HSCT, the cohort without HPV disease had markedly improved NK cell engraftment when compared with their counterparts with HPV disease.
Investigators have proposed that in γc receptor deficiency, dendritic cells may remain dysfunctional post-HSCT and thus permissive to HPV infection despite donor T-cell engraftment.3 No specific data supports this theory. Our study revealed a lower incidence of HPV-disease than in other cohorts likely related to the lower mean age of our HPV-negative cohort and excellent engraftment of T, B and NK cells in our entire cohort. We propose that poor NK cell engraftment and function represents a more likely contributor to the development of severe HPV disease in our 4 patients. It is possible that pre-HSCT myeloablation improved NK cell engraftment and function, allowing for improved response to HPV exposure in later life and thus disease limitation. With improvements in early diagnosis and curative treatment in SCID, it is imperative to review and determine the etiology of long term adverse events like severe HPV disease following transplant.
Supplementary Material
Acknowledgments
We would like to thank our patients and their families for their photographs and participation.
Funding Source: This work was supported by NIH-AI067946 to JSO
Footnotes
Conflict-of-Interest Disclosure: None
Contributor Information
Qurat-ul-Ain Kamili, Clinical Fellow, Section of Immunology, Allergy and Rheumatology, Division of Internal Medicine, Baylor College of Medicine, Houston, TX.
Filiz O Seeborg, Assistant Professor of Pediatrics, Department of Pediatrics, Section Immunology, Allergy and Rheumatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Kapil Saxena, Medical Student Year 2, Baylor College of Medicine, Houston, TX.
Sarah K Nicholas, Clinical Instructor in Pediatrics, Department of Pediatrics, Section Immunology, Allergy and Rheumatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Pinaki P Banerjee, Assistant Professor of Pediatrics, Department of Pediatrics, Texas Childrens Hospital, Center for Human Immunobiology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Laura S Angelo, Assistant Professor of Pediatrics, Department of Pediatrics, Texas Childrens Hospital, Center for Human Immunobiology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Emily M Mace, Post-doctoral Fellow, Department of Pediatrics, Texas Childrens Hospital, Center for Human Immunobiology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Lisa R Forbes, Assistant Professor of Pediatrics, Department of Pediatrics, Section Immunology, Allergy and Rheumatology, Baylor College of Medicine, Texas Children’s Hospital, Center for Human Immunobiology Medical Director, Houston, TX.
Caridad Martinez, Assistant Professor of Pediatrics, Texas Children’s Cancer Center, Department of Pediatrics, Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX.
Teresa S Wright, Assistant Professor Departments of Dermatology and Pediatrics, Section of Pediatric Dermatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
Jordan S. Orange, Professor of Pediatrics, Chief, Immunology, Allergy and Rheumatology. Director, Center for Human Immunobiology, Texas Children’s Hospital, Professor of Pediatrics, Pathology and Immunology, Associate Vice Chair, Clinical Affairs, Baylor College of Medicine, Houston, TX.
Imelda Celine Hanson, Professor of Pediatrics, Department of Pediatrics, Section Immunology, Allergy and Rheumatology, Baylor College of Medicine, Texas Children’s Hospital, Houston, TX.
References
- 1.Buckley RH. Transplantation of hematopoietic stem cells in human severe combined immunodeficiency: Long term outcomes. Immunol Res. 2011;49(1–3):25–43. doi: 10.1007/s12026-010-8191-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gennery AR, member of the Inborn Errors Working Party for the European Group for Blood and Marrow Transplantation and European Society for Immunodeficiency Transplantation of hematopoietic stem cells and long-term survival for primary immunodeficiency’s in Europe: Entering a new century, do we do better? J Allergy Clan Immune. 2010;126(1–3):602–610. doi: 10.1016/j.jaci.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 3.Laffort C, Le Deist F, Favre M, Caillat-Zucman S, Radford-Weiss I, Debre M, et al. Severe cutaneous papillomavirus disease after haematopoietic stem-cell transplantation in patients with severe combined immune deficiency caused by common γc cytokine receptor subunit or JAK-3 deficiency. Lancet. 2004;363(9426):2051–4. doi: 10.1016/S0140-6736(04)16457-X. [DOI] [PubMed] [Google Scholar]
- 4.Gaspar HB, Harwood C, Leigh I, Thrasher AJ. Severe cutaneous papillomavirus disease after haematopoietic stem-cell transplantation in patients with severe combined immunodeficiency. Br J Haematol. 2004;127(2):232–3. doi: 10.1111/j.1365-2141.2004.05176.x. [DOI] [PubMed] [Google Scholar]
- 5.Holland SM, Leiding JW. Warts and all: human papilloma virus in primary immunodeficiencies. J Allergy Clin Immunol. 2012;130(5):1030–48. doi: 10.1016/j.jaci.2012.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Orange JS. NK cell deficiency. J Allergy Clin Immunol. 2013;132:515–25. doi: 10.1016/j.jaci.2013.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orange JS, Brodeur SR, Jain A, Bonilla FA, Schneider LC, Kretschmer R, et al. Deficient natural killer cell cytotoxicity in patients with IKK-gamma/NEMO mutations. J Clin Invest. 2002;109(11):1501–9. doi: 10.1172/JCI14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011;121(4):1535–48. doi: 10.1172/JCI44862. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


