Abstract
Objective:
To investigate differences in clinical presentation, electrodiagnostic measures, CSF changes, and treatment outcome measures in HIV-infected and HIV-uninfected patients with chronic inflammatory demyelinating polyneuropathy (CIDP).
Methods:
A retrospective analysis of medical records of all patients meeting the European Federation of Neurology diagnostic criteria for idiopathic CIDP was performed in 2 neuromuscular units in Kwa-Zulu Natal between 2003 and 2015.
Results:
Eighty-four patients were included in the study; 39 were HIV-infected and 45 were HIV-uninfected. Among the HIV-infected patients, the majority were younger, were female, and had a monophasic progressive illness. Eighty-six percent (86%) were corticosteroid-responsive and 76% were in remission within 6–12 months requiring no further treatment. Among the HIV- uninfected patients, the majority were older, were male, and had a relapsing-remitting course. Twenty-seven percent (27%) were corticosteroid-responsive, 95% required combination therapy, and 33% were not in remission by 18 months on therapy.
Conclusion:
This study shows that HIV-infected patients with CIDP were younger, were more often female, displayed a monophasic progressive course, were highly steroid-responsive, and went into remission within 12 months of corticosteroid initiation.
Chronic inflammatory demyelinating polyneuropathy (CIDP) is an acquired demyelinating immune-mediated neuropathy. It is the most common treatable immune-mediated chronic neuropathy worldwide, with a prevalence ranging from 1 to 9 cases per 100,000.1–6
CIDP is considered an autoimmune disorder in which an aberrant immune response is directed towards components of the myelin sheath. However, the exact immunopathogenesis of the disease remains unknown. The response to immunosuppressive treatment is variable.7,8 Presently there is no biomarker to predict response to therapy.1
CIDP is known to occur in the setting of HIV, as are other immune-mediated neurologic disorders including a wide spectrum of neuropathies.9–16 It is commoner than acute inflammatory demyelinating polyneuropathy (AIDP).11,13,16–18 Despite the above, the clinical presentation, primary treatment outcomes, and electrophysiologic and histologic findings of CIDP in the setting of HIV are limited to case series and case reports.19–25 Treatment recommendations include IV immunoglobulin (IVIg) or plasma exchange to limit the risk of infections with corticosteroid use.7,16,17,26,27 Cost and coexistence of HIV-associated renal disease limits IVIg use and plasma exchange when treating CIDP.28,29 Locally, we manage HIV-infected patients with CIDP with corticosteroid therapy unless contraindicated.
Our experience suggests that HIV-infected patients with CIDP have a benign course, with few or no relapses, and respond rapidly to corticosteroid monotherapy. This suggests that the immune mechanisms in HIV-infected patients may aid recovery. The correlation between CD4 counts, viral load, and recovery is unknown.
We compared CIDP in HIV-infected and HIV-uninfected patients and describe differences between the 2 categories.
METHODS
The study was a retrospective chart review of a cohort of patients with idiopathic immune-mediated CIDP from the neuromuscular clinics at Inkosi Albert Luthuli Central Hospital (IALCH) in Durban and Greys Hospital in Pietermaritzburg from 2003 to 2014. The 2 units are the only neuromuscular units in the province and provide a neurologic service to approximately 11 million people. South Africa has the highest prevalence of HIV in the world, with an estimate of 6.3 million people living with HIV in 2014. Kwa-Zulu Natal (KZN) comprises 40% of the HIV burden in South Africa.30 The study was approved by the University of KZN Biomedical Research Ethics Committee (ethics reference number: BE 272/15).
Patients fulfilling the clinical, electrodiagnostic, and CSF criteria of the European Federation of Neurologic Sciences (ENFS)/Peripheral Nerve Society (PNS) for CIDP were included in the study (see supplementary data at Neurology.org/nn). Patients were excluded if they did not meet the diagnostic criteria for CIDP, clinical presentation was suggestive of AIDP, secondary causes were identified, or their HIV status was unknown. Data extracted included age; sex; race; duration, onset, and course of the disease; clinical presentation; antiretroviral therapy (ART) in HIV-infected patients; response to therapy, described as time to respond, number of relapses, and time to remission; degree of functional recovery, scored as Overall Disability Sum Score (ODSS) and the Inflammatory Neuropathy Cause and Treatment (INCAT) scale prior to and after treatment at 3–6 monthly intervals up to 18 months follow-up; side effects of treatment; electrophysiologic and CSF results; CD4 counts; and viral loads.
The cohort was divided into 2 categories: HIV-infected and HIV-uninfected. Within each category, patients were further classified as being corticosteroid-responsive, IVIg-responsive, or requiring combination therapy (figure 1). Definitions for the above terms including remission, lack of efficacy, as well as the management protocol followed by the neurology units at IALCH and Greys Hospital are included in the supplementary data.
Figure 1. Trial profile.
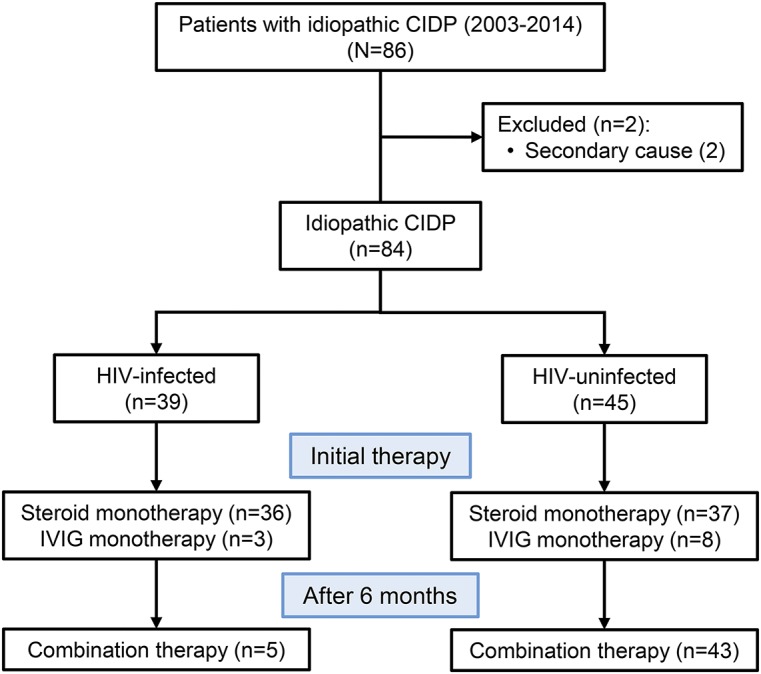
CIDP = chronic inflammatory demyelinating polyneuropathy; IVIg = IV immunoglobulin.
Statistical methods.
Data were entered in Microsoft Excel and analyzed using Prism Software. Descriptive statistics such as percentages, interquartile ranges (IQR), medians, and p values were used to summarize the results in the HIV-infected and HIV-uninfected patients. Tests used to calculate the above included Fisher exact test for categorical variables, Mann-Whitney U test for continuous variables, and Spearman test for correlation between CD4 counts, viral loads, and functional scores. A p value of <0.05 was regarded as significant.
RESULTS
Eighty-six patients fulfilled the criteria for definite CIDP; 2 were excluded due to the presence of an immunoglobulin M paraprotein. There were 44 men and 40 women. The median age was 39.5 years (IQR 27–66). The ethnic distribution was 56 black Africans (66.7%), 22 Indians (26%), and 6 whites (7.1%).
The associated etiologies in table 1 show that 10 of the 84 (11.9%) patients had type 2 diabetes mellitus (T2 DM); 2 had pulmonary tuberculosis many months after the diagnosis of CIDP, probably due to long-term immunosuppression from corticosteroid use; and 2 had epilepsy. In the remaining patients, no secondary cause was identified. There was no difference in the clinical characteristics between patients with and without T2 DM (table e-1). These patients were included in the cohort.
Table 1.
Baseline demographic features of the HIV-infected and HIV-uninfected chronic inflammatory demyelinating polyneuropathy (CIDP) categories
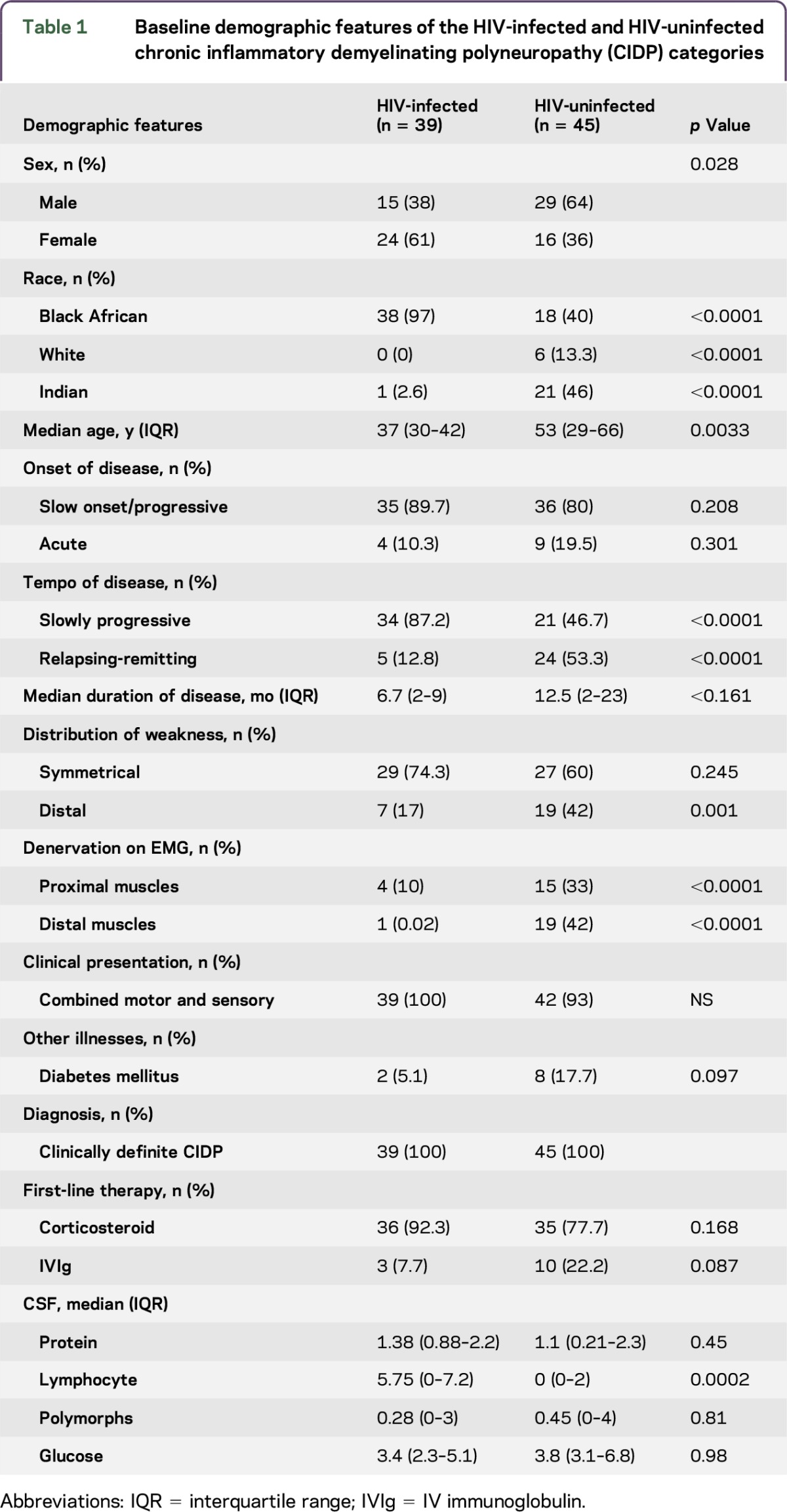
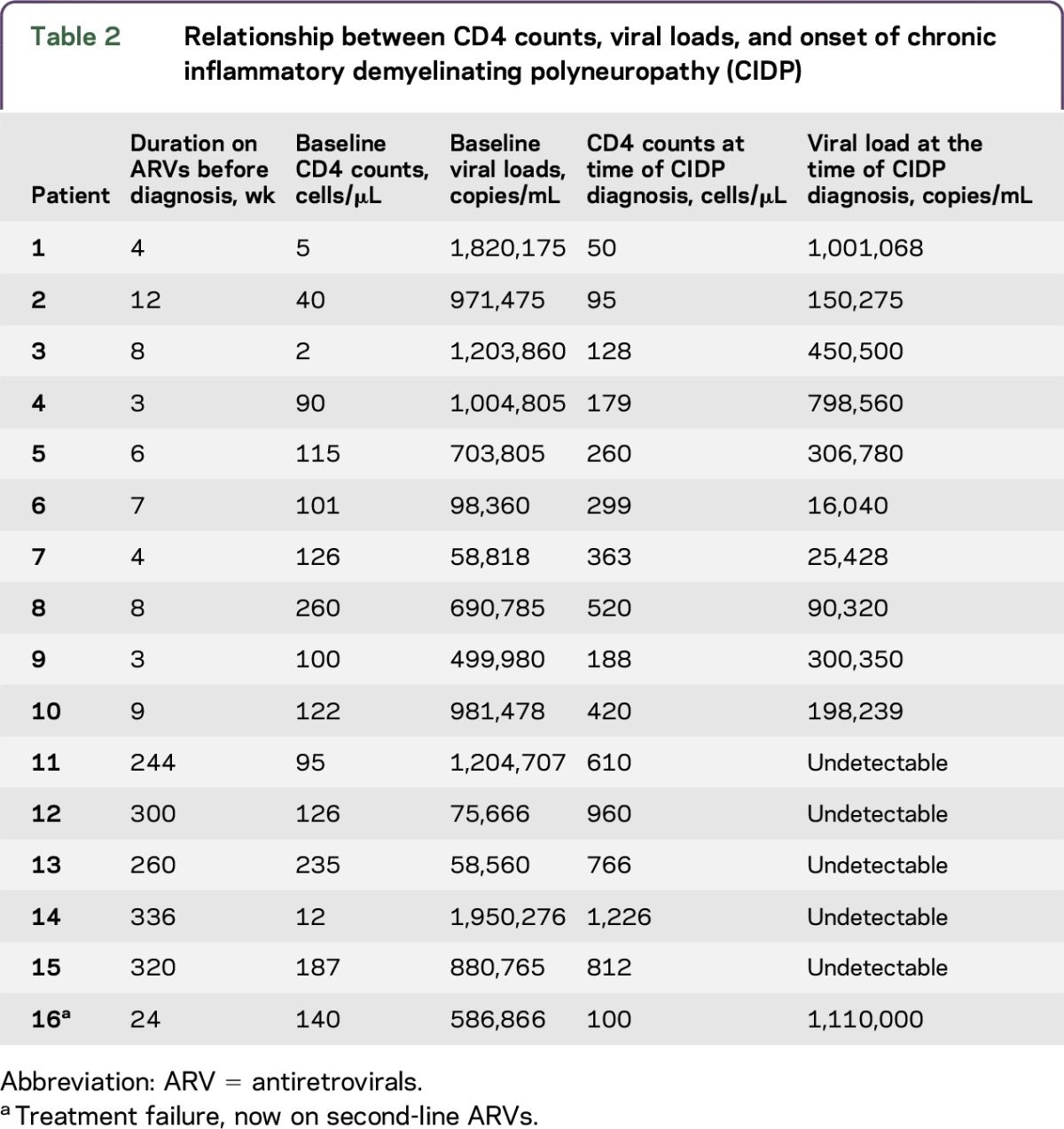
Thirty-nine of the 84 patients (46.5%) were HIV-infected and 45 (53.5%) were HIV-uninfected. Of the 39 HIV-infected patients, 38 were black Africans (97.5%) and 1 was Indian (2.5%). Sixty-one percent were female. Median age was 37 years (IQR 30–42 years). Compared to the HIV-uninfected patients, the HIV-infected patients were younger and had a female preponderance, with p values of 0.0033 and 0.028, respectively. The median CD4 count was 384 cells/mm3 (IQR 126–423 cells/mm3), median viral load was 440 copies/mL (IQR 0–34,650 copies/mL). Twenty-three (59%) patients were ART-naive at the time of CIDP diagnosis and 16 (41%) were on ARTs. Ten (62%) of the patients who were on ARTs received treatment for a median duration of 6.5 weeks (IQR 4–8). Patient 16 had treatment failure requiring second-line therapy. Five patients (31%) received ARTs for more than 5 years (table 2, patients 11–15).
Table 2.
Relationship between CD4 counts, viral loads, and onset of chronic inflammatory demyelinating polyneuropathy (CIDP)
As shown in table 1, 87.2% of the HIV-infected and 46.7% of the HIV-uninfected patients presented with a monophasic progressive course (p ≤ 0.0001). Median duration of illness among the HIV-infected and HIV-uninfected patients was 6.7 months (IQR 2–9 months) and 12.5 months (IQR 2–23 months), respectively (p = 0.16). Relapses frequencies were 12.8% among HIV-infected patients compared to 53.3% among HIV-uninfected patients (p ≤ 0.0001). Four (10%) HIV-infected patients and 9 (20%) HIV-uninfected patients had an acute presentation (p = 0.31). The majority of patients in both categories had a slowly progressive onset (p = 0.21). Clinical signs were symmetrical in 74% and 60% of HIV-infected and HIV-uninfected patients, respectively (p = 0.245). Forty-two percent of HIV-uninfected patients and 17% of HIV-infected patients had distal weakness (p = 0.001). In both categories, the majority of patients presented with a combined sensory motor presentation. There was no difference in other clinical signs such as cranial nerve involvement, truncal weakness, tremor, or thickened nerves. No patients presented with respiratory, bulbar, or autonomic signs or symptoms.
CSF analysis (table 1) revealed a difference in lymphocyte counts between the 2 categories, with a median cell count of 5.75 cells/μL (IQR 0–7.2 cells/μL) among HIV-infected patients and 0 cells/μL (IQR 0–2 cells/μL) among the HIV-uninfected patients (p = 0.0002). Protein levels, polymorph counts, and glucose levels were not different. T2 DM was associated with raised protein levels in 90% of patients. Albumino-cytologic dissociation was present in 60% of HIV-uninfected patients and 25% of the HIV-infected patients.
Treatment, treatment outcomes, and side effects of therapy are described in table 3 and figures e-1 and e-2. Three HIV-infected patients (2 with T2 DM, 1 hypertensive) received IVIg as first-line therapy and responded within 3 months. There were no relapses or side effects.
Table 3.
Response to therapy, relapses, treatment at remission, and side effects to therapy in the HIV-infected and HIV-uninfected category
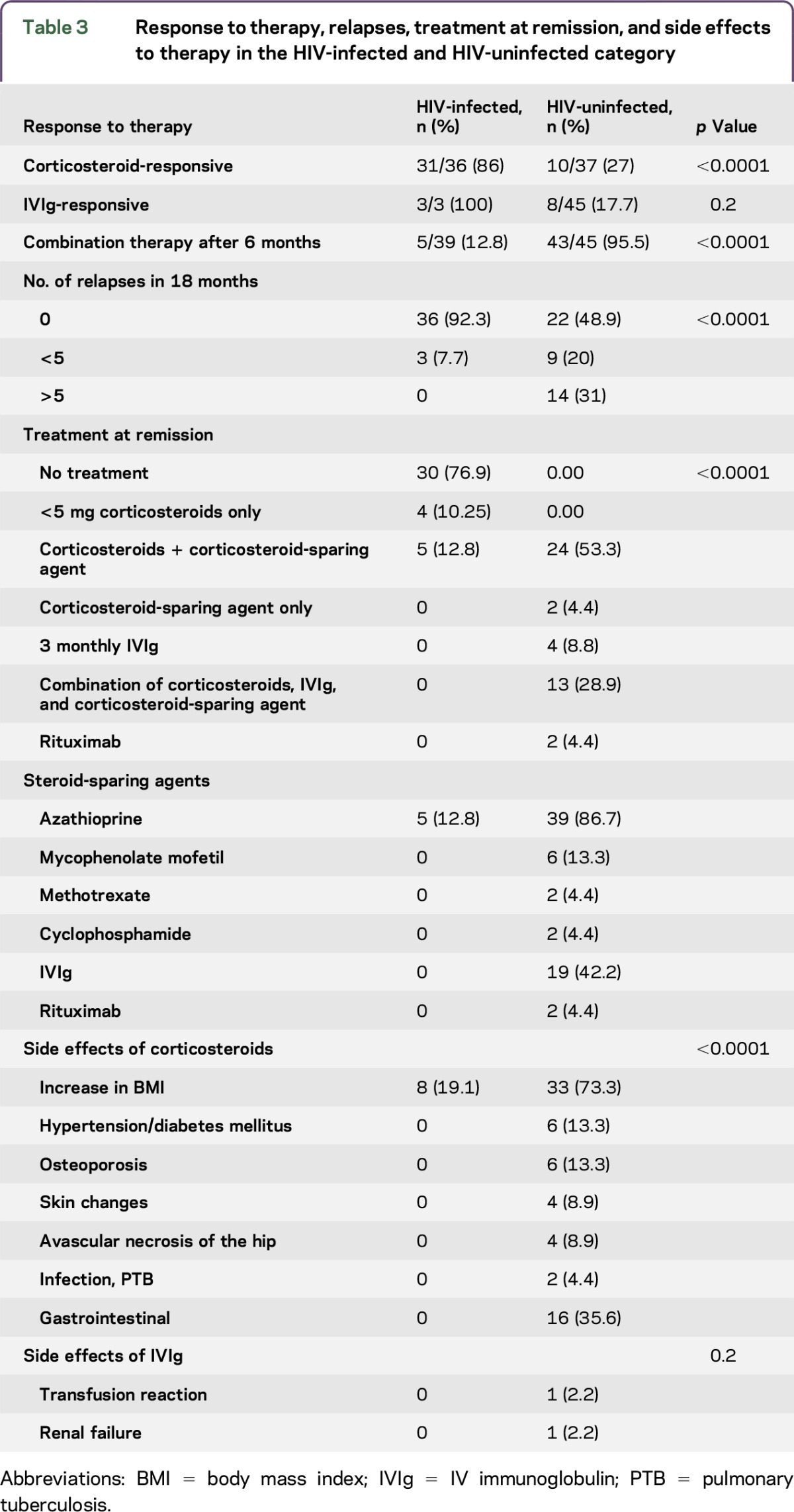
Eighty-six percent of the HIV-infected patients were corticosteroid-responsive. Fifty-six percent responded within the first 3 months, 30.7% in 3–6 months, and 12.8% in the 6- to 12-month period (figure e-2). All HIV-infected patients showed a corticosteroid response by 12 months. Seventy-six percent of the corticosteroid-responsive HIV-infected patients were in remission within the first 6 months (figure e-1) and 24% were in remission by 12 months. Seventy-seven percent of the HIV-infected patients were treatment-free at remission, 4 patients were on less than 5 mg prednisone per day, and 5 patients were on a combination of azathioprine and less than 5 mg prednisone per day. Eight of the HIV-infected patients developed an increased body mass index (BMI) during corticosteroid therapy compared to baseline. No other side effects were documented.
The functional assessment scores (INCAT and ODSS) improved significantly from high scores at presentation (patients being quadriplegic) to being almost fully functional by 6–12 months and normal by 18 months (table e-2).
Ninety-two percent of patients had no relapses in the 18-month follow-up period. Only 3 patients had fewer than 5 relapses during follow-up (table 3).
Twenty-seven percent of the HIV-uninfected patients were corticosteroid-responsive. Of the 8 patients with T2 DM on IVIg, only 25% responded within 3 months. Ninety-five percent of the HIV-uninfected patients required combination therapy (table 3). Twenty-two percent of patients responded to first-line therapy by 6 months, 17.8% by 12 months, 28.9% by 12–18 months, and 33.3% showed no response to therapy (figure e-1). Twenty-four percent went into remission by 6–12 months, 20% by 12–18 months, and 11% after 18 months. Forty-four percent were not in remission by 18-month follow-up (figure e-2). Various combinations of therapy listed in table 3 were used to aid remission in the above patients.
The HIV-uninfected patients experienced more side effects to corticosteroids compared to the HIV-infected patients (p < 0.0001). This included an increased BMI, development of a metabolic syndrome, osteoporosis, avascular necrosis of the hip, skin changes, infection, and gastrointestinal disturbances. Two patients had side effects to IVIg; 1 patient with T2 DM developed renal failure and another had a transfusion reaction (table 3). Treatment had to be discontinued. Despite therapy, the functional assessment scores in the above category showed minimal improvement after 18 months.
Among the HIV-infected patients, improving CD4 counts and decreasing HIV viral loads at 18 months did not correlate with functional recovery. Electrophysiologic studies showed no significant differences in distal motor latencies, conduction velocities, conduction block, temporal dispersion, or F-waves between the 2 categories (table e-3). However, there was a significant difference in the degree and distribution of denervation on EMG seen in the 2 categories. Only 12.8% of HIV-infected patients showed denervation in mainly proximal muscles and 75% of HIV-uninfected patients showed denervation in mainly distal muscles (table 1).
DISCUSSION
HIV-infected patients showed a rapid response to corticosteroid immunotherapy with minimal relapses. In contrast, the HIV-uninfected patients were refractory to treatment. The largest study is a prospective case series consisting of 23 patients (10 HIV-infected and 13 HIV-uninfected).19 This study reported no clinical or electrophysiologic differences between the 2 categories and no comparisons were made regarding treatment. Currently there are no comparative studies in the literature regarding treatment outcomes between HIV-infected and HIV-uninfected patients with CIDP. The current study showed significant differences in treatment outcomes, age, sex, and disease progression.
CIDP usually shows a male predominance, ranging from 1.31:1 to 2.8:1 in HIV-uninfected patients.19,31 Among the HIV-infected patients, the male: female ratio was reversed. This reversal in the sex ratio in HIV-infected patients was reported in one other study.19
The median age at onset among HIV-uninfected patients compares well to previous reported epidemiologic studies.3,6,32 Among HIV-infected patients, the median age at onset was significantly younger. The female preponderance and younger age at onset of HIV-infected patients with CIDP is an artefact of the sample and unlikely related to sex or age susceptibility as 60% of HIV-infected people in South Africa are young black females (Statistics South Africa, 2015).
The majority of HIV-infected patients presented with a monophasic slowly progressive course, whereas the HIV-uninfected patients presented with a relapsing-remitting course. This may relate to possible undefined differences in the underlying immune mechanisms in the 2 categories.
The mean CSF protein levels were slightly higher among the HIV-infected patients. This is in contrast to the findings of a recent study, but compatible with a previous case series consisting of 7 patients, 6 of whom had raised protein levels.15,19 High CSF protein levels reflect an immune-mediated disruption of the blood–nerve barrier at the level of the spinal roots, which correlates with proximal, possibly root involvement among the HIV-infected patients. However, the difference in protein levels did not reach statistical significance, possibly due to the short duration of the disease, small numbers, and unregulated immunity among the HIV-infected patients. As in previous studies, the HIV-infected patients had higher CSF lymphocyte counts, which are most likely related to CSF viremia rather than CIDP-related immunologic changes. This CSF finding is consistent with other studies.33,34 Despite 59% of HIV-infected patients being on ARTs, a significant number still had ongoing CSF lymphocytosis. Many of these patients had a suppressed plasma viral load. This may represent inadequate penetration of ART into the CSF space or CSF HIV resistance resulting in ongoing CSF viral replication.35
The response to corticosteroid monotherapy among HIV-infected patients was clearly demonstrated within 3 months. Most of the HIV-infected patients were in remission by 6 months. This short duration of corticosteroid therapy seems to be a safe and cost-effective option in HIV-infected patients. Current literature states that IVIg or plasma exchange is the preferred choice due to the risk of infection with corticosteroid therapy.8,20,22,27,29 However, in this study, the risk of infection was negligible with short duration of therapy. The only significant side effect documented among the HIV-infected patients was an increase in BMI. All patients had BMI documented at baseline and thereafter 3 monthly while on corticosteroids. Only HIV-uninfected patients had bone density scans as they were on long-term corticosteroids. This was not necessary among the HIV-infected patients as their duration of treatment was short.
The varying response to corticosteroid monotherapy in the 2 categories of patients suggests that the immunopathogenesis is different. Autoantibodies targeting noncompact myelin, which includes the nodes of Ranvier, paranode, and juxtaparanode regions, rather than major myelin protein such as P0, PMP22, may select for a relatively benign course.1 Support for antibodies in the pathogenesis was demonstrated by a good response to plasma exchange.25 A decline in HIV T-regulatory CD4 cells potentiates the emergence of autoimmune phenomena.36,37 ARTs result in immune reconstitution and upregulation of the total number of CD4 T-regulatory cells, which may contribute to remission. There is a single case report of CIDP resolving with ARTs alone.21 In our study, ARTs may have expedited recovery by restoring immune function.
In patients diagnosed as HIV-infected at CIDP diagnosis, ARTs and corticosteroids were commenced simultaneously. No deterioration occurred in this category. Concomitant use of corticosteroids may have potentially curtailed the immune reconstitution inflammatory syndrome (IRIS). This may be a potential avenue for future therapy in patients being initiated on ARTs. The 10 patients who had low baseline CD4 counts and high viral loads (table 2, patients 1–10) may have had CIDP as an IRIS phenomenon as they were commenced on ARTs for a short period. Patient 16, who developed immunologic and virologic failure, may have had an IRIS response when commencing second-line therapy. Presently there are no markers to predict who will develop this complication.
Five patients on long-term ARTs (table 2, patients 11–15) had complete immune reconstitution and behaved as HIV-uninfected patients with prolonged refractory disease and poor response to corticosteroids. Furthermore, the long duration of disease prior to presentation and presence of denervation on EMG indicating axonal damage (tables 1and e-4) may account for a poor response to corticosteroids.
Although AIDP was a potential diagnosis, this was discounted as all patients met the ENFS/PNS clinical and electrodiagnostic criteria for CIDP, progressed beyond 12 weeks, had typical electrodiagnostic findings for demyelination (table e-2), had no prior flulike illness or diarrheal illness, had an insidious onset, and had no bulbar, respiratory, or autonomic symptoms, and none showed spontaneous recovery during the initial 3 months of progression. The speculation that recovery may have occurred despite corticosteroid therapy can only be answered in a prospective study comparing placebo with corticosteroids. Nonetheless, patients with suspected AIDP and spontaneous recovery during the initial 3 months were excluded from the study. Furthermore, AIDP was unlikely in the 13 patients with acute-onset CIDP, as the 4 HIV-infected patients progressed beyond 12 weeks and responded to corticosteroid therapy within 4 weeks and the 9 HIV-uninfected patients showed a poor response to corticosteroid monotherapy in the first 6 months and required combination immunosuppressive therapy.
During the above study period, we have seen approximately 100–120 HIV- infected patients with AIDP at our neurology institute, who behaved differently from our HIV-infected patients with CIDP. All patients presented acutely and 70% had a preceding flu or diarrheal illness. Approximately 30% of patients required intensive care unit admission for bulbar/respiratory involvement or autonomic instability. Spontaneous recovery was seen in about 5% of these patients. All patients were treated with IVIg and not steroids. Therefore we concluded that our cohort of patients had CIDP rather than AIDP and were corticosteroid responsive rather than manifesting spontaneous recovery.
Limitations of the study are that it is a retrospective study; numbers are small and have tertiary care setting bias. Other limitations are that many patients may have been missed as a result of not being referred or being misdiagnosed.
This study suggests that treated HIV-infected patients with CIDP have a short duration of disease, have a benign course, and are highly corticosteroid-responsive compared to their HIV-uninfected counterparts. Although we satisfy the ENFS criteria for CIDP, the quick recovery and the relatively benign monophasic course makes AIDP a possibility. Perhaps the course and progression of CIDP in HIV-infected individuals is different and revision of criteria for the diagnosis of CIDP in HIV-infected individuals is required. This study also shows that corticosteroids are a cost-effective and safe option in HIV-infected patients with CIDP, especially in a resource-limited setting. Further prospective studies confirming a rapid corticosteroid response in HIV-infected patients with CIDP as well as unraveling the immune mechanisms responsible for CIDP in these patients is required to better define future therapy.
ACKNOWLEDGMENT
Statistical analysis was conducted by Susaan Marais, PhD, University of KwaZulu-Natal.
GLOSSARY
- AIDP
acute inflammatory demyelinating polyneuropathy
- ART
antiretroviral therapy
- BMI
body mass index
- CIDP
chronic inflammatory demyelinating polyneuropathy
- ENFS
European Federation of Neurologic Sciences
- IALCH
Inkosi Albert Luthuli Central Hospital
- INCAT
Inflammatory Neuropathy Cause and Treatment
- IQR
interquartile range
- IRIS
immune reconstitution inflammatory syndrome
- IVIg
IV immunoglobulin
- KZN
Kwa-Zulu Natal
- ODSS
Overall Disability Sum Score
- PNS
Peripheral Nerve Society
- T2 DM
type 2 diabetes mellitus
Footnotes
Supplemental data at Neurology.org/nn
AUTHOR CONTRIBUTIONS
Kaminie Moodley: developed concept, collected and analyzed data, generated manuscript. Vinod B. Patel: developed the concept, assisted with protocol development, analysis of data, and editing manuscript. P.L.A. Bill: reviewing manuscript, study concept or design, acquisition of data.
STUDY FUNDING
No targeted funding.
DISCLOSURES
The authors report no disclosures. Go to Neurology.org/nn for full disclosure forms.
REFERENCES
- 1.Mathey EK, Park SB, Hughes RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy: from pathology to phenotype. J Neurol Neurosurg Psychiatry 2015;86:973–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rajabally Y, Vital A, Ferrer X, et al. Chronic inflammatory demyelinating polyneuropathy caused by HIV infection in a patient with asymptomatic CMT 1A. J Peripher Nerv Syst 2000;5:158–162. [DOI] [PubMed] [Google Scholar]
- 3.Manji H. Neuropathy in HIV infection. Curr Opin Neurol 2000;13:589–592. [DOI] [PubMed] [Google Scholar]
- 4.McLeod JG, Pollard JD, Macaskill P, Mohamed A, Spring P, Khurana V. Prevalence of chronic inflammatory demyelinating polyneuropathy in New South Wales, Australia. Ann Neurol 1999;46:910–913. [PubMed] [Google Scholar]
- 5.Mahdi-Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. Eur J Neurol 2014;21:28–33. [DOI] [PubMed] [Google Scholar]
- 6.Knopf L, Menkes DL. Comorbid HIV and myasthenia gravis: case report and review of the literature. J Clin Neuromuscul Dis 2010;12:80–84. [DOI] [PubMed] [Google Scholar]
- 7.Bright RJ, Wilkinson J, Coventry BJ. Therapeutic options for chronic inflammatory demyelinating polyradiculoneuropathy: a systematic review. BMC Neurol 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dalakas MC; Medscape. Advances in the diagnosis, pathogenesis and treatment of CIDP. Nat Rev Neurol 2011;7:507–517. [DOI] [PubMed] [Google Scholar]
- 9.Dimachkie MM, Barohn RJ. Chronic inflammatory demyelinating polyneuropathy. Curr Treat Options Neurol 2013;15:350–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanche P, Diaz E, Gombert B, Sicard D, Rivoal O, Brezin A. Devic's neuromyelitis optica and HIV-1 infection. J Neurol Neurosurg Psychiatry 2000;68:795–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schutz SG, Robinson-Papp J. HIV-related neuropathy: current perspectives. HIV AIDS 2013;5:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrari S, Vento S, Monaco S, et al. Human immunodeficiency virus-associated peripheral neuropathies. Mayo Clin Proc 2006;81:213–219. [DOI] [PubMed] [Google Scholar]
- 13.Centner CM, Bateman KJ, Heckmann JM. Manifestations of HIV infection in the peripheral nervous system. Lancet Neurol 2013;12:295–309. [DOI] [PubMed] [Google Scholar]
- 14.Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst 2001;6:21–27. [DOI] [PubMed] [Google Scholar]
- 15.Cornblath DR, Hoke A. Recent advances in HIV neuropathy. Curr Opin Neurol 2006;19:446–450. [DOI] [PubMed] [Google Scholar]
- 16.Robinson-Papp J, Simpson DM. Neuromuscular diseases associated with HIV-1 infection. Muscle Nerve 2009;40:1043–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hahn K, Husstedt IW; Arendt GfdDN-A-A. HIV-associated neuropathies [in German]. Nervenarzt 2010;81:409–417. [DOI] [PubMed] [Google Scholar]
- 18.Miller RG, Parry GJ, Pfaeffl W, Lang W, Lippert R, Kiprov D. The spectrum of peripheral neuropathy associated with ARC and AIDS. Muscle Nerve 1988;11:857–863. [DOI] [PubMed] [Google Scholar]
- 19.Mochan A, Anderson D, Modi G. CIDP in a HIV endemic population: a prospective case series from Johannesburg, South Africa. J Neurol Sci 2016;363:39–42. [DOI] [PubMed] [Google Scholar]
- 20.Cornblath DR, McArthur JC, Kennedy PG, Witte AS, Griffin JW. Inflammatory demyelinating peripheral neuropathies associated with human T-cell lymphotropic virus type III infection. Ann Neurol 1987;21:32–40. [DOI] [PubMed] [Google Scholar]
- 21.Kume K, Ikeda K, Kamada M, Touge T, Deguchi K, Masaki T. Successful treatment of HIV-associated chronic inflammatory demyelinating polyneuropathy by early initiation of highly active anti-retroviral therapy [in Japanese]. Rinsho Shinkeigaku 2013;53:362–366. [DOI] [PubMed] [Google Scholar]
- 22.Vital A, Beylot M, Vital C, Delors B, Bloch B, Julien J. Morphological findings on peripheral nerve biopsies in 15 patients with human immunodeficiency virus infection. Acta Neuropathol 1992;83:618–623. [DOI] [PubMed] [Google Scholar]
- 23.Przedborski S, Liesnard C, Voordecker P, et al. Inflammatory demyelinating polyradiculoneuropathy associated with human immunodeficiency virus infection. J Neurol 1988;235:359–361. [DOI] [PubMed] [Google Scholar]
- 24.Chaunu MP, Ratinahirana H, Raphael M, et al. The spectrum of changes on 20 nerve biopsies in patients with HIV infection. Muscle Nerve 1989;12:452–459. [DOI] [PubMed] [Google Scholar]
- 25.Kiprov D, Pfaeffl W, Parry G, Lippert R, Lang W, Miller R. Antibody-mediated peripheral neuropathies associated with ARC and AIDS: successful treatment with plasmapheresis. J Clin Apher 1988;4:3–7. [DOI] [PubMed] [Google Scholar]
- 26.Abstracts of the joint meeting of the Italian peripheral nerve study group and the British peripheral nerve society: Trieste, Italy: April 8–10, 2010. J Peripher Nerv Syst 2010;15(suppl 1):1–40. [DOI] [PubMed] [Google Scholar]
- 27.Chimowitz MI, Audet AM, Hallet A, Kelly JJ Jr. HIV-associated CIDP. Muscle Nerve 1989;12:695–696. [DOI] [PubMed] [Google Scholar]
- 28.Ross MJ, Klotman PE. HIV-associated nephropathy. AIDS 2004;18:1089–1099. [DOI] [PubMed] [Google Scholar]
- 29.Lu TC, Ross M. HIV-associated nephropathy: a brief review. Mt Sinai J Med 2005;72:193–199. [PubMed] [Google Scholar]
- 30.Statistics SA. Statistical Release P0302: Mid-Year Population Estimates 2015 [online]. Available at: https://www.statssa.gov.za/publications/P0302/P03022015.pdf. Accessed July 1, 2016. [Google Scholar]
- 31.Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy: clinical characteristics, course, and recommendations for diagnostic criteria. Arch Neurol 1989;46:878–884. [DOI] [PubMed] [Google Scholar]
- 32.McCombe PA, Pollard JD, McLeod JG. Chronic inflammatory demyelinating polyradiculoneuropathy: a clinical and electrophysiological study of 92 cases. Brain 1987;110:1617–1630. [DOI] [PubMed] [Google Scholar]
- 33.Gisslen M, Fuchs D, Svennerholm B, Hagberg L. Cerebrospinal fluid viral load, intrathecal immunoactivation, and cerebrospinal fluid monocytic cell count in HIV-1 infection. J Acquir Immune Def Syndr 1999;21:271–276. [DOI] [PubMed] [Google Scholar]
- 34.Marshall DW, Brey RL, Cahill WT, Houk RW, Zajac RA, Boswell RN. Spectrum of cerebrospinal fluid findings in various stages of human immunodeficiency virus infection. Arch Neurol 1988;45:954–958. [DOI] [PubMed] [Google Scholar]
- 35.Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis 2010;50:773–778. [DOI] [PubMed] [Google Scholar]
- 36.Eggena MP, Barugahare B, Jones N, et al. Depletion of regulatory T cells in HIV infection is associated with immune activation. J Immunol 2005;174:4407–4414. [DOI] [PubMed] [Google Scholar]
- 37.Zandman-Goddard G, Shoenfeld Y. HIV and autoimmunity. Autoimmun Rev 2002;1:329–337. [DOI] [PubMed] [Google Scholar]


